Abstract
Polyphenols have beneficial neurological effects delaying cognitive and motor decline in aging due to their antioxidant, antiinflammatory and neuroprotective properties. These effects could be related to SIRT1 activation (implicated in synaptic plasticity, memory and inflammation) and monoaminergic synthesis modulation. In this work, we studied in old male rats, the in vivo effects of long-term administration of different polyphenols (silymarin, quercetin and naringenin; 20 mg/kg/day i.p, 4 weeks) (Sprague-Dawley, 18 months) on cognition and motor coordination. We also analyzed in different brain regions: tryptophan hydroxylase (TPH) and tyrosine hydroxylase (TH) activities, which mediate central monoaminergic neurotransmitters synthesis; and immunoreactivities of SIRT1 and NF-κB (total and acetylated forms). Results indicated that chronic polyphenolic treatments showed restorative effects on cognition and motor coordination consistently with the biochemical and molecular results. Polyphenols reversed the age-induced deficits in monoaminergic neurotransmitters (serotonin, noradrenaline, and dopamine), increasing TPH and TH activity. In addition, polyphenolic treatments increased SIRT1 levels and decreased NF-κB levels in hippocampus. These results confirm polyphenolic treatments as a valuable potential therapeutic strategy for attenuating inflamm-aging and brain function decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging of the world population and a concomitant increase in age-related diseases highlights the need to identify potential points of intervention to improve the quality of life in older adults. Aging leads to numerous changes in the physiology of the brain, such as synaptic dysfunction and cognitive alterations, in part due to an increase in oxidative stress (Harman 1956) and neuroinflammation (Salminen et al. 2008), accompanied by reduced levels of endogenous antioxidants (Bishop et al. 2010; Venkataraman et al. 2013). In vivo studies have demonstrated the importance of dietary antioxidants as melatonin (Esteban et al. 2010a; Moranta et al. 2014), tocopherol (Ramis et al. 2016) and polyphenols as resveratrol (Sarubbo et al. 2015) limiting some effects of aging. Silymarin, quercetin and naringenin are naturally occurring polyphenols found in citric and red fruits, vegetables, cereals and tea. They are used as a dietary supplement by their putative multiple positive health effects (Pandey and Rizvi 2009). These effects have been attributed to the antioxidant and anti-inflammatory properties described for silymarin (Nencini et al. 2007), quercetin (Chondrogianni et al. 2010) and naringenin (Hirai et al. 2007), which possibly allow them to exert neuroprotection (see Scalbert et al. 2005 for more information).
Brain functions as memory and motor coordination are intimately regulated by neuromodulatory systems, including the noradrenergic (Lemon et al. 2009; Murchison et al. 2004), serotonergic (Meneses 1999), and dopaminergic (Lemon and Manahan-Vaughan 2006; Li et al. 2003) systems. In fact, some of the cognitive and motor impairments associated with aging are explained by alterations in monoamine levels in cognitive-related brain regions (Esteban et al. 2010a, b; Ramis et al. 2016; Sarubbo et al. 2015; Tsunemi et al. 2005). The antioxidant properties of polyphenols may protect the enzymes involved in the synthesis and metabolism of monoamines (i.e., tryptophan hydroxylase (TPH), tyrosine hydroxylase (TH) and monoaminooxidase (MAO)) against oxidative damage, as it was suggested for resveratrol treatment (Sarubbo et al. 2015). Moreover, neuroprotective effects of polyphenols in neurological disorders like Alzheimer (Khan et al. 2012) or cerebral ischemic damage (Della-Morte et al. 2009) have been linked to SIRT1 activation. Actually, SIRT1 has a direct role in regulating normal brain function such as plasticity and memory (Gao et al. 2010), as well as it has been demonstrated that SIRT1 regulates NF-κB signaling, modulating proinflammatory responses (Salminen et al. 2008) and cell survival (Yeung et al. 2004). Consequently, SIRT1 may be a valuable potential therapeutic target to improve the older people’s quality of life throughout treating central nervous system disorders (see Yang et al. 2015 for knowing the current understanding of SIRT1 in regulating immune responses). Thus, the aim of this study was to evaluate the in vivo effects in aged rats of chronic silymarin, quercetin and naringenin treatments at cognitive (working and episodic memory, spatial learning) and motor level, to analyze whether the observed changes are related to modulation of synthesis and metabolism of monoamines (5-HT, NA, and DA), but also SIRT1 and NF-κB levels.
Materials and Methods
Animals and Treatments
Young (3 months) and old (18 months) male Sprague-Dawley rats (Charles River, Spain) were housed individually in standard cages under controlled environmental conditions (20° ± 2 °C; 70% humidity, and 12-h light/dark cycle, lights on at 08.00 h) with free access to standard food (Panlab A04, Spain) and tap water. All procedures were performed during light period and in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Directive 86/609/EEC) and the Bioethical Committee of the University. As the aim of this study was to determine the effects of polyphenols in aging, old male rats were chronically treated, during 28 days with 20 mg/kg/day, i.p of silymarin (n = 5), quercetin (n = 7) and naringenin (n = 6) dissolved in corn oil, or vehicle (corn oil, 1 ml/kg/day, i.p, n = 5) in the old control group. Young rats (3 month, n = 8) also received vehicle (i.p.) as old control group. Doses were set from a pilot study presented at the 9th FENS Forum of Neuroscience (Milan, Italy 2014). Rats were sacrificed by decapitation the next day after the last polyphenol or vehicle administration. Sacrifice was performed at 8:00 AM (during dark/light change). In order to analyze monoamines synthesis, all rats received a single administration of NSD 1015 (which is a central aromatic amino acid DOPA decarboxylase inhibitor) (100 mg/kg, i.p.) 30 min before sacrifice (under red light) to measure the in vivo activity of TPH and TH, through accumulation of 5-hydroxytryptophan (5-HTP) and dihydroxyphenylalanine (DOPA) respectively during these 30 min (see below). Brains were quickly removed and dissected on an ice-cold plate to separate the following entire regions: hippocampus, striatum (caudate-putamen) and pineal gland, which were immediately frozen in liquid nitrogen and stored at −80 °C until assays.
Behavioural Tests
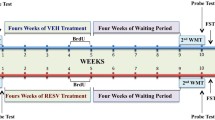
Behavioural test were conducted between 9:00 to 13:00 h, three times during treatments: before at the start of treatment, in the middle, and at the end of treatment period (Fig. 1). In order to avoid temporal overlaps between tests, they were conducted in the following order: the first day Barnes maze, the next day object recognition and rotarod, and after 24 or 48 h (depending on the age of rats) radial maze was conducted. In this way, there were avoided interferences between tests, not only for performing the test itself but also by the preparation periods (eg fasting in radial maze). The experiments were conducted in 3 runs of 10–11 animals, each displaced 2 days from the previous one. Ramis MR performed the Radial maze test and Sarubbo F performed all other behavioural tests do to overlap between different animal runs. The day where the rats were tested, the administration of polyphenols took place after the performance on the test (Fig. 1).
