Abstract
Oxycodone is an important therapeutic agent in the treatment of pain. However, its excessive use in medical practice, as well as escalating rates of illegal sharing and high potential for abuse, has generated a public health crisis in North America. The objective of this review is to discuss the prevalence, clinical utility, pharmacology, pharmacokinetics/pharmacodynamics, and addictive properties of oxycodone. Oxycodone has an abuse liability that is similar to other opioids, and in some cases, even greater. The introduction to oxycodone use can come from peers at school, during the treatment of pain, or from the black market. Implementing approaches that increase training of doctors in conjunction with more information for patients, as well as employing harm reduction strategies, diversion resistant products, and drug monitoring programs, may help to reduce the risk of oxycodone misuse and abuse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oxycodone Abuse
Substance abuse and addiction are public health concerns that affect society in multiple areas, including health care, crime rates, policy, and most importantly lead to a loss of productivity and quality of life for those affected (SAMHSA 2013). One concern has been the rising rates of prescription opioid use. Prescription opioids are important therapeutic agents in the treatment of pain. However, the overprescription of opioids, in combination with escalating rates of illegal sharing and their high potential for abuse and addiction has led to a public health crisis. In a World Drug Report published by the United Nations, Canada and the USA have the highest rate of prescription opioid use in the world (United Nations 2016). The increase in opioid abuse appears to be concurrent with the increase in the number of prescription opioid medications written and dispensed (Johnston et al. 2017).
Of particular concern has been the rising rate of oxycodone use. It has been reported that the consumption of oxycodone increased by almost 500% from 1999 to 2011 (Jones 2013). The National Survey on Drug Use and Health in 2015 revealed that in the USA, approximately 27.9 million people aged 12 or older (10.4% of the population) used oxycodone products (Hughes et al. 2015). From this, 4.3 million people aged 12 or older reported misusing these products in the past year, representing 1.6% of the population aged 12 or older. At the same time, the increase in opioid abuse has increased the number of emergency room non-medical visits, overdose deaths, and incidences of neonatal abstinence syndrome (SAMSA 2011; Patrick et al. 2012; Sproule et al. 2009; Canadian Centre on Substance Abuse 2015; Gomes and Juurlink 2016).
Pharmacology
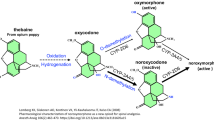
Oxycodone (6-deoxy-7,8-dihydro-14-hydroxy-3-O-smethyl-6-oxomorphine) is a semisynthetic opioid analgesic that is a derivative of the opioid alkaloid thebaine which is found in opium poppies. Some common street names of oxycodone include hillbilly heroin, perc, and OC. It is an active ingredient in a number of formulations which include intravenous injections, immediate-release solutions/capsules (Percocet, Percodan, OXY IR, OXY FAST), and extended-release preparations (OxyContin). Similar to other opioids, oxycodone induces pharmacological effects that include analgesia, euphoria, feelings of relaxation (Walsh et al. 2008; Jones et al. 2011; Colucci et al. 2014), and adverse side effects including respiratory depression, constipation, cough suppression, and sedation (Drug Enforcement Administration 2014; Walsh et al. 2008; Jones et al. 2011; Colucci et al. 2014).
Oxycodone has agonist activity on the mu (μ), delta (δ), and kappa (Κ) opioid receptors (Ross and Smith 1997). These receptors belong to the GTP binding regulatory proteins, (Goodman and Gilman 2001). Opioid receptors can be found in regions of the brain that regulate pain perception/analgesia (i.e., the cingulate cortex, insula, thalamus, dorsal horn of the spinal cord, periaqueductal gray matter), in brain regions involved in euphoria (nucleus accumbens, ventral tegmental area), as well as other organs in the body (Basbaum and Jessell 2013).Oxycodone is effective in eliminating neuropathic, somatic, and visceral pain (Kahan et al. 2006; Riley et al. 2008). The analgesic effects of opioids come from their ability to inhibit ascending transmission of nociceptive information from dorsal horn of the spinal cord and to activate inhibitory pain circuits that descend from the brain to the spinal cord (Goodman and Gilman’s, 2011).
