Abstract
Rationale
One of the adaptive abilities of the brain is the generation of a strategy to optimize acquisition of information, i.e., learning. In this study, we explored the role of environmental conditions (the light–dark cycle) and of the endocannabinoid anandamide in rats to select a strategy to solve the Barnes maze (BM).
Objectives
To determine the effects of manipulating the cannabinergic system on a spatial task in relation to the light–dark cycle.
Materials and methods
Rats received an intrahippocampal or intrastriatal administration of anandamide, AM251, or their combination at two different points of the light–dark cycle (1300 and 0100 hours), and their performance in the BM was evaluated. In addition, we determined the expression of the cannabinoid 1 receptor (CB1R) in the hippocampus and striatum throughout the light–dark cycle.
Results
Results indicate that rats solved the BM by using a spatial strategy during the light phase and a procedural (serial) strategy during the dark phase of the cycle. CB1R expression varied in the hippocampus, being higher at 1300 hours and lower at 0100 hours, whereas its expression remained unchanged in the striatum.
Conclusions
Changes in the brain, which include changes in the endocannabinoid system, prompt it to use different strategies (spatial and procedural, or others not evaluated in this study) to cope with the environmental demands. These cerebral changes are adaptive responses to the light–dark cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cannabinergic system has been implicated in many physiological processes, such as control of movement (Ameri 1999), food intake (Hao et al. 2000; Martinez-Gonzalez et al. 2004; Soria-Gomez et al. 2007), sexual behavior (Martinez-Gonzalez et al. 2004), sleep (Murillo-Rodriguez et al. 1998, 2001), learning and memory (Murillo-Rodriguez et al. 1998, 2001; Hampson and Deadwyler 1998). One of the reasons for this high variety of cannabinergic functions is the widespread cannabinoid 1 receptor (CB1R) expression in almost all the central nervous system (Tsuo et al. 1998; Pettit et al. 1998; Egertova and Elphik 2000). We reported that the CB1R (protein and mRNA) fluctuates in the pons of rats after the light–dark cycle, exhibiting the highest concentration during light (Martinez-Vargas et al. 2003). On the other hand, it has been shown that the amount of the endogenous cannabinoids, anandamide (ANA) and 2-arachidonoyl-glycerol (2-AG), also fluctuates according to the light–dark cycle in the nucleus accumbens, the prefrontal cortex, the hippocampus, the hypothalamus, and the striatum of rats (Valenti et al. 2004; Murillo-Rodriguez et al. 2006). These variations in the amount of endocannabinoids and their receptors suggest that the level of modulation given by the cannabinergic system could vary according to the hour of the day. In this work, we focus on the potential modulation on memory systems (hippocampus and striatum).
During acquisition of information, individuals utilize different strategies that make this process much more efficient. We do not know how a specific strategy from a repertoire of strategies is chosen to contend with a specific problem. However, there is an extensive literature involving specific brain structures in the regulation of specific strategies. For example, a procedural strategy is regulated by the striatum, whereas a spatial strategy is regulated by the hippocampus (Packard and McGaugh 1992, 1996; White and McDonald 2002). If these structures are actually playing these roles, questions we may ask are, when does one structure govern the behavioral output and which are the mechanisms subserving the selection? To obtain some responses to this matter, we studied the effect of the light–dark cycle and the effect of endocannabinoids on the solution of the Barnes maze (BM), a task that can be solved by using different strategies (Harrison et al. 2006).
The rationale to study the dependence of the strategy selection on the light–dark cycle was based on the observation that mental efficiency changes along the day (from 0800 to 2300 hours) in humans (Folkard 1975, 1979; May et al. 2005). These changes have been attributed to changes in the strategy to acquire the information but not to changes in the capacity to process information (Folkard 1975, 1979; May et al. 2005). Another study shows that the recall of implicit and explicit information in humans depends on the time of the day tests are applied, suggesting different circadian fluctuations for explicit and implicit retrieval (May et al. 2005). Moreover, studies performed in animals have also supported that the solution of different memory tasks depends strongly on the light–dark cycle (Chaudhury and Colwell 2002; Ralph et al. 2002).
On the other hand, it has been shown that both Δ-9 tetrahydrocannabinol (THC) and ANA deteriorate memory consolidation in several behavioral paradigms (Lichtman and Martin 1996; Varvel et al. 2001; Murillo-Rodriguez et al. 1998, 2001). However, it has also been suggested that THC might induce a shift in the strategy to solve the task (Hampson and Deadwyler 1998), potentially depending on the availability of the receptor during the course of the day (Martinez-Vargas et al. 2003) or of the endogenous ligand (Valenti et al. 2004; Murillo-Rodriguez et al. 2006).
Based on these THC and ANA effects on memory, the diurnal variations of the CB1 expression, and the level of endocannabinoids and the diurnal variations of behavior, we hypothesize that the cannabinergic system modulates the performance on a memory task depending on the light–dark cycle. We decided to analyze whether ANA administered directly into the hippocampus or into the dorsolateral striatum affects the performance of rats in the BM and that such an effect depends on the light–dark cycle. In addition, to further characterize the potential role of the endocannabinoid system in this process, we determined the diurnal variations in the expression of the CB1R in the striatum and hippocampus.
