Abstract
Background
Several endocrine therapies are available for postmenopausal women with hormone receptor-positive (HR +) advanced breast cancer (ABC). Given the absence of direct comparisons between fulvestrant and cyclin-dependent kinase 4/6 inhibitors (CDK4/6is) in combination with aromatase inhibitors (AIs), which are both used as standard first-line treatments for ABC, an indirect comparison using a network meta-analysis may be advantageous for decision making.
Objective
We performed a network meta-analysis to compare the efficacies of fulvestrant and CDK4/6is plus AIs as the first-line treatment of postmenopausal breast cancer patients.
Patients and Methods
In order to compare these treatments, we searched the PubMed, Cochrane Library, and EMBASE databases for randomized controlled trials of first-line endocrine treatment for advanced or metastatic breast cancer until October 2018. We included a total of 11 eligible trials with 5448 patients. The hazard ratios (HRs) for the efficacies of the different treatments were used as inputs in the network meta-analysis.
Results
In the overall analysis, CDK4/6is plus AIs, including palbociclib plus letrozole, ribociclib plus letrozole, and abemaciclib plus nonsteroidal AI (letrozole or anastrozole), are all superior to 500 mg fulvestrant (HR = 0.50, 95% confidence interval [CI] 0.37–0.68; HR = 0.50, 95% CI 0.35–0.71; and HR = 0.49, 95% CI 0.34–0.71; respectively).
Conclusions
Within the limitations of this network meta-analysis, the comparison indicates that CDK4/6is plus AIs might represent a better option for HR+ ABC as a first-line endocrine treatment compared with fulvestrant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several endocrine therapies are available for postmenopausal women with hormone receptor-positive advanced breast cancer, including fulvestrant and cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in combination with aromatase inhibitors (AIs). |
In the current network meta-analysis with progression-free survival/time to progression as the outcome measure, CDK4/6 inhibitors plus AIs are all superior to 500 mg fulvestrant. |
1 Introduction
Breast cancer is the most frequently diagnosed cancer worldwide, and it is the leading cause of cancer-related death among women [1]. For post-menopausal, hormone receptor-positive (HR+) advanced breast cancer patients, endocrine therapy with an aromatase inhibitor (AI) is recommended because of longer disease control compared with tamoxifen [2]. Recently, several combination regimens of AIs and the cyclin-dependent kinase 4/6 (CDK4/6) inhibitors palbociclib [3,4,5,6], ribociclib [7], and abemaciclib [8] have been approved by the US Food and Drug Administration (FDA) and other regulatory authorities around the world for the first-line treatment of these patients. Other endocrine options in this setting include fulvestrant and anastrozole plus fulvestrant.
Preclinical studies have demonstrated that CDK4/6 inhibitors are active in estrogen receptor-positive (ER+) breast cancer [9], and subsequent clinical trials have established the clinical use of CDK4/6 inhibitors: PALOMA-1 [3] was a randomized phase II study that evaluated the efficacy of palbociclib plus letrozole versus letrozole as the first-line treatment for ER+, HER2-negative (HER2−) breast cancer patients. Median progression-free survival (PFS) was increased to 20.2 months with the combination regimen compared with 10.2 months for letrozole alone (hazard ratio [HR] = 0.488; 95% confidence interval [CI] 0.319–0.748). The phase III PALOMA-2 trial [4,5,6] compared these two regimens in 666 postmenopausal women who had not received prior treatment for advanced disease. The median PFS was 24.8 months in the palbociclib-letrozole group compared with 14.5 months in the placebo-letrozole group (HR = 0.58; 95% CI 0.46–0.72; p < 0.001). The MONALEESA-2 trial [7] assessed the efficacy and safety of ribociclib combined with letrozole compared to placebo plus letrozole as first-line treatment in 668 postmenopausal women with HR+, HER2− advanced breast cancer, and the results showed that PFS was significantly longer in the ribociclib group than in the placebo group (HR = 0.56; 95% CI 0.43–0.72; p < 0.001). Abemaciclib, another CDK4/6 inhibitor, demonstrated a significantly prolonged PFS in combination with a nonsteroidal AI (letrozole or anastrozole) compared to a placebo with nonsteroidal AI (HR = 0.54; 95% CI 0.41–0.72; p = 0.000021) as first-line treatment of HR+, HER2− advanced breast cancer in the MONARCH-3 trial [8].
