Abstract
Zingiber cassumunar Roxb. rhizome extract has high potential as an antimicrobial agent, which can be applied in various food production processes and plays role in inhibiting various foodborne pathogenic microorganisms. The purpose of this research was to study the mechanism and stability of the extract’s antimicrobial activity, especially when observed from the degradation of microbial cells. Specimens were extracted by maceration method with three types of solvents with different polarities and steam distillation. The 25% extract using ethyl acetate solvent showed antimicrobial activity against Gram-negative bacteria (Enterobacter spp. and Pseudomonas spp.); Gram-positive bacteria (Listeria monocytogenes); and fungi (Rhizopus oryzae and Penicillium spp.) in well-diffusion assays. The antimicrobial activity of the extract was lower than several commercially used antibiotics such as antibacterial (penicillin G and streptomycin) and antifungal (nystatin) but exhibited broad inhibition spectrum against pathogen samples. The antimicrobial activity of the extract will increase at a low pH condition (pH 4–5) and is stable in 1–4% salt solution. Although the inhibitory activity was decreased slightly upon heating, the antimicrobial activity was stable up to 15 min of exposure to high temperatures (80○C and 100○C). Antimicrobial activity of the extract was reported to be higher in a spheroplast and protoplast condition. Leakage of metal ions such as calcium and potassium ions indicated that the activity of the extract could interfere with cell permeability so that the cells become lysed. The extract caused some damage to the bacteria and fungi cell bodies such as holes, curls, shrinkage, elongation, and swelling.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms are one of the main contributors that can affect the shelf life of a food product because they can quickly multiply either in the food product itself or caused by contamination in the process and distribution of the food product [1, 2]. If in general there are various microorganisms that spoil food products, contamination of pathogenic microorganisms becomes something very dangerous because the toxins produced are the main suspect of various diseases caused by damage to food products [3,4,5].
Herbs and spices come from various parts of plants such as leaves, roots, tubers, rhizomes, stems, seeds and flowers, which have the potential to have health benefits, provide aroma, enhance flavor, and be able to preserve food ingredients [6,7,8,9]. Zingiber cassumunar Roxb. (Fig. 1) is a species of the Zingiberaceae family that grows in South and Southeast Asia [10, 11]. Z. cassumunar is also known as plai in Thailand and bulei in China [12]. Traditionally, Z. cassumunar is used as herbal medicine which has various properties such as antiseptic, anti-infective and anti-inflammatory [13, 14]. Z. cassumunar has the potential to be an antimicrobial agent which is useful for extending the shelf life of food products [15, 16]. Han et al. [12] stated that various phytochemical compound of Z. cassumunar rhizome extract such as phenylbutenoids, sesquiterpenoids, monoterpenoids, curcuminoids, benzaldehydes, and quinones; contributed to its bioactive potency. The presence of curcumin as one of the active compounds in Z. cassumunar makes it potential to be used as a natural dye and natural antioxidant [17].
Extraction is a way to isolate, purify, and separate the desired substance from a substrate with the help of a solvent to form different phases [18, 19]. The choice of extraction method for separating active components is very important to obtain the desired optimal extract, especially related to the polarity of substances in the substrate, primarily specimens derived from plants [20]. The extraction methods which commonly used to obtain extracts of plant parts are liquid–liquid extraction and solid–liquid extraction using solvents that have different polarities such as various types of alcohol, ethyl acetate, acetone, diethyl ether, hexane, and water [21, 22].
Maceration extraction is a solid–liquid extraction with the percolation concept, in which the extraction is carried out by immersing the specimen in a continuous circulation of the solvent for a certain time [22, 23]. Maceration extraction can be optimized by setting the specimen size, temperature, degree of acidity, and time [24, 25]. Maceration extraction is suitable for extracting active components that are not heat resistant (thermolabile) because it does not require the use of relatively high temperatures which of course are adjusted to the solvent, while the weakness of this method is that it usually uses a large number of test specimens and solvents and requires a longer extraction time [23, 26].
Steam distillation is an alternative extraction method that is widely used to extract aromatic and volatile compounds derived from plants, especially those with high boiling points [27,28,29,30]. Dry steam is used as an extracting agent to extract plant specimens, so that the volatile compounds carried in the steam can be condensed into essential oils [31, 32]. Hydrodistillation is an extraction method similar to steam distillation, but instead of using dry steam as the extracting agent, it uses boiling water [32]. The weakness of steam distillation aside from the use of high temperatures which can degrade some aromatic compounds is the carrying of polar (water soluble) volatile compounds into the extracting agent so that the extraction results are not extensive, even though the condensate water which may carry the polar volatile compounds can be re-distilled [28, 33].
Extraction of essential oils from Z. cassumunar rhizomes using the hydrodistillation method has a yield of about 0.95% [15]. The active components in Z. cassumunar rhizome essential oil are dominated by sabinene (~ 40%) and terpinene-4-ol (~ 30%) [13, 34]. Sabinene and terpinene are active components that contribute to antimicrobial activity [35]. Essential oil from Z. cassumunar leaves with a concentration of 500 ppm (mg/l) can inhibit the growth of the fungus Botrytis cinerea (a pathogen in fruits) up to 100% [36]. Essential oil from Z. cassumunar rhizome can inhibit various types of Gram-positive bacteria (Propionibacterium acnes, Staphylococcus epidermidis, and Bacillus subtilis), Gram-negative bacteria (Escherichia coli, Salmonella Thypi, and Klebsiella pneumoniae), and yeast (Candida albicans and Cryptococcus neoformans) [13]. The phenylbutanoid content in Z. cassumunar rhizome essential oil can inhibit Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Pseudomonas aeruginosa) [37].
This study used several types of foodborne pathogenic microorganisms representing Gram-negative bacteria (Enterobacter spp. and Pseudomonas spp.), Gram-positive bacteria (Listeria monocytogenes), and fungi (Rhizopus oryzae and Penicillium spp.) which are commonly found in various food products. Enterobacter spp. is a Gram-negative, facultative anaerobic, motile, and rod-shaped bacterium that can grow optimally in the temperature range of 30–37○C and is known as a pathogenic bacterium [38, 39]. Enterobacter cloacae is one of the pathogenic bacteria in plants (contributing to the damage of agricultural products such as onions, papayas and cassavas) which can cause bacteraemia, respiratory tract infections and urinary tract infections [40]. Yersinia enterocolitica is also a genus of Enterobacteriaceae which is a pathogen in dairy and meat products, and can cause yersiniosis in the digestive tract with symptoms such as diarrhea and intestinal inflammation [41, 42]. Pseudomonas spp. is a Gram-negative, aerobic, motile, and rod-shaped bacterium that can grow in a wide temperature range between 4–42○C with optimal growth temperatures above 20○C [43,44,45]. Several species of Pseudomonas spp. that often become pathogens in various dairy products include Pseudomonas fluorescens and Pseudomonas aeruginosa can cause infections in humans with serious burns, low immunity, and cystic fibrosis [43, 44, 46]. Listeria monocytogenes is a Gram-positive pathogenic bacterium in the form of a rod that grows in a wide temperature range between 1–45○C with an optimal temperature between 30–37○C [47, 48]. L. monocytogenes contaminates many ready-to-eat foods such as processed meat products and unpasteurized dairy products which can cause listeriosis with symptoms of fever, nausea, muscle aches and diarrhea [48, 49].
Rhizopus oryzae is a filamentous fungi from the zygomycota phylum which has a growth temperature in the range of 30○C which contaminates many food products because it reproduces easily due to its high metabolic activity [50, 51]. Even though R. oryzae is a type of fermented fungi such as in tempe products, its massive growth causes it to become a pathogen in food products such as post-harvest rot in apples and root rot in mulberries [52,53,54]. The disease caused by R. oryzae contamination is mucormycosis, which is caused by inhalation of spores which causes tissue damage due to lack of blood supply which results in bleeding in some soft tissues such as the digestive tract and skin [55]. Penicillium spp. is a fungi that is widely found in the human environment, where spread from the soil to the air and functions as a decomposer [56, 57]. Penicillium spp. is a blue-green fungi of ascomycota with a granular surface and capable of producing an extracellular enzyme system which can potentially hydrolyze lignocellulose [58, 59]. Penicillium spp. grows in a temperature range of 4–37○C with an optimal temperature of 24○C often causing root rot in agricultural or plantation products [60]. As a pathogen, Penicillium spp. produce mycotoxin which is a toxic secondary metabolite for humans and animals such as Penicillium verrucosum and Penicillium viridicatum which produce ochratoxin [59].
Although many researches about the phytochemical bioactivity of Z. cassumunar extract had been done before, there was no study that examined its antimicrobial activity mechanism and stability on various proper environment condition based on commonly used food product criteria such as, pH, temperature, salt solution. Other than that, a comparison study with commercial antibiotics and antifungal shall show the extract’s potency as natural antimicrobial agent. Based on the description of the benefits and potency of Z. cassumunar, further research was needed to study the antimicrobial mechanism of Z. cassumunar extract against the damage to foodborne pathogenic microbial cells and the stability of the antimicrobial activity of Z. cassumunar extract against environmental conditions such as pH, salt solution, and temperature; so that the application of the extract in food products and as a natural antimicrobial agent could be optimized.
