Abstract
Background
To compare the efficacy and toxicity of the combination of cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors and nonsteroidal aromatase inhibitors (AI) versus AI alone as first-line therapy for patients with advanced hormone receptor-positive breast cancer.
Materials and methods
Phase III randomized clinical trials (RCT) were identified after a systematic review of electronic databases. A random-effect model was used to determine the pooled hazard ratio (HR) for progression-free survival (PFS) using the inverse-variance method. The Mantel–Haenszel method was used to calculate the pooled odds ratio (OR) for overall response, clinical benefit rate and treatment-related side effects. Heterogeneity was measured using the tau-squared and I2 statistics.
Results
After a systematic search, three phase III RCT (n = 1827) were included. The use of CDK 4/6 inhibitors (abemaciclib, palbociclib, and ribociclib) in combination with an AI was significantly associated with longer PFS compared to the use of letrozole or anastrozole alone (HR: 0.57; 95% CI 0.50–0.65; p < 0.00001), with no significant heterogeneity among trials. Similarly, overall response rate and clinical benefit rate were higher for patients who received the combination therapy than for patients allocated to AI alone. Grade 3 or higher treatment-related side effects were more frequently reported for patients who received CDK 4/6 inhibitors (OR: 7.51; 95% CI 6.01–9.38; p < 0.00001), these included mainly neutropenia, leukopenia and anemia.
Conclusion
The addition of CDK 4/6 inhibitors (either abemaciclib, palbociclib, or ribociclib) to an AI (anastrozole or letrozole) significantly improved PFS, overall response rate, and clinical benefit rate in comparison with a nonsteroidal AI alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy in women and represents a leading cause of cancer-specific mortality worldwide [1]. Among post-menopausal women, Hormone Receptor (HR)-positive and Human Epidermal Growth Factor Receptor type 2 (HER2)-negative breast cancer is the most frequent subtype [2]. Similarly, almost two-thirds of patients with metastatic breast cancer have HR-positive tumors, and it is estimated that around 25% of patients with HR-positive breast cancer will eventually relapse [2,3,4].
Until recently, the backbone of treatment for post-menopausal women with a metastatic HR-positive and Her-2-negative breast cancer without visceral crisis has been based on endocrine therapy with steroidal (exemestane) or nonsteroidal (anastrozole or letrozole) aromatase inhibitors (AIs), estrogen receptor antagonists (fulvestrant), and selective estrogen receptor modulators (tamoxifen) [5]. However, intrinsic and acquired resistance to hormonal blockade is responsible for relapse and eventually death of patients. Therefore, multiple studies are exploring new strategies to overcome that resistance and to improve the outcomes of these patients. Everolimus administered in combination with exemestane is the first signal transduction inhibitor and non-cytotoxic agent approved in a second-line setting for estrogen receptor-positive, Her-2-negative post-menopausal metastatic patients that target outside the estrogen-receptor signaling pathway [6]. Lately, there has been a significant interest for therapies targeting the CDK4/6-D-type cyclin–RB pathway and clinical studies support the significant role for the cyclin-dependent kinase 4/6 (CDK 4/6) inhibitors administered in combination with endocrine therapy in the treatment of metastatic HR-positive BC [7]. CDK4/6–Cyclin D complex allows the cell cycle progression from phase G1 to S through the phosphorylation of the retinoblastoma (Rb) gene product [8]. Hence, pre-clinical data on cancer cell lines panel demonstrated that the inhibition of CDK4/6–Cyclin D complex promotes G1 arrest which leads to senescence [9, 10]. Although these agents belong to the same drug class, previous studies have acknowledged some differences in their pleiotropic effects, pharmacodynamics, and pharmacokinetics [11]. To date, there is no formal head to head comparison among these inhibitors. Thus, the aim of this systematic review and meta-analysis is to evaluate and compare the efficacy and safety of the CDK 4/6 inhibitors used in combination with an AI as first-line treatment for metastatic HR-positive, HER2-negative breast cancer patients.
