Abstract
The influence of repeated freeze–thaw (F-T) treatments (0, 1, 3 and 5) on the functional and structural properties of myofibrillar protein (MP) from mirror carp (Cyprinus carpio L.) was investigated according to the changes in rheological properties, particle distribution, Zeta (ζ)-potential, molecular force, Fourier transform infrared (FTIR) spectra and sulfhydryl content. After F-T treatment with five cycles, a significant decrease in the storage modulus values (G'), loss modulus values (G"), ζ- potential, total sulfhydryl content and reactive sulfhydryl content of MP was observed (P < 0.05). The particle size increased within the third F-T cycles, but it decreased in the fifth F-T treatments (P < 0.05). The hydrogen bonds, hydrophobic interactions and disulfide bonds of protein were broken during multiple F-T treatments. In addition, as the F-T cycles increased, the α-helix and β-turn contents decreased (P < 0.05), and the β-sheet and random coil content increased (P < 0.05). Therefore, repeated F-T treatments damaged the structure of MP and reduced the functional properties of MP from mirror carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mirror carp (Cyprinus carpio L.) is a popular freshwater fish that is typically characterized by fewer scales, tender meat, fast growth, high protein content and unsaturated fatty acids. Due to its abundant nutrients, the fish is easily susceptible to spoilage after the capture [1, 2]. Frozen storage is an effective way to preserve the quality and extend the shelf-life of fish and fish products [3, 4]. Freezing, frozen storage and thawing could result in a loss of product quality associated with protein damage [3, 5], especially in terms of flavour, texture and colour, due to ice crystal formation, mechanical damage, as well as the aggregation and degeneration of protein [6].
During storage, transportation and retail, repeated F-T treatments usually occur due to the imperfect cold chain technology and temperature fluctuation [7], which may lead to some harmful physical and physicochemical changes in fish products [8,9,10]. Moreover, during repeated F-T treatments, the location and recrystallization of ice crystals destroy muscle cells, resulting in mechanical damage and mass loss of muscle tissue due to the muscle dehydration [11, 12]. These are not conducive to the functional and structural properties of fish. Du et al. [13] reported that huge and uneven ice crystals were formed during repeated F-T treatments, which damaged cells and fibrous tissue, leading to the destruction of muscle fiber structure. F-T treatments were found to affect the biochemical and physicochemical properties of catfish fillets, resulting in the destabilization of their muscle structure [8]. Zhang et al. [9] demonstrated that F-T treatment with different cycles caused protein denaturation, leading to the secondary and tertiary structure of the MP changing, which damaged the microstructure of muscle tissue.
The effect of F-T treatment with different cycles on meat quality has been analysed by many scholars [7, 14, 15]. Actually, the change in the quality of meat is mainly induced by the change in protein function [11], which is affected by the change in protein structure [10, 16]. Many processing methods, such as high pressures (> 100 MPa) [17] and high-intensity ultrasound [18], can cause protein unfolding, which affects the functional properties of the protein (mean particle size, zeta potential, dynamic rheological property, etc.). There have been some studies on the effects of freezing and frozen storage on the structure or function of surimi [19], but little research has examined the influence of F-T treatments on the functional and structural properties of MP from fish. Therefore, the influence of repeated F-T treatments on the functional and structural properties of MP from mirror carp was investigated.
Materials and Methods
Sample preparation
Six fresh mirror carps (1250 ± 100 g) were purchased from a local market (Harbin, Heilongjiang, China) and quickly killed by the trained shop staff. The scales, heads, bones, internal organs and skins were removed, and mirror carps were transported to the laboratory with an ice bag within 1 h. We selected the back muscle of the fish, and the samples were sliced into 2 × 2 × 2 cm3 fillets. The samples were frozen in a refrigerator at -25 °C for 7 days and then thawed in a 4 °C refrigerator for 12 h. The first F-T treatment was completed when the core temperature was 0–4 °C. Each treatment was applied to randomly selected samples (5 from 20) for analysis. The third and fifth F-T treatments were frozen and thawed according to the above condition.
MP extraction
Myofibrillar protein (MP) was extracted from mirror carp according to the procedure reported by Guo et al. [20] with slight modifications. The samples were centrifuged at 6,500 g at 4 °C for 15 min. The protein concentration was measured by the standard bovine serum albumin method.
Rheological properties
Dynamic thermo-mechanical analysis (DTMA) of MP samples was performed with a DHR-3 rheometer (Model Bohlin VOR Bohlin Instruments, Inc., Cranbury, NJ, USA) as described by Li et al. [21]. Samples were loaded in the 1 mm gap between the two parallel plates (diameter of upper plate = 4 cm). Gels were formed by heating the protein solutions from 30 to 85 °C at a rate of 1 °C/min. During the heating process, the samples were continually sheared in an oscillatory mode at a fixed frequency of 1 Hz with a maximum strain of 0.02. The strain level was used to ensure a linear viscoelastic range (stress versus strain) that allowed adequate measurement sensitivity while maintaining the gel integrity, as established in preliminary trials. The dynamic rheological properties were described in terms of storage modulus (G′) and loss modulus (G″).