Fulvestrant is a selective ER degrader that is approved for the treatment of postmenopausal women with ER+ advanced breast cancer. The phase III CONFIRM study demonstrated an improvement in PFS and overall survival (OS) for 500 mg fulvestrant compared with 250 mg fulvestrant [10, 11]. The FIRST [12,13,14] trial is a phase II study that demonstrated the clinical benefit rate was similar for fulvestrant (500 mg) and anastrozole (1 mg) at 72.5% versus 67.0%, respectively (odds ratio = 1.30; 95% CI 0.72–2.38; p = 0.386). Median time to progression (TTP) was 23.4 months for fulvestrant versus 13.1 months for anastrozole, yielding a 34% reduction in risk of progression (HR = 0.66; 95% CI 0.47–0.92; p = 0.01). The phase III FALCON [15] study enrolled 462 patients and demonstrated that fulvestrant exhibits superior efficacy compared with anastrozole for patients with HR + metastatic breast cancer in this indication. PFS was significantly increased in the fulvestrant group (HR = 0.797, 95% CI 0.637–0.999, p = 0.0486).
These data support both the use of a CDK4/6 inhibitor plus AI and use of fulvestrant in postmenopausal women with advanced breast cancer, but a direct head-to-head comparison of these agents would be challenging. Network meta-analysis is a method that can be used to perform indirect treatment comparisons that may predict the relative efficacy of different treatment regimens [16, 17] when there are no prospective controlled study data available.
2 Methods
2.1 Literature and Search Strategy
A systematic review of published data was conducted in October 2018 to identify randomized controlled trials as input to compare CDK4/6 inhibitors plus AI with fulvestrant as the first-line treatment of hormonal therapies for advanced breast cancer in postmenopausal women. Studies that included either a CDK4/6 inhibitor or fulvestrant for advanced or metastatic breast cancer were identified from a database search of PubMed, Cochrane Library, and EMBASE. A prespecified search strategy was employed using terms applicable to the population of interest. Search terms included breast or mammary and disease descriptors (cancer, oncology, tumor, or carcinoma) as well as metastasis, advanced, and recurrent. Search terms for treatments included aromatase inhibitors, letrozole, anastrozole, tamoxifen, CDK4/6 inhibitor, palbociclib, ribociclib, abemaciclib, and fulvestrant.
2.2 Selection Criteria and Data Extraction
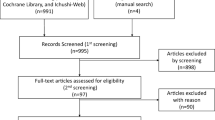
Eleven studies were selected based on population, intervention, comparison, outcome, and study design. The study selection flowchart is shown in Fig. 1. Each potential study identified was independently evaluated by two reviewers to ensure its relevance based on the predetermined criteria. We used the Cochrane Collaboration’s tool [18] for assessing the risk of bias. The quality of the trials was assessed through Review Manager, and the results are shown as Fig. 2. Trial participants included HR + (ER + and/or progesterone receptor-positive [PR +]) postmenopausal women with advanced or metastatic breast cancer who had not received previous endocrine treatment for advanced disease. The analysis assessed relative efficacy (OS/PFS/TTP) between a CDK4/6 inhibitor plus AI and fulvestrant in the full patient population of the studies that were included in the network. Data extracted from each trial included first author’s name, study design, patient population, information on the intervention (dose and treatment duration), characteristics of participants (such as median age, ER or PR status, HER2 status, prior treatment, disease sites, etc.) and outcome measures.
2.3 Statistical Analysis
The HRs for PFS/TTP/OS were collected from published reports. The mean log HR and its standard error were calculated and inputted into the model [19]. The Bayesian approach was used to perform indirect treatment comparisons between CDK4/6 inhibitor plus AI and fulvestrant. The model parameters were estimated using the Markov chain Monte Carlo (MCMC) software WinBUGS (version 1.4.3). Two chains were performed in this analysis and were run simultaneously with different initial values. The WinBUGS model ran 50,000 iterations of each chain, and the first 5000 iterations were a burn-in, which indicated that convergence was already achieved. Data were analyzed using a fixed-effect model because the deviance information criterion (DIC) of the model was lower compared with that of a random-effect model. Median HRs and the 95% CIs, which are based on the 2.5 and 97.5 percentiles from the distribution of the calculated data, are presented for the HR. The data suggest a difference between the treatments if the 95% CI does not include 1.
3 Results
Fifteen articles (11 randomized controlled trials) including a total of 5448 participants with HR + advanced breast cancer were included in the analysis. All studies were phase II or III randomized multicenter trials with postmenopausal breast cancer women. The network with the connections between the comparators is shown in Fig. 3 [3, 4, 7, 8, 10,11,12,13,14,15, 20,21,22,23,24]. Details of the individual study designs are shown in Table 1 and the patient characteristics in Table 2. Most patients were ER + or/and PR + , and some of the articles did not provide data on HER2 status. The trials also differed in regard to the percentage of prior adjuvant chemotherapy, prior adjuvant hormonal therapy, and the visceral involvement. All the studies reported PFS/TTP outcomes, and only a portion of studies reported OS. Additionally, the objective response rate is shown in Table 3.
