Abstract
From its journey from milk through to its end use, Mozzarella cheese undergoes significant transformations in its makeup of components and their structural arrangement. The typical Mozzarella processing steps each alter the structural configuration of the system. The colloidal dispersion of proteins, fat, lactose and minerals that is milk experiences physical, thermal, chemical, biological and ionic induced changes to its composition and structure throughout the manufacturing process and storage. This review critically evaluates the literature related to the structural changes occurring as a result of each step in Mozzarella cheese production process. Emphasis is placed on the role of each step and the induced transformations at the micro and macro scale in the system. Additionally, the review also looks at the changes that occur as a result of storage. This evolution in structure culminates in the creation of an end product with a bi-continuous gel structure that has a desired functionality in its end use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mozzarella cheese is one of the most consumed cheeses worldwide [1]. In the US in 2008 Mozzarella was the most consumed cheese with a consumption of 10.7 pounds per capita (4.85 kg). Mozzarella plays a large role in a considerable number of world markets, in particular the North American market [2]. It is common for cheese plants to manufacture in excess of 100,000 kg of pizza cheese daily [3]. This scale of manufacture has led to a need for precise control over the manufacturing process as well as an in depth understanding of how changes in the process impact on the structure, functionality and the composition of the cheese.
Originating in Italy, Mozzarella cheese has evolved from a regional cheese of southern Italy [4] to being one consumed internationally. Traditional Mozzarella cheese is made from buffalo milk [5]. However, the majority of the Mozzarella produced today is manufactured from pasteurised, part skimmed bovine milk [6]. Mozzarella is a member of the Pasta filata, or stretched curd, family of cheeses. The stretching of the curd gives the cheese a unique fibrous texture [6,7,8,9].
Prior to World War II Mozzarella was only consumed in small amounts outside of Italy; however, after the war there was an explosion in the popularity of Mozzarella due to the demand for Italian style products like pizza [7]. More than 70% of all Mozzarella consumed in the US is used on pizzas [5, 10]. The Mozzarella used as a pizza topping differs significantly to the traditional fresh Mozzarella cheese in both functionality and appearance.
The US has classified Mozzarella into four different categories based on composition: Mozzarella, low moisture Mozzarella, part skim Mozzarella and low moisture part skim Mozzarella [11]. The Low Moisture Part Skim (LMPS) Mozzarella is the most common variety due to its prominence on foods such as pizzas where it is used for its functional properties (Table 1).
The most common variation of Mozzarella cheese is that used for cooking purposes, in particular as a topping for pizzas [13]. This pizza cheese is often referred to as a low-moisture part-skimmed (LMPS) Mozzarella due to its lower fat and moisture content [7]. The cheese is produced from pasteurised cows’ milk with a fat content of about 1.8% and generally a combination of thermophilic cultures such as Lactobacillus ssp. and S. thermophilus [6]. Although the majority of the Mozzarella produced worldwide use thermophilic cultures, there are a few exceptions where mesophilic starters are used [3]. The choice of starter cultures depend on the acidification rate required in the curd through the cheese making process [4]. LMPS Mozzarella differs from table Mozzarella due to its lower fat content and the addition of Lactobacillus which is generally not added to table Mozzarella [6]. The addition of Lactobacillus is due to the higher acidification rate needed in pizza Mozzarella to achieve the required moisture content [3].
LMPS Mozzarella composition consists of between 30 and 40% fat to dry weight and a moisture content ranging from 45 to 52% [11]. Due to the allowable range in both the fat and moisture contents for LMPS Mozzarella, there is a degree of variation in the functional properties within the classification. Other types of Mozzarella can be used on pizzas, however, there are issues regarding the functional properties for this use. The food service industry rarely uses Mozzarella with moisture contents higher than 52% due to issues regarding shredding, matting and shorter shelf life [14].
Traditional Italian Mozzarella is a fresh unripened cheese that has a milky taste and is high in moisture [1]. However, Mozzarella used as a pizza cheese generally undergoes a period of ripening to attain a desired level of functionality [7]. During the initial period after manufacture, Mozzarella undergoes a complex set of physicochemical changes that affects the structure and functionality of the cheese [15]. It differs from most cheeses in the fact that it is consumed in a molten state the majority of the time. Due to this fact, the melting properties of Mozzarella are critical to product performance as well as consumer acceptance [16].
Processing Steps and Subsequent Structural Changes During Mozzarella Manufacture
The manufacturing procedure used to produce Mozzarella cheese plays an important role in determining the structure of the product. The processes that the cheese undergoes during manufacture have evolved from relatively crude procedures to incorporating cutting edge technical processes.
The structure of a food product is one of its most important attributes due to it being a major factor in the behaviour and functional properties of the food. Foods have a hierarchical structure from the macro level down to the micro structural level. Each processing step impacts on the structure, and hence functionality, of a product to some extent [17].
Milk
Milk is a colloidal dispersion of proteins existing in a dynamic equilibrium with fat and lactose distributed in water [17]. Bovine milk comprises between 30 and 35 g of protein per litre [18]. Bovine milk contains two distinct groups of proteins, the caseins and the whey proteins [19]. The caseins comprise approximately 80% of the protein content in bovine milk and are made up of four principal proteins: αs1, αs2, β and κ casein, in a ratio approximately 40:10:35:12 respectively, along with a number of minor proteins [20]. Casein can bind to calcium due to phosphoserine residues present in their structure [21]. Each of the different caseins has a different number of phosphoserine residues capable of binding calcium with αs1 containing 8, αs2 containing 10–13, β casein containing 5 and κ casein containing 1 phosphoserine residue per mole [22].
Approximately 95% of the casein in bovine milk is present in the form of micelles, colloidal particles [23]. These micelles are roughly spherical in shape and range in diameters from 50 to 500 nm [24]. Horne [24] states, casein micelles have a highly hydrated, open structure with levels of water between 2 and 3 g per gram of protein [25]. The reason that micelles contain more water than they do casein, due to the spaces that exist between the casein particles [26]. On a dry basis the micelles are approximately 94% protein with the other 6% being colloidal calcium phosphate (CCP), a collection of small ions mainly comprising calcium and phosphate [23]. The amorphous calcium phosphate link to the casein at the caseins ester phosphate groups [27]. αs1, αs2 and β casein are all insoluble at the calcium concentration present in milk, thus they arrange in such a way that the calcium soluble κ casein stabilises the micelle [23, 28]. This is done in such a way that the micelles have a surface layer rich in κ casein that protrudes into the surrounding medium.