Particle distribution
MP was dissolved in sodium phosphate buffer solution (50 mmol/L, pH 6.5, 0.1 mol/L NaCl) (1:5, w:v). The buffer solution was prepared with 1 mol/L HCl, NaOH particles, NaCl powder and PIPES powder (the concentration was close to 100%). Finally, a buffer solution containing 0.1 mol/L NaCl and 50 mmol/L PIPES was obtained. Particle size of MP was measured by an integrated-laser light scattering instrument (Mastersizer 2000, Malvern Instruments Co. Ltd., Worcestershire, UK). Relative refractive index and absorption were set as 0.538 and 0.622, respectively. d4,3 is the mean diameter in volume whereas d3,2 is the mean diameter in surface called “Sauter diameter”. The mean diameter in surface (d3,2) was calculated by the following equation:
The mean diameter in volume (d4,3) was calculated by the following equation:
where ni is the number of droplets of diameter di.
dv,0.5 is the size for which 50% of the sample particles have a lower size and 50% have a upper size. dv,0.1 is the size for which 10% of the sample particles have a lower size and dv,0.9 is the particle size for which 90% of the sample particles have a lower size. These indexes of protein were analysed using Malvern Mastersizer software (version 5.12c, Malvern Instruments Co. Ltd., Worcestershire, UK).
Zeta-potential
The Zeta-potentials (ζ-potential) of different F-T cycles were evaluated at room temperature by a Nano ZS series Particle Analyzer (Malvern Instruments Co. Ltd., Worcestershire, UK) as described by Long et al. [22]. Samples were diluted with deionized water until the protein content was 0.2 mg/mL. One milliliter of each diluted sample was put in a visibly clear disposable zeta cell (Model DTS 1060C, Malvern Instruments Co. Ltd., Worcestershire, UK) without any air bubbles. The equilibrium time was 1 min.
Molecular force
The major molecular forces of MP gel were measured as described by Jiang and Xiong [23] with slight modification. Different dissolving solutions were used; 50 mM sodium phosphate (pH 7.0) was added to 8 M urea, 0.5% (w/v) Sodium dodecyl sulfate (SDS) and 0.25% (v/v) β-mercaptoethanol (β-ME), respectively. The solutions were homogenized (13,500 rpm, 20 s) and heated at 80 °C for 1 h, cooled down, then centrifuged (10,000 g, 15 min) to measure protein solubility.
MP Secondary structure
The method for determining the MP secondary structure was referred to Sow and Yang [24] with a slight modification. The dissociation of myofibrils into subunits of MP was studied in water by recording the IR absorption data between 4000 and 500 cm−1 using a Nicolet 6700 spectrophotometer (Thermo Fisher Scientific, Madison, WI, USA) at room temperature (approximately 22 °C). Before determination, the sample suspensions were freeze-dried, mixed with potassium bromide in a certain proportion, and pelleted.
For peak height and location of peak, amide A, amide I, amide II and amide III were selected for quantitative measurement of maximum peak height and location (wavenumber) using Spectrum software (version 5.0.1, PerkinElmer). Baseline of each band was defined by software, and the corrected peak height was taken as the absorbance difference from the peak to the baseline.
Deconvolution of amide I was also studied for further quantitative analysis. The spectra region between 1700 and 1600 cm−1 was selected as amide I band, of which each unprocessed spectra were cut and baseline corrected. Fourier self deconvolution was performed using the Spectrum software (version 5.0.1, PerkinElmer) with line narrowing factor, gamma, set at 1.0 and the smoothing length width set at 50%-60% by using Bessel type smoothing function. The deconvoluted amide I band was then fitted using Origin Pro 9 (OriginLab, Northampton, MA, USA). The final fitting quality of the curve had corrected R2 value greater than 0.99.
Sulfhydryl content
Total sulfhydryl (SH) content
Total sulfhydryl (SH) contents were determined by the method of Ellman [25] and Xia et al. [10]. To 4.5 mL aliquots of the supernatant was added 0.5 mL of Ellman’s reagent (10 mM DTNB). The absorbance at 412 nm was recorded on a TU-1800 Spectrophotometer (Puxi Instrument Company, Beijing, China) and the SH concentration was calculated using a molar extinction coefficient of 13,600 M−l cm−l. The protein content of the soluble protein fractions was determined by the Biuret methods.
Reactive sulfhydryl (SH) content
The reactive SH contents were determined according to Xia et al. [10]. The reactive SH value was calculated as follows:
A412 and A532 represented the absorbance of the solution at 412 nm and 532 nm, respectively.