Time schedule for experimental design. Old male rats were chronically treated, during 28 days with different polyphenols (20 mg/kg/day, i.p): quercetin (n = 7), naringenin (n = 6), or silymarin (n = 5) dissolved in vehicle (corn oil). Old control rats (n = 5) young rats (3 month, n = 8) were chronically treated with vehicle (corn oil, 1 ml/kg/day, i.p,). Rats were sacrificed by decapitation the next day at 8:00 AM and whole brain samples were dissected and immediately frozen at −80 °C. All animals received NSD 1015 (100 mg/kg, i.p.) 30 min before sacrifice by decapitation to analyse monoamines syntheses. Behavioural test were conducted between 9:00 to 13:00 h, three times during chronic treatments: before the start of treatment (except Barnes test), in the middle, and at the end of treatment period. In order to avoid temporal overlaps between tests, they were conducted in the following order: the first day Barnes maze (BM; one day for familiarization), the next day object recognition (OR) and rotarod (RR)(both test with four days for familiarization), and after 24 or 48 h (depending on the age/weight of rats) radial maze (RM) was conducted. Three different experimental groups of 10–11 animals were sequentially conducted with a two days delay. Polyphenol and vehicle were administered at 9:00 AM except days devoted to behavioural tests which were administered after performing the tests
Radial Maze Test
Radial maze test was used to test spatial working memory in rats as previously reported (Sarubbo et al. 2015). The 8-arm radial maze (Panlab) consisted of an octagonal central platform (32 cm diameter) with eight equally spaced radial arms. To test working memory, rats were allowed to make an arm choice to obtain small pieces of food pellets (Panlab A04, Spain) until all eight arms had been visited, in case rats do not finish the task, the maximum time given was 20 min. Animals were submitted to fasting before each radial maze test to achieve the same motivational level in the different groups: 24 h for young rats or 48 h for old rats to achieve similar body weight loss, as described previously (Sharma et al. 2010). The maze was set in an experimental room with several external visual cues. The movements of the animals were monitored via digital video tracking system (LE 8300 with software SEDACOM v 1.3, Panlab). The sum of non-visited arms and re-entry into arms was scored as working memory errors.
Novel Object Recognition Test
The novel object recognition test is a method to measure episodic-like memory in rodents, which assesses their natural propensity to explore the novel object as their natural propensity to the novelty (Antunes and Biala 2012). It was performed in an open field device. It was conducted before the start, in the middle and in the end of treatment period. The test consists of three phases: habituation, familiarization, and test phase (Ramis et al. 2013; 2016). In the habituation phase, each animal is allowed to freely explore the open field in the absence of objects for 10 min, daily, during 4 consecutive days. On day 5, each animal was placed in the apparatus and allowed to explore for 1 min for re-habituation. During the familiarization phase, animals were placed in the centre of the apparatus with two identical objects (e.g. plastic blue 6 × 6 cm with cubes shapes) always placed in the same location by using velcro into the base of the objects, 16 cm away from the walls and allowed to explore both objects until 10 min had elapsed. Object exploration was considered when the animal’s nose or mouth was in contact with the object. After the delay (10 min), for the test phase, the animals were placed back in the open field with one object familiar (the same as the previous phase) and one novel object (e.g. plastic red 8 × 4 cm with bolus shape). The time spent exploring the objects was recorded until 10 min had elapsed. The objects had no natural significance for animals, since they are made of the same material in different shapes, and had never been associated with reinforcement or location. Moreover, during training session both objects are novel, so that the time spent on both objects should be similar, which was verified in each session. In this way, the test phase reflects the preference for novelty. The objects and the apparatus were cleaned with ethanol solution between trials. In addition, anxiety and exploratory drive of the animals were measured by recording the number of times the rats passed through the different areas of the open field and the number of urinations and defecations.
Visuospatial Learning in Barnes Maze Test
The maze consists of a circular disk (130 cm diameter) elevated above the floor with 18 holes equidistantly located around the perimeter. Under one of these holes, there is a black box or target not visually discriminated from the centre of the maze. The maze was set in an experimental room with several visual cues to serve as reference points for locating the target. Bright light was used as a stimulus to find the target, accentuating the natural agoraphobia of rats (Barrett et al. 2009). Previous studies indicated that performance on this task requires spatial stimuli recognition mediated by the hippocampus (Deacon et al. 2002). Animals were habituated to the maze, allowing them to freely explore the maze the day before the test in one session (familiarization phase). Each rat was placed in the middle of the platform, the light was switched on and the animal had 3 min to escape by hiding in the target. If the rat did not escape, it was manually placed in the target box, where it remained for 1 min. Once the animal is inside the box the light was turned off. Between tests and trials the whole apparatus was cleaned with ethanol 90%, to avoid the presence of olfactory or solid traces between animals. The day of the test, each rat performed four trials separated by 10 min. The trial finished when the animal entered the target or after 3 min, when animal was manually placed into the target box and remained there for 1 min. The time spent exploring the maze until reaching the target was denominated latency. Exploration of the non-target hole was counted as an error. Latency and errors were analysed as a percent respect to young rats in order to compare the effect of aging and treatments. Three different strategies to search the target were considered (Rueda-Orozco et al. 2008): direct (rats go directly to the target), serial (rats explore holes in sequence), or random (any other pattern to reach the target).
Motor Coordination in Rotarod Test
Motor ability and balance were evaluated on the rotarod treadmill (Panlab). Animals were submitted to training sessions during 4 days prior to test (one session/day) on a rotarod at a constant speed of 4 rpm until their performance was stabilized. In the test phase, the rats were placed on the rotarod in acceleration mode (from 4 to 40 rpm over a period of 60 s) for recording the latency to fall. Each rat repeated the test five times, leaving a few minutes for recovery.
TPH Activity (Synthesis of 5-HT) and TH Activity (Synthesis of DA and NA)
Transformation of tryptophan into 5-HTP is the limiting step in 5-HT synthesis which requires the TPH-2 isoform enzyme in most of the brain, and TPH-1 isoform enzyme in the pineal gland (Walther et al. 2003). Regarding catecholamine (NA and DA) synthesis, the limiting step is the transformation of tyrosine into DOPA which requires the TH enzyme. The in vivo activities of these rate-limiting enzymes were determined by measuring the accumulation of 5-HTP and DOPA within 30 min after inhibition of the aromatic L-amino acid decarboxylase by a maximally effective dose of NSD 1015 (3-hydroxybenzylhydrazine HCl, 100 mg/kg, i.p.). Accumulation of 5-HTP indicates 5-HT synthesis in all brain regions, whereas DOPA accumulation indicates synthesis of NA in the hippocampus and synthesis of DA in the striatum. This method also enables to quantify the pool of 5-HT, DA or NA unaffected by recent synthesis and primarily stored within neurons. Furthermore, this method allows determining levels of some monoaminergic metabolites that can reveal recent use of these neurotransmitters: 5-hydroxyindoleacetic (5-HIAA) and homovalinic acid (HVA) (Moranta et al. 2009; Sastre-Coll et al. 1999). All these compounds (monoamines, precursors and metabolites) were determined simultaneously by high-performance liquid chromatography (HPLC) with electrochemical detection. Brain regions (one of the two hippocampus, one of the two striatum, and the pineal gland) were homogenized with an Ultra-Turrax homogenizer (Type Tp 18/10, Janke and Kunkel, Germany) in 1 mL of 0.4 M HClO4, 0.01% K2EDTA and 0.1% Na2S2O5; and the homogenate was centrifuged at 40,000 g for 15 min at 4 °C. The resulting supernatant was filtered (Millex LH, Millipore) and aliquots were injected into the HPLC system on a reversed-phase column (Spherisorb S3 ODS1 C18; 3- μm particle size range) coupled to a Tracer ODS2 C18 pre-column (2–5 μm particle size range, Teknokroma, Spain). The mobile phase consisted of 0.1 M KH2PO4; 2.1 mM octane sulfonic acid; 0.1 mM K2EDTA; 2 mM NaCl and 12% methanol (pH 2.7–2.8, adjusted with 85% H3PO4), which was pumped at a flow rate of 0.8 ml/min with a Waters M-600 controller solvent delivery system (Waters, Spain). The compounds were electrochemically detected by a cell with a glassy working carbon electrode with an applied oxidation potential of +0.75 V against an in situ Ag/AgCl reference electrode (Waters M-2465 Electrochemical Detector). The output current was monitored by an interphase (Waters busSAT/IN Module) connected to a PC. The concentrations of the compounds in a given sample were calculated by interpolating the corresponding peak height into a parallel standard curve using the software Empower Pro (Waters).