Casein is far more stable in the form of micelles than if they were not organised into this form [29]. This micellar arrangement means that milk is able to withstand physiological changes to a greater extent than many other biological systems. The micelles contain roughly 65% of the 30 mM of calcium present in milk and only 10% is present as free calcium ions [30].
There has been much debate surrounding the internal structure of the casein micelles [25, 31, 32]. A number of different models have been proposed over the last 40 years to explain the structure of the micelles [31]. The models fall into three main models: the sub-micelle model [33, 34], the dual binding model [21, 35] and the nanocluster model [36,37,38]. These models have evolved over time due to the advancement in techniques capable of measuring the structure & compositional makeup of the micelles [31]. The oldest of these models, and most frequently cited, is the submicelle model [39]. This model proposed that the micelles were composed of smaller proteinaceous subunits, or submicelles [24]. The permutations of the submicelle model have included models where the submicelles are variable or constant size, composition and internal structure [40]. The proposed models suggest that the submicelles have a hydrophobic interior and hydrophilic exterior and are held together to form casein micelles by calcium phosphate cross bridges or protein interactions [31]. The dual binding model suggests that the casein molecules are a block of copolymers that form micelles through polymerisation reactions through hydrophobic interactions or across calcium phosphate [35]. It considers that the κ casein is a polymer chain terminator, stating this as the reason for its presence on the surface of the micelle [41]. The nanocluster model describes the interior as a flexible array of casein molecules that are interlinked with calcium phosphate ‘nanoclusters’ [17, 29]. This model is derived from observations that the phosphopeptides in casein bind to and stabilise small domains of calcium phosphate [42]. The formation of a micellar structure could occur due to crosslinking of these calcium phosphate nanoclusters by the highly phosphorylated αs1 & αs2 casein [37].
With the nanocluster model becoming the most favoured model a number of proposed versions of the model have been suggested including an interlocking lattice [43] and the existence of water channels within the structure [38]. During the cheese making process the micelles undergo a number of changes. However, after cheese manufacture there is still some level of the substructure that remains [44].
The fat globules that are dispersed in raw milk vary in size between 0.1–10 μm with approximately 90% existing between the size of 1 and 8 μm [17]. These dispersed globules of fat are surrounded by a lipoprotein membrane, milk fat globule membrane (MFGM) [45]. The MFGM stabilises the fat globules from coalescing and also protects them from lipases present in milk [46].
Pre-Treatment of Milk
The pre-treatment of milk for Mozzarella making is a relatively new phenomenon coinciding with the occurrence of large scale manufacture. There are still numerous manufacturers who do not pre-treat their milk; however, the majority have a pre-treatment step for reasons related to safety and consistency. Pasteurisation has become a step in the pre-treatment of milk for cheese manufacture due to a number of countries and governing bodies having restrictions on the use of raw milk [47]. Pasteurisation is the heat treatment of raw milk to minimise the health hazards associated with pathogens [48, 49].
Pasteurisation is generally done by passing milk through a plate heat exchanger so that it is exposed to a high temperature for a relatively short time; 72 °C for 15 s is standard practice to kill pathogens present in the milk [50]. There are a number of different chemical and other changes that occur during heating; the extent of the changes depends on both the temperature and duration [26]. Low temperature pasteurisation (e.g. 15 s at 74 °C) kills most microorganisms and inactivates some enzymes, high temperature pasteurisation (e.g. 15 s at 90 °C) kills all vegetative microorganisms, most enzymes are inactivated, whey proteins denature (in particular beta lactoglobulin) and –SH groups become exposed which promotes disulphide linkages. The process of pasteurisation alters the indigenous microflora of milk which enables the quality of cheese produced to be more uniform [51]. However, care needs to be taken when pasteurising milk as it can damage the coagulability and curd forming ability of the milk.
One of the most important attributes in the manufacture of quality products is consistency [52]. Variation in milk composition can occur for a number of reasons including the breed of cow that the milk comes from as well as the season [53]. This is due to different breeds of cow producing milk that differs in composition as well as the natural variation that occurs in composition throughout the year [49]. Standardisation acts as a means of ensuring the starting point of the cheesemaking process is consistent between batches [54]. This compositional adjustment of the milk is done to attain a desired composition of the cheese [50]. Generally this is achieved by centrifugal separation of the raw milk into skim milk and cream. The skim milk stream is then combined with whole milk or the cream stream to obtain the desired level of casein and fat [26]. Another method that is utilised in standardisation is the addition of non-fat dried (NFD) milk to the cheese milk prior to processing [55].
Recently ultrafiltration has also been utilised to adjust the protein content of the milk [50, 56]. The concentration of protein in the milk is achieved as low molecular weight molecules such as salts, water and lactose pass through the membrane resulting in the concentration of the remaining components [57]. This permeate can then be added to milk prior to cheese making to control the protein content. Standardisation of milk is used to try and maintain compositional uniformity between batches of cheese manufactured.
Acidification
The acidification of the milk has a large impact in most of the key steps in the manufacture of cheese [58]. Acidification can be done by the use of starter cultures, direct acidification or a mixture of both [15]. In acidification using starter cultures, the milk is inoculated with a thermophilic starter culture, which is generally composed of a mixture of both rod and cocci bacteria [5]. Thermophilic starters are used to a greater extent globally in the production of pizza cheese than mesophilic starters as it makes it easier to obtain the desired moisture content between 48 and 52% [59].
Starter selection is determined by the desired rate of acidification in the later stages of the cheese making process [4]. Most manufacturers utilise Streptococcus salivarius spp. thermophilus, cocci, alone or in combination with Lactobacillus delbrueckii ssp. bulgaricus, rods, [4] or L. helveticus [59]. S. thermophilus is weakly proteolytic and therefore is unable to produce enough free amino acids and small peptides from casein to sustain optimal growth and acidification in milk alone [5]. The use of L. bulgaricus, which is more proteolytic, thus producing more free amino acids and peptides which stimulate the growth of S. thermophilus. The ratio of these rod to cocci starters (L. bulgaricus & S. thermophiles) in the manufacture of Mozzarella influences the functional properties of the cheese due to its influence on structure [60]. This is due to the early stages of acid production being dominated by S. thermophilus, while L. bulgaricus is more dominant during the latter stages of Mozzarella production. Changes to these rod:cocci ratio will change the rate of acid production which will influence the structure of the curd and thus the functional properties of the cheese. The role of the starter is to convert lactose to lactic acid and by doing so reduce the pH of the milk [9].