Materials and Methods
Materials
The materials used in this study consisted of main raw materials, analytical materials (including microbiological and physicochemical analysis), and microorganism specimens. The main raw material was Z. cassumunar rhizome obtained from local farmers in the Klaten district, Central Java, Indonesia. The materials used for the physicochemical analysis included various solvents namely water, hexane and ethyl acetate obtained from Bratachem. Materials for analysis included NaCl from Dolphin, where KH2PO4 solution, HCl, NaOH, Nutrient Agar media, Nutrient Broth, Potato Dextrose Agar, Potato Dextrose Broth, and Tween-80 obtained from Merck; Penicillin-G antibiotics and Streptomycin Sulphate antibiotics obtained from Meiji, and Nystatin antifungal from Novell. The microorganism specimens used were pure cultures of Gram-negative bacteria (Enterobacter spp. and Pseudomonas spp.), pure cultures of Gram-positive bacteria (L. monocytogenes), and fungi cultures (R. oryzae and Penicillium spp.) obtained from SEAFAST (South-East Asian Food and Agriculture Science and Technology) Center, Institut Pertanian Bogor, Indonesia.
Research Methods
The general research procedures included the extraction process, analysis of antimicrobial activity, and testing of advanced mechanisms such as damage to the bacterial cell wall (spheroplast and protoplast), stability of the extract against the environment, comparison of extract activity with antibiotics, leakage of metal ions, and analysis of damage to cell morphology. The research flowchart can be seen in Fig. 2.
Extraction by Maceration Method
Maceration extraction was carried out by adopting the method from Houghton & Raman [23] and Farooq et al. [61]. Fresh Z. cassumunar rhizomes were washed, reduced in size and water content using chopper and oven (Memmert) at 40○C for about 72 h. Drying was considered complete when the Z. cassumunar rhizome shrunk and could be broken easily. The results of this drying process were ground into a coarse powder with blender (Phillips) to be then extracted.
Extraction was carried out using three types of solvents representing different polarities, namely water (polar, dipole moment 9.2 D), ethyl acetate (semipolar, dipole moment 4.4 D), and hexane (non-polar, dipole moment 0.0 D) [62]. The amount of 100 g of Z. cassumunar rhizome powder specimens were mixed in 600 ml of solvent and stirred in an incubator shaker (Memmert) for 24 h at 37○C. Filtration was done by filter paper and vacuum pump (Buchi). Extracts derived from water solvents were concentrated with a water bath (Memmert) for 72 h at 50○C. Extracts derived from ethyl acetate and hexane were concentrated with a rotary evaporator (Buchi) for 1 h at 50○C. All of these extracts were used in analyzing the antimicrobial activity with the well-diffusion method.
Qualitative Phytochemical Analysis of Z. cassumunar Rhizome Maceration Extract
Phytochemical analysis was carried out using methods from Harborne [63] to qualitatively determine the active components of Z. cassumunar rhizome extract which have antimicrobial activity, primarily against pathogenic microorganisms. The active components tested included tannins, alkaloids, flavonoids, saponins, terpenoids, steroids, phenolics, and glycosides. The stages of phytochemical testing methods ar as followed:
-
a.
Tannin analysis
1 ml of extract sample was put into a test tube and then added 1% gelatin solution. The test results show positive when a white precipitate forms
-
b.
Alkaloid analysis
As much as 1 ml of the extract sample was concentrated into the spot plate and then added 3 drops of dragendorf reagent. The test results show positive when it forms orange red.
-
c.
Flavonoid analysis
1 ml of extract sample was added with a few drops of concentrated H2SO4. The test result is positive when yellow (flavones and isoflavones), orange (flavonoids), and red (chalcones) are formed.
-
d.
Saponin analysis
1 ml of the extract sample was concentrated and put into a test tube and then added 10 ml of hot water. The solution was cooled and shaken for 10 seconds and will froth which lasts for 10 minutes if saponins are present.
-
e.
Terpenoid analysis
1 mg of dried Z. cassumunar rhizome powder was dissolved in 2 ml of chloroform and added with 10 drops of anhydrous acetic acid and 3 drops of concentrated sulfuric acid. The solution was shaken slowly and left for a few minutes. The test result is positive if a red or purple color is formed.
-
f.
Steroid analysis
1 g of dried Z. cassumunar rhizome powder sample was added with 3 drops of anhydrous acetic acid and 1 drop of concentrated sulfuric acid. The test was positive if a green or blue color is formed that is stable for about 1 minute.
-
g.
Phenolic analysis
1 g of dried Z. cassumunar rhizome powder sample was dissolved in 10 ml of ethanol and filtered to obtain the filtrate. The filtrate is dripped with 5 drops of 10% NaOH. The test was positive if a red color is formed which is stable for about 1 minute.
-
h.
Glycosides analysis
1 ml of Z. cassumunar rhizome extract sample was evaporated and dissolved with 5 ml of acetic anhydrous acid and added with 10 drops of concentrated sulphuric acid. A positive result was indicated by the formation of a green or blue colour.
Extraction by Steam Distillation Method
Steam distillation method was adopted from Wan et al. [64] and Ayub et al. [65]. The treatment for Z. cassumunar rhizomes samples were the same as the maceration extraction method. After going through the drying and grinding process, 1000 g of Z. cassumunar rhizome powder was steam-distillate for 3 h to get its volatile compounds. Water was heated to ~ 100○C to produce steam which was used for distillation. Water vapour and essential oils would separate when condensed. The addition of anhydrous sodium sulphate was carried out to dehydrate essential oils.
Analysing the Antimicrobial Activity of Z. cassumunar R. Rhizome Extract Using the Well-Diffusion Method
The test was carried out to determine the optimal extract concentration which showed antimicrobial activity. Optimal inhibition is shown from the smallest extract concentration which is able to produce a diameter of inhibition that is not different from other concentrations. Well-diffusion method was carried out according to Magaldi et al. [66].
The microbial culture to be tested was refreshened up by inoculation into 10 ml of nutrient broth (for bacteria) and potato dextrose broth (for fungi) and incubated for 24 h at 37○C (for bacteria) and 30○C (for fungi). The cultures were then re-inoculated into 9 ml of nutrient broth or potato dextrose broth and incubated until they reached the exponential phase (8 h for bacteria and 24 h for fungi). Before analysing the antimicrobial activity of the extract, the cultures were incubated (8 h for bacteria and 24 h for fungi) to reach at least 106 CFU/ml microbial density. By doing this, the potency of antimicrobial activity could be analysed against similar amount and density of each microbial cultures (~ 106 CFU/ml).
Microbial suspension of 1 ml was inoculated with liquid nutrient agar (for bacteria) and liquid potato dextrose agar (for fungi) at a stable temperature and poured into petri dishes to solidify. Tween-80 (0.5%) were added to the agar media to optimized the extract’s difussion in non polaric phase (hexane solvent maceration and essential oil). After the media becomes solid, holes are made with a diameter of 6 mm. Z. cassumunar rhizome extract as much as 60 µl was added with different concentrations (control 0%, 5%, 10%, 15%, 20%, 25% (w/v). Incubation was carried out at 37○C for 24 h (for bacteria) and 48 h (for fungi). Control specimens were pure solvents without extract addition such as water, ethyl acetate, and hexane (according to each maceration extraction types). For essential oil specimen, the control was hexane (non-polar solvent), to accommodate its polarity. The inhibition diameter was measured based on the clear area formed around the well using a caliper.The test was repeated three times.
Determination of Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC)
Tests were carried out to determine the minimum concentration of the extract sufficient to inhibit microbial growth [67]. The MIC calculation is determined by natural logarithmic (Ln) of concentration of Z. cassumunar rhizome extract on the X axis; against the squared value of inhibition diameter on the Y axis. The intersection of the curve with the X axis gives the value of natural logarithmic of MBC or MFC. The concentration of MBC or MFC is the anti-Ln of the intersection value. MIC is 25% of the MBC or MFC value. The example of calculating MBC and MIC of Enterobacter spp. is showed in Fig. 3.
Stability Analysis of Selected Extract Antimicrobial Activity Against pH
The Z. cassumunar extract was tested for stability at various pH levels by dissolving it in a solution of KH2PO4 (which was made by dissolving 17 g of KH2PO4 in 250 ml of distilled water) with the degree of acidity adjusted (starting from 4, 5, 6, 7, and 8) using HCl and NaOH. The extract was dissolved at the chosen concentration in a KH2PO4 solution whose degree of acidity had been adjusted and then tested again for its antimicrobial activity using the well-diffusion method.
Stability Analysis of Selected Extract Antimicrobial Activity Against Salt Solution
The selected Z. cassumunar rhizome extract was added to a saturated salt solution with a concentration of 0 (control), 1, 2, 3, and 4% (w/v). The extract was again tested for its antimicrobial activity using the well-diffusion method.
Stability Analysis of Selected Extract Antimicrobial Activity Against Heat Treatment
Z. cassumunar rhizome extract was heated at 80○C and 100○C for 0 (control without heating) 5, 10, and 15 min. The extract was again tested for its antimicrobial activity using the well-diffusion method.