Western Blot Analysis
The remaining hippocampus from animals was homogenized in 1:15 weight/volume of homogenization buffer (50 mM Tris-HCl, pH 7,5; 1 mM EDTA; 2% SDS) in the presence of a protease inhibitor cocktail (Pierce). Extracts were sonicated three times for 5 s, and then they were mixed 1:1 with loading Laëmmli buffer. Protein samples (20 μg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (3 MM Whatman). Immunoblot analyses were performed by using the antibodies: anti-SIRT1 (rabbit polyclonal, 1:1000 dilution, Millipore); anti-NF-κB p65 (rabbit polyclonal, 1:1000 dilution, Santa Cruz); anti-NF-κB p65 (acetyl K310) (rabbit polyclonal, 1:1000 dilution, Abcam); anti-α-tubulin (mouse monoclonal, 1:5000, Sigma). Proteins were detected with the ECL Western Blotting Substrate (Pierce). The chemiluminescence bans were digitalized with GeneGnome XRQ (Syngene, USA) and analyzed with the public domain software ImageJ (Rasband, 1997–2016).
Drugs and Reagents
Drugs and reagents were purchased from Sigma-Aldrich (a part of the Merck group, Germany).
Statistics
Results are expressed as means ± S.E.M. of values. Two-way ANOVA followed by Fisher’s LSD test was used for statistical evaluation of behavioural test in each experimental group throughout the chronic treatments. One-way ANOVA followed by Newman-Keul test was used for other statistical evaluations. Level of significance was set at p ≤ 0.05. Chi-square test was used to analyse strategies in Barnes maze test. Data were analyzed with the program Graph-Pad Prism, version 5.0.
Results
Effects of Chronic Polyphenols Treatments on the Deterioration of Spatial Working Memory of old Rats in Radial Maze Test
As expected, aging was characterized by a deterioration in spatial working memory analyzed by the radial maze test (Fig. 2a, b). Old rats spent more time and made more errors than young rats during the task. However, old animals chronically treated with silymarin, quercetin and naringenin (20 mg/kg, i.p. daily) improved working memory; which was already observed at the middle of the treatment and more clearly at the end. Rats chronically treated with silymarin, quercetin and naringenin decreased their performance time and number of errors; showing performance time and errors similar to young animals in the radial maze test. These animals spent less time (30–60%) and made fewer errors (34–54%) during the task than rats treated with vehicle respect to the beginning of treatment (Fig. 2a, b). In order to ensure a correct working memory analysis and a correct interpretation of results from radial maze test, it was also analysed the motor activity as the ratio between the distance and the exploration time. No differences in motor activity were observed between groups (data not shown). Note that there were not observed acute effects for these polyphenols in the radial maze; analyzed 1 h after the an acute i.p. administration (20 mg/kg; data not shown, presented at the 9th FENS Forum of Neuroscience).
Radial Maze Test. Effects of silymarin (n = 5), quercetin (n = 7) and naringenin (n = 6) (20 mg/kg/day, i.p) in old rats on the eight-arm radial maze task, compared with young (n = 8) and old (n = 5) vehicle (vh) groups. Bars represent means ± SEM derived from the time spent to complete the task a, and the sum of errors made during the task b. For performance time a, two way ANOVA detected a significant main effect for the treatment: F(4, 66) = 13.76, p < 0.0001; but also effect for period of treatment: F(2, 66) = 5.86, p = 0.0045; and interaction of both: F(8, 66) = 2.38, p = 0.0257. Fisher’s LSD post-hoc test detected significant differences respect young control group with Old vehicle group at start, middle, and end of treatment (***p ≤ 0.001); and with silymarin (***p ≤ 0.001), quercetin and naringenin (**p ≤ 0.01, both) treated groups only before the chronic treatment. Fisher’s LSD test also detected significant differences respect old vehicle group with polyphenol treated groups at middle († p ≤ 0.05 for silymarin and †† p ≤ 0.01 for quercetin and naringenin) and end of treatment († p ≤ 0.05 for silymarin and ††† p ≤ 0.001 for quercetin and naringenin). For errors b, two way ANOVA detected a similar significant effects for the treatment: F(4, 66) = 12.57, p < 0.0001; period of treatment: F(2, 66) = 18.41, p < 0.0001; but also for interaction of both: F(8, 66) = 3.88, p < 0.0001. Fisher’s LSD post-hoc test detected significant differences respect young control group with Old vehicle group at middle (*p ≤ 0.05) and end of treatment (***p ≤ 0.001); and with quercetin (*p ≤ 0.05 at middle and end of treatment). Ficher’s LSD test also detected significant differences respect old vehicle group with polyphenol treated groups at middle (†† p ≤ 0.001 for naringenin and ††† p ≤ 0.001 for silymarin and quercetin) and end of treatment (†† p ≤ 0.01 for silymarin and ††† p ≤ 0.001 for quercetin and naringenin)
Effects of Chronic Polyphenols Treatments on the Deterioration of Episodic Memory of old Rats on the Novel Object Recognition Test
Episodic-like memory was analyzed using the novel object recognition test (Fig. 3a, b). During familiarization phase, animals did not show any preference for an object (left or right, both identical), regardless of treatment performed (data not shown). However, during test phase, young animals explored the novel object longer than the familiar one; according to the rodent’s novelty preference behaviour; but old control rats did not showed statistical differences in the time spend exploring both objects. In contrast, old rats chronically treated with polyphenols explore the novel object for more time than the familiar one, in some cases already at the middle of the treatment period, and in all cases at the end of treatment period (Fig. 3a, b). On the other hand, the level of anxiety and exploration in the open field (areas visited, urinations and defecations) was not affected by treatments since no differences were observed between the old control group and the groups of animals treated with polyphenols (data not shown). Thus, increased exploration of the novel object in old animals treated with polyphenols seems to be more related to an improvement in memory than to an increase in exploration in general.