Statistical analysis
The results were evaluated by three independent experiments, and the relevant traits of each batch of samples were determined in triplicate. They were shown as the mean values ± standard deviations by the Statistix 8.1 software package. Variance (ANOVA) was used to analysis the data, and Duncan was used to compare the significance of the date (P < 0.05). The graphs were made with SigmaPlot 10.5.
Result and discussion
Rheological property
The rheological characteristics of the MP suspensions during the gelation process reflect the quality of proteins. In particular, storage modulus (G', the elastic response of the gelling material) is a measure of the deformation energy stored in the sample during the shear process, which represents the elastic behavior of a sample [26]. The influence of repeated F-T treatments on the storage modulus (G') and loss modulus (G") of MP is shown in Fig.1
The G' of all samples started to rise at the beginning of heating and produced a peak at 46 °C. From 46 °C to 54 °C, the G' decreased rapidly, then increased again until 80 °C. This rheological pattern is a typical characteristic of muscle protein and was in agreement with that reported in the literature [27] for MP, reflecting the transitions of heavy meromyosin and light meromyosin. It indicated that the temporary network structure of gels formed in the intermediate temperature range by the association of heavy meromyosin (myosin head) was disrupted later when light meromyosin (myosin tail) underwent unfolding, resulting in the rheological transition in the 45–60 °C temperature range. The first development stage of G' was caused by the unfolding of heavy meromyosin and denaturation of the light meromyosin [28]. The protein molecule unfolding led to the second development stage of G' arises. At the same temperature, the G' progressively declined with the F-T treatments increased, indicating that the decrease in elasticity was owing to the increased F-T treatments. The lower G' value was related to protein degradation and weaker gel forming ability, and the decrease in transition temperatures indicated the formation of protein aggregation and protein instability [29].
As shown in Fig. 1B, the changes in rheologic properties were also represented by the change in loss modulus (G") with temperature, which describes the viscosity of the system [30]. The G" showed the same trend as the G'. The G" reached its maximum value at 46 °C. Then, the G" dropped significantly when the temperature rose from 46 to 54 °C and then tended to flatten up to 80 °C. During the heating of gel formation, the G' was consistently higher than the G", indicating that elasticity had an advantage over viscosity. A possible interpretation for the deleterious effect of F-T processes on dynamic rheological properties of MP is that the F-T treatments caused structural changes, protein denaturation, and excessive protein aggregation and insolubilization [31].
Particle distribution
The particle size is a vital parameter for the physical and storage stability of the protein. The change in particle size of MP induced by the repeated F-T treatments is shown in Table 1. The particle size distribution of MP in F-T treatment with different cycles exhibited a monomodal distribution. d4,3 is the mean diameter in volume whereas d3,2 is the mean diameter in surface called “Sauter diameter”. the d4,3 and d3,2 of the sample were increased by 170.8% and 296.0% compared with the fresh sample after five F-T treatments. The increase in particle size may be due to the penetration of water molecules into the protein where they form ice crystals during the freezing–thawing process, resulting in producing stronger mechanical swelling to destroy the structure of the protein, causing the particles to swell [32]. The changes in the mean particle diameters were primarily caused by myofibrillar protein unfolding and an increase in protein surface area. Additionally, the disulphide bridges, hydrogen bonds, and hydrophobic interactions between proteins were also strengthened, which promoted the aggregation of MP and increased the average particle size [33]. In addition, the peak span decreased with the increase of F-T treatments, which indicated that the large molecular weight protein was formed in the protein solution.
As shown in Table 1, dV,0.1, dV,0.5 and dV,0.9 demonstrate that the cumulative distributions of particle size were 10%, 50% and 90%, respectively. The median diameter (dV,0.5) of fresh sample protein was 24.41 μm, while the protein particle size increased with the F-T treatments increased. After five F-T treatments, the median diameter of protein was the largest (125.08 μm). The variation trends of protein particle size of dV,0.1 and dV,0.9 were similar to dV,0.5, which significantly increased (P < 0.05). Xia et al. [10] showed that F-T treatments could accelerate protein oxidation and change the MP structure. The increase in particle size might be due to the formation of ice crystals, which reduces the availability of liquid water, increases the ionic strength of cells, and accelerates protein denaturation and aggregation [34].