Comparison Between Selected Extract Antimicrobial Activity Against Antibiotics
The selected concentration of Z. cassumunar rhizome extract was used as a control to be compared with the microbial inhibition performance of 3 types of antibiotics namely penicillin and streptomycin (antibacterial); and nystatin (antifungal). The concentration of the antibiotics used was varied to 10, 50, and 100 ppm. Antibiotic solutions were made by diluting each different antibiotic powder used in this study into 1 L of water according to the desired concentration such as 10 mg/l for 10 ppm solution; 50 mg/l for 50 ppm solution, and 100 mg/l for 100 ppm solution. Antimicrobial activity testing was again carried out using the well-diffusion method.
Bacterial Protoplast and Spheroplast Analysis
Spheroplast analysis was performed for Gram-negative bacteria (Enterobacter spp. and Pseudomonas spp.) and protoplast for Gram-positive bacteria (L. monocytogenes). Spheroplast testing was carried out by inoculating the bacterial culture on nutrient agar which had been incubated for 24 h, and the colony were suspended in sterile 10 mM tris HCl buffer containing 1 mmol EDTA, 0.5 mol/l sucrose, and 50 µg/l lysozyme then incubated for 30 min. The process was continued by centrifugation for 15 min at room temperature at 6000 rpm and resuspended in 1 ml of 10 mM sterile tris HCl buffer without lysozyme. Controls were intact cells suspended in only 1 ml of 10 mM sterile trisHCl buffer. Antimicrobial activity analysis was again carried out using the well-diffusion method.
Protoplast analysis was carried out by inoculating the bacterial culture on NA which was incubated for 24 h, and suspended in sterile 10 mM tris HCl buffer containing 0.01 mol/l MgCl2, 0.5 mol/l sucrose, and 50 µg/l lysozyme and then incubated for 30 min. The process was continued by centrifugation for 15 min at room temperature at 6000 rpm and resuspended in 1 ml of 10 mM sterile tris HCl buffer without lysozyme. Controls were intact cells suspended only in 1 ml of 10 mM sterile tris HCl buffer. Antimicrobial activity analysis was again carried out using the well diffusion method. The entire process of making pheroplast and protoplast were carried out using the methods from Willams & Gledhill [68].
Leakage of Metal Ions in Microbial Cells
Analysis of metal ion leakage was carried out according to Giokas et al. [69], using an atomic absorption spectrophotometer (Shimadzu). Selected extracts were contacted with microbial pellets so that metal ion leakage could be identified as a sign of lysis in microbial cells. The metal ion leaks to be detected are Ca2+ and K+. The sample was destructed in the selected solvent. Solutions containing metal ions were evaporated using acetylene until they were reduced in gaseous form. The cathode lamp will emit energy at a certain wavelength (according to the desired metal ion) which is absorbed by the metal ions evaporated using acetylene. It is the amount of energy absorbed that reflects the amount of metal ion that is evaporated.
Damage Analysis of Microbial Cell Morphology
Tests were carried out using a scanning electron microscope (Hitachi) at a magnification of up to 10,000 × (on bacterial samples) and 7500 × (on fungal samples) to see damage to microbial cells. Sample preparation was carried out by preparing microbial cultures in nutrient broth (bacteria) or potato dextrose broth (fungi). Z. cassumunar rhizome extract was added according to the selected concentration and homogenized with a vortex for incubation. The microbial suspension was put into an Eppendorf tube to be separated by centrifugation at 1550 rpm for 10 min. The precipitate was fixed with a primer made of 20% glutaraldehyde in 0.2 M sodium cacodylate. The suspension was allowed to stand for 2 h at 4○C and then precipitated again by centrifugation and the remaining solution could be discarded.
Rinsing was carried out by dissolving the sample in sodium cacodylate buffer solution and incubating it for 10 min at 4○C and then purifying the suspension using a centrifuge. Rinsing was carried out 2 times. The sample was then dissolved in tannic acid (0.2 g of tannic acid in 10 ml of sodium cacodylate buffer) and left for 24 h. The samples were again rinsed with sodium cacodylate buffer 2 times.
Sample drying was carried out by dissolving the sample in alcohol with concentrations ranging from 50, 60, 70, 80, 90, and 100% (2 times repetition) with the addition of tert-butanol. The suspension is separated by centrifugation so that the solution can be removed and a little tert-butanol can be added to the bacterial precipitate to thicken it. The sample suspension is applied slowly using a loop on the cover glass, and attached to the stub. The stub was plated with gold for 5 min using ion plating and then tested using scanning electron microscope. The microscopy process was carried out using method from Bozzola & Russell [70].
Statistical Analysis
Data analysis for the influence of the variable type of solvent, extract concentration, stability of the extract on pH, temperature, and salt was carried out using a factorial completely randomized design (CRD) method (two factors for solvent and extract concentration variables and one factor for the variable of pH, temperature, and salt) and Duncan's test.
Result and Discussion
Sample Preparation
Fresh Z. cassumunar rhizome first lowered its water content using a dryer at 40○C. Reducing the water content is important to avoid enzymatic reactions that may occur in fresh rhizomes and potentially degrade bioactive components when extraction is carried out [71]. The water content of fresh Z. cassumunar was 61.89 ± 1.88%. After reducing the water content, the dried Z. cassumunar was ground into powder. Z. cassumunar powder has a moisture content of 10.48 ± 0.35%. Refinement of the sample is carried out with the aim of obtaining more extract results due to the greater surface area so that it can produce more dissolved components when extracted.
In general, the process of reducing the water content is carried out at a temperature of 60○C, so the use of low temperatures (20–40○C) in the treatment of specimens aims to reduce the risk of degradation of various phenolic components [72]. There was a 12.2% decrease in essential oil at around 60–65○C, and a 5.3% reduction in oleoresin at 60○C from the extraction process of ginger (Zingiber officinale) rhizome [73]. This certainly contributes to obtaining a lower water content than heating to a higher temperature (60○C) such as ginger rhizome with a water content of 81.3% [73].
Extraction
Maceration extraction was carried out using three solvents with different polarities (hexane, ethyl acetate, and water) to extract the bioactive components whose solubility matched the polarity of the solvents. Essential oil extraction was carried out by water-based steam distillation method.
Maceration and Qualitative Phytochemical Analysis
A ratio of 1:6 between sample and solvent was chosen so that the sample can be submerged properly and has a low viscosity so that the bioactive components can be extracted optimally both when soaking and shaking with an incubator shaker. The sample solution is filtered with the help of a vacuum pump and the filtrate is evaporated to remove the solvent to produce an extract in a more concentrated form. The extract is stored in a concentrated container and at refrigerator temperature (4○C) to maintain the stability of the bioactive components because high light intensity and increased temperature have the potential to reduce the activity of the components in the extract [74,75,76].
The results of the water extract were obtained in the form of a brownish-dark yellow solid (Fig. 4a). The results of the ethyl acetate extract were obtained in the form of a dark yellow viscous liquid (Fig. 4b). The results of the hexane extract were obtained in the form of a light yellow viscous liquid (Fig. 4c). The resulting extract yields were 13.02 ± 0.37% (water), 4.92 ± 0.28% (ethyl acetate), and 2.60 ± 0.18% (hexane). The bioactive components in Zingiberaceae rhizomes are generally polar, as in the extraction of the rhizome of Curcuma zedoaria which produced a yield of 6.85% in polar solvents compared to 1.21% in non-polar solvents [77]. The magnitude of the yield value of an extract does not necessarily reflect the magnitude of the activity of a bioactive component of the extract [78, 79].
Qualitative phytochemical analysis was carried out on the extract results with three types of solvents. The three types of solvents are capable of extracting bioactive compounds in different intensities. These compounds include phenolic acids, glycosides, flavonoids, triterpenoids, saponins, and alkaloids (Table 1). Phenolic compounds are one of the aromatic compounds that are polar and easily soluble in water which are often found bound to sugars as glycosides [80, 81]. Flavonoid compounds are derivatives of polyphenols which contain the most in plant extracts and contribute as antioxidants, anti-inflammatory, anticancer, and antimicrobial by modulating cellular enzymatic functions such as anthocyanin color pigments which are water soluble [82,83,84,85]. Triterpenoid compounds are active plant compounds that have alcohol groups and contribute to antimicrobial activity, one of which is terpinene [86,87,88]. Saponins are often found as glycosides of the triterpenoid group [89]. Alkaloids are secondary components of plants that have nitrogen groups and are widely available in the form of salts or organic acids [90, 91]. Tannins are anti-nutrient components that contribute to inhibiting the absorption of iron in the body, even so, tannins have the potential to be good antioxidants, such as (-)-epigallocatechin-3-gallate (EGCG) [92]. Tannins are common in Zingiberaceae, however, the absence of tannins in the phytochemical analysis of Z. cassumunar could be a contribution from the relatively young age of Z. cassumunar and the amount of CO2 in the environment where Z. cassumunar was planted below 400 µmol/mol [93]. Steroids are usually found as active components in Zingiberaceae extracts, but the results of the phytochemical analysis in this study did not detect the presence of steroids in Z. cassumunar extract. This also occurred in a study by Hamad et al. [94] which stated that steroids and tannins were not detected in ginger extract (Z. officinale) extracted with water at 90○C. The study by Han et al. [12] also revealed that various phytochemical compounds have the potency of becoming antimicrobial agent and many medicinal properties such as anticancer, anti-inflammatory, anti-asthma, and antioxidant.