In the mid-sixties a breakthrough was made in the form of using direct acidification in place of lactic fermenters in the production of Mozzarella [15]. There are a number of advantages of using a direct acidification including reduced processing time, lower costs as well as providing a better means of standardising the characteristics of the cheese [61, 62]. Another factor noted regarding the initial work done on direct acidification was that it produced Mozzarella that was suitable for use on pizzas immediately after manufacture [15]. However, if direct acidification is used completely in place of starter cultures there are implications in regards to the browning of the cheese when baked [63]. This is due to starter cultures producing small peptides and amino acids that are able to react with residual sugars in the system when subjected to heat. Therefore, the directly acidified Mozzarella will undergo browning to a lesser extent than cheese made with starter cultures.
One of the key purposes of acidifying milk prior to cheesemaking is to reduce the final calcium content of the cheese [64]. This occurs due to the disassociation of colloidal calcium phosphate as the pH reduces [17, 65]. During the acidification process, calcium phosphate solubilises and proteins disassociate from the micelles. The release of proteins from the casein micelle was found to be temperature dependent with lower levels of protein released at higher temperatures [66]. The maximum protein disassociation occurs at a pH of approximately 5.5 [67].
During the initial stages of acidification when the pH of the milk is lowered from its initial pH to a pH of 5.9, there is a progressive decrease in the casein particles hydration [68]. This is coupled with a decrease in the apparent particle radius of the casein micelle [69]. However, as the pH is dropped further, the apparent particle radius of the micelle increases back to its original size by about a pH of 5.2 [69]. This return to initial size occurs as the hydration of the micelle returns to approximately its initial value [68]. This is a result of the casein micelles undergoing structural reorganisation during the acidification [67]. This is due to less calcium binding and ion pairing taking place with the reduction in the number of negatively charged amino acid side groups as the pH drops.
The stability of the external κ casein layer of the casein micelle remains relatively stable down to a pH of 5 [37]. After this point, the κ casein layer collapses allowing the micelles to aggregate. However, cheese making utilises coagulants such as chymosin to cleave off the κ casein to promote aggregation at a desired pH. The use of chemical acidifiers over starter cultures is widely used as it reduces production costs as well as providing a method of standardising the characteristics of the cheese [61]. Direct acidification also allows for lower calcium and higher moisture contents to be achieved [70]. The lower calcium content causes the formation of hydrated and swollen paracasein fibres on stretching causing the cheese to be drier, softer and gummy in texture as a young cheese. The type of acid used in direct acidification can influence the calcium content and rheology of the cheese [71]. NMR spectroscopy identified that the mobility of the casein micelle does not change with pH [72].
Coagulation
A number of different coagulants are now used to make cheese [5]. The coagulant that has been traditionally used in cheese manufacture is rennet which is derived from the abomasum of young milk fed calves [25, 50]. Rennet is primarily made up of the enzyme chymosin [73] but also contains other enzymes such as pepsin in smaller quantities. Chymosin has an isoelectric point of between 4.5 and 4.7 [74]. Due to the increasing demand for clotting enzymes in the 1960’s and the limited supply of calf stomachs for rennet, several substitutes were identified [75], including pepsin [76], proteinases from different fungi (The most common from Rhizomucor miehei [77]), chymosin [78], as well as recombinant or genetically engineered chymosin [75]. The use of pepsin as a rennet substitute has several defects attributed to its use such as extensive proteolysis [79] and producing a coagulum with a more open structure than chymosin, allowing a greater amount of fat loss, as well as producing a cheese with a much softer body [79].
The addition of coagulants to milk has a dramatic effect on the structure of the system. The conversion that milk undergoes during coagulation can be classified as a two stage process [73]. The primary stage of the rennet action is the production of para-κ-casein and soluble glycomacropeptides. This reaction takes place when the coagulation enzyme hydrolyses the κ casein at the Phe105-Met106 site which is several times more susceptible to acid proteinases than any other peptide bond in the milk system [80]. Although some coagulants do not attack the Phe105-Met106 site, the majority used commercially in cheese making do attack this site. The majority of the glycomacropeptides are lost into the whey whilst the para-κ-casein remains attached to the exterior of the micelle [77]. The αs1, αs2 and β caseins are not hydrolysed during coagulation but may be hydrolysed during the ripening of Mozzarella.
There are a number of factors that have been found to affect the hydrolysis of κ casein by rennet. These include the pH, ionic strength, temperature, degree of glycosylation and the heat treatment of milk [20]. Heating milk above 60 °C has been proven to have an adverse effect on the coagulation if the milk is exposed to the heat for a sufficient duration [81]. If the heat treatment is severe, over 90 °C for 10 min, milk does not coagulate. One explanation for this is that the heat treatment changes β-lactoglobulin so that it interferes with the casein micelles hindering the rennet action [82]. This interference occurs due to the formation of a complex between β-lactoglobulin and κ casein [83]. Alternatively, the cleavage of the κ casein did occur, however, the β-lactoglobulin binds with the para κ casein interfering with aggregation through steric hinderance [84, 85].
Altering the pH of the milk affects the hydrolysis of the κ casein [86]. The optimal pH for chymosin activity in milk is pH 6.0, so reducing the pH from the natural pH in milk increases the rate of hydrolysis [87]. The charge on the micelles also decreases as the pH is dropped from the natural pH of milk [86]. The net effect of reducing the pH of milk on the hydrolysis of κ casein is that the coagulation time decreases [88]. The ionic strength of the milk has an effect on the coagulation time [88]. As the ionic strength is reduced so is the coagulation time [88, 89]. This is a result of higher ionic strengths causing a screening of the positive charge on the κ casein and the negative charge of the chymosin, restricting enzyme-substrate attraction [88, 90]. This is the general case, however, calcium, which contributes to ionic strength, plays an additional role in coagulation as discussed below.
The secondary or non-enzymic phase of coagulation involves the aggregation of the casein micelles [91]. The stability of the micelle is due to the net negative charge as well as steric repulsions of the κ casein [28]. The hydrolysis of the κ casein destabilises the casein micelle due to a reduction in the zeta potential as well as reducing the intermicellular repulsions caused by the removal of protruding peptides [80]. The reduction of the zeta potential is due to the release of the glycomacropeptides which diffuse away from the micelle [28]. The reduction in the steric hindrance allows the paracasein micelles to aggregate together [92]. This aggregation of the micelles occurs when approximately 85% of the κ casein has been hydrolysed [20]. However, individual micelles do not participate in gelation, the formation of a 3-dimentional space filled gel [25], until approximately 97% of the κ casein has been hydrolysed. In order to promote coagulation at a lower degree of κ hydrolysis, a lower pH can be used as well as increasing the temperature of the system [80]. Micelles stay discrete until approximately 60% of the visual coagulation time, at which point they begin to aggregate into chain like structures. These chain-like structures continue to aggregate forming clumps, clusters and eventually a gel like network [28]. The strength of the gel, curd tension, is an important factor in terms of the cheese yield [73]. The curd tension is affected by the same variables as the coagulation.