Materials and methods
Search strategy and study selection
We followed the PRISMA statement for reporting systematic reviews and meta-analyses (checklist available as supplementary file) [12]. Two authors independently examined the abstracts retrieved by a search strategy in electronic databases (MEDLINE, EMBASE and The Cochrane Central Register of Controlled Trials) from October 2007 to October 2017 (Supplementary file). The research was conducted on October 3rd, 2017. Proceedings of the American Society of Clinical Oncology (ASCO) Annual Meeting, San Antonio Breast Cancer Annual Symposium, and the European Society of Medical Oncology Annual Meeting were also queried from 2012 to 2017 for relevant abstracts. In cases where a report of the same trial was obtained, the most recent results were included (corresponding to longer follow-up). Then, the authors examined full-text articles of potentially eligible studies according to the eligibility criteria. Disagreements on the inclusion of selected trials were resolved in discussions with another author.
Eligibility criteria
We decided to include only phase III randomized controlled trials (RCT) that reported the comparison of CDK 4/6 inhibitors plus hormonal treatment versus hormonal treatment alone as first-line therapy in metastatic HR-positive, HER2-negative breast cancer. We excluded trials with incomplete data.
Outcomes
The primary outcome was progression-free survival (PFS), calculated from the date of randomization to the date of progression (defined by the Response Evaluation Criteria in Solid Tumors “RECIST” 1.1 criteria [13] or death). The secondary outcomes were: (1) objective response rate (ORR): defined as the percentage of patients with complete or partial response as per RECIST 1.1 criteria (as assessed in all randomly assigned patients); and (2) clinical benefit (CBR): defined as a confirmed complete or partial response or stable disease lasting 24 weeks or more. We also evaluated the safety of each arm in all patients who received at least one dose of the study treatment. Adverse drug reactions (ADR) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Quality assessment
The risk of bias was evaluated by the authors using the Cochrane Collaboration Tool [11]. Publication bias was visually examined in a funnel plot. The risk of bias was categorized as ‘low risk’, ‘high risk’, or as ‘unclear risk’.
Data collection and statistical analysis
For the primary efficacy outcome (PFS) we reported the hazard ratio (HR) with a corresponding 95% confidence interval (95% CI). For the association of the odds of overall response and clinical benefit rate we employed the Mantel–Haenszel odds ratio and its corresponding 95% CI. We used a random-effect model for the efficacy measures according to the DerSimonian–Laird method. The pooled hazard ratios and pooled odds ratios were calculated according to the inverse-variance method, as described by Parmar et al. [14]. Heterogeneity was determined by the Tau-squared and I2 statistics. Data analysis was performed using RevMan 5.3 software.
Role of funding source
No funding source had any role in study design, data analysis, or writing of this manuscript.
Results
Study selection
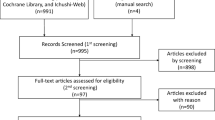
Through the search strategy, we identified three trials [15,16,17] that compared 1106 patients treated with the combination of a CDK 4/6 inhibitor (abemaciclib, palbociclib or ribociclib) plus an aromatase inhibitor versus 721 patients treated with an aromatase inhibitor alone (letrozole 2.5 mg daily or anastrozole 1 mg per day on a continuous schedule). The PRISMA flow diagram for study inclusion is shown in Fig. 1.
Description of studies and patients
Table 1 summarizes the main characteristics of each trial. Palbociclib and ribociclib were tested in combination with letrozole 2.5 mg/day in the PALOMA-2 and MONALEESA-2 trials, respectively [15, 16]. Abemaciclib was used in combination with anastrozole 1 mg/day (19.9%) or letrozole 2.5 mg/day (79.1%) (as per physician’s choice) in the MONARCH-3 trial [16]. The primary outcome was PFS in all trials (Table 2). Table 1 also resumes the main characteristics of patients for each trial.
Outcomes
Objective response
The odds of objective response were significantly higher with the combination of any CDK 4/6 inhibitor plus AI versus AI alone (Mantel–Haenszel OR: 1.75; 95% CI 1.41–2.18). We did not detect significant heterogeneity regarding this outcome (Tau2: 0.01; I2: 0%; p = 0.72)(Fig. 3).
Clinical benefit
The addition of a CDK 4/6 inhibitor plus an AI significantly increased the odds of clinical benefit (Mantel–Haenszel OR: 1.81; 95% CI 1.40–2.34). We did not detect heterogeneity regarding this specific outcome (Tau2 < 0.01; I2: 0%; p = 0.52) (Fig. 4).