There is some debate surrounding whether the μ- or Κ-opioid receptor mediate oxycodone’s central effects. A subset of researchers argue that oxycodone’s effects on analgesia are not facilitated by the μ-opioid receptor but instead the Κ-opioid receptor (Ross and Smith 1997; Ross et al. 2000). This is based on studies that demonstrate that the Κ-opioid receptor antagonist, norbinaltorphimine, is able to attenuate the antinociceptive activity of oxycodone; whereas, the δ-selective opioid receptor antagonist, naltrinole, and μ1-selective opioid receptor antagonist (naloxohazine) had little effects. Moreover, Neilsen et al. (2000) showed that in morphine tolerant rats, there is a low degree of cross-tolerance to intravenous oxycodone administration. On the basis of these studies, it was suggested that oxycodone’s effects are mediated by mechanisms other than μ-opioid activation. But, Beardsley and colleagues argued that oxycodone’s pharmacological activity is mediated by μ as opposed to either Κ or δ receptors. Beardsley et al. (2004) found that only beta-FNA, a μ-opioid antagonist, was able to block the antinociceptive effects of oxycodone in mice during the tail-flick test. Also, it was reported that oxycodone dose-dependently suppressed signs of spontaneous withdrawal in morphine-dependent monkeys indicating that it is able to induce cross-dependence with morphine (Beardsley et al. 2004). Further, they argue that Κ-opioid agonists are not self-administered; hence, it cannot be possible that oxycodone is predominately Κ-opioid mediated, since oxycodone is self-administered. Furthermore, when oxycodone was tested in rats trained to discriminate heroin (a μ-opioid agonist) from vehicle, it engendered a complete and dose-dependent generalization (Beardsley et al. 2004), indicating that oxycodone and heroin share overlapping subjective discriminative properties.
Pharmacodynamics and Pharmacokinetic
Oxycodone is metabolized in the liver by enzymes CYP3A4 and CYP2D6, which creates noroxycodone and oxymorphone, respectively. Noroxycodone is a weak opioid agonist, and oxymorphone is present in small amounts, so their effects are mostly negligible (Smith 2009). Instead, the primary effects are predominantly mediated through the parent compound, oxycodone. The behavioral effects of oxycodone can last up to 5 h and in the extended-release formulation can have effects that last up to 8–12 h (Drug Enforcement Administration 2014).
The abuse liability of a substance is partly a function of its route of administration. Different routes of administration can lead to greater intoxication and can therefore increase risk of addiction and overdose. After oral administration, oxycodone has a bioavailability that ranges from 60 to 80% compared to morphine’s 40%, and the plasma protein binding of oxycodone in human serum is 45%, which is not too different of that of morphine which is 35% (Leow et al. 1993; Kokki et al. 2004, 2012; Osborne et al. 1990). As a result, compared to oxycodone, a twofold higher dose of oral controlled-release morphine is needed to receive same antinociceptive effects (Silvasti et al. 1998). Similarly, in rats, after subcutaneous and intraperitoneal administration, oxycodone was respectively 2 and 4× more potent than morphine (Pöyhiä and Kalso 1992).When given epidurally, morphine appears equipotent to oxycodone in providing pain relief (Yanagidate 2004; Sng et al. 2016). However, the opposite was observed when the drugs were administered intrathecally with morphine being 14 times more potent than oxycodone (Pöyhiä and Kalso 1992). Oxycodone has lower affinity for the μ-opioid receptor than morphine; however, oxycodone crosses the blood brain barrier more effectively than morphine and has a higher cerebral distribution by volume (Weele et al. 2014; Olkkola et al. 2013), which may contribute the observed differences in potency. Although, when morphine binds to the receptor is more efficacious than oxycodone, or in other words morphine activates the receptor more than oxycodone does (Thompson et al. 2004; Lalovic et al. 2006).
Addictive Properties
Although oxycodone is an efficacious pain reliever, the abuse of and addiction to oxycodone has become a major public health concern. The following subsections will describe the effects of oxycodone on characteristics that are common to most addictive substances, including: reinforcement, withdrawal, relapse, cognitive deficits, as well as neurobiological alterations.
Reinforcement
All drugs of abuse can act as reinforcers, that is they increase the strength of responses that lead to their access (Everitt and Robbins 2005). Various studies have shown that non-dependent recreational opioid users who received oxycodone reported drug-liking, euphoria, and a willingness to take the drug again (Colucci et al. 2014; Zacny and Drum 2010; Stoops et al. 2010; Walsh et al. 2008). When drug use is chronic, it can lead to a loss of control over drug-taking, and overtime become compulsive and habitual (Ostlund and Balleine 2008).
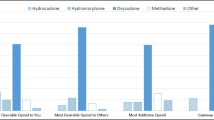
Oxycodone has shown to produce reinforcing effects similar in magnitude to other opioids like heroin and morphine with substantially less negative effects (Comer et al. 2008). The differences in the positive and negative effects produced by the different classes of opioids may differentially contribute to their addiction liability. This is supported by drug-choice procedures, which have found that when given the choice between high doses of morphine and oxycodone, non-dependent participants strongly prefer oxycodone (Comer et al. 2013). Similarly, Babalonis et al. (2013) found that if given the chance to work for money and oxycodone, non-dependent prescription opioid abusers worked significantly harder to obtain high doses of oxycodone. And at lower doses of oxycodone, the amount of work performed to obtain oxycodone and money was similar (Babalonis et al. 2013). In the same individuals, oxycodone produced greater ratings of “drug liking” and “highs,” compared to placebo and codeine (Babalonis et al. 2013). These data suggest that although oxycodone is similar to other opioids in many ways, it possesses reinforcing properties that set it apart; this may then contribute to its increased abuse potential.