Materials and methods
Subjects
For all the experiments, Wistar albino male rats (weight, 250–300 g) were used. They were housed individually in a temperature-controlled environment with a 12-h light/dark cycle (lights on at 8:00 a.m.) and ad libitum access to food and water. Rats were housed individually 5 days before the surgery and throughout the entire experiment.
Drugs
Arachidonylethanolamide (ANA) and the CB1R antagonist AM251 were obtained from Sigma Aldrich and Cayman Chemical, respectively. The concentrations for intrahippocampal and intrastriatal administrations were 0.5 μg/1 μl (ANA0.5μg), 1 μg/1 μl (ANA1μg), and 2 μg/1 μl (ANA2μg) of ANA dissolved in dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS; 30%) and 1.6 μg/1 μl of AM251 dissolved in DMSO in PBS (30%; equimolar concentration to the ANA1μg concentration).
Surgery
Ten days before the behavioral training, rats were implanted bilaterally under anesthesia (cocktail: ketamine 66 mg/kg + xylazine 0.26 mg/kg + acepromazine 1.3 mg/kg) with guide cannulae (23 gauge) directed to either hippocampus (P = 4, L = 2.5, V = 2.2; Fig. 1a) or either dorsolateral striatum (a = 0.2, L = 3.0, V = 3.0; Fig. 1b). The injector protruded 1 mm from the tip of the guide cannula. Three supporting stainless steel screws were implanted into the skull. The brains were prepared for histological analysis with cresyl violet staining to verify the correct placement of the injector (Fig. 1a,b)
Barnes maze
This maze (Barnes 1979) consists of a 150-cm-diameter and 3.5-cm-thick circular wooden disk, elevated 90 cm above the floor. Forty holes, 7 cm in diameter, were equidistantly located around the perimeter and centered 5 cm from the edge. A black wooden escape tunnel (10 × 10 × 30 cm) was placed beneath one hole, selected randomly for each rat, the position of the escape tunnel remained in the same spatial place for the entire training.
BM solution
To solve this maze, rats are placed inside a cylindrical chamber located at the center of the maze. When they are set free from this chamber, rats start searching randomly for the escape tunnel. While the training progresses, rats switch from random searching to one of two strategies, spatial or serial.
Spatial strategy
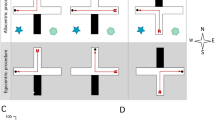
For a strategy to be considered spatial, rats had to comply with the following criteria: Rats had to go directly to the target zone (two holes on each side of the target hole, five holes total) and keep exploring there (Fig. 1c). If rats explored a hole outside this range, the strategy was not considered spatial. Neither the time nor the number of errors was taken into account to qualify a strategy as spatial.
Serial strategy
For a strategy to be considered serial, rats had to comply with the following criteria: They had to explore holes in sequence (one after the previous adjacent) and following one direction. If these criteria were not reached, the strategy was not considered serial. Rats could start exploring at any hole, but if they searched the target zone, only there the strategy was considered spatial. Neither the time nor the number of errors was taken into account to qualify this strategy as serial (Fig. 1d). Any other way to approach the target hole was considered a random behavior (Fig. 1e). We also quantified the total time of performance in the maze to reach the target hole. Each nontarget hole visited was considered an error, and the number of holes between the first hole explored and the target one was considered the “distance.” At the end, as a complementary measure of behavior, we plotted the percentage of errors made in the target zone.
Training
Animals were trained to solve the BM during one session on each of the five successive days at 1300 or 0100 hours. Each session consisted of four trials, for a total of 20 trials in 5 days. On the first trial, rats were placed into the escape tunnel for 1 min. At the end of this time, rats were placed at the center of the BM, inside the cylindrical chamber, and a white noise (90 dB) was delivered by a speaker located 1 m above the center of the maze, affixed to the room’s ceiling. Rats were left in this chamber for 10 s and then set free to explore the maze and find the escape tunnel. The trial ended when the rat entered the escape tunnel or once 4 min had elapsed. When the rat entered the escape tunnel, the white noise was turned off, and the rat was allowed to stay in the tunnel for 1 min. The escape tunnel was always located beneath a hole positioned in the same place with regard to the room. In this way, although we turned the BM around, for each rat in every single trial, the escape tunnel was always in the same place. The position of the escape tunnel was selected randomly for each rat at the beginning of the training. Light (approximately 50 lx) was always on during the training session, regardless of the phase of the cycle. The training session lasted 20 min at the most.
Immediately after the end of the last trial of each day, rats were injected directly into the hippocampus or dorsolateral striatum with the corresponding treatment. ANA administration was performed at the end of each training session because endocannabinoids are involved in plastic processes, they might affect memory consolidation, and ANA is quickly inactivated by the cellular enzymes (for review, see Bisogno et al. 2005); in this way, we were able to avoid undesirable effects on locomotor activity and memory evocation on the next day. A videotape of each rat’s performance was recorded for off-line analysis.