The coagulation of the rennet altered micelles is dependent on the concentration of calcium ions [20, 62]. These calcium ions may act by crosslinking micelles with serine phosphate residues or charge neutralisation [73]. Colloidal calcium phosphate (CCP) is also important in the coagulation process as if the level of CCP falls below 20%, coagulation will not occur [80]. This is due to the removal of CCP causing micelle dissociation and may cause the charge on the micelles to increase. The reduction in CCP can be offset by increasing the concentration of calcium ions [20]. If there are no free calcium ions the destabilised micelles will not aggregate [28].
Coagulation of renneted micelles is also dependent of the temperature of the milk, with normal bovine milk not coagulating at temperature below 18 °C unless the calcium content is increased [73]. The type of coagulant used has an impact on the rate of micelle aggregation as well as the development of gel strength [80].
Dehydration
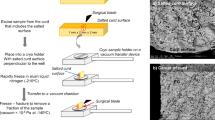
The dehydration process can be divided into a number of key steps: cutting, cooking, draining, Cheddaring and milling.
Once the curd has reached a desired degree of firmness the gel is usually cut or broken [73]. The cutting of the gel enhances syneresis [5, 93]. The gap between the blades used to cut the gel is instrumental in controlling the syneresis related to cutting, with smaller gaps cutting the gel to a greater extent which enhances syneresis. The amount of cutting is dependent on the type of cheese that is being manufactured, with low moisture cheeses cut to a greater degree than high moisture cheeses. As the Mozzarella used on pizzas has a moisture content generally in the bounds of between 48 and 52%, it is generally cut with blades that are widely spaced, leading to a lower degree of cutting in Mozzarella than lower moisture cheeses. Structurally cutting breaks apart the gel allowing the fluid entrapped within to flow.
Due to the majority of Mozzarella being produced using thermophilic starter cultures, the cooking temperature of the cheese is about 41 °C [3]. This is done as it provides a desirable temperature for the starter culture to convert lactose into lactic acid. The cooking of the curd and whey mixture plays a fundamental role in the control of syneresis by influencing curd shrinkage and acid development [50]. The cooking temperature used in the manufacture of the Mozzarella curd is an easily adjustable parameter that has an effect on a number of variables [94]. When the cooking temperature is increased there is a subsequent loss in moisture, reduced rate of proteolysis and an increase in the apparent viscosity. The impact that the cooking of the curd has on the functional properties of the Mozzarella depends on the heat stability of the coagulant as well as the amount and activity of the starter.
The temperature of cooking will have an effect on the structure of the curd. At the temperature of cooking, the hydrophobic interactions within the casein will strengthen [95] which leads to the protein matrix contracting and forcing some entrapped water out into a free state [96]. In addition to changes to the protein, cooking causes the fat globules to aggregate; however, the milk fat globule membranes that surround the globule remain relatively intact. The milk fat globules with intact membranes exist as non-interacting particles within the cheese matrix. The intact milk fat globule membrane may be one of the reasons for poor functional properties of heated Mozzarella immediately following manufacture.
The draining step in the manufacture of Mozzarella cheese essentially is the process that separates the curd from the whey [54, 97]. This simply separates the liquid fraction from the solid gel structure. The pH at drainage is the key factor affecting the demineralisation of the curd [98]. The effect of this on calcium is that the majority of the non-micellar calcium is lost in the whey while the micellar calcium is retained within the curd [64]. The altering of the pH at which the whey is drained impacts the level of calcium lost in the whey. Hence, the pH at drainage determines the functional stability of Mozzarella curd for the stretching process as well as the properties of the finished cheese [71, 99]. The draining occurs once a desired temperature has been reached, referred to as the draw pH [98]. This pH depends on the type of cheese being produced and the desired composition of the cheese. There are four major steps in the drainage: a) additional whey is expelled from the curd grains b) the curd grains deform c) curd grains partially fused together c) whey flows out of the curd bed [97, 100].
The Cheddaring process allows the curd to knit together to form curd granules [17, 50]. This occurs when the para-casein micelles fuse together to form a continuous protein phase [17]. The fusion of these curd grains into a coherent mass is an essential part of the formation of most cheeses [101].
Salting
Salting of the cheese curd is a critical step in the cheese making process [50]. The amount of salt and the method of addition of salt to the cheese play an important role in key characteristics of the cheese [102]. The salting of Mozzarella can occur either in the form of dry salting or brine salting [59]. Dry salting is where salted is added directly to the curd prior to the stretching in Mozzarella production. Prior to dry salting, the curd is generally milled to maximise the surface area exposed to the salt. The dry salting of the curd is a means of controlling the moisture content as it promotes syneresis. The Mellow is the process that occurs once the salt has been added to the curd. It involves the mixing and uptake of salt as well as the subsequent moisture loss [50].
The other method of salting, brine salting, occurs after the Mozzarella has been stretched, moulded into the desired shape and has been cooled in water [5]. Brine salted Mozzarella has a salt gradient that is highest at the surface and lowest in the centre of blocks of cheese [103]. The brine that the cheese in soaked in needs to have the pH and the calcium content adjusted so as to prevent leaching of calcium and lactic acid out of the cheese which result in defects to the cheese [102], these defects can affect further processing and the quality of the cheese. The salt in the cheese is mainly present in the aqueous phase [102].
The salting of the curd results in an increase in the level of protein hydration within the gel matrix [104,105,106]. The degree of hydration of the protein matrix has a significant influence on the texture and functional properties of cheese [107, 108]. The salting and subsequent hydration results in a swelling of the protein matrix [104] and leads to a reduction in the level of expressible serum from the curd [109]. The increased level of hydration can be viewed as being caused by the interaction of sodium ions with the casein and the sodium displacement of calcium from the casein [107, 110]. This has been Illustrated through studies that have identified an increase in calcium in expressible serum following salting [107].
Stretching
Stretching refers to the process in which the Mozzarella curd is subjected to thermo-mechanical treatment involving the application of shear stress to the plasticized curd [3, 70]. The stretching process gives Mozzarella its unique structure and fibrous texture [6,7,8] which results in functionality related to its melt properties [106]. Industrially the curd is generally plasticized and kneaded in hot water or a dilute brine solution [70]. This is generally performed using mechanical mixers with single or multiple screws to knead the curd in the hot water which is controlled by steam injection [3, 111].