Oxycodone also has reinforcing properties in animals. It is readily self-administered in rodents (Leri and Burns 2005; Zhang et al. 2009; Wade et al. 2015; Secci et al. 2016) and monkeys (Beardsley et al. 2004). Similar to other opioids, self-administration of oxycodone shows an escalation of lever pressing over time and increases in the amount of work performed (motivation) to obtain the reinforcer (Wade et al. 2015). It also produces a robust conditioned place preference (Kirkpatrick and Bryant 2015; Campbell et al. 2012; Rutten et al. 2011), which indicates the ability of the drug to produce a preference for contexts associated with positive affective experiences. Further, related to its abuse potential, is the ability of oxycodone to produce locomotor sensitization. Sensitization refers to the enhanced psychomotor response to a stimulus after repeated exposure to that stimulus. It has been shown that repeated treatment with oxycodone facilitates psychomotor sensitization (Leri and Burns 2005; Niikura et al. 2013; Liu et al. 2005; Emery et al. 2015, 2016). Since there is considerable homology in the neurocircuitry of sensitization and drug-seeking (for review, see Steketee and Kalivas 2011) and so the sensitizing properties of drugs of abuse are thought to be relevant to compulsive drug-seeking and drug-craving behavior.
Withdrawal
The prolonged use of oxycodone can lead to the development of withdrawal. Similar to other opioids, oxycodone has shown to produce withdrawal symptoms in both humans (Mars et al. 2014; Wong et al. 2015) and animals (Wiebelhaus et al. 2016; Hutchinson et al. 2009). In humans, these symptoms can be physical (writhing, tremors, nausea, muscle aches, sweating, weight loss, cramping) and/or motivational (dysphoric mood, craving) (Carmichael and Lee 2010). In animals, withdrawal symptoms are characterized by increases in jumps, paw tremors, and loss of body weight (Enga et al. 2016). The desire to relieve withdrawal distress can add to motivation to continue opioid use in both humans (Wise and Koob 2014) and animals (Gerak et al. 2009).
Relapse
The risk of relapse during periods of abstinence poses a major challenge in the treatment of opiate drug addiction (Gossop et al. 1989; Gossop et al. 2002). Given the magnitude of the oxycodone abuse problem, it is surprising that little research has been performed to characterize oxycodone relapse. In the research available, similar to other drugs of abuse, oxycodone has shown to induce relapse after exposure to drug-paired cues, stress, and/or re-exposure to the drug itself (Leri and Burns 2005; Campbell et al. 2012; Grella et al. 2011).
Cognitive Deficits
Those brain regions that underlie addiction overlap with those involved in cognitive processing (i.e., learning, memory, flexible decision making, impulsivity) (for review, see Gould 2010). And exposure to oxycodone has shown to influence some of these processes. For example, pre-clinical research has revealed that animals with repeated exposure to oxycodone are impaired on behavioral flexibility tasks. More specifically, rodents that had been previously exposed to oxycodone showed diminished ability to change a dominant response which was no longer useful to a response that was useful (Seip-Cammack and Shapiro 2014). This is interesting, because one hypothesis purports that drugs of abuse impair flexible decision-making by altering mechanisms in the brain that compute reward expectancies, which may then result in behavior that is compulsive and habitual. In fact, oxycodone exposure has shown to alter the function of the hippocampus (Zhang et al. 2015), which is thought to be necessary to respond to the environment in a flexible manner (Vilà-Balló et al. 2017). Davis et al. (2010) showed that female rats that were treated with oxycodone produced offspring that had impaired spatial learning (Davis et al. 2010). These data suggest repeated oxycodone exposure can cause cognitive deficits; this could in turn have effects on the ability of individuals to control drug-taking and drug-seeking.
Neurobiology
The opioid system is heavily linked to the mesolimbic dopamine (DA) system (Johnson and North 1992). One of the G-protein-coupled receptors, the μ-opioid receptor, is found throughout the mesolimbic system and modulates DA release in the nucleus accumbens, affecting responses to drug reward (Le Merrer et al. 2009). Opioids act on the mesolimbic DA system through inhibition of GABA interneurons in the ventral tegmental area (VTA) which exert an inhibitory control on DA neurons in the nucleus accumbens. Hence, opioids indirectly activate the DA system through GABA inhibition (Johnson and North 1992). The role of DA signaling is multifaceted, it has been shown to be involved in learning and predicting the occurrence of rewards (Schultz and Dickinson 2000), ascribing incentive salience to neural representations of rewards and cues (Berridge and Robinson 1998), and has roles in attention, sensorimotor behavior, or effort which then influences reward pursuit (Ikemoto and Panksepp 1999; Horvitz 2000).