ζ-potential
The stability of the colloidal dispersion system and the surface charge nature of the particles in the solution can be characterized by the zeta potential of the MP. The absolute value of ζ-potential is an important index to measure the degree of electrostatic interaction [35]. The change in ζ-potential of MP induced by the F-T treatment with different cycles is shown in Table 1. The absolute ζ-potential value of protein declined significantly from 26.87 mV (fresh sample) to 16.50 mV after five F-T cycles (P < 0.05). The decrease of the absolute value of the potential indicated that the repulsion between the molecules of the protein weakened and the aggregation between proteins increased, thereby increasing the particle size of the MP. Besides, it may be that the F-T treatments destroyed the cell membrane, leading to the outflow of exudates. The exudates usually contain amino acids and some soluble enzymes. In addition, the exudates also contain ions to enhance conductivity. Increased ionic concentration may lead to the compression of the electrical double layer and a corresponding reduction in zeta potential. A higher voltage to which the exudates were subjected led to more ionic substances effused from the cell membrane so that the impedance modulus decreased with an increased amount of conducting ions [36]. The low absolute value of the ζ-potential will reduce the repulsion between particles in the system, and hydrophobic interactions or van der Waals forces will promote the aggregation and flocculation of proteins, thereby damaging the stable state of the solution [37]. Therefore, the changes in conduction properties resulting from different F-T treatments could be related to impedance property.
Molecular force
Molecular forces such as covalent and non-covalent bonds are used to immobilize three-dimensional network of MP gel. There were hydrogen bonds, hydrophobic interactions and disulfide bonds during gel formation [38]. Therefore, by adding certain chemical solvents to the protein gel, the constituent protein that promotes gel formation could dissolve, thereby explaining the change in the balance forces in the gels [39]. The change in solubility of MP gel in the different solvents during F-T treatment with different cycles is described in Fig.2A. The solubility of MP added with urea decreased significantly with increased F-T treatments (P < 0.05). This may be because the reformation of ice crystals during repeated F-T treatments destroyed the intermolecular hydrogen bonds, which were one of the important forces to maintain the stability of the MP gel. Urea could break hydrogen bonds between molecules, which caused MP to aggregate to form larger protein particles. This was consistent with the result of Diao et al. [39], who noted that gels dissolved in 8 M urea due to the destruction of intermolecular hydrogen bonds.
With the addition of SDS reagents into the MP, the solubility was increased with increased F-T treatments (P < 0.05), which indicated that hydrophobic interactions also played an important role in MP gels. The results showed that with the increase of F-T treatments, the hydrophobic groups become more exposed, which contributes to the increase in hydrophobicity, and the unfolded protein molecules aggregate to form a gel during the gel formation process.
In addition to the non-covalent bonds mentioned above (hydrogen bonds and hydrophobic interactions), MP gel formation also relies on covalent bonds (disulfide bonds). There was no significant change in the solubility of protein samples after the first and third F-T treatments. And the solubility of the protein added with β-ME increased significantly after five F-T treatments (P < 0.05), which showed that multiple F-T treatments could promote the formation of disulfide bonds. This is because with the increase of the number of F-T cycles, the degree of MP oxidation is deepened and the generation of disulfide bonds is increased. In conclusion, covalent bonds might also play a major role in the formation of MP gel.
MP Secondary structure
FTIR can be used to analyse the change in the secondary structure of protein through baseline correction, deconvolution, second-order derivative and fitting analysis of absorbance curves [40]. As shown in Fig.3A, the MP contained amides A, B, I, II and III at the wavelengths of 3300 cm−1, 3100 cm−1, 1654 cm−1, 1537 cm−1 and 1307 cm−1, which are related to hydrogen bonds, C = O stretching vibration, N–H stretching vibration and bending, C-N stretching and N–H deformation from amide linkages, respectively [41]. The major amide bands (amide A, B, I, II, III) were present in every spectra with some variations in wavenumber and peak height (Fig. S1). During repeated F-T treatments, there was no significant difference in the wavenumber of amide A, B, I, II, III of all samples. In addition, the peak height of amide A, B, I, II, III could be reduced by F-T processes. The reduction in the intensity of amide A, B, I, II, III bands were associated with the greater loss of molecular order [24]. Mejri et al. [42] indicated that the amide I band (1600–1700 cm−1) in FTIR spectra, which was related to secondary structure, mainly caused by C = O stretching vibration, centred at 1650 cm−1 and consisted of multiple overlapping component bands due to many protein fragments with different structures. Meanwhile, the wavelengths of amide I and II of MP decreased slightly compared to fresh samples with the increase of the number of F-T treatments. This result demonstrated that repeated F-T treatments would destroy the secondary structure of the protein.
