Abstract
Background
Drug–drug interactions are a major concern in oncology and may potentially affect the outcome of patients with cancer.
Objective
In this study, we aimed to determine whether the concomitant use of statins, metformin, or proton pump inhibitors affects survival in patients with metastatic renal cell carcinoma treated with first-line combination therapies.
Methods
Medical records of patients with documented metastatic renal cell carcinoma between January 2016 and November 2021 were reviewed at 17 participating centers. This research was conducted in ten institutions, including both referral centers and local hospitals. Patients were assessed for overall survival, progression-free survival, and overall clinical benefit. Univariate and multivariate analyses were conducted to explore the association of variables of interest with overall survival and progression-free survival.
Results
A total of 304 patients receiving dual immunotherapy (51%) or immunotherapy/vascular endothelial growth factor-tyrosine kinase inhibitor (49%) combinations were eligible for inclusion in this retrospective study. Statin use was a significant prognostic factor for longer overall survival in a univariate analysis (hazard ratio 0.48, 95% confidence interval 0.26–0.87; p = 0.016) and a multivariate analysis (hazard ratio 0.48, 95% confidence interval 0.31–0.74; p < 0.001) and was significantly associated with an overall clinical benefit (83% in statin users vs 71% in non-users; p = 0.045). Otherwise, the use of metformin or proton pump inhibitors did not affect the outcome of these patients.
Conclusions
Our study suggests a prognostic impact of statin use in patients receiving first-line immuno-oncology combinations. The mechanism of this interaction warrants further elucidation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Drug–drug interactions are a major concern in oncology and may potentially affect the outcome of patients with cancer. |
In this study, we aimed to determine whether the concomitant use of statins, metformin, or proton pump inhibitors affects survival in patients with metastatic renal cell carcinoma treated with first-line combination therapies. |
Our study suggests a prognostic impact of statin use in patients receiving first-line immuno-oncology combinations. |
1 Introduction
The incidence of renal cell carcinoma (RCC) is increasing worldwide, with approximately 76,000 new cases estimated in 2021 in the USA [1, 2]. The prognosis of patients with metastatic RCC (mRCC) has been completely changed by the advent of immune checkpoint inhibitors. These agents are directed against the cytotoxic T-lymphocyte antigen-4 or programmed cell death-1 axis and are able to counteract tumor-induced T-cell immune suppression, a key mechanism for RCC growth and metastasis [3, 4]. A variety of immuno-oncology (IO) combinations have been developed for patients with mRCC, rapidly becoming the new standard of care as first-line therapy. These combinations consist of dual IO-IO agents (anti-programmed cell death-1 nivolumab plus anti-cytotoxic T-lymphocyte antigen-4 ipilimumab) or in IO plus anti-vascular endothelial growth factor tyrosine kinase inhibitors, such as pembrolizumab plus axitinib, avelumab plus axitinib, nivolumab plus cabozantinib, or pembrolizumab plus lenvatinib [5,6,7,8,9].
The majority of patients with RCC receive concomitant medications for the treatment of cancer-related symptoms or co-morbidities. Drug–drug interactions have become an area of increasing interest. This evidence can be explained by the ability of some concomitant medications to exert immune-modulatory effects, thus influencing the efficacy of immunotherapy in this context [10]. A more classic example is represented by steroids, whose immunosuppressive capability, which might dampen the activity of immunotherapies, has resulted in their more routine use to manage immune-related adverse effects [11]. Two other examples are represented by antibiotic treatment, which has demonstrated to influence the efficacy of immune checkpoint inhibitors and to modulate acute and chronic pain by altering the normal bacterial microenvironment in several retrospective and few prospective studies [12,13,14,15] and by proton pump inhibitors (PPIs), which seem to not affect the outcome of patients treated with immunotherapy [16, 17]. Based on this scenario, we aimed to first assess the impact of commonly used concomitant medications, such as statins, metformin, and PPIs, on the outcome of patients with mRCC treated with first-line combination therapies.