Novel objects recognition test. Long-term effects of silymarin (n = 5), quercetin (n = 7) and naringenin (n = 6) (20 mg/kg/day, i.p) in old rats on the test phase of the novel object recognition test, compared with young (n = 8) and old (n = 5) vehicle (vh) groups. Bars represent means ± SEM of the time spent exploring the novel and the familiar object (N = novel object, F = familiar object) a the difference in time spent exploring the novel and the familiar object (N-F) b. T-test detected significant differences in a comparing the time exploring the novel and the familiar objects (## p ≤ 0.01, ### p ≤ 0.001). In b, Two way ANOVA detected a significant main effect for the treatment: F(4, 66) = 7.91, p < 0.0001; but also an effect for period of treatment: F(2, 66) = 3.67, p = 0.0309; and interaction of both: F(8, 66) = 2.45, p = 0.0217. Fisher’s LSD post-hoc test detected significant differences respect young control group with Old vehicle group at start (*p ≤ 0.05), middle (**p ≤ 0.01), and end of treatment (***p ≤ 0.001); and with silymarin, quercetin and naringenin treated groups at star (*p ≤ 0.05, all); and naringenin at middle of treatment (*p ≤ 0.05). Fisher’s LSD test also detected significant differences respect old vehicle group with silymarin treated group at the middle of treatment (†† p ≤ 0.01) and all polyphenol treated groups at end of treatment (†† p ≤ 0.01 for silymarin and ††† p ≤ 0.001 for quercetin and naringenin)
Effects of Chronic Polyphenols Treatments on the Deterioration of Spatial Learning of old Rats in Barnes Maze Test
Spatial learning was assessed with the Barnes maze test at the middle and at the end of treatment period (Fig. 4). Throughout training period it was detected a general improvement in spatial learning; which was observed by a decrease in time and number of errors and by an increase in the use of direct strategy (data not shown). In any case, and as expected, test results showed an impairment in old rats respect to young rats in the parameters previously mentioned. However, at the end of treatments with silymarin, quercetin and naringenin old animals decreased significantly the time (77%, 44% and 54.3% respectively) and the number of errors (92.3%, 74.4% and 80.8% respectively) respect to the old control group. In quercetin and naringenin treated rats improvements in time were observed on midtreatment. The improvement in spatial learning in polyphenol treated rats was due to the predominant use of direct and serial strategy (as it happened in young rats). In contrast, old control rats followed a random strategy (Fig. 4c).
Barnes maze test. Effects of silymarin (n = 5), quercetin (n = 7) and naringenin (n = 6) (20 mg/kg/day, i.p) on Barnes maze test in old rats, compared with young (n = 8) and old (n = 5) vehicle (vh) groups, in the middle and at the end of the treatment. Bars represent means ± SEM derived from Total latency a and total errors b. For latency a, two way ANOVA detected a significant effect only for the treatment: F(4, 53) = 5.78, p < 0.001; but not effect for period of treatment: F(1, 53) = 2.8, p = 0.1002; and interaction of both: F(4, 53) = 1.33, p = 0.271. Fisher’s LSD post-hoc test detected significant differences respect young control group with Old vehicle group at middle (***p ≤ 0.001) and end of treatment (*p ≤ 0.05). Fisher’s LSD test also detected significant differences respect old vehicle group with polyphenol treated groups at middle († p ≤ 0.05 for silymarin, †† p ≤ 0.01 for quercetin, and ††† p ≤ 0.001 for naringenin) and end of treatment (†† p ≤ 0.01 for silymarin, p = 0.0894 for quercetin and † p ≤ 0.05 for naringenin). For errors b, two way ANOVA also detected a significant effect only for the treatment: F(4, 53) = 3.08, p < 0.05; but not effect for period of treatment: F(1, 53) = 2.14, p = 0.1495; and interaction of both: F(4, 53) = 0.77, p = 0.5483. Fisher’s LSD post-hoc test detected significant differences respect young control group with Old vehicle group at middle of treatment (*p ≤ 0.05). Fisher’s LSD test also detected significant differences respect old vehicle group with polyphenol treated groups at middle († p ≤ 0.05 for naringenin and p = 0.0747 for quercetin) and end of treatment († p ≤ 0.05 for silymarin and naringenin, and p = 0.0649 for quercetin). c Strategy percentage. Chi-square test detected significant differences respect young control group with old control animals (#χ2 = 8.57, df = 2, p < 0.05 at middle and ###χ2 = 15.96, df = 2, p < 0.001 end of treatment) and Silymarin group only at middle of treatment (#χ2 = 8.42, df = 2, p < 0,05); And between old treated animals only at the end of chronic treatment. In old animals treated with Silymarin ($$$χ2 = 21.01, df = 2, p < 0.001), Quercetin ($$$χ2 = 27.7, df = 2, p < 0.001), and Naringenin ($$$Chi-square: χ2 = 22.3, df = 2, p < 0.001) respect Old control group treated with vehicle
Effects of Chronic Polyphenols Treatments on the Deterioration of Motor Coordination in old Rats in Rotarod Test
Figure 5 shows the permanence time (s) in rotarod apparatus. Results showed that aging causes impairment in motor coordination, as it is showed by the decrease in permanence time on rotarod of old rats respect to young rats. Nevertheless, at the end of treatments with silymarin, quercetin and naringenin old rats not only managed to increase their permanence time on the wheel respect to the start of treatments, they also improved respect to old control rats (38%, 43% and 53%, respectively).
Rotarod test. Effects of silymarin (n = 5), quercetin (n = 7) and naringenin (n = 6) (20 mg/kg/day, i.p) in old rats on the rotarod test, compared with young (n = 8) and old (n = 5) vehicle (vh) groups. Bars represent the mean ± SEM of the permanence time on the apparatus. Two way ANOVA detected a significant main effect for the treatment: F(4, 66) = 26.83, p < 0.001; but also an effect for period of treatment: F(2, 66) = 8.383, p < 0.001. Fisher’s LSD post-hoc test detected significant differences respect young control group with Old vehicle group and the different polyphenol treated groups (***p ≤ 0.001 for all at any period of treatment, except **p ≤ 0.01 for naringenin at the end of treatment). Fisher’s LSD test also detected significant differences respect old vehicle group with naringenin treated group at the middle of treatment († p ≤ 0.05) and all polyphenol treated groups at end of treatment († p ≤ 0.05)
Effects of Chronic Polyphenols Treatments in old Rats on 5-HT Synthesis and Metabolism in Hippocampus, Striatum and Pineal Gland
Old rats showed a reduction in 5-HTP accumulation, 5-HT content and 5-HIAA level in hippocampus, striatum and prominently in pineal gland (Fig. 6), revealing a reduced activity of TPH-2 and TPH-1 due to aging. Chronic silymarin, quercetin and naringenin treatments in old rats increased similarly the TPH activity (measured as 5-HTP accumulation) in hippocampus (37%, 52% and 53%;), striatum (36%, 44% and 53%;) and pineal gland (239%, 288% and 238%). Consequently, the mean value of 5-HT content also increased after polyphenolic treatments (hippocampus: 44%, 48%, and 49%; striatum: 77%, 68% and 57%; pineal gland 323%, 307% and 145%, respectively). In contrast with these results, polyphenols reduced 5-HIAA levels in hippocampus and striatum respect to old control rats (with exception of sylimarin in hippocampus), suggesting an inhibitory effect on MAO-A enzime (Fig. 6). This effect was not seen in pineal gland where the effect of age reduced drastically 5-HIAA levels to limit of detection of the HPLC-ED detector used.