The secondary structure of the protein is composed of the α-helix, β-sheet, β-turn and aperiodic structures [43]. The different secondary structures and their band positions correspond to: α-helix (from 1645 to 1662 cm−1), β-sheet (from 1692 to 1770 cm−1 and 1615 to 1638 cm−1), β-turn (from 1662 to 1692 cm−1) and random coil (from 1638 to 1645 cm−1) [44]. After five F-T treatments, noticeable reductions in the absorption of α-helix and β-turn components and increases in the absorption of β-sheets and random coils were observed (Fig. 3B). The contents of various secondary structures are shown in Table 2. For the MP of mirror carp after five F-T treatments, the amount of α-helix and β-turn decreased significantly by 9.08% and 4.93%, and the amount of β-sheet and random coil increased by 6.04% and 7.97%, respectively. The decrease in helical structure likely occurred at the expense of random coil and β-turn as the proportion of these two components increased after five F-T treatments [24]. These changes are consistent with the reorganization of MP from an ordered rigid structure to a disordered flexible structure, β-turns are commonly associated with a highly ordered protein structure, whereas the β-sheet and random coil structures have been described as typical of flexible and open structures. Furthermore, the increased β-sheet region is related to the increase in protein–protein interactions between exposed hydrophobic regions, promoting the formation of intermolecular β-sheet structures [45]. Our ζ-potential results also indicated that a lower ζ-potential could reduce the repulsion between particles in the system, thereby disrupting the steady state of the solution. Zhao et al. [46] illustrated that the change in protein secondary structure might be due to hydrogen bonds being broken by physical effects included the water redistribution, ice recrystallization and protein damage during repeated F-T treatments. In addition, the F-T treatments can accelerate protein oxidation, and the secondary structure of the protein is destroyed with the increase of the degree of protein oxidation. However, after five F-T treatments, no relevant proteolytic and composition changes were found, indicating that the protein stability of white shrimp (Litopenaeus vannamei) myofibrillar protein was higher than that of seafood [47].
Sulfhydryl content
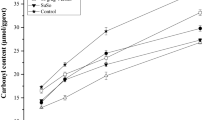
SH group is a kind of secondary bond, which maintains the tertiary structure of the protein [48]. The change in total SH content and reactive SH content of MP during F-T treatment with different cycles is shown in Fig. 2B. The total SH content and reactive SH content of the sample significantly decreased from F-T treatment with 0 cycle (64.50 μmol/g and 6.33 μmol/g) to F-T treatment with 5 cycles (42.60 μmol/g and 1.97 μmol/g) (P < 0.05). The alteration in MP during frozen conditions causes the loss of functional properties such as SH groups. The decreased SH contents could be due to aggregation or denaturation of MP as a consequence of the oxidation of cysteine thiol group's and interchange reactions in disulfide bonds [10]. It has been previously reported that cysteine is more prone to oxidation due to its presence at the catalytic center of the proteins [49]. Xia et al. [10] speculated that the decrease in SH content might be owing to the formation of disulfide bonds either within polypeptides or between polypeptides. In addition, the decreased SH content also might be explained by some polymerization reactions, resulting in the formation of disulfide bonds [46]. Meanwhile, the spatial structure of the protein was also changed, and the change in the SH content was also related to the decrease in the α-helix content [50]. Zhang et al. [51] reported that the change in the SH content could reflect the degree of the change in the tertiary and quaternary structure of the protein.
Schematic mechanism of protein structure changes during repeated freeze–thaw treatments
The schematic model is shown in Fig.4. MP is a kind of multi-component protein, and myosin plays a major role in the process of gelation. The gel formation process of myosin can be regarded as the process of denaturation, aggregation and formation of the gel network structure of natural proteins. During this process, myosin unwinding and aggregation occur simultaneously [52]. The original myosin structure is mainly composed of myosin heavy chain (MHC) and myosin light chain (MLC). The helices and sheets structure mainly reside in MHC [30]. The rod-like tail portion of myosin is a coiled-coil structure of α-helix (maintained by hydrogen bonding) with many hydrophobic residues [53]. At the same time, many sulfhydryl groups are also present in the MHC and MLC of myosin [30, 54].
For fresh myosin from mirror carp, the most percentage of the secondary structure was α-helix. With the increase of the number of F-T treatments, the percentage of α-helix decreased, and the percentage of β-turn and random increased. The decline of α-helix structure was attributed mainly to structural changes (unfolding) in the tail of myosin during the frozen process. The changes of the secondary structure of myosin suggested that the stabilities of the myosin structure reduced greatly after freezing. Hydrophobic residues embedded in myosin molecules were exposed [55]. In addition, the transition of liquid water to solid ice during the F-T process leads to the increase of ionic strength and the decrease of pH, which eventually leads to the dissociation of MLC and the exposure of SH [30]. During the process of gel formation, the enhancement of hydrophobic interactions and covalent cross-linking (the conversion of SH to disulfide bonds) lead to protein aggregation, which ultimately leads to a decrease in the functional properties of MP during repeated F-T treatments.
Conclusions
F-T treatment with several cycles causes the changes in protein structure that affect the functional properties of protein from mirror carp. F-T treatment with several cycles had a detrimental effect on dynamic rheological properties and led to the weakening of the intermolecular forces of the protein gels. In addition, protein aggregation, which was accelerated by increased F-T treatment, led to a conspicuous decrease in particle size and an increase in zeta potential. F-T treatment accelerated a decrease in the content of α-helix, β-turn, the total SH and reactive SH, as well as an increase in the content of β-sheet and random coil, resulting in changes in the protein structure. Therefore, it is necessary to prevent temperature fluctuations and minimize the number of repeated F-T treatments during the transportation and storage of fish.