In the second experiment, two different groups of rats were trained in exactly the same way as previously described, except that, in this case, drug administration was conducted 2 h after the end instead of immediately after the training session. This noncontingent administration would allow us to determine whether the effects of the treatments were related to consolidation of memory or not.
Cannabinoid receptor determination
Western blotting
To assess the CB1R protein expression, tissue samples were collected at six different time points of the light–dark cycle (0900, 1300, 1700, 2100, 0100, and 0500 hours; n = 10 rats per point). Samples were processed independently. Samples were homogenized in PBS with protease inhibitors and centrifuged at 600×g, 10 min, 4°C. The supernatant was centrifuged at 39,000×g, 15 min, 4°C. Twelve percent analytical sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed as described elsewhere (Laemmli 1970). Briefly, resuspended precipitate of tissue homogenates (15 μg protein) was mixed 1:1 with sample Laemmli buffer and heated (95°C, 5 min) before loading on a 0.75-mm-thick gel. Samples were subjected to electrophoresis (150 V, 2 h), and gels were transferred onto a nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) at 100 V for 1 h at 4°C. The membrane was incubated with 3% PBS-Tween, 10% nonfat dry milk, and 2% normal goat serum for 30 min at room temperature (rt), followed by incubation with anti-CB1R (Cayman Chemical Company, Ann Arbor, MI, USA) 1:1,600 overnight at 4°C. The blot was washed with PBS-Tween (three times, 5 min/each), then incubated 1 h at rt in a 1:2,000 dilution of goat anti-rabbit IgG horseradish peroxidase conjugate, and developed with diaminobenzidine (0.5 mg/ml in PBS plus 0.3 μl/ml 30% H2O2). Density of bands was analyzed using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
RNA extraction
To detect changes in CB1R mRNA expression, samples were collected at the same six time points of the Western blot assay (n = 5 per point). Total RNA was extracted using the TRIzol method (Life Technologies, Rockville, MD, USA). In brief, 100 mg of tissue was homogenized in 1 ml of TRIzol; 200 μl of chloroform was added and mixed in a vortex. Two phases were obtained by centrifuging at 12,000×μg for 10 min. The aqueous phase was recovered, and 0.5 ml of isopropanol was added. Total RNA was obtained by centrifugation at 12,000×μg for 10 min. Its integrity was confirmed by running an aliquot on a 1% agarose gel.
Reverse transcriptase-polymerase chain reaction
RNA (2 μg) was reverse transcribed using One Step System (Life Technologies) according to manufacturer’s recommendations, adding a step of DNAse treatment. Briefly, 2 μg of total RNA was incubated with DNAse (RNAse free), 1 U in 10 μl of the appropriate buffer solution, for 15 min at rt, adding 1 μl of 25 mM ethylenediaminetetraacetic acid, and heating at 65°C for 10 min to stop the reaction. This mixture was used for reverse transcriptase (RT), adding 25 μl of buffer 2X (0.4 mM of each dNTP, 2.4 mM MgSO4), 1 μl RT/thermus aquaticus polymerase mixture, MgCl2, CB1RR antisense primer: 5′atgctgttgtctagaggctg-3′ (10 μM, 1 μl), and water to adjust to 50 μl. RT was performed at 42°C for 30 min and stopped by heating 5 min at 94°C. Polymerase chain reaction (PCR) conditions were as follows: CB1RR sense primer, 5′-catcatcatccacacgtcag-3′; CB1RR antisense primer, 5′-atgctgttgtctagaggctg-3′; 33 cycles; denaturing, 45 s, 94°C; annealing, 45 s, 53°C; and elongation 1 min, 72°C, as previously described (Martinez-Vargas et al. 2003). We also amplified cyclophilin cDNA to assess RNA quality. We used primers, 5′-agacgccgctgtctcttttcg-3′ and 5′-ccacacagtcggagatggtgatc-3′, as previously described (Martinez-Vargas et al. 2003). In all assays, RNA samples without RT were run to exclude DNA contamination.
To evaluate the significant changes of the CB1R (protein and mRNA) throughout the day, the mean value of the protein amount of each rat for each time point was calculated and compared against the mean of an arbitrarily chosen point in the curve (0900 hours).
Immunohistochemistry
Naive rats were deeply anesthetized and transcardially perfused at two time points of the dark–light cycle (0100 and 1300 hours; N = 5 rats per point). The brains were removed and processed for immunohistochemistry. All sections were processed at the same time and under the same conditions. Coronal sections (50 μm) were cut by means of a cryostat. Sections were collected, one out of five, and were selected with reference to Paxinos and Watson’s (1986) atlas. Tissue sections were incubated at rt with 0.075% H2O2 in PBS for 20 min to block the endogenous peroxidase. Then, sections were blocked in 5% normal goat serum/PBS with 0.3% Triton X-100 (TPBS). Upon completion of this part of the procedure, sections were incubated for 48 h at 4°C with CB1R antibody (Santa Cruz, CA, USA; 1:100 in TPBS). Bound antibodies were revealed by using the avidin–biotin peroxidase complex method (Vector Laboratories). Once sections were mounted and prepared, a photograph of each was taken under the exact same parameters of amplification, illumination, and contrast. Analysis of the CB1R immunoreactivity was performed in the dorsolateral region of the striatum and in the granular layer of the dentate gyrus (DG), CA1 and CA3 hippocampal regions. Each region was referred to a cell-free area (hippocampus–stratum radiatum and striatum–corpus callosum) in the same section to correct against background.