Steam Distillation
Extraction by steam distillation was carried out using fresh Z. cassumunar rhizome. The result of steam distillation is liquid essential oil which has a transparent light yellow color with a yield of 3.15 ± 0.19% (Fig. 4d). The most active ingredients in the essential oil of the rhizome of Z. cassumunar are sabinene (~ 40%) and terpinene-4-ol (~ 30%) which actively contribute to antimicrobial activity [13, 34, 35].
Antimicrobial Activity Testing Using Well Diffusion Methods
Antimicrobial activity analysis was carried out with four different extractions where three of them used maceration methods with different solvents (water, ethyl acetate, and hexane) and steam distillation extraction (which produces essential oils). The extract concentrations used were 5, 10, 15, 20, and 25% (w/v) for the tested microorganisms. Control specimens (which are solvents without extract) proofed that inhibition occured not caused by the influence of the solvent, but by the bioactive content obtained from the extract. Initial density of microbial specimens were calculated according to its incubation time. Table 2 showed the intial density of microbial specimens which on average ~ 106 CFU/ml. The amount of microbial density is important to know the potency of antimicrobial activity against the similar amount of microorganisms. Initial density of microbial specimens were in the range of 6.32 – 6.90 log CFU/ml. Calculation of antimicrobial activity is indicated by the area of the clear zone or the diameter of the inhibition. Even though in the phytochemical tests the extracts showed positive results for various phytochemical components (Table 1), the test results showed that most of the water, hexane, and steam distillation extracts did not have an inhibition zone, or if there was, the inhibition zone was present in the fungi specimens with the higher extract concentration. This may be due to the less concentration of the extract so that its antimicrobial activity has not been detected. Essential oil itself consists of aromatic and volatile compounds which are the result of lipophilic extraction which tend to be non-polar and insoluble in water [95,96,97]. Most of the essential oil compositions consist of terpenes and terpenoids which are quite effective in acting as antifungals [36, 98, 99]. At the concentrations used, it can be seen that the antifungal activity of R. oryzae and Penicillium spp. occurs, but in bacteria the antibacterial activity has not been seen so it is likely that the concentration used is not large enough. Figures 5, 6, 7, 8, and 9 show this phenomenon.
Semipolar extract with ethyl acetate solvent showed antimicrobial activity on all microbial specimens (Gram-negative, Gram-positive, and fungi) with concentrations ranging from 5–25%. If we look at the absence of activity in the hexane and water extracts, this may be due to the ethyl acetate extract being able to extract polar and nonpolar compounds, and there is a combination of activity between these compounds so that, even at low concentrations used, antimicrobial activity has been detected. Several studies have shown antimicrobial activity using extracts with ethyl acetate solvents, including: mangrove leaf extract Avicennia officinalis as much as 250 µg/ml can inhibit the growth of Enterobacter aerogenes and P. aeruginosa with inhibition diameters of 14 and 22 mm [100]; 50 and 100 µg/ml of Ulmus wallichiana tree bark extract could inhibit the growth of P. aeruginosa with an inhibition diameter of 10 and 32 mm [101]; Moringa oleifera twig extract 100% can inhibit the growth of L. monocytogenes with an inhibition diameter of about 6.6 mm [102]; Aconitum chasmanthum rhizome extract 5 – 15% inhibited the growth of Penicillium notatum by 55.34 – 78.21% mycelial inhibition [103]; 100% Chamaecyparis obtusa trunk and twig extract can inhibit the growth of R. oryzae with an inhibition diameter of about 25.3 – 26.7 mm [104].
Figures 5 and 6 show the antimicrobial activity of ethyl acetate extract against Gram-negative bacteria Enterobacter spp. (0.78–3.53 mm) and Pseudomonas spp. (1.95–3.55 mm) with an increasing trend of inhibition as the concentration of ethyl acetate solvent extract increased. Figure 7 shows the antimicrobial activity of the ethyl acetate extract against Gram-positive bacteria L. monocytogenes (2.38–7.50 mm) with an increasing trend of inhibition as the concentration of the ethyl acetate solvent extract increases. Inhibition in Gram-negative bacteria tends to be smaller because the structure of the cell wall of Gram-negative bacteria is more complex consisting of a thin layer of lipopolysaccharide, protein, phospholipids, and peptidoglycan, resulting in penetration of the extract into the cell more difficult when compared to Gram-positive bacteria, even some Gram-negative bacteria are able to make defense enzymes in the periplasm to degrade several types of antibiotics such as penicillin [105].
Figures 8 and 9 show the antimicrobial activity of ethyl acetate extract against R. oryzae (1.18–8.18 mm) and Penicillium spp. (3.60–7.28 mm) with an increasing trend of inhibition along with increasing concentration of ethyl acetate solvent extract. Fungi are eukaryotic organisms with complex cell walls where one of the constituents of the cell membrane is a sterol component in the form of ergosterol [106]. This resulted in the inhibition of non-polar extracts with hexane and essential oils in R. oryzae (7.53–11.63 mm for hexane extraction and 2.43–3.95 mm for essential oils) and Penicillium spp. (1.45–2.20 mm for essential oils).
The antimicrobial activity analysis showed that the ethyl acetate maceration extract had broader inhibition range against different types of foodborne pathogenic microorganisms. The higher the concentration used, also showed a more consistent inhibition against all microorganism specimens. Based on the explanation and statistical calculations, the maceration extract using ethyl acetate solvent with a concentration of 25% is the selected extract to be used for further testing.
Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC)
Table 3 shows that the ethyl acetate extract of Z. cassumunar rhizome has good activity against Gram-negative bacteria (2.93 and 5.99% MBC), Gram-positive (5.41% MBC) and fungi (3.61 and 6.08% MFC). The MIC concentration of the ethyl acetate extract of Z. cassumunar rhizome starts from 0.75–1.52%. Pithayanukul et al. [13] explained that the MBC of Z. cassumunar extract using the micro-dilution method was 0.62–2.5% and the MFC was 0.31–1.25%. This is thought to be due to the fact that the extract used in the study of Pithayanukul et al. [13] may be purer. Even though the MBC and MFC value were around ~ 3–6%, the usage of 25% concentration was due to the significance level of microorganism specimens’ inhibition.
Stability Analysis of Extract Antimicrobial Activity Against pH
The pH range used in this test is 4–8 by considering the optimum pH for the growth of non-extremophilic bacteria in the range 5–9 and acid-fast bacteria in the range 3–4. pH 3 and pH 9 are considered rarely used or not suitable for food products nowadays (usually related to palatability), so it is not included in the test [107,108,109].
Figure 10 shows that in general, the higher the pH used to dilute the extract, the smaller the diameter of inhibition, and even inhibition tends not to occur at pH 7 and 8 (occurring in Pseudomonas spp., R. oryzae, and Penicillium spp.). The lower the pH used (at pH 4 and pH 5) indicates a higher inhibition diameter (5.80–8.78 mm). Various studies have shown that the antimicrobial activity of an extract to be used as a preservative will be more stable at low pH [78, 110,111,112]. This is related to the degradation caused by the ionization of the active extract components to the pH of the environment where if pH < pKa then protonation of the extract compounds occurs so that the extract compounds becomes more cationic and not easily ionized, whereas if pH > pKa then deprotonation occurs the extract compounds become more anionic so that they are easily ionized which is characterized by a decrease in the activity of antimicrobial extracts at higher pH [113, 114]. On the other hand, microorganisms themselves try to maintain their internal balance, one of which is with a pH balance, where if the environmental pH is lower than the cell's pH, then positive ions (protons) will more easily enter the cell through a natural osmosis process, so that larger energy is needed to remove the excess ions into the environment with higher concentrations which of course can have a negative impact on cell metabolism such as denaturation of cell membranes, proteins, enzymes, and damage to nucleic acids, and lead to the lack of development of cell growth [115,116,117,118].
Based on this explanation, the ethyl acetate extract of Z. cassumunar rhizome with a concentration of 25% has better stability in environmental conditions with a lower pH (pH 4 and pH 5) so that it has the potential to be applied in various suitable food products.
Stability Analysis of Extract Antimicrobial Activity Against Salt Solution
The salt concentrations used in this test were 1, 2, 3, and 4% (w/v) of a 25% concentration of ethyl acetate extract solution. The control is a salt solution without extract with a concentration according to the test variable. The choice of salt concentration is based on the consideration that as a salty taste enhancer (not as a preservative) the salt concentration used in various food products is in the range of 0.5–5% for food in general, and ~ 7% for young cheese products [119]. A solution with a salt concentration of ~ 16.5% can inhibit most foodborne pathogenic microbes because this concentration can reach a water activity value of ~ 0.90, where in general pathogenic microorganisms grow with a water activity level of ≤ 0.90 such as L. monocytogenes (0.90), Salmonella spp. (0.93), Clostridium botulinum I (0.94), Bacillus cereus (0.95), C. botulinum II (0.97), and Campylobacter spp. (0.98) [120].
Figure 11 shows that the extracts which were contacted with various salt concentrations remained stable in inhibiting the test microorganisms with almost uniform inhibition diameter ranges for different microorganisms (3.98–8.98 mm). If the salt concentration used does not function as a preservative agent, then the presence of the salt will be complementary to the antimicrobial activity derived from plant extracts [121,122,123].