Influence of repeated freeze-thaw treatments on the solubility (A) and sulfhydryl content (B) of mirror carp myofibrillar protein gel in different solvents. F0, F1, F3, and F5 represent samples subjected to 0, 1, 3, 5 freeze–thaw cycles, respectively. The means for the same solvent with different lowercase letters (A-D/a-d) differ significantly (P < 0.05)
References
Q.X. Sun, F.D. Sun, X.F. Xia, H.H. Xu, B.H. Kong, The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason Sonochem 51, 281–291 (2019)
R.C. Baptista, C.N. Horita, A.S. Sant’Ana, Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: a review. Food Res. Int 127, 108762 (2019)
M. Korzeniowska, I.W.Y. Cheung, E.C.Y. Li-Chan, Effects of fish protein hydrolysate and freeze-thaw treatment on physicochemical and gel properties of natural actomyosin from pacific cod. Food Chem 138(2–3), 1967–1975 (2013)
F.F. Li, B. Wang, B.H. Kong, S. Shi, X.F. Xia, Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll 97, 105223 (2019)
X. Zhao, J. Wu, L. Chen, H.S. Yang, Effect of vacuum impregnated fish gelatin and grape seed extract on metabolite profiles of tilapia (Oreochromis niloticus) fillets during storage. Food Chem 293, 418–428 (2019)
X.F. Xia, B.H. Kong, J. Liu, X.P. Diao, Q. Liu, Influence of different thawing methods on physicochemical changes and protein oxidation of porcine longissimus muscle. LWT-Food Sci. Technol 46(1), 280–286 (2012)
S. Ali, W. Zhang, N. Rajput, M.A. Khan, C.B. Li, G.H. Zhou, Effect of multiple freeze-thaw cycles on the quality of chicken breast meat. Food Chem 173, 808–814 (2015)
S. Benjakul, F. Bauer, Biochemical and physicochemical changes in catfish (Silurus glanis Linne) muscle as influenced by different freeze-thaw cycles. Food Chem 72(2), 207–217 (2001)
M.C. Zhang, F.F. Li, X.P. Diao, B.H. Kong, X.F. Xia, Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci 133, 10–18 (2017)
X.F. Xia, B.H. Kong, Q. Liu, J. Liu, Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze-thaw cycles. Meat Sci 83(2), 239–245 (2009)
J. Qi, C.B. Li, Y.J. Chen, F.F. Gao, X.L. Xu, G.H. Zhou, Changes in meat quality of ovine longissimus dorsi muscle in response to repeated freeze and thaw. Meat Sci 92(4), 619–626 (2012)
G.P. Hong, M.J. Choi, Comparison of the quality characteristics of abalone processed by high-pressure sub-zero temperature and pressure-shift freezing. Innov. Food Sci. Emerg. 33, 19–25 (2016)
X. Du, P. Chang, J.Y. Tian, B.H. Kong, F.D. Sun, X.F. Xia, Effect of ice structuring protein on the quality, thermal stability and oxidation of mirror carp (Cyprinus carpio L.) induced by freeze-thaw cycles. LWT-Food Sci. Technol 124, 109–140 (2020)
T. Baygar, Y. Alparslan, Ş Çakli, Effects of multiple freezing and refrigerator thawing cycles on the quality changes of sea bass (Dicentrarchus labrax). Iranian J. Fisher. Sci 12(2), 289–300 (2013)
B. Wang, F.F. Li, N. Pan, B.H. Kong, X.F. Xia, Effect of ice structuring protein on the quality of quick-frozen patties subjected to multiple freeze-thaw cycles. Meat Sci. 172, 108335 (2021)
X. Du, H.J. Li, C.H. Dong, Y.M. Ren, N. Pan, B.H. Kong, H.Y. Liu, X.F. Xia, Effect of ice structuring protein on the microstructure and myofibrillar protein structure of mirror carp (Cyprinus carpio L.) induced by freeze-thaw processes. LWT-Food Sci. Technol 139, 110570 (2021)
F.T. Saricaoglu, O. Gul, A. Besir, I. Atalar, Effect of high pressure homogenization (HPH) on functional and rheological properties of hazelnut meal proteins obtained from hazelnut oil industry by-products. J. Food Eng 233, 98–108 (2018)
Z. Zhang, J.M. Regenstein, P. Zhou, Y. Yang, Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem 34, 960–967 (2017)
X.Y. Liu, T. Zhang, Y. Xue, C.H. Xue, Changes of structural and physical properties of semi-gel from alaska pollock surimi during 4 °C storage. Food Hydrocoll 87, 772–782 (2019)