Statistical analyses
For the time performance and total errors in the BM, a two-way analysis of variance (ANOVA) test was used (treatment X day of training); Student–Newman–Keuls method was used to conduct pair-wise comparisons among the groups. Strategy was analyzed using a two-way ANOVA test (treatment X strategy); Student–Newman–Keuls method was used as post hoc test. All treatments in the hippocampus were analyzed together, but independently from treatments in the striatum. Distribution of errors was analyzed with a one-way ANOVA and all pair-wise multiple comparison procedures (LSD). Distance was analyzed using one-way ANOVA on ranks and Dunn’s method as post hoc test. Significant differences for the CB1R immunohistochemistry, RT/PCR, and Western blot were obtained by using a one-way ANOVA test and a Bonferroni post hoc test. All analyses were performed considering p < 0.05 as statistically significant.
Results
Intrahippocampal administration of ANA and AM251
There was a significant effect of day of training for all treatments during the light and dark phase of the cycle that reflected the decrease in the time required to solve the maze and the total errors along the sessions (Fig. 2a, Time Light F = 40.523, p < 0.001; Time Dark F = 43.232, p < 0.001; Fig. 2b, Errors Light F = 5.994, p < 0.001; Errors Dark F = 8.535, p < 0.001). Even in this condition, there was no effect of treatment on time of performance or total errors during the light or dark phase (Fig. 2a,b).
Intrahippocampal administration of ANA (dose-response curve), ANA + AM251, and AM251. Time of performance (a) and total errors (b) during the light and dark phase of the cycle. Distance with respect to the target-hole during the light (white bars) and dark (black bars) phase of the cycle (c). d Percentage of errors inside the target zone (dashed area in the maze inset on the right). Comparison of strategies between control and experimental groups during the light (e) and dark (f) phase of the cycle. Significant differences are indicated by lines under each plot; doted lines in c and d represent differences between control groups in light and dark cycle
Our results indicate that rats receiving the vehicle and training at 0100 or at 1300 hours used a similar amount of time to solve the BM (Fig. 2a), albeit using different strategies (Fig. 2e,f). In all figures, where strategies are illustrated, data are presented as the mean value of the last 2 days of training, when the performance time reaches the lowest values. Those rats trained at 1300 hours (light phase) learned to solve the BM using a spatial strategy with respect to rats trained at 0100 hours (p < 0.001). In addition, the median of the distance was significantly shorter during the light than during the dark phase (Fig. 2c; between groups F = 5, p < 0.05), the percentage of errors within the target zone was significantly higher (Fig. 2d; p < 0.001). In contrast, those rats trained at 0100 hours (dark phase) exhibited a serial strategy with respect to rats trained at 1300 hours (Fig. 2f); consistently, the median of the distance was far from the target hole (Fig. 2c), and the percentage of errors on the target area was lower.
As shown in Fig. 2, significant differences emerged between groups with respect to the use of different strategies depending on the light–dark cycle (Treatment x Strategy F = 10.223, p < 0.001). Particularly ANA1μg and ANA2μg administered during the light phase significantly decreased spatial strategy (ANA1μg p < 0.001, ANA2μg p = 0.003). ANA1μg significantly increased random strategy (p = 0.009; Fig. 2e). Concurrently, the median of the distance was located significantly far from the target hole (Fig. 2c; between groups F = 5, p < 0.001; Cont vs ANA1 p < 0.05; Cont vs ANA2 p < 0.05), and the distribution of errors in the target zone decreased (Fig. 2d; between groups F = 3.992, p < 0.001; Cont vs ANA1 p = 0.015; Cont vs ANA2 p = 0.021). No effects of ANA0.5μg were detected at 1300 or 0100 hours on strategy (Fig. 2f), distance (Fig. 2c), or distribution of errors (Fig. 2d). These data indicate that rats under higher doses of ANA loose their ability to exhibit a spatial strategy.
During the dark phase, no significant effect of ANA was detected on strategy (Fig. 2f), distance (Fig. 2c), or distribution of errors (Fig. 2d).
The effect of ANA1µg on spatial strategy during the light phase was prevented by the administration of the CB1R antagonist AM251; no significant differences were detected against control group in strategy, (Fig. 2e), distance (Fig. 2c), or distribution of errors (Fig. 2d). The same dose of AM251 used to block the effects of ANA produced no effects when administered alone during the light phase of the cycle (Fig. 2a,c,d,e); however, during the dark phase, it decreased significantly the expression of the serial strategy and increased spatial strategy (Fig. 2e; serial p < 0.001, spatial p = 0.001); in agreement with this, the distribution of errors was also increased in the target area (Fig. 2d; p = 0.039).