The forest plot of the HRs for the comparators of interest relative to 500 mg fulvestrant is presented in Fig. 4. Palbociclib plus letrozole, ribociclib plus letrozole, and abemaciclib plus nonsteroidal AI are all more effective for PFS/TTP compared with 500 mg fulvestrant (HR = 0.50 95% CI 0.37–0.68; HR = 0.50, 95% CI 0.35–0.71; and HR = 0.49, 95% CI 0.34–0.71; respectively). Only letrozole exhibited a value of 1 in the HR forest plot (HR = 0.90, 95% CI 0.71–1.15). The remaining endocrine treatments, including 250 mg fulvestrant, tamoxifen and anastrozole, are significantly less effective than 500 mg fulvestrant (HR = 1.36, 95% CI 1.18–1.57; HR = 1.29, 95% CI 1.07–1.54; and HR = 1.19, 95% CI 1.02–1.40; respectively). Adverse events are summarized in Table 4 to compare fulvestrant 500 mg with CDK4/6 inhibitors plus AIs.
4 Discussion
Fulvestrant is considered a reasonable first-line option for advanced breast cancer, and so are CDK4/6 inhibitors. Therefore, the optimal choice of first-line treatment is not clear. In this article, we used a network meta-analysis to compare the efficacies of CDK4/6 inhibitors plus AIs and fulvestrant 500 mg. Our analysis includes abemaciclib, which was not included in a previous analysis [25]. However, both articles have shown that the CDK4/6 inhibitors combined with AIs show improved PFS/TTP compared to fulvestrant 500 mg as first-line endocrine therapy for advanced breast cancer. As far as OS is concerned, fulvestrant 500 mg is better than fulvestrant 250 mg and anastrozole according to the FIRST and FALCON trials. This makes fulvestrant 500 mg the standard treatment in clinical practice. In contrast, the PALOMA-1 trial showed no statistical difference for OS, and the MONARCH-3 trial of abemaciclib only provided numerical results without p values, both due to the limited follow-up of the studies to date.
Regarding the side effect profiles of these treatments, grade 3 or higher treatment-related side effects for CDK4/6 inhibitors mainly include neutropenia, leukopenia, and anemia [26]. Fulvestrant is generally well tolerated, with gastrointestinal symptoms, hot flashes, and skeletal muscle symptoms, similar to anastrozole. In addition to the efficacy and side effects, we also consider the cost of a medicine when making a treatment decision, and CDK4/6 inhibitors are currently much more expensive than fulvestrant.
In terms of the mechanisms of action, cyclin-dependent kinase inhibitors prevent unchecked cell division by blocking CDK4/6 binding to cyclin D1 and their kinase activity [27]. Fulvestrant competitively binds to the ER and downregulates ER via functional blockade and increased turnover. The PALOMA-1 study demonstrated that palbociclib has activity when combined with endocrine therapy. The PALOMA-2 study is designed to further confirm the efficacy of palbociclib as a first-line treatment. The PALOMA-3 [28] trial assigned advanced HR+, HER2− breast cancer patients who had relapsed or progressed during prior endocrine therapy to receive palbociclib and fulvestrant or placebo and fulvestrant. This study supports the idea that the cyclin D1–CDK4–CDK6 complex is a key downstream effector in HR+ breast cancer [29]. More interestingly, one study [30] showed that CDK6 is highly expressed in fulvestrant-resistant breast cancer cells and that palbociclib is effective in inhibiting the growth of ER+ breast cancer cells with high expression of CDK6 that respond poorly to fulvestrant alone. These findings provide preclinical and clinical evidence for the use of CDK6 as a predictive biomarker of response to fulvestrant treatment in ER+ metastatic breast cancer, and might help select patients who may benefit from combination targeted therapy with CDK4/6 inhibitors and fulvestrant.
The heterogeneity of the patient populations included in our analysis, especially with regard to previous adjuvant treatment, is an area of concern. The FALCON trial enrolled locally advanced or metastatic breast cancer patients who had not received previous endocrine therapy. By contrast, the CDK4/6 inhibitor trials could include patients who had received adjuvant or neoadjuvant endocrine therapy. Therefore, these conditions may weaken the efficacy of CDK4/6 inhibitors to some extent. Also, the populations included in the CDK4/6 trials had substantially higher levels of previous chemotherapy (39–48%) or hormonal therapy (33–58%) than the fulvestrant trials (13–26% and 0–25%, respectively), which also might have an impact on the results. Another problem involves endocrine resistance. Only the PALOMA-1 trial reported data from subgroup analysis of patients who relapsed within 12 months from the end of adjuvant treatment, and the HR for this group was 0.765 (95% CI 0.232–2.523). Considering that most postmenopausal breast cancer patients, including primary or secondary endocrine resistant patients, will receive AIs during adjuvant therapy, it remains unclear whether the CDK4/6 inhibitors are still beneficial for these patients.