Treatment-related side effects
As shown in Table 2, the number of serious adverse side effects was higher in patients allocated to the combination treatment in comparison with patients who received any AI alone. The odds of having any grade 3 or 4 treatment-related side effect were significantly higher in patients receiving the experimental combination (Mantel–Haenszel OR: 7.51; 95% CI 6.01–9.38) (Fig. 5). The most common all-grade toxicity was neutropenia ranging from 66.5% (palbociclib) to 21.1% (abemaciclib), followed by leukopenia and anemia. Nonetheless, rates of febrile neutropenia were low, ranging from 1.8% (palbociclib) to 0.3% (abemaciclib). Some toxicities were only reported for specific CDK 4/6 inhibitors. For example, abemaciclib was associated with grade 3 increased blood creatinine in 7 patients (2.1%), and ribociclib use was linked to QT prolongation of more than 60 ms in 9 patients (2.7%).
Subgroup analyses
The PFS analyses according to age, ethnicity, performance status, and disease setting (de novo metastatic versus recurrent metastatic) are presented as supplementary files. The combination treatment resulted in significant benefit for all abovementioned subgroups in comparison with the use of an AI alone. We found no heterogeneity in all the pre-specified subgroups, with the exception of race. Specifically, patients from Asia exhibited a higher benefit of the experimental treatment (HR: 0.38; 95% CI 0.26–0.54) versus patients from other part of the world (HR: 0.55; 95% CI 0.45–0.67).
Risk of bias
The risk of bias assessment is presented in a supplementary file.
All included trials were double blind with low risk of selection, performance, attrition, detection, and reporting bias. We did not detect evidence of substantial publication bias in the funnel plot analysis (Supplementary file).
Discussion
This systematic review and meta-analysis summarizes available data from published phase III randomized clinical trials regarding the PFS benefit of first-line therapy when adding CDK 4/6 inhibitors to an AI in patients with HR-positive, HER2-negative breast cancer [15,16,17].
Overall, our statistical approach showed an increase of the PFS for patients treated with the combination of abemaciclib, palbociclib or ribociclib and an aromatase inhibitor in comparison to those treated with an AI alone. This benefit was shown for all the aforementioned agents with no evidence of substantial heterogeneity among trials. Furthermore, the rates of clinical benefit and objective response were very similar among the included CDK 4/6 inhibitors. Notably, the median progression-free survival was almost 25 months, which is approximately 10 months longer than that seen with other therapies in the first-line setting, such as anastrozole [18], letrozole, [19], tamoxifen [18, 19], exemestane [20], and fulvestrant [21].
Although the overall efficacy of abemaciclib, palbociclib and ribociclib was very similar, the pattern of side effects was different among these agents. As shown in Fig. 5, rates of grade 3 or 4 adverse events were more frequently reported in patients treated with ribociclib (83.2%), followed by palbociclib (75.7%), and abemaciclib (27.5%). However, the rate of treatment modification due to adverse events, serious adverse events and deaths due to adverse events was more frequently reported in patients treated with abemaciclib compared to those treated with ribociclib and palbociclib. Interestingly, the most common grade 3 and 4 adverse events observed in patients treated with palbociclib and ribociclib were neutropenia and leukopenia, and this was not associated with a clinically meaningful risk of infections or febrile neutropenia. Indeed, previous pre-clinical models have suggested that CDK inhibitors induce bone marrow suppression through a reversible cell cycle arrest. In contrast, chemotherapeutic cytotoxic agents induce DNA damage and apoptosis [22]. This might explain why the grade 3 and 4 neutropenia mediated by CDK inhibitors were not associated with a proportional increase in the risk of febrile neutropenia. Although it is not possible to draw definite conclusions from this indirect comparison, we can make the hypothesis that this different pattern of toxicity observed for each experimental drug might be explained by a different potency to the selective inhibition of CDK 4/6 and different mechanisms of toxicity [23]. For instance, increased blood creatinine was only reported for abemaciclib due to its inhibitory effect on renal tubular secretion of creatinine [17]. This agent was also associated with higher rates of grade 3 and 4 diarrhea. Analogously, QT prolongation was only described for ribociclib.