One hypothesis is that the difference in addiction liability of different drugs of abuse might be a result of differences in DA activity. A study by Weele et al. (2014) profiled differences in how oxycodone and morphine affected DA activity. They utilized fast-scan cyclic voltammetry and microdialysis combined with mass spectrometry to reveal that during the first minute following a morphine infusion there was an increase in extracellular DA and GABA; however, this increase in GABA concentration was not observed after an oxycodone infusion. Since GABA has shown to inhibit DA activity, it offers an explanation as to why phasic DA release activity for morphine returned to baseline following the first minute, but persisted for oxycodone throughout the 15-min measurement period; it would also explain why there was a greater amplitude of phasic DA (DA transients) for oxycodone (Weele et al. 2014). Increases in DA transients are thought to be associated with increased addiction liability, and so it is possible that oxycodone has greater addictive liability, which would also explain the earlier study reported where oxycodone was more reinforcing than morphine in a drug-choice procedure (Comer et al. 2013).This increased responsiveness to DA has also been demonstrated in studies where mice pre-treated with oxycodone show greater locomotor supersensitivity to quinpirole (a D2/D3 DA receptor agonist) than morphine pre-treated mice (Emery et al. 2015). Overall, these results suggest oxycodone exerts effects on the DA system that are different from other opioids.
Chronic exposure to oxycodone has also shown to produce structural and functional changes in regions of the brain that mediate affect, impulse, reward, and motivation which are dysregulated in addiction (Upadhyay et al. 2010). For example, in prescription opioid-dependent subjects, where oxycodone was the commonly consumed prescription opioid in the last 30 days, a significant volumetric loss in the amygdala, decreased anisotropy in axonal, efferent and afferent pathways of the amygdala, and decreased functional connectivity in the brain networks in the anterior insula, amygdala, and nucleus accumbens was observed (Upadhyay et al. 2010).
Pathways to Oxycodone Addiction
Oxycodone use occurs in both medical and non-medical users. The purpose of this section is to review the possible motives, sources, and routes to abuse.
Adolescents
The nonmedical use of oxycodone products among adolescents has been a growing public health concern over the last decade. The National Institute of Drug Abuse (2016) reported that the prevalence of OxyContin use in Grades 8, 10, and 12 is no longer increasing and has stabilized. There are various estimates on the amount of non-medical use of prescription opioids in adolescents, and some studies have revealed that up to 24% of adolescents have used oxycodone products (Wu et al. 2008). Oxycodone use is especially troublesome in this age group, because during this developmental period, the brain is highly plastic, and the prefrontal cortex which is important for executive control is still under development (Casey et al. 2008). Therefore, the use of drugs during this period can be even more detrimental (Squeglia et al. 2009).
It was reported that most prescription opioid abuse is initiated with oxycodone products, and the age of first use for these products is on average 15 years old (Vosburg et al. 2016). The misuse of prescription opiates by adolescents tends to be largely experimental (Mccabe et al. 2007); however, a number of other reasons have also been indicated such as to get high, or to stop pain. One study identified that the belief that the drug is “controllable” and “safe” compared to illicit drugs, as well as the ease of access of oxycodone formulations from friends and family’s medicine cabinets are also factors that have significantly contributed to the misuse of oxycodone (Katz and Hays 2004). This increase in prescription opioid misuse has also increased the overdose rates among young adults, who experienced a greater increase in rates of death from opioid analgesics than any other age group (Frank et al. 2015). Furthermore, those adolescents who have previously abused prescription opioids, heroin, or have become addicted to prescription opioids reported oxycodone as their preferred prescription opioid (Vosburg et al. 2016; Osgood et al. 2012). Pre-clinical studies have also shown that oxycodone exposure during adolescence increases sensitivity to drugs later on in life (Sanchez et al. 2016).