2 Patients and Methods
2.1 Study Population
We retrospectively analyzed data from patients aged ≥18 years with a histologically confirmed diagnosis of RCC and a histologically and/or radiologically diagnosis of metastatic disease, treated with first-line immune-oncology combinations. First-line therapies included nivolumab plus ipilimumab, pembrolizumab plus axitinib, axitinib plus avelumab, nivolumab plus cabozantinib, or pembrolizumab plus lenvatinib.
This international real-world study collected data from 17 international institutions from Italy, Spain, and the USA involved in the treatment of patients with RCC. Data collection included data from 1 January, 2016 to 30 November, 2021.We retrospectively reviewed data from paper and electronic charts. For each patient, we collected data including: tumor histology, nephrectomy status, initial Eastern Cooperative Oncology Group performance status, International Metastatic RCC Database Consortium (IMDC) criteria, sites of metastases, and concomitant use of statins, PPIs, or metformin. Patients without sufficient data on tumor assessment and response to first-line therapy were excluded from this analysis.
First-line therapy was generally performed till the evidence of radiological tumor progression on computed tomography or magnetic resonance imaging scans, unacceptable adverse events, or death. Follow-up was generally performed by periodical physical and laboratory assessment every 4–6 weeks. Imaging was carried out according to standard local procedures every 8–12 weeks. Dose reductions and treatment interruptions were managed basing on standard guidelines according to the type and severity of drug-related adverse events. Statin, metformin, and PPI users were defined as those who had been prescribed continuous oral medications over a period of ≥ 30 days and were receiving at least one of these concomitant medications at the time of first-line therapy.
2.2 Study Endpoints
The primary objective of our retrospective study was to assess the influence on the oncological outcome of concomitant statins, PPIs, or metformin in patients treated with first-line immune combinations for advanced RCC. Tumor radiological assessment was led according to the RECIST 1.1 criteria [18] and data on tumor response (complete or partial responses, stable or progressive disease) were collected and analyzed.
Overall survival (OS) was defined as the time from the start of treatment to death or lost at follow-up. Progression-free survival (PFS) was defined as the time from the start of treatment to progression or death from any cause, whichever occurred first. Patients without a tumor progression to a following line of treatment or death or lost at follow-up at the time of analysis were censored at their last follow-up date. Overall clinical benefit (OCB) was defined by the percentage of patients who experienced complete responses (CR), partial responses (PR), or stable diseases (SD).
2.3 Statistical Analysis
Progression-free survival and OS were estimated by using the Kaplan–Meier method with Rothman’s 95% confidence intervals (CIs), and comparisons were performed by the log-rank test. Cox proportional hazards models were used for univariate and multivariate analyses. Categorical endpoints were compared by the chi-square test. Significance levels were set at a 0.05 value and all p values were two sided. The statistical analysis was performed using MedCalc version 19.6.4 (MedCalc Software, Mariakerke, Belgium).
3 Results
3.1 Study Population
Three hundred and four patients were included in our retrospective study. The median age was 65 years (range 29−89 years); 224 patients (74%) were male (Table 1). Tumor histology was clear cell in 261 subjects (86%). Tumor histology among the 43 patients with non-clear RCC included 12 patients (4%) with papillary RCC, six (2%) with sarcomatoid differentiation, six (2%) with unclassified tumors, four (1%) with poorly differentiated disease, three (1%) with chromophobe RCC, and 12 (4%) patients with other non-clear cell histologies. The number of metastatic sites was two or more in 220 patients (72%). Lung (69%), lymph nodes (54%), and bone (31%) were the most common metastatic sites (Table 1). According to IMDC criteria, 51 patients (17%) had favorable-risk features, 184 (61%) had intermediate-risk features, and 69 (22%) had poor-risk features (Table 1).
First-line therapy consisted of dual immunotherapy in 155 patients (51%); 149 (49%) patients treated with the IO-TKI combination, 110 (36%) received pembrolizumab plus axitinib, 24 (8%) received axitinib plus avelumab, eight (3%) received pembrolizumab plus lenvatinib, and seven (2%) received nivolumab plus cabozantinib. Eighty-seven patients (29%) received second-line therapies, which consisted of sunitinib or cabozantinib in 90% of patients (Table 1).