Serotonergic System. Effect of chronic silymarin (n = 5), quercetin (n = 7) and naringenin (n = 6) treatments on serotonergic system in hippocampus, striatum, and pineal gland. Bars represent (mean ± SEM in ng/g of wet tissue or total pineal gland) 5-HTP accumulation (during 30 min after decarboxylase inhibition), 5-HT tissue content, and 5-HIAA metabolite levels. One-way ANOVA followed by Newman-Keuls test was used for statistical evaluation: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to young rats; † p ≤ 0.05, †† p ≤ 0.01, ††† p ≤ 0.001 compared to old control rats. Statistic differences were found for 5-HTP accumulation (hippocampus: F(4, 26) = 11.7, p < 0.0001; striatum: F(4, 26) = 3.69, p < 0.0301; pineal gland: F(4, 26) = 7.85, p = 0,0003); 5-HT content (hippocampus: F(4, 26) = 3.05, p = 0.002; striatum: F(4, 26) = 4.82, p = 0.01; pineal gland: F(4, 26) = 47.46, p < 0,0001) and 5-HT content (hippocampus: F(4, 26) = 8.17, p = 0.0002; striatum: F(4, 26) = 7.8, p = 0.0003)
Effects of Chronic Polyphenols Treatments in old Rats on Noradrenaline Synthesis and Metabolism in Hippocampus
Aging decreased DOPA accumulation (synthesis of NA by TH) and NA content in hippocampus (Fig. 7a). Silymarin, quercetin and naringenin treatments in old rats increased DOPA accumulation (71%, 133% and 169%, respectively) and NA content (39%, 29% and 37%, respectively) respect to old control rats (Fig. 7a). HPLC method used do not allow detection of any NA metabolite.
Catecholaminergic system. Effect of chronic silymarin (n = 5), quercetin (n = 7) and naringenin (n = 6) treatment on catecholaminergic system in hippocampus and striatum. Bars represent DOPA accumulation (during 30 min after decarboxylase inhibition), DA or NA tissue content, and HVA metabolites levels in striatum. One-way ANOVA followed by Newman-Keuls test was used for statistical evaluation: **p ≤ 0.01, ***p ≤ 0.001 compared to young rats; † p ≤ 0.05, †† p ≤ 0.01, ††† p ≤ 0.001 compared to old control rats. Statistic differences were found for DOPA accumulation (hippocampus: F(4, 26) = 12.39, p < 0.0001; striatum: F(4, 26) = 58.22, p < 0.0001) and for NA content (hippocampus: F(4, 26) = 7.57, p = 0.0004) in; DA content (striatum: F(4, 26) = 55.03, p < 0.0001)
Effects of Chronic Polyphenols Treatments in old Rats on Dopamine Synthesis and Metabolism in Striatum
An age-related decrease in DOPA accumulation (TH activity), DA content and HVA levels in striatum was observed (Fig. 7b). Meanwhile, silymarin, quercetin and naringenin treated old rats increased similarly DOPA accumulation (63%, 57% and 42%, respectively) and DA content (28%, 38% and 39%, respectively) respect to old control rats, while HVA metabolite was not significantly modified, which suggested a possible attenuation of MAO activity by polyphenols (Fig. 7b).
Effects of Polyphenols on SIRT1 and NF-κB Immunoreactivity in Hippocampus
SIRT1 protein (110 kDa) level in hippocampus of old rats was significantly lower than in young rats (35% reduction, Fig. 8a). In contrast, the immunoreactivity for SIRT1 from old rats chronically treated with silymarin, quercetin and naringenin was significantly higher than in old control rats, reaching values close to those of young rats (77%, 86% and 93%, respectively Fig. 8a); indicating the ability of polyphenols to increase SIRT1 protein levels. Interestingly, in all SIRT1 western blots were also detected a secondary band of 75 kDa, which was also modulated by different experimental conditions. Previous studies have shown that this 75 kDa band could correspond to a cleaved form of SIRT1 (Oppenheimer et al. 2012). The immunoreactivity for this 75 kDa band also decreased in aged hippocampus, but less than SIRT1 band (110 kDa), only suffered a 14% decrease respect to young animals (Fig. 8a). Silymarin, quercetin and naringenin treatments also increased the immunorreactivity of this 75 kDa band to levels close to those of young rats (100%, 115% and 113% respect young rats levels, respectively).
Western blot. Effects of chronic silymarin (n = 5), quercetin (n = 7) and naringenin (n = 6) treatments on SIRT1 (110 and 75 kDa) a and NF-κB acetylated, total and NF-κB acetylated respect to NF-κB total levels b in hippocampus, compared with young and old (vh) rats. Protein levels were normalized to α-tubulin content. Bars represent mean ± SEM of 4 experiments, and they are express as percentage relative to the young (vh) group. One-way ANOVA followed by Newman-Keuls test detected significant differences for SIRT1 (110 kDa: F(4, 26) = 15.03; p < 0.0001; 75 kDa: F(4, 26) = 7.19, p = 0.0005), NF-κB acetylated levels (F(4, 26) = 47.56; p < 0.0001), NF-κB total levels (F(4, 26) = 77.91; p < 0.0001) and NF-κB acetylated respect to total levels (F(4, 26) = 3.026; p < 0.05) comparing with young rats (*p ≤ 0.05, **p ≤ 0.01,*** p ≤ 0.001) and old rats († p ≤ 0.05, ††† p ≤ 0.001). Below graphs are showed a representative immunoblot of each analyzed protein
Additionally, these changes in SIRT1 observed were accompanied by changes in NF-κB levels. SIRT1 reductions observed in old rats were accompanied by an incresase in NF-κB acetylated immunoreactivity (16%); meanwhile, aging did not modify NF-κB total levels in hippocampus (Fig. 8b). In contrast, polyphenols treatments decreased levels of both NF-κB total and acetylated form, respect to both young and old control rats. These chronic treatments decreased NF-κB acetylated levels in old rats reaching values below those of young rats (silymarin 74%, quercetin 81% and naringenin 58%); but polyphenols treatments also reduced the immunoreactivity for NF-κB total in hippocampus below those of young rats (silymarin 37.38%, quercetin 36.84% and naringenin 49.72%, respectively).