“Street Addicts”
Oxycodone has become a popular street drug through its ability to induce a heroin-like euphoria (Comer et al. 2008). Sellers et al. (2006) conducted a field study regarding the relative abuse potential of opioid formulations (fentanyl, hydromorphone, and oxycodone) in Canada and found using the Opiate Attractiveness Scale, oxycodone was the most attractive. In one study of opioid-dependent patients, 37% of patients reported receiving opioids solely from physician prescriptions, 26% from both a prescription and the “street,” and 21% from the street (Canadian Centre on Substance Abuse 2015). In another study, data was collected from various prescription drug abuser samples which indicated that street dealers are a primary source for unlawfully obtaining prescription drugs, reaching 70–80% among street drug users, treatment clients, and methadone maintenance patients (Cicero et al. 2011; Inciardi et al. 2010). It was also found that the main source of oxycodone differed according to the primary motivation for use. For example, those who used oxycodone for euphoria or withdrawal were twice as likely to use a dealer as those whose primary motivation was pain relief (Davis and Johnson 2008).
Researchers have also characterized how route of administration affects non-medical use of oxycodone. It was found that the rates of unemployment were more than double in intravenous (IV) users than that of intra-nasal (IN) users (Jones et al. 2011). Although in this sample recreational use of oxycodone began a number of years later (mid- to late 30s), all participants reported recreational drug use beginning in their early to mid-teens (IN: 16 and IV: 13), where marijuana was typically their first drug of abuse, 84%. A majority of the users reported purchasing oxycodone on the street (IV: 48%, IN: 68%) or from an individual with a prescription (IV: 48%, IN: 24%) (Jones et al. 2011). Comparatively, few IV abusers indicated that they obtained oxycodone from a prescription that they themselves obtained from a physician (4%); whereas, this number was 16% for the IN group (Jones et al. 2011). Overall this suggests that the abuse patterns of IV and IN users are different, and might require different treatment interventions.
One concern is that the use of these prescription opioids will lead to illicit drug use. And in fact, of those that were abusing opioids, a majority reported that their first opioid was a prescription drug (oxycodone or fentanyl) (Cicero et al. 2014). Grau et al. (2007) also found that individuals who initiated non-medical opioid use with oxycodone had a greater incidence of transitioning to heroin use or injection of drugs. Many oxycodone pill users reported that a consideration for using heroin was that heroin was more easily available than prescription opioid pills and/or heroin was cheaper (Mars et al. 2014; Cicero et al. 2014; Kuehn 2013). Oxycodone is an expensive street drug, an 80-mg OxyContin pill retails for approximately $6 at a pharmacy, but sells for around $80 on the street (Dasgupta et al. 2013), whereas the street price for 80 mg of heroin was approximately $45 (Ciccarone et al. 2009; Ciccarone 2009).
Pain Patients
Physiological Dependence
Individuals who take opioids as prescribed for pain management are still vulnerable to developing drug dependence and/or tolerance. These changes typically occur as a result of counter adaptations expressed in opioid receptors and their intracellular signaling cascades (Henriksen and Willoch 2008). It is however possible to be dependent or tolerant to particular effects of oxycodone in the absence of addiction. And while withdrawal is one of the criteria that can be met for an individual to be diagnosed with opioid use disorder, it is not one that needs to be met. Moreover, it is not considered to be met in those individuals who are taking opioids under appropriate medical supervision (American Psychiatric Association 2013).
Opioid addiction as a cause of appropriate medical management of pain is rare, and some studies suggest that opiate addicts and pain patients are largely separate populations. For example, Edlund et al. (2007) studied over 15,000 veterans who were prescribed opioids for pain and maintenance for 3 months. They reported that only 2% of the study population developed opioid abuse (specific statistics with respect to the various classes of prescription opioids were not available). In another study, non-abusers of prescription opioids only self-administered oxycodone when they judged that they required pain relief, whereas those identified as prescription opioid abusers administered oxycodone in the presence and/or absence of pain, even though both groups reported similar subjective effects for oxycodone (Comer et al. 2010). In addition, animal studies have also shown that opioids are less rewarding in the presence of ongoing pain (Narita et al. 2007; Betourne et al. 2008).Taken together, these findings suggest that addiction related outcomes for oxycodone may be different in pain patients. That being said, the relief from pain is a form of reinforcement itself, and so the transition to use outside of appropriate suggested medical consumption is a risk. Hence, careful medical supervision for those using oxycodone (or other prescription opioids) is still necessary.
Sell, Diversion, and Other Opioids
A subset of individuals, who are prescribed oxycodone, inappropriately use or divert them. According to the Substance Abuse and Mental Health Services Administration (SAMHSA), approximately 70% of those abusing pain relieving medications received them illicitly and less than 20% acquire the drugs directly through a prescription from a doctor. There are several sources of drug diversion: (1) theft of medications in transit; (2) theft of drugs by employees; (3) stolen or forged prescriptions; and (4) doctor shopping (Smith and Woody 2005). In a study by Rigg et al. (2012), an in-depth semi-structured interview was conducted with a diverse sample of prescription drug dealers. They found that drug dealers relied on a wide variety of diversion methods, which included visiting multiple pain clinics, stealing medications from pharmacies, and purchasing medications from patients (Rigg et al. 2012). One of the most common methods of diversion is transfer of prescription opioids by patients who have received legitimate prescriptions to family members or friends who are trying to self-medicate generic pain (Mccabe et al. 2007; Volkow & Mclellan 2016). Finally, approximately 7–10% of diversion occurs among patients who feign pain to acquire prescription opioids (Gwira et al. 2014).