Concomitant use of statins, PPIs, or metformin was reported in 31%, 50%, and 17% of patients, respectively (Table 2). Patients’ characteristics are summarized in Tables 1 and 2. The use of PPIs was significantly more frequent in patients treated with the IO plus TKI combination than the IO plus IO combination (Table 1). No significant differences were found in terms of clinicopathological features based on the concomitant use of statins, PPIs, or metformin (Table 1) or stratified by the type of first-line combination, except for the IMDC group distribution (Table 2).
3.2 Survival Analysis in the Overall Study Population
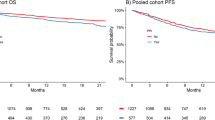
The median follow-up time from RCC diagnosis was 35.8 months (range 33.9−39.5 months). The median OS from the start of first-line therapy was 36.5 months (95% CI 28.1−36.5). In 176 patients (58%), first-line therapy was ongoing at the time of the data cut-off. Sixty-six patients (22%) had died at the time of the data cut-off.
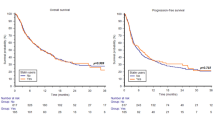
A significant difference was found in terms of sex, with male patients showing longer OS compared with female patients (36.5 months, 95% CI 29.6−50.0 vs not reached [NR], 95% CI 28.1−36.5, p = 0.019, Fig. 1). Nephrectomy was associated with longer OS (NR, 95% CI NR−NR vs 28.1 months, 95% CI 12.5−36.5, p < 0.001, Fig. 1). The median OS was 36.5 months (95% CI 36.5−36.5), 31.4 months (95% CI 27.3−31.4), and NR (95% CI NR−NR) in patients with good, intermediate, and poor IMDC risk criteria, respectively (p = 0.004, Fig. 1).
Statin users, compared with non-users, showed longer OS (NR, 95% CI NR−NR vs 28.1 months, 95% CI 27.3−31.4, p = 0.014, Fig. 1). Otherwise, concomitant use of PPIs (31.4 months, 95% CI 22.3−31.4 vs 36.5 months in non-users, 95% CI 27.3−36.5, p = 0.315, Fig. 1) or metformin (NR, 95% CI NR−NR vs 31.4 months in non-users, 95% CI 28.0−36.5, p = 0.889, Fig. 1) was not significantly associated with OS.
The median PFS from the start of first-line therapy was 16.30 months (95% CI 9.3−22.6), without reporting a significant difference between sexes (15.2 months in male patients, 95% CI 9.2−23.1, 21.5 months in female patients, 95% CI 5.9−22.6, p = 0.763). As for OS, nephrectomy (21.6 months, 95% CI 15.2−26.7 vs 8.4 months, 95% CI 6.0−11.3, p = 0.001, Fig. 2), and IMDC criteria (NR, 95% CI NR−NR in the good risk subgroup; 15.2 months, 95% CI 8.5−21.6 in patients with intermediate risk criteria; 9.2 months, 95% CI 3.9−26.7 in patients with poor risk features, p = 0.004, Fig. 2) were associated with PFS. Furthermore, two or more metastatic sites was correlated with the worst PFS (9.9 months, 95% CI 8.1−16.4, vs 23.1 months, 95% CI 17.0−23.2, p = 0.026, Fig. 2).
Similarly, concomitant statin use was correlated with longer PFS (NR, 95% CI NR−NR vs 11.3 months, 95% CI 8.3−16.3, p < 0.001, Fig. 2), in contrast to both PPIs (23.1 months, 95% CI 8.1−23.2 vs 11.5 months, 95% CI 9.0−17.0, p = 0.288, Fig. 2) or metformin (NR, 95% CI NR−NR vs 12.3 months, 95% CI 9.0−21.5, p = 0.369, Fig. 2).