Our study has several limitations. First, given the lack of OS results, the analysis did not calculate the HR of OS. Some trial publications stated that OS data were immature at the time of analysis because of the insufficient follow-up time. For example, the PALOMA-2 trial and the MONALEESA-2 trial did not present OS results, and the MONARCH-3 trial only provided the HR of OS without p values. The TTP/PFS can provide some evidence for clinical practice. However, without OS results, the evidence may not be sufficiently powerful to support decision making between the use of a CDK4/6 inhibitor with an AI versus treatment with fulvestrant. Second, we did not perform a subgroup analysis, because of the lack of additional data, such as lung metastasis, etc. As a result, this lack of information will influence clinical decision making. Third, PFS and TTP are two different outcome measures that we included in this study. TTP is defined as the time from randomization until objective tumor progression. TTP does not include deaths, whereas PFS does, leading to different results.
Finally, there are many ongoing clinical trials that will provide additional data in this field. For example, PALOMA-4 (NCT02297438) is a phase III study that compares palbociclib plus letrozole with placebo plus letrozole in Asian people, with an estimated primary completion date of March 2019. Another study is MONARCH plus (NCT02763566), which is a randomized, double-blind, phase III study to compare nonsteroidal AI (anastrozole or letrozole) plus abemaciclib (or plus placebo) with fulvestrant plus abemaciclib (or plus placebo) in postmenopausal women with HR+, HER2− locoregionally recurrent or metastatic breast cancer. The estimated study completion date is November 27, 2019. We are looking forward to these results because the study compares a CDK4/6 inhibitor to fulvestrant head-to-head as the first-line treatment. Moreover, we want to assess whether fulvestrant plus a CDK4/6 inhibitor is superior to AI plus fulvestrant, but no current clinical trial is addressing this question.
In conclusion, the network meta-analysis demonstrates that CDK4/6 inhibitors may represent an effective first-line treatment compared with fulvestrant (500 mg) for HR + advanced breast cancer. However, additional clinical studies are needed to provide more evidence.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Cardoso F, et al. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):9–11.
Finn RS, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35.
Finn RS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–36.
Rugo HS, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol. 2018;29(4):888–94.
Turner NC, et al. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann Oncol. 2018;29(3):669–80.
Hortobagyi GN, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–48.
Goetz MP, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–46.
Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat. 2017;166(1):41–54.
Di Leo A, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28(30):4594–600.
Di Leo A, et al. Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst. 2014;106(1):337.
Robertson JF, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27(27):4530–5.
Robertson JF, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat. 2012;136(2):503–11.
Ellis MJ, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol. 2015;33(32):3781–7.
Robertson JFR, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005.
Sutton A, et al. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. 2008;26(9):753–67.
Glenny A, et al. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9(26):1–134.
Chandler J, et al. (eds) (2016) Cochrane methods 2016. Cochrane library 78.
Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol. 2010;10:54.
Howell A, et al. Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol. 2004;22(9):1605–13.
Nabholtz JM, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol. 2000;18(22):3758–67.
Bonneterre J, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the tamoxifen or arimidex randomized group efficacy and tolerability study. J Clin Oncol. 2000;18(22):3748–57.
Mouridsen H, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19(10):2596–606.
Mouridsen H, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21(11):2101–9.
Zhang T, et al. Effect of first-line endocrine therapy in patients with hormone-sensitive advanced breast cancer: a network meta-analysis. Onco Targets Ther. 2018;11:2647–56.
Ramos-Esquivel A, et al. Cyclin-dependent kinase 4/6 inhibitors as first-line treatment for post-menopausal metastatic hormone receptor-positive breast cancer patients: a systematic review and meta-analysis of phase III randomized clinical trials. Breast Cancer. 2018;25(4):479–88.
Scott SC, Lee SS, Abraham J. Mechanisms of therapeutic CDK4/6 inhibition in breast cancer. Semin Oncol. 2017;44(6):385–94.
Turner NC, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373:209–19.
Musgrove EA, et al. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–72.
Alves CL, et al. High CDK6 protects cells from fulvestrant-mediated apoptosis and is a predictor of resistance to fulvestrant in estrogen receptor-positive metastatic breast cancer. Clin Cancer Res. 2016;22(22):5514–26.
Acknowledgements
The authors would like to thank all working group members for their contribution to this study and express their heartfelt gratitude to their mentor and research team.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of Interest
Qianqian Guo, Xiaojie Lin, Lingling Ye, Rui Xu, Yan Dai1, Yuzhu Zhang, and Qianjun Chen declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Guo, Q., Lin, X., Ye, L. et al. Comparative Efficacy of CDK4/6 Inhibitors Plus Aromatase Inhibitors Versus Fulvestrant for the First-Line Treatment of Hormone Receptor-Positive Advanced Breast Cancer: A Network Meta-Analysis. Targ Oncol 14, 139–148 (2019). https://doi.org/10.1007/s11523-019-00633-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00633-9