Intrastriatal administration of ANA and AM251
All treatments during the light and dark phase of the cycle caused a significant effect on the day of training that reflected the decrease in the time to solve the maze and the total number of errors along the sessions (Fig. 3a, Time Light F = 28.976, p < 0.001; Time Dark F = 39.449, p < 0.001; Fig. 3b, Errors Light F = 5.004, p < 0.001; Errors Dark F = 8.130, p < 0.001). There was no effect of treatment on time of performance or total errors during the light or dark phase (Fig. 3a,b). No differences were detected among treatments regarding strategies, distance, and distribution of errors during the light phase.
Intrastriatal administration of ANA, ANA + AM251, and AM251. Time of performance (a) and total errors (b) during the light and dark phase of the cycle. Distance with respect to the target-hole during the light (white bars) and dark (black bars) phase of the cycle (c). d Percentage of errors inside the target zone (dashed area in the maze inset on the right). Comparison of strategies between control and experimental groups during the light (e) and dark (f) phase of the cycle. Significant differences are indicated by lines under each plot; doted lines in c and d represent differences between control groups in light and dark cycle
On the other hand, during the dark phase of the cycle, the serial strategy was significantly decreased by all treatments (ANA1μg, p = 0.016; ANA2μg, p = 0.010; ANA–AM251, p = 0.002; AM251, p = 0.016) except with ANA0.5μg (p = 0.321) as illustrated in Fig. 3f. No changes were observed on random or spatial strategy (Fig. 3f). In this case, it is again remarkable that the effect of ANA administration was effective only in one phase of the cycle (dark phase). Another surprising result is that the ANA effect on behavior during the dark phase was not reversed by AM251 administration (Fig. 3f). In this experiment, the combination ANA–AM251 and AM251 alone also decreased serial strategy. These results suggest that activation, as much as inactivation, of the cannabinergic system affects striatal functionality, and this effect depends on the light–dark cycle.
Intrastriatal and intrahippocampal noncontingent administration of ANA
A group of rats was implanted in the hippocampus and trained during the light phase of the cycle, while another group was implanted in the striatum and trained during the dark phase. ANA1μg was injected 2 h after (ANA1μg/2ha) the end of each session every day of training, except on the fifth one. We decided to use only these groups because the main effects of ANA in the hippocampus were observed during the light phase of the cycle, and in the striatum during the dark phase. Data obtained from this group were compared against the control group and the group of ANA1μg injected immediately after the end of each session. Groups trained and receiving ANA1μg/2ha exhibited a normal learning curve that reflected the decrease in the time required to solve the maze and the total number of errors along the sessions (Fig. 4a; Hippocampus: Time F = 23.763, p < 0.001; Errors F = 5.205, p < 0.001; Striatum: Time F = 21.711, p < 0.001; Errors F = 4.704, p = 0.001). All the differences that we had observed in rats injected with ANA immediately after the training were missing in these rats injected 2 h after the end of the session, in both the hippocampus in the light phase and the striatum in the dark phase, as depicted in Fig. 4b,c, and d (Hippocampus: Distance ANA1μg/2ha vs ANA1μg, DifofRank = 38.394, p < 0.05; ANA1μg/2ha vs Cont, DifofRank = 3.252, p > 0.05; Dist.of.Errors ANA1μg/2ha vs ANA1μg, p = 0.042; ANA1μg/2ha vs Cont p = 0.664; Spatial ANA1μg/2ha vs ANA1μg p < 0.001; ANA1μg/2ha vs Cont p = 0.448; Serial ANA1μg/2ha vs ANA1μg, p = 0.967, ANA1μg/2ha vs Cont p = 0.252; Random ANA1μg/2ha vs ANA1μg, p = 0.003; ANA1μg/2ha vs Cont, p = 0.649; Striatum Distance between groups F = 2, p = 0.448; Dist.of.Errors between groups F = 0.233, p = 0.774; Spatial ANA1μg/2ha vs ANA1μg, p = 0.913; ANA1μg/2ha vs Cont, p = 0.939; Serial ANA1μg/2ha vs ANA1μg, p = 0.043, ANA1μg/2ha vs Cont, p = 0.818; Random ANA1μg/2ha vs ANA1μg, p = 0.123; ANA1μg /2ha vs Cont, p = 0.878).
Noncontingent administration of ANA into the hippocampus and striatum (ANA1μg/2ha). (a) Performance time (left) and total errors (right). (b) Distance with respect to the target-hole and percentage of errors inside the target zone (c) for rats trained under intrahippocampal ANA during the light phase (white bars) and intrastriatal ANA during the dark phase (gray bars). (d) Strategies for rats trained under intrahippocampal ANA during the light phase (clear circles) and intrastriatal ANA during the dark phase (dark circles). Doted lines represent the median and means for the control group. No effect is observed when ANA is administered 2 h after the training with respect to control, but when compared against ANA immediately after training, significant differences can be observed. Significant differences are indicated by lines under each plot
CB1R expression
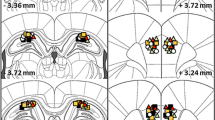
Expression of the CB1R protein in the hippocampus underwent changes along the 24 h, specifically 1300 hours, was significantly different from 0100 hours (p < 0.001) and 0500 hours (p < 0.009), while remaining unchanged in the striatum (Fig. 5a). On the other hand, expression of the CB1R mRNA showed an important variation in the hippocampus, specifically 2100 hours was significantly different from 1700, 1300, 0900, and 05:00 hours (p < 0.001) while remaining unchanged in the striatum (Fig. 5b). The immunohistochemistry assay revealed significant differences in the dorsolateral striatum (p < 0.023) and DG (p < 0.005), whereas CA1 and CA3 did not change (Fig. 6).