In the univariate analysis, sex (HR = 0.55, 95% CI 0.33−0.91, p = 0.021), nephrectomy (HR = 0.38, 95% CI 0.23−0.63, p < 0.001), IMDC criteria (HR = 1.90, 95% CI 1.26−2.86, p = 0.002), and statin use (HR = 0.48, 95% CI 0.26−0.87, p = 0.016) were significantly associated with OS. Their prognostic role was confirmed in a multivariate analysis (Table 3). Regarding PFS, nephrectomy, two or more metastatic sites, IMDC criteria, and statin use were significant predictors in both univariate and multivariate analyses (Table 3).
3.3 Response to Therapy
In the overall study population, responses to first-line therapy consisted of eight CR (3%), 128 PR (42%), 94 SD (31%), and progressive disease (PD) as best responses in 74 patients (24%), with an OCB of 76% (Fig. 3). Overall clinical benefit was 83% (CR = 2%, PR = 51%, SD = 30%, PD = 17%) in statin users and 71% (CR = 2%, PR = 38%, SD = 31%, PD = 29%) in non-users (p = 0.045, Fig. 3). No significant differences were found between PPI users (OCB = 74%, CR = 4%, PR = 37%, SD = 33%, PD = 26%) and non-users (OCB = 76%, CR = 1%, PR = 47%, SD = 28%, PD = 24%, p = 0.639, Fig. 3). Similarly, in the 53 metformin users, OCB was 68% (CR = 4%, PR = 49%, SD = 15%, PD = 32%) compared with 77% (CR = 2%, PR = 41%, SD = 34%, PD = 23%) in non-users (p = 0.150, Fig. 3).
3.4 Role of Concomitant Statins, PPIs, or Metformin in Patients with RCC Stratified by First-Line IO-IO or IO-TKI Combinations
In patients treated with the first-line IO-IO combination, the median OS and PFS were NR (95% CI NR−NR) and 11.3 months (95% CI 7.2−16.4), respectively. Statin administration was significantly associated with PFS (21.6 months, 95% CI 7.8−21.6, vs 9.2 months, 95% CI 5.9−15.2, p = 0.038, Fig. S1 of the Electronic Supplementary Material [ESM]) but not with OS (NR, 95% CI NR−NR vs NR, 95% CI NR−NR, p = 0.710, Fig. S1 of the ESM). The concomitant use of PPIs was associated with neither OS (28.0 months, 95% CI 18.4−28.0 vs NR, 95% CI NR−NR, p = 0.118, Fig. S1 of the ESM) nor PFS (8.1 months, 95% CI 6.4−21.6 vs 11.5, 95% CI 7.1−17.0, p = 0.618, Fig. S1 of the ESM). Similarly, metformin intake did not correlate with OS (NR, 95% CI NR−NR vs NR, 95% CI NR−NR, p = 0.229, Fig. S1 of the ESM) and PFS (21.6 months, 95% CI 3.1−21.6, vs 9.9 months, 95% CI 7.5−16.4, p = 0.618, Fig. S1 of the ESM).
Patients treated with the IO-IO combination reported 5% CR, 40% PR, 26% SD, and 29% PD (OCB = 71%). No significant differences were found between patients treated or not treated with concomitant medications (statins: OCB = 75% vs 69%, p = 0.488; PPIs: OCB = 67% vs 73%, p = 0.581; metformin: OCB = 60% vs 74%, p = 0.142).
In patients treated with the IO-TKI combinations, the median OS and PFS were 31.4 (95% CI 28.1−36.5) and 23.2 months (95% CI 10.8−23.2), respectively. Statin use was associated with both improved OS (NR, 95% CI NR−NR, vs 28.1, 95% CI 27.3−31.4, p < 0.001, Fig. S2 of the ESM) and PFS (NR, 95% CI NR−NR, vs 23.1, 95% CI 8.5−23.2, p = 0.006, Fig. S2 of the ESM). Otherwise, no significant differences were observed between PPI users and non-users (median OS: NR, 95% CI NR−NR, vs 28.1, 95% CI 27.3−36.5, p = 0.703; median PFS: NR, 95% CI NR−NR, vs 10.8, 95% CI 8.5−10.8, p = 0.805; Fig. S2 of the ESM) or between metformin users and non-users (median OS: NR, 95% CI NR−NR, vs 31.4, 95% CI 27.3−36.5, p = 0.183; median PFS: NR, 95% CI NR−NR, vs 23.2, 95% CI 9.0−23.2, p = 0.387; Fig. S2 of the ESM).