Discussion
Although not all the molecular mechanisms involved in the aging process are well known, oxidative stress (Harman 1956) and inflammaging (Franceschi et al. 2000; Salminen et al. 2008a, b) have been identified as the leading causes. In this regard, polyphenols due to their antioxidant (Halliwell et al. 1997; Khurana et al. 2013) and anti-inflammatory properties (Tangney and Rasmussen 2013), has been pointed out as key molecules in the prevention of brain aging. Oxidative damage and inflammaging are two processes, which suffer a feedback among themselves, having different effects on brain during aging such as the declive in functionality of TPH and TH enzymes due to their inefficient phosphorylation after ROS injury (De la Cruz et al. 1996; Hussain and Mitra 2000). Altogether brings a marked reduction in monoamines levels such as NA, DA, and 5-HT (Esteban et al. 2010a, b; Sarubbo et al. 2015; Tsunemi et al. 2005) which are believed to be partially responsible for impairments in memory and motor coordination (Collier et al. 2004; Cools 2011; Haider et al. 2014; Ramis et al. 2016; Sarubbo et al. 2015), and for the prevalence of neurodegenerative diseases during senescence (Hussain and Mitra 2000). Results from the present work demonstrated that chronic administration of polyphenols (silymarin, quercetin and naringenin) to old rats can improve their cognitive and motor functions as well as restore TPH-1, TPH-2 and TH activity. As Figs. 6 and 7 show, monoamine levels (NA, DA and 5HT) are also increased in regions directly involved in cognitive and motor processes such as the hippocampus and the striatum; and also in the pineal gland, involved in the control of circadian rhythms; which also directly affect the cognitive functions. These results are in line to those observed from other polyphenols such as resveratrol (Sarubbo et al. 2015) and other antioxidants such as tocopherol (Ramis et al. 2016). These studies explained the positive effects of polyphenols as a consequence of the antioxidant protection of polyphenols on the enzymes involve in monoamines synthesis, which help to increase the monoamine levels contributing to the improve in cognitive function. Similarly to what is described in these other works, we have not observed significant effect from acute administration of any of the polyphenols tested in the present work (tested 1 h after i.p administration, 20 mg/kg), at least in the radial maze performance (data not shown, but presented at 9th FENS Forum). Suggesting a long-term adaptive mechanism for polyphenol neuroprotective effects, rather than an acute or fast effect. This is support by the fact that, cognitive performance in the different behavioral test is better at the end of the chronic treatments, comparing with the middle of these chronic treatments.
Additionally, It is noteworthy that there were not detected differences in anxiety or exploratory drive by effect of polyphenols treatments as it was seen in the analyses of Radial test and Novel object recognition test. This support the idea that the cognitive improvement after polyphenol treatments is due to enhancement of memory and learning capacities and not consequence of other factors that could influence the performance of behavioural test. Moreover, and in the same line of the previous statement, although there was deterioration in motor coordination due to aging, this did not affect the performance of the behavioural tests, since no differences in motor activity were detected during the performance of these behavioural tests.
The molecular mechanism behind these beneficial effects of polyphenols is still unknown. However, some proteins and signalling pathways have been higlighted as to be involved in it. The first one is the enzyme SIRT1, which has been associated with a neuroprotective function in a myriad of neuronal injury and neurodegeneration paradigms (Ng et al. 2015). The function of SIRT1 is related to the regulation of memory (Gao et al. 2010), and therefore some studies have found that reduction in the expression of SIRT1 has negative consequences on cognitive abilities (Adler et al. 2007; Michán et al. 2010), as well as this decrease contributes to the development of neurodegenerative diseases associated with aging such as Alzheimer and Parkinson (Herskovits and Guarente 2013 and 2014; Qin et al. 2006). Results of the present work corroborate that aging reduces SIRT1 levels in hippocampus (Quintas et al. 2012). The present work also demonstrates that silymarin, quercetin and naringenin can increase SIRT1 levels in aged rats. Although it has been shown in vitro that resveratrol can activate SIRT1 directly through an allosteric mechanism (Hubbard et al. 2013), in vivo this direct effect has not been observed and it is not well known how polyphenols increase SIRT1 activity in vivo. It seems that oxidative stress is a key event, because it reduces SIRT1 mRNA levels inducing inhibition of SIRT1 expression (Yamakuchi et al. 2008). In addition, cysteine residues from SIRT1 are vulnerable to oxidation affecting both the activity of SIRT1 and its degradation by the proteasome (Cai et al. 2012; Furukawa et al. 2007). Therefore, we hypothesize that polyphenols due to their antioxidant properties may avoid the consequences of oxidative damage, protecting SIRT1 enzyme.
SIRT1 modulation could contribute to modulate another important action mechanism for polyphenol effects, NF-κB signaling pathway. Many age-related deleterious effects observed in the brain could be related with inflamm-aging processes, in which NF-κB signaling plays a highly relevant role (Adler et al. 2007; Chen and Greene 2003). It is of crucial importance the finding that the efficiency of the NF-κB signalling might be regulated by SIRT1 longevity factor (Adler et al. 2007; Salminen et al. 2008; Yeung et al. 2004). SIRT1 interacts with the RelA/p65 subunit of NF-κB and inhibits transcription by deacetylating RelA/p65 at lysine 310 (Chen et al. 2005; Kauppinen et al. 2013; Xie et al. 2013; Yeung et al. 2004). Thus, the age-related decrease in SIRT1 activity enhances the NF-κB signaling, which supports inflammatory responses in brain (Salminen et al. 2013). In this regard, we detected an increase in NF-κB acetylated levels in hippocampus of old rats without significant changes in total protein levels of NF-κB. Interestingly, all polyphenolic treatments produced a prominent decrease in NF-κB acetylated level in hippocampus from aged rats; with a greater effect for naringenin. But these effects were accompanied by a decrease in NF-κB total levels, coinciding with previous results from old rats fed with diet rich in polyphenols (Goyarzu et al. 2004). Results suggest that changes in NF-κB acetylated levels are mainly due to changes in total protein levels rather than changes in post-transcriptional modifications. The pharmacological inhibition of NF-κB has been shown to prevent the occurrence of different types of inflammation in mouse models of premature aging (Gillum et al. 2011; Osorio et al. 2012; Tilstra et al. 2012; Yao et al. 2012; Zhang et al. 2010). Consistently, our results suggest that polyphenolic treatments could lead to a downregulation of the NF-κB signaling pathway through a SIRT1 mediated mechanism (direct or indirect); which would contribute to neuroprotection by reducing the proinflammatory state in the hippocampus of aged rats.
In addition, it has been discovered under proinflammatory conditions an alternative mechanism of action for SIRT1. Oppenheimer et al. (2012) discovered that exposing human osteoarthritic chondrocytes to inflammatory factors (like TNF-α) generates a stable and enzymatically inactive SIRT1 fragment (75 kDa) resistant to further degradation; which in turn prevents cell death avoiding apoptosis. In this regard, we have observed for the first time in brain the 75 kDa fragment of SIRT1, which was conserved by aging and reduced by polyphenolic treatments in hippocampus. The increase in SIRT1 fragmentation during aging could reveal a cell mechanism to protect them from inflamm-aging; and polyphenols would reduce SIRT1 fragmentation as a consequence of proinflammatory state reduction in the brain. Current efforts are underway to clarify this.