CB1R protein expression in the hippocampus and striatum during the light–dark cycle (a). Mean ± SEM of densitometry obtained from the 53-kDa band. In the hippocampus, a significant increase is observed during the light phase of the cycle (p < 0.05). In the striatum, the highest concentration occurs at 0900 hours, while the lowest occurs at 2100 hours, albeit the changes are not significant (note that the y axis does not start at the origin). CB1R mRNA expression in the hippocampus and striatum during the light–dark cycle (b). Histogram of densitometry obtained from the mRNA band. There is an increase in the hippocampus during the light phase of the cycle (p < 0.05). In the striatum, the concentrations remained unchanged during the cycle. Curves are expressed as a double plot for a better appreciation of the cycle
Representative sections for the immunohistochemistry in CA3 (a, e), CA1 (b, f), DG (c, g), and dorsolateral striatum (d, h) at 1300 hours (light phase) and 0100 hours (dark phase). Note the different expressions of CB1R in the DG (c, g) and the striatum (d, h). i Histogram of densitometry obtained from each structure at 1300 vs 0100 hours
Discussion
Our results indicate that rats learned to solve the BM by selecting one of two strategies, depending on the light–dark cycle, that is, spatial during the light and serial during the dark phase (Figs. 2 and 3f). However, the capacity to solve the maze remained unchanged because rats utilized a similar time to solve the task (Figs. 2 and 3a), whether they were performing during the dark or the light phase of the cycle. This idea was previously put forth in different tasks by Folkard in humans (Folkard 1975, 1979) and by Yanovski in rats (Yanovski et al. 1986). Based on the extensive literature supporting the involvement of the hippocampus in the control of spatial strategies and of the striatum in the control of procedural strategies (Packard and McGaugh 1992, 1996; White and McDonald 2002; Packard and Teather 1998; Packard and Knowlton 2002) such as the serial one, these data suggest that these two brain structures take control, one at the time, of the way the subject approaches the information depending on the hour of the day.
We are providing two lines of evidence to support the notion that the cannabinergic system is implicated in the change of strategies, depending on the light–dark cycle. First, we have shown by three different techniques that the expression of CB1R in the hippocampus changes depending on the light–dark cycle, while the striatum is more stable. These data suggest that the switching of strategies might result from the activity of the cannabinergic and, potentially, of other inhibitory systems. However, these variations in the cannabinergic system may be crucial in the hippocampus but not in the striatum that, as aforementioned, remains stable. This is not the first time the cannabinergic system is described as showing diurnal variations. Valenti et al. (2004) and Murillo-Rodriguez et al. (2006) have shown that 2-AG and ANA fluctuate and, more importantly, these authors reported that 2-AG concentration is higher in the hippocampus and striatum during the light phase of the cycle, whereas ANA is higher during the dark phase. This is an intriguing condition that deserves further investigation. We have, at present, no data to support any comments to this respect. However, these findings prompt us to clarify the participation of 2-AG and its possible interaction with ANA in memory consolidation.
Second, changes in CB1R expression in the hippocampus are reflected on behavior, as can be implied from the fact that administration of ANA causes dose-dependent effects only during the light phase of the cycle, when CB1R expression is higher, and that these effects are blocked by the antagonist AM251 (Fig. 2). The latter is correlated with a decreased CB1R expression. This idea is complemented by the fact that AM251 administered alone during the dark phase in the hippocampus facilitates the expression of a spatial strategy (Fig. 2), suggesting that the CB1 receptor is implicated in blocking the expression of spatial strategy during the dark phase. This evidence and the fact that ANA does not show significantly different effects during the dark phase of the cycle also sustain the hypothesis that the differential expression of CB1R is physiologically significant for the brain in spite of what seem to be subtle changes in CB1R expression.
Numerous studies have suggested that the distribution of the cannabinergic receptor in the hippocampus is predominantly presynaptic in both GABAergic (Katona et al. 1999, 2000; Hoffman and Lupica 2000; Wilson and Nicoll 2001; Alger 2004) and glutamatergic terminals (Shen et al. 1996; Ameri et al. 1999; Alger 2002; Katona et al. 2006). In the case of the hippocampus, administration of ANA during the light phase (higher CB1R concentrations) would lead to an imbalanced modulation of glutamate and GABA release, two important systems regulating the function of the hippocampus. Very likely, during the dark phase, to reach this imbalance would probably require higher doses of the cannabinoid agonist. In any case, the hippocampus showed a different sensitivity to cannabinoids depending on the light–dark cycle.