In the patients treated with IO-TKI combinations, we registered one CR (1%), 65 PR (44%), 54 SD (36%), and 29 PD (19%), with on OCB of 81%. A significant difference was found between statin users and non-users (OCB = 90% vs 76%, p = 0.046), while no differences were reported with the other two concomitant medications (PPIs: OCB = 79% vs 83%, p = 0.598; metformin: OCB = 74% vs 82%, p = 0.385).
4 Discussion
Concomitant medications are known to potentially influence the outcomes of patients with cancer treated with immunotherapy or targeted therapy. The mechanisms underlying this evidence include drug–drug interactions, activity on gut microbiota, and alterations of drug pharmacokinetics [10, 19]. In this scenario, we investigated the impact of concomitant statins, PPIs, or metformin in patients with mRCC treated with first-line IO-IO or IO-TKI combinations. In our analysis, we showed that statin exposure was associated with longer OS and PFS and improved OCB, while PPIs and metformin did not affect the outcome in patients with mRCC. These results are in line with the results from the meta-analysis published by Luo et al. [20], in which statin intake was a predictor of OS in RCC, bladder cancer, and prostate cancer. Statin administration has shown antitumor activity in both in vitro and in vivo RCC models [21] and has been associated with increased OS in patients who underwent surgery for localized RCC [22,23,24]. Statin users showed better outcomes when treated with anti-vascular endothelial growth factor or mammalian target of rapamycin inhibitors for mRCC but not with interferon-α [25]. Furthermore, a pilot trial led by Manoukian et al. [26] designed to assess the efficacy and safety of combining a bisphosphonate and a statin in patients with RCC metastatic to bone observed treatment responses in 4 of the 11 patients enrolled, with the development of sclerosis in lytic bone lesions and improved deoxypyridinoline and N-telopeptide bone biomarkers. Moreover, our results are also in line with those recently published by our group on the positive impact of statin use in patients with mRCC treated with nivolumab [27].
Our study presents several limitations, mainly owing to its retrospective nature. First, we did not perform a centralized review of radiological imaging. Second, we did not have available data on both comorbidities and concomitant use of other medications that could influence the efficacy of first-line therapy. Furthermore, dose, type, and duration of concomitant statins, PPIs, or metformin as well as patients’ cholesterol levels were not available at the time of the data analysis.
Nevertheless, our data clearly show that statin administration is associated with the outcome of patients with mRCC treated with first-line immune-oncology combinations. A better comprehension of this interaction and a prospective validation of these data are needed in order to optimize the outcome of these patients and guide clinicians’ choices in daily clinical practice.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Santoni M, Piva F, Porta C, Bracarda S, Heng DY, Matrana MR, et al. Artificial neural networks as a way to predict future kidney cancer incidence in the United States. Clin Genitourin Cancer. 2021;19(2):e84-91.
Massari F, Santoni M, Ciccarese C, Santini D, Alfieri S, Martignoni G, et al. PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat Rev. 2015;41(2):114–21.
Santoni M, Buti S, Conti A, Porta C, Procopio G, Sternberg CN, et al. Prognostic significance of host immune status in patients with late relapsing renal cell carcinoma treated with targeted therapy. Target Oncol. 2015;10(4):517–22.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, CheckMate 214 Investigators, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41. https://doi.org/10.1056/NEJMoa2026982.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Eng J Med. 2021;384:1289–300.
Guven DC, Kavgaci G, Aktepe OH, Yildirim HC, Sahin TK, Aksoy S, et al. The burden of polypharmacy and drug-drug interactions in older cancer patients treated with immunotherapy. J Oncol Pharm Pract. 2022;28(4):785–93.