Based on this explanation, the ethyl acetate extract of Z. cassumunar rhizome with a concentration of 25% has stability in a salt solution of up to 4% and has the potential to be applied in various suitable food products.
Stability Analysis of Extract Antimicrobial Activity Against Heat Treatment
Heating is one of the most widely used processes in the processing of food products. The use of temperatures of 80○C and 100○C and time variables of 5, 10 and 15 min are representative of food product processing processes with high temperatures such as pasteurization, steaming, blanching and boiling [124,125,126]. Figure 12 shows that the extract was able to show diameter inhibition both at 80○C (3.13–5.00 mm) and at 100○C (3.43–5.90 mm). The stability of the antimicrobial activity of the extract against the tested parameters of temperature and time was indicated by the absence of significant differences from statistical data testing. The active components of plant extracts, especially those that are polar such as phenolic compounds, remain stable at a heating temperature of 100○C for 30 min [123]. Research conducted by Lampe [127] also strengthens this statement that the antioxidant activity (which usually cannot withstand high temperatures) of Z. officinale extract is also stable after boiling for 30 min, so it is possible that it also has the same potential as its antimicrobial activity.
Based on this explanation, the ethyl acetate extract of Z. cassumunar rhizome with a concentration of 25% has stability in the heating process up to 100○C for 15 min and has the potential to be applied in various suitable food products.
Comparison Between Extract Antimicrobial Activity Against Antibiotics
The three types of antibiotics used in this study were antibacterial (penicillin and streptomycin) and antifungal (nystatin) which are commonly used in medical activities with variable concentrations of 10, 50 and 100 ppm [128, 129]. Figure 13 shows a comparison of the antimicrobial activity of Z. cassumunar extract with the antibiotics used. Penicillin is an antibiotic that can inhibit bacterial cell wall synthesis (not antifungal). Penicillin-G which is used in this study is a natural penicillin that has limited activity against Gram-negative bacteria, so the diameter of inhibition in testing against Enterobacter spp. and Pseudomonas spp. did not appear [130]. The cell wall structure of Gram-negative bacteria is more complex and more hydrophobic due to the presence of a lipopolysaccharide layer, which makes the diffusion of antibiotics such as penicillin G more difficult [105, 130].
Streptomycin is an aminoglycoside antibiotic that has a broad spectrum in inhibiting various types of bacteria (not antifungal) both Gram-negative (such as the Enterobactericeae families and P. aeruginosa) and Gram-positive (such as the Staphylococcaceae families and L. monocytogenes) which can inhibit protein synthesis by interfering the coding of RNA in ribosomes so that it form abnormal proteins that can disrupt cell metabolism [131,132,133,134,135]. This is shown from the results of testing for Gram-negative and Gram-positive bacteria in Fig. 13 which shows inhibition.
Nystatin is an antifungal that binds to ergosterol in the fungal cell wall so that it has a direct impact on cell permability and has the potential to cause lysis [136]. Nystatin is an antifungal with a broad spectrum of inhibition, so it is widely used for the treatment of otomycosis caused by fungi and yeasts such as Aspergillus spp., Rhizopus spp., Penicillium spp., and Candida spp. [137]. Figure 13 shows the inhibitory ability of nystatin in the fungi specimens used but not in the bacterial specimens.
The ethyl acetate extract of Z. cassumunar rhizome has a broad spectrum of inhibition against Gram-negative, Gram-positive, or fungi, although from the concentration used, the inhibition is not as big as the antibiotics used. Based on this explanation, the ethyl acetate extract of Z. cassumunar rhizome with a concentration of 25% has the potential to be used for various food product applications because it has a broad spectrum of inhibition.
Bacterial Protoplast and Spheroplast Analysis
Protoplast (derivatives of Gram-positive bacteria) and spheroplast (derivatives of Gram-negative bacteria) is a condition when a bacterial cell does not have a cell wall where in the protoplast the cell does not have an outer membrane but has a plasma membrane while in the spheroplast, the cell still has an outer membrane and a plasma membrane [105, 138, 139]. To make protoplast and spheroplast cells, lysozyme assistance is needed as a catalyst to degrade peptidoglycan so that the cell wall will dissolve, therefore to help prevent cells from breaking easily due to osmotic imbalance, buffers such as Tris–HCl and MgCl2 are needed to maintain isotonic environmental conditions [105, 139,140,141,142]. If the cell walls of Gram-positive bacteria can be directly degraded with lysozyme, for Gram-negative bacteria with more complex cell walls, chelating agents such as EDTA are needed to degrade the lipopolysaccharide structure by binding to metal cations such as Ca2+ which are abundant in the lipopolysaccharide layer [143, 144].
Analysis of the antimicrobial activity of Z. cassumunar extract aims to determine the direct impact of the extract's activity on the cytoplasm of bacterial cells which no longer have cell walls. Figure 14 shows that the inhibition is as predicted to be larger in diameter in protoplast and spheroplast bacterial cells (6.13–8.93 mm). This indicates that the active components in the extract react directly to bacterial metabolism.
Leakage of Metal Ions in Microorganism Cells
The metal ions tested are the ions that are needed predominantly in the metabolism of microorganisms such as Ca2+ and K+. Ca2+ ion is an ion that is important in the preparation of the cell wall of microorganisms which is used as a buffer to maintain the osmotic balance of cells with their environment [145, 146]. In Gram-negative bacteria with more complex cell walls, Ca2+ is one of the metal ions that is used as a constituent of the lipopolysaccharide layer [144]. In fungi, Ca2+ is one of the micronutrients needed directly to send transduction signals needed to carry out metabolism besides being a component of the cell wall [106]. K+ plays an important role in the cell transport system and contributes to the cytoplasmic homeostatic balance of microbial cells which is related to enzyme activity and ribosome performance [147,148,149,150]. In fungi (and bacteria) apart from maintaining osmosis stability, K+ is also a macronutrient needed to carry out metabolism [106].
Table 4 shows the leakage of Ca2+ and K+ ions in the microorganism specimens that were in contact with Z. cassumunar extract. Leakage of K+ ions (607.41–11,110.58 mg/l in bacteria and 221.52–237.92 mg/l in fungi) is greater than Ca2+ ions (10.05–11.39 mg/l in bacteria and 4.91–6.86 mg/l in fungi) indicating that the presence of K+ is more dominant as a macronutrient in microorganism cells. In addition, by leaking K+ which plays a role in transportation and cell homeostasis, of course, it will inhibit cell metabolism and make it easier for cells to lyse. Based on this explanation, the mechanism of antimicrobial activity of Z. cassumunar extract can be proven by the leakage of Ca2+ and K+ ions.
Microbial Cell Morphology Damage
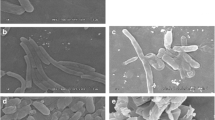
Figure 15a, b, and c show the images of how the effect of extract activity on the cell morphology of Enterobacter spp., Pseudomonas spp., and L. monocytogenes bacteria. Part ‘1’ shows the control bacterial cells that were not in contact with the extract, while part ‘2’ shows the bacterial cells that have been in contact with the extract where swelling is seen as indicated by the presence of lumps (shown by arrows) which indicates that the cell permeability is disturbed resulting in leakage and cell lysis.
Figure 15d and e show how the effect of extract activity on cell morphology of R. oryzae and Penicillium spp. Part '1' shows the control fungal cells that were not in contact with the extract, while part '2' shows the fungi cells that have been contacted with the extract where shrinkage occurs in the fungal mycelium (shown by arrows) indicating plasmolysis in the fungal cells. Based on this explanation, the mechanism of antimicrobial activity of the ethyl acetate extract of Z. cassumunar rhizome at a concentration of 25% focuses on destroying the cell wall which disrupts cell permeability.
Conclusion
Maceration extraction using ethyl acetate solvent was the most effective extraction method to extract the active components from Z. cassumunar rhizome. This was shown by its antimicrobial activity which has a broad spectrum against pathogens that inhibit Gram-negative bacteria (Enterobacter spp. and Pseudomonas spp.), Gram-positive bacteria (L. monocytogenes), and fungi (R. oryzae and Penicillium spp.). Statistical calculations showed that the concentration of 25% is the selected extract for further testing. Extracts with water and hexane solvents did not show inhibition on all types of bacteria and Penicillium spp., but there was inhibition on R. oryzae. Z. cassumunar essential oil showed inhibition on all types of fungi.
The MIC value in the microbial specimens used was 0.75–1.52%, the MBC value in bacterial cells was 2.93–5.99%, and the MFC in fungi cells was 3.61–6.08%. The antimicrobial activity of Z. cassumunar extract is influenced by the difference in pH where it is more stable at lower pH (around pH 4 and pH 5). The antimicrobial activity of Z. cassumunar extract is stable in different salt concentrations used and stable at heating temperatures up to 100○C. The antimicrobial activity of 25% Z. cassumunar extract has a broad spectrum of inhibition but is not as strong as the activity of the compared antibiotics (penicillin G, streptomycin, and nystatin) at concentrations of 10, 50, and 100 ppm.
Tests for antimicrobial activity were carried out on protoplast and spheroplast bacterial cells which matched the predictions of having a greater inhibitory effect than their vegetative cells. This was also clarified by the results of observing the morphology of the microorganism cells that were in contact with the extract, where swelling and rupture of the microorganism cells occurred. This indicates that the mechanism of the extract's antimicrobial activity is related to the destruction of the cell walls resulting in leakage of metal ions (Ca2+ and K+) which interfere with cell permeability.