The plasticisation and stretching is governed by the level of casein associated calcium at the time of stretching, which is in turn governed by the total calcium content and the pH of the curd [70]. The ability to plasticise is determined by the amount of casein associated calcium phosphate that is available to crosslink the amorphous para-casein matrix when heat is applied to the curd [3, 112,113,114,115]. There are two key conditions that are needed to be controlled for optimal stretching of the curd [70]. The first is that the curd needs to be sufficiently acidified and demineralised. The second condition for optimal stretching is the heat transfer between the curd and the brine during the stretching process.
Stretching can be considered a two stage process [3]. The first stage involves the milled curd entering the hot water, settling at the bottom and increasing in temperature so that it becomes a plastic workable consistency. As part of this the curd is worked in hot water or brine and attains a temperature generally between 55 and 65 °C [116]. In this temperature range hydrophobic interactions are at their maximum strength [117]. As the temperature of the curd increases there will be a corresponding increase in the strength of the hydrophobic interactions within the protein matrix. The strengthening hydrophobic interactions will result in the protein matrix contracting as the hydrophobic regions within the interior of the protein get closer in proximity, forcing some of the water out into a free state in the interstitial spaces surrounding the fat globules [118].
The second stage of the process refers to the kneading and stretching that occurs as the plasticised curd is worked by the augers. During the stretching process the amorphous para-casein matrix of the curd is aligned into fibres that are roughly parallel with channels of fat globules and free serum between them [70]. The serum channels contain water, residual proteins, minerals, fat globules and bacterial cells [44]. The heating that occurs during the stretching reduces the activity of residual rennet in the curd which reduces the extent of primary proteolysis during ripening [119].
A problem that can occur with the use of screws stretching the curd is that at high screw speeds tearing of the curd can occur [120]. The tearing is caused by the curd not being fully plasticised as it doesn’t have time to get up to an adequate temperature. The heterogeneous quasi-laminar structure created during the stretching process is instrumental in a number of the key functional properties of Mozzarella [70].
Free serum has been identified following the stretching process of Mozzarella cheese curd, however, whether this is caused by the heating of the curd, the shearing of the curd or a combination of the two is unknown.
Effect of Storage
Although it is considered an unripened cheese, Mozzarella does undergo significant structural changes during storage that have a dramatic effect on the functionality of the cheese [7]. Immediately after manufacture, low moisture Mozzarella cheese does not possess the desired functional properties for its application [3]. This fresh Mozzarella has a tough, fibrous texture and does not stretch or flow well when melted. This is due to the thick paracasein fibres that are formed during stretching being initially hydrophobic, favouring strong protein-protein interactions which resist flow and stretching [70].
Mozzarella undergoes significant structural and functional changes during the first few weeks of aging whereby it becomes softer and becomes more stretchable [5]. During this short period of aging, some of the β-casein partially disassociates from the casein matrix and becomes the main intact casein in the serum phase [44]. This is due to lessening in the hydrophobic forces in the matrix.
Proteins become less aggregated and become more hydrated during maturation [121]. This protein swelling is enhanced by high salt and low ionic calcium concentrations which lead to an increase in the tendency for the cheese to melt [44]. This is likely to be caused by the reduction in protein-protein interactions, resulting from the swelling, calcium solubilisation and proteolysis, increasing the ease of which the protein aggregates flow [122, 123]. The increase in hydration of proteins causes the amount of expressible serum and quantifiable free water, identified through NMR measurements, to decrease dramatically over the first few weeks of aging [110, 118]. Salt promotes structural swelling of the protein matrix and casein solubilisation through peptizing [104]. Lowering the level of calcium present in the cheese will increase the peptising action of salt [124].
Although short term aging of Mozzarella is needed due to it improving the shreddability and allowing the water binding capacity to increase, aging for a period beyond 2–3 weeks generally results in a progressive deterioration in the shredding characteristics [125]. This is due to the texture progressively becoming soft and gummy making shredding difficult. How the Mozzarella is stored after processing has an effect on the functionality of the cheese [126]. The length of refrigerated and frozen storage has an effect on the textural properties of the Mozzarella [127].
Another significant factor affecting the structure of Mozzarella is the proteolytic breakdown that occurs during aging [77, 103, 128]. Proteolysis occurs as residual enzymes present in the cheese hydrolyse the casein causing a breakdown in the protein matrix [129]. The amount of residual coagulant impacts on the proteolytic breakdown that occurs during aging [5]. This is influenced by the stretching process where the curd is exposed to heat and shear. The additional heat treatment largely inactivates the chymosin present in the curd, while the more heat stable plasmin remains active [112]. Plasmin predominantly hydrolyses β casein [130] resulting in the breakdown of β casein in most Mozzarella’s being greater than that of αs1-casein [103]. This preferential breakdown of β-casein is due in part to the complex protease-protease inhibitory system of plasmin in milk [131]. Plasmin in milk exists in its zymogen form, plasminogen [132], and its conversion and activity governed by a number of activators and inhibitors [131]. Both the plasminogen activator inhibitor and the plasmin inhibitor are affected by heat [131] while the plasminogen activator is heat stable [133]. This allows the conversion of plasmin from plasminogen to occur and in the absence of an inhibitor the plasmin can hydrolyse the casein in the cheese. Plasmin is the main enzyme responsible for the breakdown of β-casein in cheese [134].
Using electrophoresis it was found that Mozzarella had a greater percentage of intact casein than either Cheddar or Gouda cheeses [135]. This is likely to be due to the temperature treatment that occurs to the curd during the thermomechanical stretching step during the manufacture process.
Freezing Mozzarella is of commercial interest due to the ability to arrest physicochemical changes during the ripening process as well as a means of extending shelf-life [136]. There have been a number of defects associated with thawed cheese after it has been subjected to conventional freezing [137]. For Mozzarella these defects include poor cohesiveness, discolouration, fat leakage, watery surface and an acid flavour [138]. When cheese is subjected to conventional freezing where heat is removed progressively, the nucleation of water begins at the surface of the cheese and an ice front forms, progressing towards the centre [106, 137]. As the ice front advances, the solute concentration in the remaining liquid becomes more concentrated as they are excluded from the ice front. Due to this there are a number of changes that can occur in the cheese as a result of freezing. The expansion of water as it changes to ice crystals weakens the protein matrix leading to the cheese having a more porous structure and being softer [139]. However, this effect is only significant if sufficient bulk water is present, as in fresh Mozzarella prior to water absorption into the protein phase [44].
The temperature and speed used in freezing Mozzarella is an important factor in the melting properties of the cheese [126]. If the freezing rate is sufficiently fast, large crystals will not form and the protein matrix may not be disrupted enough to impact on texture [140]. A slower rate of freezing has been shown to increase the meltability of Mozzarella, likely due to large ice crystals damaging the protein matrix [126]. The form in which Mozzarella is frozen, in terms of being in block form or shredded, also has an effect on the stretch and melting properties. The stretching properties of the Mozzarella were greatest in shredded cheese due to the rapid freezing limiting the size of the ice crystals.