Inappropriate Prescription
Inappropriate prescribing practices have likewise contributed to an increase in the availability of opioids to the general public. The rise in the rate of oxycodone prescriptions between 1995 and 2004 has been correlated with increases in non-medical use, Emergency Room Department visits, and unintentional opioid death (Wisniewski et al. 2008; Dhalla et al. 2009). Factors contributing to this trend include a lack of consensus among doctors regarding appropriate use and dosing of medications as well as for-profit clinics whose physicians may prescribe opioid products beyond what is necessary (CDC 2014).
Prevention and Treatments
It would be unrealistic to eliminate oxycodone medications altogether, because they are extremely effective for treating various types of pain (Moradi et al. 2012; Riley et al. 2008). Instead, efforts need to be directed at decreasing the misuse of these medications. Some of these initiatives involve increasing information available to patients and doctors, harm reduction strategies, diversion resistant products, and use of drug monitoring programs.
Better Information
Many non-medical prescription opioid users are uninformed about factors that may lead to overdose and techniques for preventing/responding to overdose situations (Lankenau et al. 2012; Frank et al. 2015).Therefore, directives aimed at increasing awareness may help to decrease the risk of overdose deaths. In fact, it was found that drug use decreases when drugs are perceived as harmful (U.S. Cong., Senate Caucus on International Narcotics Control 2014). And since most illicit drug use starts in adolescence (SAMHSA 2017; SAMHSA 2012), programs aimed specifically at teenagers may be very effective.
Because easy access to oxycodone products from family medicine cabinets can be one source of access, it would be useful to ensure that all patients receiving opioid therapy receive education regarding the safe storage and disposal of opioid medications. The Government of Canada is also working to reduce problematic opioid use and its related harms, by proposing that the use of warning stickers (i.e., similar to those seen with cigarettes) and patient information handout be mandatory with all prescription opioids at time of sale (Health Canada 2017).
Harm Reduction/Maintenance
Harm reduction strategies are becoming a popular approach in reducing risky drug use behaviors. These programs are designed to decrease drug-related harm without requiring the cessation of drug use. They include three major programs: (a) needle exchange programs; (b) maintenance therapy; and the (c) use of naloxone.
Needle Exchange Programs
Often intravenous use of oxycodone and other opioid drugs is employed, because it produces rapid and heightened effects of the drug. An Australian study found that 95% of regular opioid abusers reported “injection” as the most common route of abuse (Peacock et al. 2015). And of those opioid-dependent individuals who enroll in methadone maintenance treatment programs, the proportion of individuals reporting injection as a route of abuse was 15% for the immediate release of oxycodone and 38% for the controlled release of oxycodone (Rosenblum et al. 2007). One problem associated with intravenous use is that it can lead to collapsed veins, skin infections, and increased risk of contracting infectious diseases (i.e., HIV and hepatitis B and C) through contaminated needles. So to help mitigate these risks, needle and syringe programs have been employed. These are interventions where sterile needles/syringes and other injecting equipment is provided to drug users.
Maintenance Programs
Opioid substitution treatment programs have also been employed to diminish the use and effects of illicit opiates (i.e., withdrawal, decrease cravings) and reduce the risk of opioid overdose.
Naloxone
Unintentional opioid overdose deaths have been a serious public health concern. They are predominately a result of oxycodone’s respiratory depressive effects. To help reduce and prevent overdose deaths, naloxone injectable and nasal spray kits have been available to be taken home by individuals, as well as to be carried by first responders. Naloxone is a μ-opioid receptor antagonist, which competitively binds to μ-opioid receptors. As a result, it can rapidly reverse the central nervous system depressive effects of opioid agonists, hence, helping to intervene in cases of opioid overdose. However, naloxone has shown to diminish opioid tolerance and may increase risk of overdose in individuals who return to illicit drug use. In fact, overdose deaths associated with oral naltrexone are three to seven times higher than those associated with methadone maintenance therapy (Gibson and Degenhardt 2007). And so, patient information about the effects of naloxone on subsequent opioid use is important.