The results of this study are one of the efforts to continue to find potential natural antimicrobials that have suitable stability for the application of various food products. Further research may be carried out to find a more specific formula for applicable action in the future. Various antimicrobial mechanisms related to cell metabolism can also be studied, such as protein and nucleic acid leakage, enzyme degradation, and inhibition of biosynthesis in microbial cells which of course can deepen, complement, and update the results of this research.
Data Availability
No datasets were generated or analysed during the current study.
References
S. Galié, C. García-Gutiérrez, E.M. Miguélez, C.J. Villar, F. Lombó, Front. Microbiol. 9, 898 (2018)
M. Pigłowski, Int. J. Environ. Res. Public Health 16, 477 (2019)
L. Pirofski, A. Casadevall, BMC Biol. 10, 6 (2012)
A. Chlebicz, K. Śliżewska, Int. J. Environ. Res. Public Health 15, 863 (2018)
J.M. Lorenzo, P.E. Munekata, R. Dominguez, M. Pateiro, J.A. Saraiva, D. Franco, in Innovative technologies for food preservation: Inactivation of spoilage and pathogenic microorganisms, ed. by F.J. Barba, A.S. Sant’Ana, V. Orlien, M. Koubaa (Academic Press, London, 2018), pp. 53–107
E.I. Opara, M. Chohan, Int. J. Mol. Sci. 15, 19183–19202 (2014)
M.E. Embuscado, J. Funct. Foods. 18, 811–819 (2015)
S.M. El-Sayed, A.M. Youssef, Heliyon. 5, e01989 (2019)
R. Vázquez-Fresno, A.R.R. Rosana, T. Sajed, T. Onookome-Okome, N.A. Wishart, D.S. Wishart, Genes Nutr. 14, 18 (2019)
A.K. Bordoloi, J. Sperkova, P.T. Leclercq, J. Essent. Oil Res. 11, 441–445 (1999)
S. Nakamura, J. Iwami, Y. Pongpiriyadacha, S. Nakashima, H. Matsuda, M. Yoshikawa, Nat. Prod. Commun. 17, 2 (2022)
A.R. Han, H. Kim, D. Piao, C.H. Jung, E.K. Seo, Molecules 26, 2377 (2021)
P. Pithayanukul, J. Tubprasert, M. Wuthi-Udomlert, Phytother. Res. 21, 164–169 (2007)
S. Koontongkaew, O. Poachanukoon, S. Sireeratawong, T. Dechatiwongse Na Ayudhya, P. Khonsung, K. Jaijoy, R. Soawakontha, M. Chanchai, Int. Sch. Res. Notices. 2014, 632608 (2014)
M.N.I. Bhuiyan, J.U. Chowdhury, J. Begum, Bangladesh. J. Pharmacol. 3, 69–73 (2008)
M. Sharifi-Rad, E.M. Varoni, B. Salehi, J. Sharifi-Rad, K.R. Matthews, S.A. Ayatollahi, F. Kobarfard, S.A. Ibrahim, D. Mnayer, Z.A. Zakaria, M. Sharifi-Rad, Z. Yousaf, M. Iriti, A. Basile, D. Rigano, Molecules 22, 2145 (2017)
M.X. Li, X. Bai, Y.P. Ma, H.X. Zhang, N. Nama, S.J. Pei, Z.Z. Du, Ind. Crops Prod. 141, 111764 (2019)
N. Ćujić, K. Šavikin, T. Janković, D. Pljevljakušić, G. Zdunić, S. Ibrić, Food Chem. 194, 135–142 (2016)
Q.W. Zhang, L.G. Lin, W.C. Ye, Chin. Med. 13, 20 (2018)
A.P. Cacique, E.S. Barbosa, G.P. de Pinho, F.O. Silvério, Cienc. Agrotecnologia. 44, e017420 (2020)
C.D. Stalikas, J. Sep. Sci. 30, 3268–3295 (2007)
U.J. Vajić, J. Grujić-Milanović, J. Živković, K. Šavikin, D. Godevac, Z. Miloradović, B. Bugarski, N. Mihailović-Stanojević, Ind. Crops Prod. 74, 912–917 (2015)
P.J. Houghton, A. Raman, in Laboratory handbook for the fractionation of natural extracts. ed. by P.J. Houghton, A. Raman (Springer, Boston, 1998), pp.22–53
O. Ghomari, F. Sounni, Y. Massaoudi, J. Ghanam, L.B. Drissi Kaitouni, M. Merzouki, M. Benlemlih, Biotechnol. Rep. 23, e00347 (2019)
N.E.H. Lezoul, M. Belkadi, F. Habibi, F. Guillén, Molecules 25, 4672 (2020)
O.R. Alara, N.H. Abdurahman, C.I. Ukaegbu, N.H. Azhari, Ind. Crops Prod. 122, 533–544 (2018)
F.J. Heredia, M.L. González-Miret, A.J. Meléndez-Martínez, I.M. Vicario, in Instrumental assessment of food sensory quality. ed. by D. Kilcast (Woodhead Publishing, Cambridge, 2013), pp.565–610
J.M. Prado, R. Vardanega, I.C.N. Debien, M.A.deA. Meireles, L.N. Gerschenson, H.B. Sowbhagya, S. Chemat, in Food waste recovery: processing technologies and industrial techniques. ed. by C.M. Galanakis (Academic Press, London, 2015), pp.127–148
M. Séquin, in Encyclopedia of Applied Plant Sciences, ed. by B. Thomas, B.G. Murray, D.J. Murphy (Academic Press, London, 2016), pp. 393–398
S.A. El-Toumy, A.A. Hussein, in Cold pressed oils: green technology, bioactive compounds, functionality, and applications. ed. by M.F. Ramadan (Academic Press, London, 2020), pp.711–718
S. Irmak, O. Erbatur, in Environmentally Compatible Food Packaging, ed. by E. Chiellini (CRC Press, Boca Raton, 2008), pp. 263–293
P. Pushpangadan, V. George, in Handbook of herbs and spices, 2nd edn., ed. by K.V. Peter (Woodhead Publishing, Oxford, 2012), pp.55–72
A. Ammann, D.C. Hinz, R.S. Addleman, C.M. Wai, B.W. Wenclawiak, Fresenius. J. Anal. Chem. 364, 650–653 (1999)
R. Mektrirat, T. Yano, S. Okonogi, W. Katip, S. Pikulkaew, Molecules 25, 613 (2020)
A. Lahmar, A. Bedoui, I. Mokdad-Bzeouich, Z. Dhaouifi, Z. Kalboussi, I. Cheraif, K. Ghedira, L. Chekir-Ghedira, Microb. Pathog. 106, 50–59 (2017)
P. Tripathi, N.K. Dubey, A.K. Shukla, World. J. Microbiol. Biotechnol. 24, 39–46 (2008)
T. Taechowisan, S. Suttichokthanakorn, W.S. Phutdhawong, J. Appl. Pharm. Sci. 8, 121–127 (2018)
L. Rogers, K. Power, P.O. Gaora, S. Fanning, in Encyclopedia of food and health. ed. by B. Caballero, P.M. Finglas, F. Toldrá (Academic Press, Oxford, 2015), pp.545–551
A. Davin-Regli, J.P. Lavigne, J.M. Pagès, Clin. Microbiol. Rev. 32, 4 (2019)
T. García-González, H.K. Sáenz-Hidalgo, H.V. Silva-Rojas, C. Morales-Nieto, T. Vancheva, R. Koebnik, G.D. Ávila-Quezada, Plant Pathol. J. 34, 1–10 (2018)
V. Gupta, P. Gulati, N. Bhagat, M.S. Dhar, J.S. Virdi, Eur. J. Clin. Microbiol. Infect. Dis. 34, 641–650 (2015)
V. Ntuli, P.M.K. Njage, E.M. Buys, J. Dairy Sci. 99, 9534–9549 (2016)
S. Arslan, A. Eyi, F. Özdemir, J. Dairy Sci. 94, 5851–5856 (2011)
L. Meng, Y. Zhang, H. Liu, S. Zhao, J. Wang, N. Zheng, Front. Microbiol. 8, 2158 (2017)
L. Ruiz-Roldán, B. Rojo-Bezares, M. de Toro, M. López, P. Toledano, C. Lozano, G. Chichón, L. Alvarez-Erviti, C. Torres, Y. Sáenz, Sci. Rep. 10, 11667 (2020)
M.F. Moradali, S. Ghods, B.H.A. Rehm, Front. Cell. Infect. Microbiol. 7, 39 (2017)
L. Dortet, L. Radoshevich, E. Veiga, P. Cossart, in Encyclopedia of microbiology. ed. by T.M. Schmidt (Elsevier, Amsterdam, 2019), pp.803–818
T. Cherifi, J. Arsenault, F. Pagotto, S. Quessy, J. Cote, K. Neira, S. Fournaise, S. Bekal, P. Fravalo, PLoS ONE 15, e0236807 (2020)
H. Zhang, J. Wang, Z. Chang, X. Liu, W. Chen, Y. Yu, X. Wang, Q. Dong, Y. Ye, X. Zhang, Front. Microbiol. 12, 729114 (2021)