On the other hand, Flowers et al. 2015 have demonstrated that NT-020 (a proprietary blend of polyphenols) can enhance performance in learning and memory tasks in aged mice by activating oxidative stress response pathways, and decrease inflammation trough the reduction of proinflammatory molecules (TNF-α, IL-1β, etc), meanwhile supports anti-inflammatory and pro-neurogenic (neurotrophines) gene expression in the hippocampus. It seems that these combinations of results agree with the results of the present work, which also found the combination of the following points: cognitive improvement, SIRT1 increased levels and reduction of inflammation after polyphenolic treatments. In addition, we found increased levels of monoamines, which can be explained in part by the regulation of inflammatory pathways and increased levels of SIRT1, which may interact reducing not only inflammation but also oxidative stress, protecting TH and TPH from ROS and cytokines. Moreover, a direct relationship between SIRT1 levels and brain monoaminergic systems modulation has been established, since SIRT1 directly regulates mood and behavior by deacetylating the NHLH2 transcription factor, which activates MAO-A transcription increasing anxiety (Libert et al. 2011; Chaudhuri et al. 2013). Results from the present work do not reflect this direct effect of SIRT1 on brain, because despite SIRT1 level increase due to polyphenol treatment, monoamine metabolites do not change. In this same regard, some polyphenols have been shown to directly inhibit MAO activity or monoamine re-uptake (Chen et al. 2015; Yañez et al. 2006), which could be a generalized mechanism of polyphenolic compounds. More studies are also needed to deep understand the relevance of SIRT1 on the effects of polyphenols on monoaminergic systems. In spite of the fact that we did not study the effects of polyphenols on adult hippocampal neurogenesis, it is very interesting the fact that Flowers et al. 2015 found an improvement in neurogenesis in aged rats after polyphenolic treatment, throughout the augment in Nrf-2 and Wnt signalling (directly involve in neurogenesis). Monoamines like serotonin and its receptors have a critical role in adult hippocampal neurogenesis, having positive impact on cell proliferation and survival of newborn neurons (Alenina and Klempin 2015). Moreover, an increase in number of neurons may lead to an increase in the number of potential monoamine synthesis sites, giving place to an increase in levels of monoamines. For these reasons, it seems that the increase in monoamine synthesis found in this work may be related to the regulation by polyphenols of several mechanisms such as oxidative stress, inflammaging and last but not less important neurogenesis, which all at the same time obviously affect positively cognition. It is necessary that research continues in this line for understanding the molecular and pharmacological network by which polyphenols can synergistically modulate all these processes meanwhile preserves cognition, avoiding aging consequences.
Finally, we have to consider that additional mechanism and/or brain regions could contribute to the observed phenotypes; since there is not a direct correlation between the polyphenol that improves to a greater extend behavioral parameters and the one that obtains a greater recovery of neurochemical and molecular parameters in the analyzed brain regions.
Conclusion
Chronic polyphenolic treatments modulate hippocampal SIRT1 and NF-κB levels, attenuating inflamm-aging and avoiding the monoamines descent in cognitive related brain regions. Therefore, polyphenols may be used as a potential therapy for restoring the impaired cognitive and motor functions that frequently accompany aging.
References
Adler A, Sinha S, Kawahara T, Zhang J, Segal E, Chang H (2007) Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev 21:3244–3257
Alenina N, Klempin F (2015) The role of serotonin in adult hippocampal neurogenesis. Behav Brain Res 277:49–57
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Barrett G, Bennie A, Trieu J, Ping S, Tsafoulis C (2009) The chronology of age-related spatial learning impairment in two rat strains, as tested by the Barnes maze. Behav Neurosci 123:533–538
Bishop N, Lu T, Yankner B (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535
Cai W, Ramdas M, Zhu L, Chen X, Striker G, Vlassara H (2012) Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci U S A 109:15888–15893
Chaudhuri AD, Yelamanchili SV, Fox HS (2013) MicroRNA-142 reduces monoamine oxidase a expression and activity in neuronal cells by downregulating SIRT1. PLoS One 8:e79579
Chen LF, Greene WC (2003) Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J Mol Med (Berl) 81:549–557
Chen J, Zhou Y, Mueller-Steiner S, Chen L, Kwon H, Yi S, Mucke L, Gan L (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem 280:40364–40374
Chen J, Lin D, Zhang C, Li G, Zhang N, Ruan L, Yan Q, Li J, Yu X, Xie X, Pang C, Cao L, Pan J, Xu Y (2015) Antidepressant-like effects of ferulic acid: involvement of serotonergic and norepinergic systems. Metab Brain Dis 30:129–136
Chondrogianni N, Kapeta S, Chinou I, Vassilatou K, Papassideri I, Gonos E (2010) Anti-ageing and rejuvenating effects of quercetin. Exp Gerontol 45:763–771
Collier T, Greene J, Felten D, Stevens S, Collier K (2004) Reduced cortical noradrenergic neurotransmission is associated with increased neophobia and impaired spatial memory in aged rats. Neurobiol Aging 25:209–221
Cools R (2011) Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol 21:402–407
De La Cruz C, Revilla E, Venero J, Ayala A, Cano J, Machado A (1996) Oxidative inactivation of tyrosine hydroxylase in substantia nigra of aged rat. Free Radic Biol Med 20:53–61
Deacon R, Rawlins J, Nicholas P (2002) Learning impairments of hippocampal-lesioned mice in a paddling pool. Behav Neurosci 116:472–478
Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. (2009) Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience 159(3):993–1002. doi:10.1016/j.neuroscience.2009.01.017
Esteban S, Garau C, Aparicio S, Moranta D, Barceló P, Fiol M, Rial R (2010a) Chronic melatonin treatment and its precursor L-tryptophan improve the monoaminergic neurotransmission and related behavior in the aged rat brain. J Pineal Res 48:170–177
Esteban S, Garau C, Aparicio S, Moranta D, Barceló P, Ramis M, Tresguerres J, Rial R (2010b) Improving effects of long-term growth hormone treatment on monoaminergic neurotransmission and related behavioral tests in aged rats. Rejuvenation Res 13:707–716
Flowers A, Lee JY, Acosta S, Hudson C, Small B, Sanberg CD, Bickford PC. (2015) NT-020 treatment reduces inflammation and augments Nrf-2 and Wnt signaling in aged rats. J Neuroinflammation. 12(1):174
Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908:244–254
Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S (2007) H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem 20:45–54
Gao J, Wang W, Mao Y, Gräff J, Guan J, Pan L (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466:1105–1109
Gillum M, Kotas M, Erion D, Kursawe R, Chatterjee P, Nead K, Muise E, Hsiao J, Frederick D, Yonemitsu S, Banks A, Qiang L, Bhanot S, Olefsky J, Sears D, Caprio S, Shulman G (2011) SirT1 regulates adipose tissue inflammation. Diabetes 60:3235–3245
Goyarzu P, Malin DH, Lau FC, Taglialatela G, Moon WD, Jennings R, Moy E, Moy D, Lippold S, Shukitt-Hale B, Joseph JA (2004) Blueberry supplemented diet: effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr Neurosci 7:75–83
Haider S, Saleem S, Perveen T, Tabassum S, Batool Z, Sadir S, Liaquat L, Madiha S (2014) Age-related learning and memory deficits in rats: role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age (Dordr) 36:9653
Halliwell B, Zentella A, Gomez E, Kershenobich D (1997) Antioxidants and human disease: a general introduction. Nutr Rev 55:S44–S49
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Herskovits AZ, Guarente L (2013) Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res 23:746–758