Diversion Resistant Products
When OxyContin pills are crushed, there is an increase in the associated positive subjective effects (Webster et al. 2012), which leads to greater misuse and abuse. In order to reduce the risk of oxycodone abuse reformulated and tamper resistant products were designed. Some of these supposed tamper resistant formulations include: ReMOXY, which is oxycodone in a gel capsule that is resistant to crushing, breaking, freezing, dissolving, and heat alteration; Targin, which is an oxycodone/naloxone combination that is less attractive for non-medical use due to reduction in euphoric feelings that accompany its use; and Oxy/niacin, which is a formulation that does not decrease analgesic effects but does increase the potential for adverse effects.
After the introduction of these reformulated versions, it was found that the abuse of the reformulated extended-release oxycodone (ERO) was 41% lower than historical abuse for original ERO (Butler et al. 2013), which may be a result of the decreased abuse liability of the reformulated oxycodone products. In comparison to the original ERO, the reformulated ERO was shown to decrease the positive subjective effects and increase the negative effects (Perrino et al. 2013; Harris et al. 2013). Although the introduction of reformulated/tamper resistant oxycodone has the potential to decrease oxycodone abuse, it may be associated with a switch to illicit drugs. It was found that the recent abuse-deterrent OxyContin was associated with a 36% decrease in the use of that medicine, but during the same time frame, it was coupled with a 42% increase in heroin use (Coplan et al. 2013; Cicero & Ellis 2015; Dart et al. 2015). In fact, nonmedical abusers of oxycodone have stated that the switch to heroin use for them was because oxycodone was not available to them (U.S. Cong., Senate Caucus on International Narcotics Control 2014). This has important implications for developing policies, as it is not ideal to shift the addiction to a more potentially dangerous opioid.
Education
The education of physicians offers another avenue that can be exploited to decrease overall oxycodone abuse. Physicians admit that they are not confident about how to prescribe opioids safely (Keller et al. 2012), and both physicians and pharmacists state that they are not confident in their ability to detect prescription drug abuse, discuss abuse issues with their patients, or discuss treatment facility options (Hagemeier et al. 2013). This suggests that medical school training on pain management and addiction needs to improve, which can leave clinicians more knowledgeable about how to manage addictions. Doctors can also employ urine drug screening to help identify illicit drug use and drug diversion (Katz and Fanciullo 2002) or look at alternate treatments for chronic pain such as NSAIDS, i.e., acetaminophen.
Prescription Drug Monitoring Program
Lastly, decreasing the amount of oxycodone that is available through sources of diversion like doctor shopping (obtaining a prescription from multiple prescribers) and pill milling (inappropriate prescribing practices by doctors) is a promising avenue. A part of the opioid problem stems from a lack of supervised medicine consumption (Bell 2010). One survey found that of those that used opioids for nonmedical purposes, approximately 20% received opioids from more than one prescriber (Jones et al. 2014). The use of online databases available to doctors and pharmacists that provide information about medical history could help to hinder the ability of individuals to acquire multiple prescriptions. In fact, drug monitoring programs have shown to decrease the number of opioid medications prescribed (Chakravarthy et al. 2011). In addition, practices like “upscheduling” where patients are not able to fill a prescription automatically, but need a new one will force patients to visit their prescribing doctor more frequently, may allow doctors to be more informed of their patient’s oxycodone use. One concern with prescription monitoring programs is their effect on patients being chronically treated for pain with opioids. The under treatment of pain could decrease pain-related outcomes such as patient function and quality of life. In addition, studies have also shown that a decrease in prescription opioid prescribing was associated with increased use of heroin (Cicero et al. 2012). So, are we just replacing one addiction for another? These are all factors that need to be considered when developing public policies and designing programs.
Conclusion
A multifaceted approach in treating the opioid crisis that includes better clinical training in the management of acute and chronic pain, patient education, diversion resistant products, and harm reduction programs are likely to help curb the epidemic of oxycodone addiction. Also, the use of programs that are tailored for specific populations (i.e., adolescents, street users) may be more effective at ameliorating the opioid abuse crisis.
References
American Psychiatric Association. (2013). Opioid use disorder. In Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Publishing.
Babalonis, S., Lofwall, M. R., Nuzzo, P. A., Siegel, A. J., & Walsh, S. L. (2013). Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug and Alcohol Dependence, 129, 116–124.
Basbaum, A.I., & Jessell, T.M. (2013). Pain. In E.R. Kandel, et al. (Eds.), Principles of neural science (5th ed.). New York: McGraw Hill.
Beardsley, P. M., Aceto, M. D., Cook, C. D., Bowman, E. R., Newman, J. L., & Harris, L. S. (2004). Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Experimental and Clinical Psychopharmacology, 12(3), 163–172.
Bell, J. (2010). The global diversion of pharmaceutical drugs. Addiction, 105(9), 1531–1537.
Berridge, K. C., & Robinson, T. E. (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research. Brain Research Reviews, 28(3), 309–369.