A striking feature of the addition of CDK 4/6 inhibitors to endocrine therapy is that it provided a consistent PFS benefit across all pre-specified subgroups including age, ethnicity, prior endocrine therapy or chemotherapy, ECOG performance status, site of metastatic disease, number of metastatic site, and disease-free interval. However, there was a trend of higher PFS in Asian patients than in other ethnicities. Although this subgroup comprised only 26.6% of all patients, it has been previously documented that patients from this ethnicity have better outcomes than White patients, probably as a result of different pharmacokinetics, efficacy or tolerance to CDK inhibitors [24].
Despite the PFS benefit shown in this analysis, overall survival data are not yet available, but given the important benefits observed point towards the possibility of a promising survival advantage. Further studies should determine if comparable benefits are observed when CDK4/6 inhibitors are used in combination with anti-Her-2 agents in HER-2 positive metastatic breast cancer, and in association with chemotherapy for triple-negative tumors. In searching for a rationale at the molecular level, pre-clinical data based on cell lines suggested that the ones sensitive to CDK 4/6 inhibitors had luminal features whereas the resistant ones had basal-like features. Furthermore, sensitivity to palbociclib was associated specifically with high levels of Rb and Cyclin D [25].
Conclusions
In conclusion, the addition of CDK 4/6 inhibitors (abemaciclib, palbociclib, or ribociclib) to an AI (anastrozole or letrozole) significantly improved PFS, ORR and CBR when compared with a nonsteroidal AI used alone, with an acceptable safety profile, similarly in three major randomized phase III clinical trials. Therefore, CDK 4/6 inhibitors represent an important therapeutic advance that changes the paradigm of first-line treatment for metastatic HR-positive and HER2-negative breast cancer.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Migliaccio I, Malorni L, Hart CD, Guarducci C, Di Leo A. Endocrine therapy considerations in postmenopausal patients with hormone receptor positive, human epidermal growth factor receptor type 2 negative advanced breast cancers. BMC Med. 2015;13(1):1–6. https://doi.org/10.1186/s12916-015-0280-0.
Group EBCTC, (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Cardoso F, Costa A, Senkus E, Aapro M, Andre F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso MJ, Carey L, et al. 3rd ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol. 2017;28:16–33. https://doi.org/10.1093/annonc/mdw544.
Rugo HS, Rumble B, Macrae E, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34:3069–103.
Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
Lu J. Palbociclib: a first-in-class CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive advanced breast cancer. J Hematol Oncol. 2015;8:98.
Coudreuse D, Nurse P. Driving the cell cycle with a minimal CDK control network. Nature. 2010;468(7327):1074–9.
Xu, et al. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J Hematol Oncol. 2017;10:97. https://doi.org/10.1186/s13045-017-0467-2.
Bilgin B, Sendur MAN, Sener Dede D, et al. A current and comprehensive review of cyclin-dependent kinase inhibitors for the treatment of metastatic breast cancer. Curr Med Res Opin. 2017;33:1559–69.
Wesierska-Gadek J, Hajek SB, Sarg B, et al. Pleiotropic effects of selective CDK inhibitors on human normal and cancer cells. Biochem Pharmacol. 2008;76:1503–14.
Higgins JP, Green S. Cochrane collaboration. Cochrane handbook for systematic reviews of interventions. 1st ed. Hoboken: Wiley; 2008.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;445:228–47.
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–934.
Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–36.
Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive advanced breast cancer. N Engl J Med. 2016;375:1738–48.
Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46.
Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18:3758–67.
Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–606.
Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–90.
Robertson JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: results from the FIRST study. J Clin Oncol. 2009;27:4530–5.
Hu W, Sung T, Jessen BA, et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res. 2016;22(8):2000–8.
Costa R, Gradishar WJ. Differences are important: breast cancer therapy in different ethnic groups. J Glob Oncol. 2017;3:281–4.
Lin Gomez S, Clarke CA, Shema SJ, et al. Disparities in breast cancer survival among asian women by ethnicity and immigrant status: a population-based study. Am J Public Health. 2010;100:861–9.
Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18:17.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ramos-Esquivel, A., Hernández-Steller, H., Savard, MF. et al. Cyclin-dependent kinase 4/6 inhibitors as first-line treatment for post-menopausal metastatic hormone receptor-positive breast cancer patients: a systematic review and meta-analysis of phase III randomized clinical trials. Breast Cancer 25, 479–488 (2018). https://doi.org/10.1007/s12282-018-0848-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0848-6