Striatal administration of ANA, AM251, or ANA-AM251 resulted in a decrease in the serial strategy and an increase in the random behavior during the dark phase (Fig. 3f). This effect may not be seen during the light phase because, as indicated by the control groups, the spatial strategy is dominant in this phase, and serial strategy is less frequently exhibited (Figs. 2 and 3). The relative stable CB1R concentration in the striatum supports the hypothesis that the hippocampus might be responsible for the change of strategy, in the sense that if the hippocampus were active, it would lead the behavior, i.e., the strategy would be spatial; whereas if the hippocampus were less active, then the striatum would lead the behavior, i.e., the strategy elected would be serial.
The fact that all our cannabinergic manipulations on the striatum brought about changes in behavior may be explained by the fact that the cannabinergic system is indispensable for striatal function, as indicated by studies on striatum’s long-term plasticity (long-term depreciation LTD) (Gerdeman et al. 2002). In this structure, strong evidence supporting the presence of the CB1R in both glutamatergic and GABAergic presynaptic terminals has been found, as well as in some postsynaptic terminals (Szabo et al. 1998; Fusco et al. 2004; Huang et al. 2001; Köfalvi et al. 2005). In the striatum, as well as in other structures, the cannabinergic system is implicated in neurotransmitter release modulation, i.e., GABA or glutamate-release via a CB1R-dependent mechanism (Köfalvi et al. 2005). Affecting the striatal cannabinergic system by blocking or enhancing CB1R activity would lead to an imbalance in striatal function, as this receptor has been proposed as fundamental for it.
In the first part of our study, we performed injections immediately after the training, pursuing to describe an effect in memory consolidation. Such an effect was partially confirmed by the fact that the treatments effectively modified the behavior in these conditions. Nevertheless, this does not exclude the possibility that ANA is affecting permanently the functionality of either the hippocampus or the striatum. The noncontingent administration of ANA confirms that ANA affects the early stages of consolidation of memory, as no effects on behavior were observed under these conditions.
We propose that the interaction between the hippocampus and the dorsolateral striatum favoring either structure to control behavior (White and McDonald 2002) results from the adaptation to the light–dark cycle. This interaction is modified by the direct administration (to the hippocampus or to the striatum) of ANA in a light–dark cycle-dependent way. It is remarkable that such a selection of strategy is an adaptive response to the presence or absence of natural light. As it has been suggested by others (Folkard 1975, 1979), the subject’s efficiency to handle information does not depend only on his capacity to store and organize it, but also on his ability to exhibit one or another strategy to acquire it. In the case of our rats, they preferred to use a spatial strategy during the light and a serial strategy during the dark phase of the cycle. We insist on remarking that the strategy selection depends on the cycle rather than on light availability, as we provided the rats with ambiance illumination during BM performance, regardless of the phase of the cycle. We would like to interpret these data suggesting that the physiological condition of the brain is tightly related to the natural changes of environmental illumination (light–dark cycle), but not to fortuitous light availability (as the light we provided for about half an hour during the BM training).
We have to admit that it has been largely demonstrated that light pulses of 100–400 lx induce a shift in phase and period of circadian rhythms in hamsters and mice, particularly when applied to animals under continuous darkness (advances or delays depending on the time of application and duration of the pulse, from 1 to 18 h; Comas et al. 2006). However, scientific literature describing the effect of light pulses in rats, in particular, Wistar albino rats, is inexistent. For this reason, ambiance light was maintained at about 50 lx during the 20 min of the experimental session. This pulse of light is below the typical level of stimulation. In addition, the studies showing advances or delays in circadian rhythms have been conducted on constant darkness; hence, circadian rhythms are in “free running.” In our experimental conditions, rats were maintained under a light–dark cycle (12:12); thus, they are synchronized to this exogenous rhythm. Hence, the 50-lx stimulation doubtfully affected the circadian rhythm.
In our study, we describe a role of endocannabinoids as part of the modulating system, whose function is to switch from one strategy to the other to solve the problem efficiently.