Y.Y. Guo, B.H. Kong, X.F. Xia, T. Yu, Q. Liu, Changes in physicochemical and protein structural properties of common carp (Cyprinus carpio) muscle subjected to different freeze-thaw cycles. J. Aquat. Food Prod. Technol 23(6), 579–590 (2014)
Y.Q. Li, B.H. Kong, X.F. Xia, Q. Liu, X.P. Diao, Structural changes of the myofibrillar proteins in common carp (Cyprinus carpio) muscle exposed to a hydroxyl radical-generating system. Process Biochem 48(5–6), 863–870 (2013)
Z. Long, M.M. Zhao, N. Liu, D.L. Liu, D.X. Sun-Waterhouse, Q.Z. Zhao, Physicochemical properties of peanut oil-based diacylglycerol and their derived oil-in-water emulsions stabilized by sodium caseinate. Food Chem 184, 105–113 (2015)
J. Jiang, Y.L. Xiong, Extreme pH treatments enhance the structure-reinforcement role of soy protein isolate and its emulsions in pork myofibrillar protein gels in the presence of microbial transglutaminase. Meat Sci 93(3), 469–476 (2013)
L.C. Sow, H.S. Yang, Effects of salt and sugar addition on the physicochemical properties and nanostructure of fish gelatin. Food Hydrocoll 45, 72–82 (2015)
G.L. Ellman, Tissue sulfhydryl groups. Archives of Biochem. Biophys 82(1), 70–77 (1959)
Z. Yang, H.J. Yang, H.S. Yang, Effects of sucrose addition on the rheology and microstructure of k-carrageenan gel. Food Hydrocoll 75, 164–173 (2018)
H.L. Wang, M. Pato, Z. Pietrasik, P. Shand, Biochemical and physicochemical properties of thermally treated natural actomyosin extracted from normal and PSE pork Longissimus muscle. Food Chem 113, 21–27 (2009)
H.L. Niu, Y. Li, J.C. Han, Q. Liu, B.H. Kong, Gelation and rheological properties of myofibrillar proteins influenced by the addition of soybean protein isolates subjected to an acidic pH treatment combined with a mild heating. Food Hydrocoll 70, 269–276 (2017)
F.F. Li, X. Du, B. Wang, N. Pan, X.F. Xia, Y.H. Bao, Inhibiting effect of ice structuring protein on the decreased gelling properties of protein from quick-frozen pork patty subjected to frozen storage. Food Chem 353, 129104 (2021)
Y.G. Zhou, H.S. Yang, Effects of calcium ion on gel properties and gelation of tilapia (Oreochromis niloticus) protein isolates processed with pH shift method. Food Chem 277, 327–335 (2019)
X.F. Xia, B.H. Kong, Y.L. Xiong, Y.M. Ren, Decreased gelling and emulsifying properties of myofibrillar protein from repeatedly frozen-thawed porcine longissimus muscle are due to protein denaturation and susceptibility to aggregation. Meat Sci 85(3), 481–486 (2010)
A.Q. Zhao, L. Yu, M. Yang, C.J. Wang, M.M. Wang, X. Bai, Effects of the combination of freeze-thawing and enzymatic hydrolysis on the microstructure and physicochemical properties of porous corn starch. Food Hydrocoll 83, 465–472 (2018)
F.F. Li, X. Du, Y.M. Ren, B.H. Kong, B. Wang, X.F. Xia, Y.H. Bao, Impact of ice structuring protein on myofibrillar protein aggregation behaviour and structural property of quick-frozen patty during frozen storage. Int. J. Biol. Macromol 178(1), 136–142 (2021)
L.C. Sow, J.M.N. Chong, Q.X. Liao, H.S. Yang, Effects of κ-carrageenan on the structure and rheological properties of fish gelatin. J. Food Eng 239, 92–103 (2018)
Y.G. Zhou, H.S. Yang, Enhancing tilapia fish myosin solubility using proline in low ionic strength solution. Food Chem. 320, 126665 (2020)
T.H. Chen, Y.P. Zhu, M.Y. Han, P. Wang, R. Wei, X.L. Xu, G.H. Zhou, Classification of chicken muscle with different freeze-thaw cycles using impedance and physicochemical properties. J. Food Eng 196, 94–100 (2017)
L.C. Sow, N.Z.Y. Toh, C.W. Wong, H.S. Yang, Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll 94, 459–467 (2019)
M.G. Wu, Y.L. Xiong, J. Chen, Rheology and microstructure of myofibrillar protein-plant lipid composite gels: Effect of emulsion droplet size and membrane type. J. Food Eng 106(4), 318–324 (2011)
X.Q. Diao, H.N. Guan, X.X. Zhao, X.P. Diao, B.H. Kong, Physicochemical and structural properties of composite gels prepared with myofibrillar protein and lard diacylglycerols. Meat Sci 121, 333–341 (2016)