Pan EY, Merl MY, Lin K. The impact of corticosteroid use during anti-PD1 treatment. J Oncol Pharm Pract. 2020;26(4):814–22.
Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7.
Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–8. https://doi.org/10.1001/jamaoncol.2019.2785.
Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29:1437–44. https://doi.org/10.1093/annonc/mdy103.
Santoni M, Miccini F, Battelli N. Gutmicrobiota, immunity and pain. Immunol Lett. 2021;229:44–7.
Peng K, Chen K, Teply BA, Yee GC, Farazi PA, Lyden ER. Impact of proton pump inhibitor use on the effectiveness of immune checkpoint inhibitors in advanced cancer patients. Ann Pharmacother. 2022;56(4):377–86.
Mollica V, Santoni M, Matrana MR, Basso U, De Giorgi U, Rizzo A, et al. Concomitant proton pump inhibitors and outcome of patients treated with nivolumab alone or plus ipilimumab for advanced renal cell carcinoma. Target Oncol. 2022;17(1):61–8.
Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7.
Fogli S, Porta C, Del Re M, Crucitta S, Gianfilippo G, Danesi R, et al. Optimizing treatment of renal cell carcinoma with VEGFR-TKIs: a comparison of clinical pharmacology and drug-drug interactions of anti-angiogenic drugs. Cancer Treat Rev. 2020;84: 101966.
Luo Y, She DL, Xiong H, Fu SJ, Yang L. The prognostic effect of statin use on urologic cancers: an updated meta-analysis of 35 observational studies. Medicine. 2015;94(36): e1523.
Okubo K, Isono M, Miyai K, Asano T, Sato A. Fluvastatin potentiates anticancer activity of vorinostat in renal cancer cells. Cancer Sci. 2020;111(1):112–26.
Neumann E, Klaiber P, Freitag K, Schwab M, Schaeffeler E, Hennenlotter J, et al. Assessment of concomitant non-oncologic medication in patients with surgically treated renal cell carcinoma: impact on prognosis, cell-cycle progression and proliferation. J Cancer Res Clin Oncol. 2019;145(7):1835–43.
Haddad AQ, Jiang L, Cadeddu JA, Lotan Y, Gahan JC, Hynan LS, et al. Statin use and serum lipid levels are associated with survival outcomes after surgery for renal cell carcinoma. Urology. 2015;86(6):1146–52.
Hamilton RJ, Morilla D, Cabrera F, Leapman M, Chen LY, Bernstein M, et al. The association between statin medication and progression after surgery for localized renal cell carcinoma. J Urol. 2014;191(4):914–9.
McKay RR, Lin X, Albiges L, Fay AP, Kaymakcalan MD, Mickey SS, et al. Statins and survival outcomes in patients with metastatic renal cell carcinoma. Eur J Cancer. 2016;52:155–62.
Manoukian GE, Tannir NM, Jonasch E, Qiao W, Haygood TM, Tu SM. Pilot trial of bone-targeted therapy combining zoledronate with fluvastatin or atorvastatin for patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2011;9(2):81–8.
Santoni M, Massari F, Matrana MR, Basso U, De Giorgi U, Aurilio G, et al. Statin use improves the efficacy of nivolumab in patients with advanced renal cell carcinoma. Eur J Cancer. 2022;172:191–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
All the authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Data are available upon reasonable request.
Code availability
Not applicable.
Author contributions
Conception and design: MS, JMC, FM, CP; acquisition of data: MS, FM; analysis and interpretation of data: all authors; drafting of the manuscript: FM, MS; statistical analysis: MS; supervision: CM, NB.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santoni, M., Molina-Cerrillo, J., Myint, Z.W. et al. Concomitant Use of Statins, Metformin, or Proton Pump Inhibitors in Patients with Advanced Renal Cell Carcinoma Treated with First-Line Combination Therapies. Targ Oncol 17, 571–581 (2022). https://doi.org/10.1007/s11523-022-00907-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00907-9