Herskovits AZ, Guarente L. (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81(3):471–483
Hirai S, Kim Y, Goto T, Kang M, Yoshimura M, Obata A, Yu R, Kawada T (2007) Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci 81:1272–1279
Hubbard BP, Gomes AP, Dai H et al (2013) Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339:1216–1219
Hussain A, Mitra A (2000) Effect of aging on tryptophan hydroxylase in rat brain: implications on serotonin level. Drug Metab Dispos 28:1038–1042
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A (2013) Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25:1939–1948
Khan M, Khan M, Khan A, Ahmed ME, Ishrat T, Tabassum R, Vaibhav K, Ahmad A, Islam F (2012) Naringenin ameliorates Alzheimer’s disease (AD)-type neurodegeneration with cognitive impairment (AD-TNDCI) caused by the intracerebroventricular - streptozotocin in rat model. Neurochem Int 61:1081–1093
Khurana S, Venkataraman K, Hollingsworth A, Piche M, Tai T (2013) Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients 5:3779–3827
Lemon N, Manahan-Vaughan D (2006) Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. Neuroscience 26:7723–7729
Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D (2009) Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on β-adrenergic receptor activation. Cereb Cortex 19:2827–2837
Li S, Cullen W, Anwyl R, Rowan M (2003) Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci 6:526–531
Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Guarente L (2011) SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 147:1459–1472
Meneses A (1999) 5-HT system and cognition. Neurosci Biobehav Rev 23:1111–1125
Michán S, Li Y, Chou M, Parrella E, Ge H, Long J, Allard J, Lewis K, Miller M, Xu W, Mervis R, Chen J, Guerin K, Smith L, McBurney M, Sinclair D, Baudry M, de Cabo R, Longo V (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30:9695–9707
Moranta D, Esteban S, García-Sevilla J (2009) Chronic treatment and withdrawal of the cannabinoid agonist WIN 55,212-2 modulate the sensitivity of presynaptic receptors involved in the regulation of monoamine syntheses in rat brain. Naunyn Schmiedeberg's Arch Pharmacol 379:61–72
Moranta D, Barceló P, Aparicio S, Garau C, Sarubbo F, Ramis M, Nicolau C, Esteban S (2014) Intake of melatonin increases tryptophan hydroxylase type 1 activity in aged rats: preliminary study. Exp Gerontol 49:1–4
Murchison C, Zhang X, Zhang W, Ouyang M, Lee A, Thomas S (2004) A distinct role for norepinephrine in memory retrieval. Cell 117:131–143
Nencini C, Giorgi G, Micheli L (2007) Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine 14:129–135
Ng F, Wijaya L, Tang B (2015) SIRT1 in the brain-connections with aging-associated disorders and lifespan. Front Cell Neurosci 9:64
Oppenheimer H, Gabay O, Meir H, Haze A, Kandel L, Liebergall M, Gagarina V, Lee E, Dvir-Ginzberg M (2012) 75-Kd Sirtuin 1 blocks tumor necrosis Factor α-mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum 64:718–728
Osorio F, Bárcena C, Soria-Valles C, Ramsay A, de Carlos F, Cobo J, Fueyo A, Freije J, López-Otín C (2012) Nuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory response. Genes Dev 26:2311–2324
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev 2:270–278
Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers J, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve A, Pasinetti G (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281:21745–21754
Quintas A, De Solís AJ, Díez-Guerra FJ, Carrascosa JM, Bogoñez E (2012) Age-associated decrease of SIRT1 expression in rat hippocampus. Prevention by late onset caloric restriction. Exp Gerontol 47:198–201
Ramis M, Sarubbo F, Sola J, Aparicio S, Garau C, Miralles A, Esteban S (2013) Cognitive improvement by acute growth hormone is mediated by NMDA and AMPA receptors and MEK pathway. Prog Neuro-Psychopharmacol Biol Psychiatry 45:11–20
Ramis M, Sarubbo F, Terrasa J, Moranta D, Aparicio S, Miralles A, Esteban S (2016) Chronic α-tocopherol increases central monoamines synthesis and improves cognitive and motor abilities in old rats. Rejuvenation Res 19:159–171
Rueda-Orozco P, Soria-Gomez E, Montes-Rodriguez CJ, Martínez-Vargas M, Galicia O, Navarro L, Prospero-García O (2008) A potential function of endocannabinoids in the selection of a navigation strategy by rats. Psychopharmacology 198:565–576
Salminen A, Ojala J, Huuskonen J, Kauppinen A, Suuronen T, Kaarniranta K (2008) Interaction of aging-associated signaling cascades: inhibition of NF-κB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci 65:1049–1058
Salminen A, Kaarniranta K, Kauppinen A (2013) Crosstalk between oxidative stress and SIRT1: impact on the aging process. Int J Mol Sci 14:3834–3859
Sarubbo F, Ramis M, Aparicio S, Ruiz L, Esteban S, Miralles A, Moranta D (2015) Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age (Dordr) 37:9777
Sastre-Coll A, Esteban S, García-Sevilla JA (1999) Effects of imidazoline receptor ligands on monoamine synthesis in the rat brain in vivo. Naunyn Schmiedeberg's Arch Pharmacol 360:50–62
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45:287–306
Sharma S, Rakoczy S, Brown-Borg H (2010) Assessment of spatial memory in mice. Life Sci 87:521–536
Tangney CC, Rasmussen HE (2013) Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep 15:324
Tilstra J, Robinson A, Wang J, Gregg S, Clauson C, Reay D, Nasto L, St Croix C, Usas A, Vo N, Huard J, Clemens P, Stolz D, Guttridge D, Watkins S, Garinis G, Wang Y, Niedernhofer L, Robbins P (2012) NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest 122:2601–2612
Tsunemi A, Utsuyama M, Seidler B, Kobayashi S, Hirokawa K (2005) Age-related decline of brain monoamines in mice is reversed to young level by Japanese herbal medicine. Neurochem Res 30:75–81
Venkataraman K, Khurana S, Tai T (2013) Oxidative stress in aging-matters of the heart and mind. Int J Mol Sci 14:17897–17925
Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M (2003) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299:76
Xie J, Zhang X, Zhang L (2013) Negative regulation of inflammation by SIRT1. Pharmacol Res 67:60–67
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 105:13421–13426
Yañez M, Fraiz N, Cano E, Orallo E (2006) Inhibitory effects of cis- and transresveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem Biofis Res Commun 344:688–695
Yang H, Bi Y, Xue L, Wang J, Lu Y, Zhang Z, Chen X, Chu Y, Yang R, Wang R, Liu G (2015) Multifaceted modulation of SIRT1 in cancer and inflammation. Crit Rev Oncog 20:49–64
Yao H, Chung S, Hwang J, Rajendrasozhan S, Sundar I, Dean D, McBurney M, Guarente L, Gu W, Rönty M, Kinnula VVL, Rahman I (2012) SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 122:2032–2045
Yeung F, Hoberg JJE, Ramsey CSC, Keller MMD, Jones DRD, Frye RRA, Mayo MMW (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380
Zhang H, Li L, Gao P, Chen H, Zhang R, Wei Y, Al E (2010) Involvement of the p65/RelA subunit of NF-κB in TNF-α-induced SIRT1 expression in vascular smooth muscle cells. Biochem Biophys Res Commun 397:569–575
Acknowledgements
Authors thank the financial contribution of: Universitat de les Illes Balears (UIB)-Govern Balear (ECT 025 09), Pont La Caixa-UIB Program (7/2014) and MINECO, Madrid, Spain (SAF2014-55903-R). F. Sarubbo was supported by a UIB predoctoral contract and MR Ramis was supported by a predoctoral FPU Spanish Ministry of Education fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest, they have been informed, and consent to manuscript publication.
Human and Animal Rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the Bioethical Committee of the University of the Balearic Islands and with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Directive 86/609/EEC).
Rights and permissions
About this article
Cite this article
Sarubbo, F., Ramis, M.R., Kienzer, C. et al. Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats. J Neuroimmune Pharmacol 13, 24–38 (2018). https://doi.org/10.1007/s11481-017-9759-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-017-9759-0