B.J. Meussen, L.H. De Graaff, J.P.M. Sanders, R.A. Weusthuis, Appl. Microbiol. Biotechnol. 94, 875–886 (2012)
T. Denardi-Souza, K.C. Massarolo, S.M. Tralamazza, E. Badiale-Furlong, CYTA. J. Food. 16, 156–164 (2017)
J.H. Kwon, J. Kim, Mycobiology. 39, 140–142 (2011)
Y. Yang, T. Kameda, H. Aoki, D.E. Nirmagustina, A. Iwamoto, N. Kato, N. Yanaka, Y. Okazaki, T. Kumrungsee, J. Funct. Foods. 49, 162–167 (2018)
B.N. Gnanesh, A. Tejaswi, G.S. Arunakumar, M. Supriya, H.B. Manojkumar, P. Tewary, J. Appl. Microbiol. 131, 360–374 (2021)
D.H. Walker, M.R. McGinnis, in Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms, ed. by L.M. McManus, R.N. Mitchell (Academic Press, Amsterdam, 2014), pp. 217–221
J.I. Pitt, in Food spoilage microorganisms, ed. by C. dW. Blackburn (Woodhead Publishing, Cambridge, 2006), pp. 437–450
C.M. Visagie, J. Houbraken, J.C. Frisvad, S.B. Hong, C.H.W. Klaassen, G. Perrone, K.A. Seifert, J. Varga, T. Yaguchi, R.A. Samson, Stud. Mycol. 78, 343–371 (2014)
D. Nigam, M. Asthana, A. Kumar, in New and Future Developments in Microbial Biotechnology and Bioengineering: Penicillium System Properties and Applications, ed. by V.K. Gupta, S. Rodriguez-Couto (Elsevier, Amsterdam, 2017), pp. 187–200
G.P. Munkvold, S. Arias, I. Taschl, C. Gruber-Dorninger, in Corn: Chemistry and Technology, ed. by S.O. Serna-Saldivar (Woodhead Publishing, Cambridge, 2018), pp. 235–287
A.N. Yadav, P. Verma, V. Kumar, P. Sangwan, S. Mishra, N. Panjiar, V.K. Gupta, A.K. Saxena, in New and Future Developments in Microbial Biotechnology and Bioengineering: Penicillium System Properties and Applications, ed. by V.K. Gupta, S. Rodriguez-Couto (Elsevier, Amsterdam, 2017), pp. 3–18
S. Farooq, S.A. Mir, M.A. Shah, A. Manickavasagan, in Plant Extracts: Applications in the Food Industry, ed. by S.A. Mir, A. Manickavasagan, M.A. Shah (Academic Press, London, 2022), pp. 23–37
H. Nawaz, M.A. Shad, N. Rehman, H. Andaleeb, N. Ullah, Braz. J. Pharm. Sci. 56, e17129 (2020)
J.B. Harborne, Phytochemical methods: a guide to modern techniques of plant analysis, 3rd edn. (Chapman and Hall Ltd., London, 1998)
N. Wan, J. Lan, Z. Wu, X. Chen, Q. Zheng, X. Gong, Separations. 9, 137 (2022)
M.A. Ayub, G. Goksen, A. Fatima, M. Zubair, M.A. Abid, M. Starowicz, Separations. 10, 27 (2023)
S. Magaldi, S. Mata-Essayag, C. Hartung De Capriles, C. Perez, M.T. Colella, C. Olaizola, Y. Ontiveros, Int. J. Infect. Dis. 8, 39–45 (2004)
S.F. Bloomfield, in Mechanisms of action of chemical biocides: Their study and Expolitation, ed. by S.P. Denyer, W.B. Hugo (WB Blackwell Scientific Publications, London, 1991), pp. 1–22.
P. Williams, L. Gledhill, in Mechanism of Action of Chemical Biocides: Their Study and Exploitation, ed. by S.P. Denyer, W.B. Hugo (WB Blackwell Scientific Publications, London, 1991), pp. 87–108.
D.L. Giokas, G.Z. Tsogas, A.G. Vlessidis, M.I. Karayannis, Anal. Chem. 76, 1302–1309 (2004)
J.J. Bozzola, L.D. Russell, Electron microscopy: principles and techniques for biologists, 2nd edn. (Jones & Bartlett Publishers, Boston, 1998)
S. Al Jitan, S.A. Alkhoori, L.F. Yousef LF, in Studies in Natural Products Chemistry, ed. by F.R.S. Atta-ur-Rahman FRS (Elsevier, Amsterdam, 2018), pp 389–417
P. Garcia-Salas, A. Morales-Soto, A. Segura-Carretero, A. Fernández-Gutiérrez, Molecules 15, 8813–8826 (2010)
E. Jayashree, R. Visvanathan, T. John Zachariah, J. Food. Sci. Technol. 51, 3190–3198 (2014)
K.M. Sim, H.J. Lee, C.W. Nho, G.N. Bae, J.H. Jung, Aerosol. Sci. Technol. 48, 324–332 (2014)
I.Y.P. Chua, P.J.H. King, K.H. Ong, S.R. Sarbini, P.H. Yiu, J. Soil Sci. Plant Nutr. 15, 605–614 (2015)
T.M. Alba, E. Tessaro, A.M. Sobottka, Braz. J. Biol. 84, e254174 (2022)
Selvy, Study of the Antibacterial Activity of Curcuma zedoaria Extract against Food Pathogenic Bacterial Cell Damage (Bachelor’s thesis, Universitas Pelita Harapan, 2008)
F.D. Gonelimali, J. Lin, W. Miao, J. Xuan, F. Charles, M. Chen, S.R. Hatab, Front. Microbiol. 9, 1639 (2018)
F.S. Shafodino, J.M. Lusilao, L.M. Mwapagha, PLoS ONE 17, e0272457 (2022)
O.R. Alara, N.H. Abdurahman, C.I. Ukaegbu, Curr. Res. Food. Sci. 4, 200–214 (2021)
Y. Zhang, X. Guo, Z. Peng, M.A. Jamali, Trends. Food Sci. Technol. 119, 215–226 (2022)
J. Valls, S. Millán, M.P. Martí, E. Borràs, L. Arola, J. Chromatogr. A 1216, 7143–7172 (2009)
J. Dai, R.J. Mumper, Molecules 15, 7313–7352 (2010)
A.N. Panche, A.D. Diwan, S.R. Chandra, J. Nutr. Sci. 5, e47 (2016)
R. Mattioli, A. Francioso, L. Mosca, P. Silva, Molecules 25, 3809 (2020)
R.T. Nzogong, F.S.T. Ndjateu, S.E. Ekom, J.A.M. Fosso, M.D. Awouafack, M. Tene, P. Tane, H. Morita, M.I. Choudhary, J.deD. Tamokou, BMC. Complement. Altern. Med. 18, 159 (2018)
P.K. Mukherjee, in Quality Control and Evaluation of Herbal Drugs, ed. by P.K. Mukherjee (Elsevier, Amsterdam, 2019), pp. 237–328
D. Hu, H. Gao, X.S. Yao, in Comprehensive Natural Products III, ed. by H.W. Liu, T.P. Begley (Elsevier, Amsterdam, 2020), pp. 577–612
Y.J. Zhao, C. Li, J. Agric. Food Chem. 66, 12155–12165 (2018)
T. Richard, H. Temsamani, E. Cantos-Villar, J.P. Monti, in Advances in Botanical Research, ed. by D. Rolin (Academic Press, London, 2013), pp. 67–98
P. Dey, A. Kundu, A. Kumar, M. Gupta, B.M. Lee, T. Bhakta, S. Dash, H.S. Kim, in Recent Advances in Natural Products Analysis, ed. by A.S. Silva, S.F. Nabavi, M. Saeedi, S.M. Nabavi (Elsevier, Amsterdam, 2020), pp. 505–567
W. Petroski, D.M. Minich, Nutrients 12, 2929 (2020)
A. Ghasemzadeh, H.Z.E. Jaafar, E. Karimi, S. Ashkani, Molecules 19, 16693 (2014)
A. Hamad, W. Anggraeni, D. Hartanti, Jurnal. Aplikasi. Teknologi. Pangan. 6, 177–183 (2017)
S. Bhattacharya, in Essential Oils in Food Preservation, Flavor and Safety, ed. by V.R. Preedy (Academic Press, Amsterdam, 2016), pp. 19–29
W. Dhifi, S. Bellili, S. Jazi, N. Bahloul, W. Mnif, Medicines. 3, 25 (2016)
J.L. Ríos, in Essential Oils in Food Preservation, Flavor and Safety, ed. by V.R. Preedy (Academic Press, Amsterdam, 2016), pp. 3–10
S.K. Kyasa, J. Chem. Educ. 97, 1966–1969 (2020)
A. Masyita, R. Mustika Sari, A. Dwi Astuti, B. Yasir, N. Rahma Rumata, T. Bin. Emran, F. Nainu, J. Simal-Gandara, Food. Chem. X. 13, 100217 (2022)
P. Lalitha, A. Parthiban, V. Sachithanandam, R. Purvaja, R. Ramesh, S. Afr, J. Bot. 142, 149–155 (2021)
K.S. Bora, A. Kumar, G. Bisht, J. Ayurveda. Integr. Med. 9, 190–194 (2018)
T.N. Minh, B.Q. Minh, T.H.M. Duc, P. Van Thinh, L.V. Anh, N.T. Dat, L. Van Nhan, N.Q. Trung, Processes 10, 563 (2022)