In most applications Mozzarella is used in a shredded form therefore many manufacturers shred the product prior to distribution. There are a number of problems concerning shredding Mozzarella related to the texture of the cheese [141]. If the cheese is too soft there are issues regarding the clogging of the cutting blades as well as the shredded cheese matting into sticky aggregates. If the cheese is too firm, shredding will result in the shattering of particles due to the brittleness of the cheese.
Conclusion
Each processing step throughout the manufacturing process has an effect on the structure of the final Mozzarella cheese produced. As it transforms from liquid milk to its final gel structure, the composition and arrangement of constituents within the system alter, affecting the functionality of the cheese in its end use. These processing steps involve thermal, physical, biological and ionic drivers that induce changes to proportions components within the system as well as to the overall structure of the system. As manufacturers look to improve their processing of Mozzarella or investigate alternative processing methods, it is of paramount importance that they understand the implications of each processing step on the microstructure of the cheese as it will affect the quality of the product produced.
References
S. Francolino, F. Locci, R. Ghiglietti, R. Iezzi, G. Mucchetti, LWT Food Sci. Technol. 43, 310 (2010)
S.C. Eliot, J.-C. Vuillemard, J.-P. Emond, J. Food Sci. 63, 1075 (1998)
P. Kindstedt, M. Caric, and S. Milanovic, in Pasta-filata Cheeses, ed. by P. F. Fox, P. L. H. McSweeney, T. M. Cogan, T. P. Guinee. Cheese: Chemistry, Physics and Microbiology, vol 2 (Academic Press, Cambridge 2004), pp. 251–277
S. Rankin, C. Chen, D. Sommer, and A. Esposito, in Mozzarella and Scamorza Cheese, ed. by Y. H. Hui. Handbook of Food Science, Technology, and Engineering, vol 4 (CRC Press, Boca Raton, 2005), pp. 150 151–150 111
P.S. Kindstedt, Crit. Rev. Food Sci. Nutr. 33, 167 (1993)
P. F. Fox, T. M. Cogan, and T. P. Guinee, (Aspen publishers, Maryland, 2000)
P.S. Kindstedt, in Cheese: Chemistry, Physics and Microbiology, ed. by P. F. Fox. (Chapman & Hall, London, 1993), p. 337
G.G. Ribero, A.C. Rubiolo, S.E. Zorrilla, J. Food Eng. 91, 516 (2009)
D. J. McMahon and C. J. Oberg, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 1041
S. Ferris and H. Palmiter, Italian-type Cheese in the USA 1987 (American Producers of Italian-type Cheese Association, 1987)
D. McMahon, C. Oberg, W. McManus, Aust. J. Dairy Technol. 48, 99 (1993)
USFDA, in Title 21, Parts 100–169 (U.S. Department of Health and Human Services, Washington DC, 1989)
P. Walstra, J. Wouters, T. Geurts, Dairy science and technology (CRC, Boca Raton, 2006) 2nd edn
N.C. Bertola, A.N. Califano, A.E. Bevilacqua, N.E. Zaritzky, J. Dairy Sci. 79, 185 (1996)
P.S. Kindstedt, Int. J. Dairy Technol. 57, 85 (2004)
P.S. Kindstedt, J.K. Rippe, C.M. Duthie, J. Dairy Sci. 72, 3117 (1989)
M.A.E. Auty, in Encyclopedia of Dairy Sciences, ed. by H. Roginski. (Elsevier, Oxford, 2002), p. 1796
H.E. Swaisgood, in Advanced Dairy Chemistry Volume 1: Proteins, ed. by P. F. Fox, P. L. H. McSweeney. (Kluwer Academic/Plenum Publishers, NY, 2003), p. 139
A. Varnam, J. Sutherland, Milk and milk products: technology, chemistry and microbiology (Aspen Publishers, Gaithersburg, 2001)
P.F. Fox, P.L.H. McSweeney, in Microbiology and Biochemistry of Cheese and Fermented Milk, ed. by B. A. Law. (Blackie Academic & Professional, London, 1997), p. 1
D.S. Horne, Curr. Opin. Colloid Interface Sci. 7, 456 (2002)
T.P. Guinee, B. O'Brien, in Technology of Cheesemaking, ed. by B. A. Law, A. Y. Tamine. (Wiley-Blackwell, Oxford, 2010), p. 96
P.F. Fox, Developments in Food Proteins 3, 69 (1984)
D.S. Horne, in Milk Proteins, ed. by A. Thompson, M. Boland, H. Singh. (Academic Press, San Diego, 2008), p. 133
D. S. Horne and J. A. Lucey, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 115
P. Walstra, R. Jenness, Dairy chemistry and physics (John Wiley & Son, NY, 1984)
T. van Vliet, P. Walstra, J. Food Eng. 22, 75 (1994)
J.A. Lucey, in Milk Proteins, ed. by A. Thompson, M. Boland, H. Singh. (Academic Press, San Diego, 2008), p. 449
C. Holt and D. Horne, Nederlands melk en Zuiveltijdschrift 50, 85 (1996)
C. Holt, D. Davies, A. Law, J. Dairy Res. 53, 557 (1986)
D.J. McMahon, W.R. McManus, J. Dairy Sci. 81, 2985 (1998)
P. Walstra, Int. Dairy J. 9, 189 (1999)
C.W. Slattery, R. Evard, Biochimica et Biophysica Acta (BBA)-Protein Structure 317, 529 (1973)
P. Walstra, J. Dairy Sci. 73, 1965 (1990)
D.S. Horne, Int. Dairy J. 8, 171 (1998)
C. Holt, Adv. Protein Chem. 43, 63 (1992)
C.G. de Kruif, C. Holt, in Advanced Dairy Chemistry Volume 1: Proteins, ed. by P. F. Fox, P. L. H. McSweeney. (Kluwer Academic/Plenum Publishers, NY, 2003), p. 233