L.C. Sow, S.J. Tan, H.S. Yang, Rheological properties and structure modification in liquid and gel of tilapia skin gelatin by the addition of low acyl gellan. Food Hydrocoll 90, 9–18 (2019)
S.N.K.M. Putra, N.H. Ishak, N.M. Sarbon, Preparation and characterization of physicochemical properties of golden apple snail (Pomacea canaliculata) protein hydrolysate as affected by different proteases. Biocatalysis and Agric. Biotechnol 13, 123–128 (2018)
M. Mejri, B. Rogé, A. Bensouissi, F. Michels, M. Mathlouthi, Effects of some additives on wheat gluten solubility: a structural approach. Food Chem 92(1), 7–15 (2005)
H.J. Li, Y.F. Hu, X.H. Zhao, W. Wan, X. Du, B.H. Kong, X.F. Xia, Effects of different ultrasound powers on the structure and stability of protein from sea cucumber gonad. LWT-Food Sci. Technol. 137, 110403 (2021)
C.M.B. Pinilla, A. Brandelli, M.E. López-Caballero, P. Montero, Md.C. Gómez-Guillén, Structural features of myofibrillar fish protein interacting with phosphatidylcholine liposomes. Food Res. Int. 137, 109687 (2020)
H.T. Liu, J.N. Zhang, H. Wang, Q. Chen, B.H. Kong, High-intensity ultrasound improves the physical stability of myofibrillar protein emulsion at low ionic strength by destroying and suppressing myosin molecular assembly. Ultrason. Sonochem 74, 105554 (2021)
J.Y. Zhao, F.J. Dong, Y.Y. Li, B.H. Kong, Q. Liu, Effect of freeze-thaw cycles on the emulsion activity and structural characteristics of soy protein isolate. Process. Biochem 50(10), 1607–1613 (2015)
H.E. Ramrez-Guerra, C.O. García-Sifuentes, R. Pacheco-Aguilar, J.C. Ramirez-Suarez, M.E. Lugo-Sánchez, S.M. Scheuren-Acevedo, The influence of ante-mortem hypoxia on the physicochemical stability of myofibrillar proteins in the muscle tissue of white shrimp (Litopenaeus vannamei) exposed to multiple freeze–thaw cycles. Eur. Food Res. Technol 235, 37–45 (2012)
Y. Tian, J.B. Du, Effect of disulfide bond and mercapto-group on structure and function of protein and analytical method. J. Appl. Clinical Pediatrics 19, 1499–1501 (2007)
D. Park, Y.L. Xiong, A.L. Alderton, T. Ooizumi, Biochemical changes in myofibrillar protein isolates exposed to three oxidizing systems. J. Agri. Food Chem 54(12), 4445–4451 (2006)
Q. Liu, Q. Chen, B.H. Kong, J.C. Han, X.Y. He, The influence of superchilling and cryoprotectants on protein oxidation and structural changes in the myofibrillar proteins of common carp (Cyprinus carpio) surimi. LWT-Food Sci. Technol 57(2), 603–611 (2014)
Z.Y. Zhang, Y.L. Yang, P. Zhou, X. Zhang, J.Y. Wang, Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem 217, 678–686 (2017)
X. Du, M.N. Zhao, N. Pan, S.P. Wang, X.F. Xia, D.J. Zhang, Tracking aggregation behaviour and gel properties induced by structural alterations in myofibrillar protein in mirror carp (Cyprinus carpio) under the synergistic effects of pH and heating. Food Chem 362, 130222 (2021)
L. Wei, L.W. Cao, S.B. Xiong, J. You, Y. Hu, R. Liu, Effects of pH on self-assembly of silver carp myosin at low temperature. Food Biosci 30, 100420 (2019)
Y.X. Xu, Y.M. Yin, R. Wang, H.L. Zhao, X.P. Li, S.M. Yi, J.R. Li, J.C. Xie, Effect of deacetylated konjac glucomannan on heat-induced structural changes and flavor binding ability of fish myosin. Food Chem 365, 130540 (2021)
Y.Q. An, J. You, S.B. Xiong, T. Yin, Short-term frozen storage enhances cross-linking that was induced by transglutaminase in surimi gels from silver carp (Hypophthalmichthys molitrix). Food Chem 257, 216–222 (2018)
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 32172273) and the Major Science and Technology Projects of “Millions” Engineering in Heilongjiang Province (2019ZX07B03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, X., Li, H., Nuerjiang, M. et al. Influence of repeated freeze–thaw treatments on the functional and structural properties of myofibrillar protein from mirror carp (Cyprinus carpio L.). Food Biophysics 16, 492–501 (2021). https://doi.org/10.1007/s11483-021-09689-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-021-09689-5