S. Rafiq, N.A. Wagay, H.O. Elansary, M.A. Malik, I.A. Bhat, Z.A. Kaloo, A. Hadi, A. Alataway, A.Z. Dewidar, A.M. El-Sabrout, K. Yessoufou, E.A. Mahmoud, Antioxidants. 11, 1052 (2022)
T. Morikawa, T. Ashitani, N. Sekine, N. Kusumoto, K. Takahashi, J. Wood Sci. 58, 544–549 (2012)
L. Samarayanake, Essential Microbiology for Dentistry.4th ed. (Churchill Livingstone Elsevier, Edinburgh , 2012)
.M. Walker, N.A. White, in Fungi: Biology and Applications, ed. by K Kavanagh (John Wiley and Sons, West Sussex, 2011), pp. 1–35
H.G. Yuk, D.L. Marshall, Appl. Environ. Microbiol. 70, 3500–3505 (2004)
E. Padan, E. Bibi, M. Ito, T.A. Krulwich, Biochim. Biophys. Acta 1717, 67–88 (2005)
Q. Jin, M.F. Kirk, Front. Environ. Sci. 6, 21 (2018)
J.H. Doughari, D. Sunday, Pharm. Biol. 46, 400–405 (2008)
A. Lopez-Malo, S.M. Alzamora, M.J. Paris, L. Lastra-Vargas, M.B. Coronel, P.L. Gómez, E. Palou, in Antimicrobials in Food, ed. by P.M. Davidson, T.M. Taylor, J.R.D. David (CRC Press, Boca Raton, 2020), pp. 527–595
A. Gavriil, E. Zilelidou, A.E. Papadopoulos, D. Siderakou, K.M. Kasiotis, S.A. Haroutounian, C. Gardeli, I. Giannenas, P.N. Skandamis, Sci. Rep. 11, 21971 (2021)
S. Natesan, V. Krishnaswami, S.T. Veedu, D.P. Mohanan, K. Ruckmani, R. Palanichamy, in Functional Chitosan: Drug Delivery and Biomedical Applications, ed. by S. Jana, S. Jana (Springer, Singapore, 2019), pp. 107–134
C. Ardean, C.M. Davidescu, N.S. Nemeş, A. Negrea, M. Ciopec, N. Duteanu, P. Negrea, D. Duda-Seiman, V. Musta, Int. J. Mol. Sci. 22, 7449 (2021)
S.S. Stadmiller, A.H. Gorensek-Benitez, A.J. Guseman, G.J. Pielak, J. Mol. Biol. 429, 1155–1161 (2017)
N. Guan, L. Liu, Appl. Microbiol. Biotechnol. 104, 51–65 (2020)
J. Rivera-Araya, A. Pollender, D. Huynh, M. Schlömann, R. Chávez, G. Levicán, Front. Microbiol. 10, 2455 (2019)
Z.A. Sajid, F. Aftab, Int. J. Agron. 2022, 5158768 (2022)
J. Rysová, Z. Šmídová, Foods. 10, 2237 (2021)
D. Christopher, C.A. Wallace, Perspect. Public. Health 134, 216–224 (2014)
A.L.M. Vigil, E. Palou, S.M. Alzamora, in Antimicrobials in Food, ed. by P.M. Davidson, T.M. Taylor, J.R.D. David (CRC Press, Boca Raton, 2005), pp. 429–451
S. Chusri, S.P. Voravuthikunchai, Lett. Appl. Microbiol. 52, 565–572 (2011)
E.S.H. Atwaa, M.R. Shahein, H.A. Radwan, N.S. Mohammed, M.A. Aloraini, N.K.A. Albezrah, M.A. Alharbi, H.H. Sayed, M.A. Daoud, E.K. Elmahallawy, Fermentation. 8, 428 (2022)
M. Aamir, M. Ovissipour, S.S. Sablani, B. Rasco, Int. J. Food Sci. 2013, 271271 (2013)
H.W. Xiao, Z. Pan, L.Z. Deng, H.M. El-Mashad, X.H. Yang, A.S. Mujumdar, Z.J. Gao, Q. Zhang, Inf. Process. Agric. 4, 101–127 (2017)
N.O. Mwebi, B.M.O. Ogendi, Cogent. Food. Agric. 6, 1834661 (2020)
J.W. Lampe, Am. J. Clin. Nutr. 78, 579S-583S (2003)
A. Agunos, D. Léger, C. Carson, Can. Vet. J. 53, 1289–1300 (2012)
R. Shukla, P. Singh, B. Prakash, N.K. Dubey, Food Control 25, 27–33 (2012)
G.M.S. Soares, L.C. Figueiredo, M. Faveri, S.C. Cortelli, P.M. Duarte, M. Feres, J. Appl. Oral Sci. 20, 295–309 (2012)
H.A. Kirst, N.E. Allen, in Comprehensive Medicinal Chemistry II, ed. by J.J. Plattner (Elsevier Science, Amsterdam, 2007), pp. 629–652
L. Maryam, A.U. Khan, Front. Microbiol. 7, 2007 (2016)
E. Germovsek, C.I. Barker, M. Sharland, Arch. Dis. Child. Educ. Pract. Ed. 102, 89–93 (2017)
J.F. Vianna, K.S. Bezerra, I.N.J. Oliveira, E.L. Albuquerque, U.L. Fulco, Phys. Chem. Chem. Phys. 21, 19192–19200 (2019)
M.M. Elsayed, R.M. Elkenany, A.I. Zakaria, B.M. Badawy, Environ. Sci. Pollut. Res. 29, 54359–54377 (2022)
R.L. Wattier, W.J. Steinbach, in Principles and Practice of Pediatric Infectious Diseases, ed. by S.S. Long, C.G. Prober, M. Fischer (Elsevier, Philadelphia, 2018), pp. 1532-1541
K. Ali, M.A. Hamed, H. Hassan, A. Esmail, A. Sheneef, Int. Arch. Otorhinolaryngol. 22, 400–403 (2018)
M.J. Santesmases, J. Hist. Biol. 49, 3–36 (2016)
H. Nishida, Int. J. Mol. Sci. 21, 7131 (2020)
M.T. Madigan, J.M. Martinko, K.S. Bender, D.H. Buckley, D.A. Stahl, Brock Biology of Microorganisms, 14th ed. (Pearson Education, Glenview, 2015)
D.M. Figueroa, H.M. Wade, K.P. Montales, D.E. Elmore, L.E.O. Darling, J. Vis. Exp. 138, e57904 (2018)
S. Takahashi, M. Mizuma, S. Kami, H. Nishida, Sci. Rep. 10, 8832 (2020)
R.J.W. Lambert, G.W. Hanlon, S.P. Denyer, J. Appl. Microbiol. 96, 244–253 (2004)
L.A. Clifton, M.W.A. Skoda, A.P. Le Brun, F. Ciesielski, I. Kuzmenko, S.A. Holt, J.H. Lakey, Langmuir 31, 404–412 (2015)
Y. Xie, L. Yang, Sci. Rep. 6, 20628 (2016)
S. Shaikh, N. Nazam, S.M.D. Rizvi, K. Ahmad, M.H. Baig, E.J. Lee, I. Choi, Int. J. Mol. Sci. 20, 2468 (2019)
W. Epstein, in Progress in nucleic acid research and molecular biology, vol. 75 (Elsevier, Amsterdam, 2003), pp.293–320
S. Ueda, Y. Kawamura, H. Iijima, M. Nakajima, T. Shirai, M. Okamoto, A. Kondo, M.Y. Hirai, T. Osanai, Sci. Rep. 6, 32354 (2016)
N. Korolev, BioEssays 43, 2000108 (2021)
J. Stautz, Y. Hellmich, M.F. Fuss, J.M. Silberberg, J.R. Devlin, R.B. Stockbridge, I. Hänelt, J. Mol. Biol. 433, 166968 (2021)
Acknowledgements
This study was conducted by the support dan research facility of Microbiology Laboratory and Chemistry Laboratory of Universitas Pelita Harapan; Indonesian Medical and Aromatic Crop Research Institute (IMACRI) of Ministry of Agriculture; Tropical Biopharmaca Research Center of Intitut Pertanian Bogor; and Research Center for Biology, Badan Riset dan Inovasi Nasional. All researchers would also like to thank all research assistants and laboratory instrument operators who have supported the success of this research.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
A.H. conceived and conducted the experiments, prepare research draft, figure and tables. A.J.N.P. conceived, designed, and supervised the research. A.L.J. supervised the research and carried out research draft. I.B. carried out research draft and did statistical analysis. B.N. assisted in laboratory works, processing raw data, proof read the research draft.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hendriko, A., Parhusip, A.J.N., Juwono, A.L. et al. Mechanism and Stability of Antimicrobial Activity of Zingiber cassumunar Roxb. Rhizome Extract Against Foodborne Pathogenic Microorganisms. Food Biophysics (2024). https://doi.org/10.1007/s11483-024-09841-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11483-024-09841-x