References
Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission focus on endocannabinoids. Prog Neurobiol 68:247–286
Alger BE (2004) Endocannabinoids: getting the message across. Proc Natl Acad Sci U S A 101:8512–8513
Ameri A (1999) The effect of cannabinoids on the brain. Progress Neurobiol 58:315–348
Ameri A, Wilhelm A, Simmet T (1999) Effects of the endogenous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. J Pharm 126:1831–1839
Barnes CA (1979) Memory deficits associated with senescence: a neurobiological and behavioral study in the rat. J Comp Physiol Psychol 93:74–104
Bisogno T, Ligresti A, Di Marzo V (2005) The endocannabinoid signaling system: biochemical aspects. Pharmacol Biochem Behav 81:224–238
Chaudhury D, Colwell CS (2002) Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res 133:95–108
Comas M, Beersma DG, Spoelstra K, Daan S (2006) Phase and period responses of the circadian system in mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms 21:362–372
Egertova M, Elphik MR (2000) Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol 422:159–171
Folkard S (1975) Diurnal variation in logical reasoning. Br J Psychol 66:1–8
Folkard S (1979) Time of day and level of processing. Mem Cogn 7:272–274
Fusco FR, Martorana A, Giampa C, De March Z, Farini D, D’Angelo V, Sancesario G, Bernardi G (2004) Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse 53:159–167
Gerdeman GL, Ronesi J, Lovinger DM (2002) Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5:446–451
Hampson RE, Deadwyler SA (1998) Role of cannabinoid receptors in memory storage. Neurobiol Dis 5:474–482
Hao S, Avraham Y, Mechoulam R, Berry EM (2000) Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur J Pharmacol 392:147–156
Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP (2006) Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem 13:809–819
Hoffman AF, Lupica CR (2000) Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus. J Neurosci 20:2470–2479
Huang CC, Lo SW, Hsu KS (2001) Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol 532:731–748
Katona I, Sperlágh B, Sik A, Köfalvi A, Vizi ES, Mackie K, Freund TF (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558
Katona I, Sperlágh B, Magloczky Z, Santha E, Köfalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF (2000) Gabaergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience 100:797–784
Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF (2006) Molecular composition of endocannabinoid system at glutamatergic synapses. J Neurosci 26:5628–5637
Köfalvi A, Rodriguez RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlag B (2005) Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci 16:2874–2884
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lichtman AH, Martin BR (1996) Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology 126:125–131
Martinez-Gonzalez D, Bonilla-Jaime H, Morales-Otal A, Henriksen SJ, Velazquez-Moctezuma J, Prospero-Garcia O (2004) Oleamide and anandamide effects on food intake and sexual behavior of rats. Neurosci Lett 364:1–6
Martinez-Vargas M, Murillo-Rodriguez E, Gonzalez Rivera R, Landa A, Mendez-Diaz M, Prospero-Garcia O, Navarro L (2003) Sleep modulates cannabinoid receptor 1 expression in the pons of rats. Neuroscience 117:197–201
May CP, Hasher L, Foong N (2005) Implicit memory, age, and time of day: paradoxical priming effects. Psychol Sci 16:96–100
Murillo-Rodriguez E, Sanchez-Alavez M, Navarro L, Martinez-Gonzalez D, Drucker-Colin R, Prospero-Garcia O (1998) Anandamide modulates sleep and memory in rats. Brain Res 812:270–274
Murillo-Rodriguez E, Giordano M, Cabeza R, Henriksen SJ, Mendez-Diaz M, Navarro L, Prospero-Garcia O (2001) Oleamide modulates memory in rats. Neurosci Lett 313:61–64
Murillo-Rodriguez E, Desarnaud F, Prospero-Garcia O (2006) Diurnal variation of arachidonoylethanolamine, palmitoylethanolamide and oleoylethanolamide in the brain of the rat. Life Sci 79:30–37
Packard MG, Knowlton BJ (2002) Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25:563–593
Packard MG, McGaugh JL (1992) Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci 106:439–446
Packard MG, McGaugh JL (1996) Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65:65–72
Packard MG, Teather LA (1998) Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiol Learn Mem 69:163–203
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic, San Diego
Pettit DAD, Harrison HP, Olson JM, Spencer RF, Cabral GA (1998) Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res 51:391–402
Ralph MR, Ko CH, Antoniadis EA, Seco P, Irani F, Presta C, McDonald RJ (2002) The significance of circadian phase for performance on a reward-based learning task in hamsters. Behav Brain Res 136:179–184
Shen M, Pieser TM, Seybold VS, Thayer SA (1996) Cannabinoid receptor agonist inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci 16:4322–4334
Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, DiMarzo V, Propsero-Garcia O (2007) Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol 151:1109–1116
Szabo B, Dörner L, Pfreundtner C, Nörenberg W, Starke K (1998) Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience 85:395–403
Tsuo K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1R receptors in the rat central nervous system. Neuroscience 83:393–411
Valenti M, Vigano D, Cascio MG, Rubino T, Steardo L, Prolaro D, Di Marzo V (2004) Differential diurnal variations of AEA and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci 61:945–950
Varvel SA, Hamm RJ, Martin BR (2001) Differential effects of delta 9-THC on spatial reference and working memory in mice. Psychopharmacology 157:142–150
Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature 410:588–592
White NM, McDonald RJ (2002) Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem 77:125–184
Yanovski JA, Adler NT, Gallistel CR (1986) Does the perception of reward magnitude of self-administered electrical brain stimulation have a circadian rhythm. Behav Neurosci 100:888–893
Acknowledgment
This research was supported by Grant 49797 from CONACYT to OPG. PER was supported by a fellowship from CONACYT and DGAPA-UNAM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rueda-Orozco, P.E., Soria-Gomez, E., Montes-Rodriguez, C.J. et al. A potential function of endocannabinoids in the selection of a navigation strategy by rats. Psychopharmacology 198, 565–576 (2008). https://doi.org/10.1007/s00213-007-0911-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0911-z