D.G. Dalgleish, Soft Matter 7, 2265 (2010)
C.G. de Kruif, T. Huppertz, V.S. Urban, A.V. Petukhov, Adv. Colloid Interf. Sci. (2012)
C. Holt, C.G. de Kruif, R. Tuinier, P.A. Timmins, Colloids Surf. Physicochem. Eng. Aspects 213, 275 (2003)
D.S. Horne, Colloids Surf. Physicochem. Eng. Aspects 213, 255 (2003)
D.G. Dalgleish, M. Corredig, Annu. Rev. Food Sci. Technol. 3, 449 (2012)
D.J. McMahon, B.S. Oommen, J. Dairy Sci. 91, 1709 (2008)
D. Everett, in Structure of Dairy Products, ed. by A. Y. Tamime. (Blackwell Publishing Ltd, Oxford, 2007), p. 170
T. Keenan and I. Mather, Advanced Dairy Chemistry Volume 2 Lipids, 137 (2006)
R. Ward, J. German, and M. Corredig, Advanced Dairy Chemistry Volume 2 Lipids, 213 (2006)
P. F. Fox and T. M. Cogan, in Factors that Affect the Quality of Cheese, ed. by P. F. Fox, P. L. H. McSweeney, T. M. Cogan, T. P. Guinee. Cheese: Chemistry, Physics and Microbiology, vol 1 (Academic Press, Cambridge, 2004), pp. 583–608
E. T. Ryser, in Encyclopedia of Dairy Sciences, edited by H. Roginski (Elsevier, Oxford, 2002), pp. 2232
R. R. Panthi, K. N. Jordan, A. L. Kelly, and J. J. Sheehan, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 23
R. J. Bennett and K. A. Johnston, in General Aspects of Cheese Technology, ed. by P. F. Fox, P. L. H. McSweeney, T. M. Cogan, T. P. Guinee. Cheese: Chemistry, Physics and Microbiology, vol 2 (Academic Press, Cambridge, 2004), pp. 23–50
P. F. Fox and P. L. H. McSweeney, in Cheese: An Overview, ed. by P. F. Fox, P. L. H. McSweeney, T. M. Cogan, T. P. Guinee. Cheese: Chemistry, Physics and Microbiology, vol 1 (Academic Press, Cambridge, 2004), pp. 1–18
F.J. Solorza, A.E. Bell, Int. J. Dairy Technol. 48, 133 (1995)
A.R. Hill, in Chemistry of Structure-Function Relationships in Cheese, ed. by E. L. Malin, M. H. Tunick. (Plenum Press, New York, 1995), p. 43
A. K. Legg, A. J. Carr, R. J. Bennett, and K. A. Johnston, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 643
P. Kindstedt, A. Hillier, J. Mayes, in Technology of cheesemaking, ed. by B. Law, A. Tamime. (Blackwell Publishing Ltd, Oxford, 2010), p. 330
V. V. Mistry and J.-L. Maubois, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 677
V.V. Mistry, in Encyclopedia of Dairy Sciences edited by J. W. Fuquay (Academic Press, San Diego, 2011), p. 618
P.F. Fox, P.L.H. McSweeney, Food Science & Technology 20, 28 (2006)
P.S. Kindstedt, in Encyclopedia of Dairy Sciences, ed. by H. Roginski. (Elsevier, Oxford, 2002), p. 386
J.J. Yun, D.M. Barbano, L.J. Kiely, P.S. Kindstedt, J. Dairy Sci. 78, 751 (1995)
M. Faccia, A. Trani, A. Di Luccia, J. Dairy Sci. 92, 4211 (2009)
P. F. Fox, T. M. Cogan, and T. P. Guinee, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 617
C.J. Oberg, A. Wang, L.V. Moyes, R.J. Brown, G.H. Richardson, J. Dairy Sci. 74, 389 (1991)
L.E. Metzger, D.M. Barbano, M.A. Rudan, P.S. Kindstedt, J. Dairy Sci. 83, 648 (2000)
J. A. Lucey, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 179
D.G. Dalgleish, A.J.R. Law, J. Dairy Res. 55, 529 (1988)
D.J. McMahon, H. Du, W.R. McManus, K.M. Larsen, J. Dairy Sci. 93, 1783 (2009)
S. Le Feunteun, F. Mariette, Macromolecules 41, 2079 (2008)
M. Alexander, M. Corredig, D.G. Dalgleish, Food Hydrocoll. 20, 325 (2006)
P. S. Kindstedt, in Low-moisture Mozzarella Cheese, ed. by P. L. H. McSweeney. Cheese Problems Solved (CRC Press, Boca Raton, 2007), pp. 298–329
B. Keller, N.F. Olson, T. Richardson, J. Dairy Sci. 57, 174 (1974)
H.S. Rollema, J.A. Brinkhuis, J. Dairy Res. 56, 417 (1989)
P.F. Fox, T.P. O'Connor, P.L.H. McSweeney, T.P. Guinee, N.M. O'Brien, Adv. Food Nutr. Res. 39, 163 (1996)
T. Kleinert, I. Lange, B. Rösicke, A. Honig, R. Schleusener, Eng. Life Sci. 8, 367 (1988)
M. Jacob, D. Jaros, H. Rohm, Int. J. Dairy Technol. 63, 370 (2010)
P.F. Fox, L. Stepaniak, Int. Dairy J. 3, 509 (1993)
P.F. Fox, P.L.H. McSweeney, Food Rev. Int. 12, 457 (1996)
H. Birkkjaer and P. Jhnk, Technological Suitability of Calf Rennet Substitutes, 194 (Bulletin of the International Dairy Federation, 1985) pp. 8–13
S.K. Garg, B.N. Johri, Food Rev. Int. 10, 313 (1994)
P.F. Fox, Dairy Industries International 52, 11 (1987)
H. Singh, S.I. Shalabi, P.F. Fox, A. Flynn, A. Barry, J. Dairy Res. 55, 205 (1988)
A. Kannan, R. Jenness, J. Dairy Sci. 44, 808 (1961)
W.H. Sawyer, J. Dairy Sci. 52, 1347 (1969)
S.G. Anema, S. Kim Lee, H. Klostermeyer, LWT Food Sci. Technol. 40, 99 (2007)
H. Singh, S.I. Shalabi, P.F. Fox, A. Flynn, A. Barry, J. Dairy Res. 55, 205 (1988)
T. Janhøj and K. B. Qvist, in The Formation of Cheese Curd Technology of cheesemaking (Wiley-Blackwell, Hoboken, 2010), pp. 130–165
A.C.M. Hooydonk, I. Boerrigter, H. Hagedoorn, Neth. Milk Dairy J. 40, 297 (1986)
C. Daviau, M.H. Famelart, A. Pierre, H. Goudedranche, J.L. Maubois, Lait 80, 397 (2000)
M.H. Famelart, F. Lepesant, F. Gaucheron, Y. Le Graet, P. Schuck, Lait 76, 445 (1996)
S. Visser, P.J. Rooijen, C.J. Slangen, Eur. J. Biochem. 108, 415 (1980)
D.G. Dalgleish, J. Dairy Res. 46, 653 (1979)
P. Walstra, H.J.M. Vandijk, T.J. Guerts, Neth. Milk Dairy J. 39, 209 (1985)
P. Walstra, H.J.M. Vandijk, Neth. Milk Dairy J. 37, 92 (1983)
J.J. Yun, L.J. Kiely, D.M. Barbano, P.S. Kindstedt, J. Dairy Sci. 76, 3664 (1993)
C.M. Bryant, D.J. McClements, Trends Food Sci. Technol. 9, 143 (1998)
J.R. Smith, J.P. Hindmarsh, A.J. Carr, M.D. Golding, D. Reid, J. Food Eng. 214, 257 (2017)
J.C. Akkerman, C.A.P. Buijsse, J. Schenk, P. Walstra, Neth. Milk Dairy J. 50, 371 (1996)
L.J. Kiely, P.S. Kindstedt, G.M. Hendricks, J.E. Levis, J.J. Yun, D.M. Barbano, H.D. Goff, M. Rousseau, Food Struct. 11, 217 (1992)
M.M. Ak, S. Gunasekaran, Cheese Rheology and Texture (CRC Press LLC, Boca Raton, 2003)
J. C. Akkerman, Drainage of Curd. Doctoral thesis, Wageningen Agricultural University, 1992
J.C. Akkerman, R.O. Lewis, P. Walstra, Neth. Milk Dairy J. 47, 137 (1993)
B.J. Sutherland, in Encyclopedia of Dairy Sciences, ed. by H. Roginski. (Elsevier, Oxford, 2002), p. 293
N.Y. Farkye, L.J. Kiely, R.D. Allshouse, P.S. Kindstedt, J. Dairy Sci. 74, 1433 (1991)
M.R. Guo, J.A. Gilmore, P.S. Kindstedt, J. Dairy Sci. 80, 3092 (1997)
A.J. Pastorino, C.L. Hansen, D.J. McMahon, J. Dairy Sci. 86, 60 (2003)
D. W. Everett and M. A. E. Auty, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 547
T. P. Guinee and P. F. Fox, in Cheese (Fourth edition) (Academic Press, San Diego, 2017), pp. 317
T. P. Guinee, in Protein in Cheese and Cheese Products: Structure-function Relationships, ed. by P. L. H. McSweeney, and P. F. Fox. Advanced Dairy Chemistry (Springer, New York, 2016), pp. 347–415
M.K. Rowney, P. Roupas, M.W. Hickey, D.W. Everett, Int. Dairy J. 14, 809 (2004)
M.R. Guo, P.S. Kindstedt, J. Dairy Sci. 78, 2099 (1995)
P. Sharma, P.A. Munro, T.T. Dessev, P.G. Wiles, R.J. Buwalda, Food Hydrocoll. 54, 266 (2016)
R.C. Lawrence, L.K. Creamer, J. Gilles, J. Dairy Sci. 70, 1748 (1987)
T. Kimura, Y. Sagara, M. Fukushima, S. Taneya, Milchwissenschaft-Milk Science International 47, 547 (1992)
J.A. Lucey, P.F. Fox, J. Dairy Sci. 76, 1714 (1993)
F. Kosikowski and V. Mistry, Cheese and Fermented Milk Foods (Edwards Brother, Inc., Ann Arbor, 1997), 3rd edn., Vol. 1, pp. 174–184
D.J. McMahon, C. Oberg, Encyclopedia of Dairy Sciences 1, 737 (2011)
G. Nemethy, H.A. Scheraga, J. Phys. Chem. 66, 1773 (1962)
S.J. Vogt, J.R. Smith, J.D. Seymour, A.J. Carr, M.D. Golding, S.L. Codd, J. Food Eng. 150, 35 (2015)
J. Lucey, in Microstructural Approaches to the Study and Improvement of Cheese and Yogurt Products, ed. by D. McClements. Understanding and Controlling the Microstructure of Complex Foods (CRC Press, Boca Raton, 2007), pp. 600–621
A. Renda, D.M. Barbano, J.J. Yun, P.S. Kindstedt, S.J. Mulvaney, J. Dairy Sci. 80, 1901 (1997)
D.J. McMahon, C.J. Oberg, Aust. J. Dairy Technol. 53, 98 (1998)
D.J. McMahon, R.L. Fife, C.J. Oberg, J. Dairy Sci. 82, 1361 (1999)
N.S. Joshi, K. Muthukumarappan, R.I. Dave, Int. J. Food Prop. 7, 239 (2004)
B.M. Paulson, D.J. McMahon, C.J. Oberg, J. Dairy Sci. 81, 2053 (1998)
P. S. Kindstedt, Advances in experimental medicine and biology (USA) 367, 27 (1995)
C.J. Oberg, R.K. Merrill, R.J. Brown, G.H. Richardson, J. Dairy Sci. 75, 1161 (1992)
M.H. Tunick, K.L. Mackey, P.W. Smith, V.H. Holsinger, Neth. Milk Dairy J. 45, 117 (1991)
L. Costabel, M.S. Pauletti, E. Hynes, J. Dairy Sci. 90, 2103 (2007)
P.F. Fox, J. Dairy Sci. 72, 1379 (1989)
N.Y. Farkye, P.F. Fox, J. Agric. Food Chem. 39, 786 (1991)
B. Ismail, S.S. Nielsen, J. Dairy Sci. 93, 4999 (2010)
M.B. Grufferty, P.F. Fox, J. Dairy Res. 55, 609 (1988)
D.D. Lu, S.S. Nielsen, J. Food Sci. 58, 1010 (1993)
E.P. Feeney, P.F. Fox, T.P. Guinee, Lait 81, 463 (2001)
L. Creamer, New Zealand Journal of Dairy Science and Technology 11, 130 (1976)
M.I. Kuo, S. Gunasekaran, LWT Food Sci. Technol. 42, 9 (2009)
D.S. Reid, H. Yan, J. Food Qual. 27, 436 (2004)
D. Johnston, Milchwissenschaft-Milk Science International 55, 559 (2000)
N.G. Graiver, N.E. Zaritzky, A.N. Califano, J. Food Sci. 69, E123 (2004)
M.A. Cervantes, D.B. Lund, N.F. Olson, J. Dairy Sci. 66, 204 (1983)
N.C. Bertola, A.N. Califano, A.E. Bevilacqua, N.E. Zaritzky, Lebensmittel-Wissenschaft und-Technologie 29, 470 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, J.R., Carr, A.J., Golding, M. et al. Mozzarella Cheese – A Review of the Structural Development During Processing. Food Biophysics 13, 1–10 (2018). https://doi.org/10.1007/s11483-017-9511-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-017-9511-6