Betourne, A., Familiades, J., Lacassagne, L., Halley, H., Cazales, M., Ducommun, B., Lassalle, J. M., Zajac, J. M., & Frances, B. (2008). Decreased motivational properties of morphine in mouse models of cancerous- or inflammatory-chronic pain: implication of supraspinal neuropeptide FF(2) receptors. Neuroscience, 157, 12–21.
Butler, S. F., Cassidy, T. A., Chilcoat, H., Black, R. A., Landau, C., Budman, S. H., & Coplan, P. M. (2013). Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. The Journal of Pain, 14(4), 351–358.
Campbell, A. T., Kwiatkowski, D., Boughner, E., & Leri, F. (2012). Effect of yohimbine stress on reacquisition of oxycodone seeking in rats. Psychopharmacology, 222(2), 247–255.
Canadian Centre on Substance Abuse (CCSA). (2015). Prescription opioids. Ottawa: Canadian Centre on Substance Abuse.
Carmichael, J. P., & Lee, M. A. (2010). Symptoms of opioid withdrawal syndrome afterswitch from oxycodone to alfentanil. Journal of Pain and Symptom Management, 40(6), e4–e6.
Casey, B. J., Jones, R. M., & Hare, T. A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–126.
Centers for Disease Control and Prevention (CDC). (2014). Opioid painkiller prescribing. Retrieved from https://www.cdc.gov/vitalsigns/opioid-prescribing/.
Chakravarthy, B., Shah, S., & Lotfipour, S. (2011). Prescription drug monitoring programs and other interventions to combat prescription opioid abuse. Western Journal of Emergency Medicine, 13(5), 422–425.
Ciccarone, D. (2009). Heroin in brown, black and white: Structural factors and medical consequences in the US heroin market. International Journal of Drug Policy, 20(3), 277–282.
Ciccarone, D., Unick, G. J., & Kraus, A. (2009). Impact of South American heroin on the US heroin market 1993–2004. International Journal of Drug Policy, 20(5), 392–401.
Cicero, T. J., & Ellis, M. S. (2015). Abuse-deterrent formulations and the prescription opioid abuse epidemic in the United States. JAMA Psychiatry, 72(5), 424.
Cicero, T. J., Kurtz, S. P., Surratt, H. L., Ibanez, G. E., Ellis, M. S., Levi-Minzi, M. A., & Inciardi, J. A. (2011). Multiple determinants of specific modes of prescription opioid diversion. Journal of Drug Issues, 41(2), 283–304.
Cicero, T. J., Ellis, M. S., & Surratt, H. L. (2012). Effect of abuse-deterrent formulation of OxyContin. The New England Journal of Medicine, 367, 187–189.
Cicero, T. J., Ellis, M. S., Surratt, H. L., & Kurtz, S. P. (2014). The changing face of heroin use in the United States. JAMA Psychiatry, 71(7), 821.
Colucci, S. V., Perrino, P. J., Shram, M., Bartlett, C., Wang, Y., & Harris, S. C. (2014). Abuse potential of intravenous oxycodone/naloxone solution in nondependent recreational drug users. Clinical Drug Investigation, 34(6), 421–429.
Comer, S. D., Sullivan, M. A., Whittington, R. A., Vosburg, S. K., & Kowalczyk, W. J. (2008). Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology, 33(5), 1179–1191.
Comer, S. D., Sullivan, M. A., Vosburg, S. K., Kowalczyk, W. J., & Houser, J. (2010). Oxycodone: Laboratory study of the relationship between pain and abuse liability. Drug and AlocoholDependence, 109, 130–138.
Comer, S. D., Metz, V. E., Cooper, Z. D., Kowalczyk, W. J., Jones, J. D., Sullivan, M. A., et al. (2013). Comparison of a drug versus money and drug versus drug self-administration choice procedure with oxycodone and morphine in opioid addicts. Behavioural Pharmacology, 24, 504–516.
Coplan, P. M., Kale, H., Sandstrom, L., Landau, C., & Chilcoat, H. D. (2013). Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse-deterrent characteristics. Pharmacoepidemiology and Drug Safety, 22(12), 1274–1282.
Dart, R. C., Surratt, H. L., Cicero, T. J., Parrino, M. W., Severtson, S. G., Bartelson, B. B., & Green, J. L. (2015). Trends in opioid analgesic abuse and mortality in the United States. New England Journal of Medicine, 372(16), 1572–1574.
Dasgupta, N., Freifeld, C., Brownstein, J. S., Menone, C. M., Surratt, H. L., Poppish, L., et al. (2013). Crowdsourcing black market prices for prescription opioids. Journal of Medical Internet Research, 15(8), e178.
Davis, W. R., & Johnson, B. D. (2008). Prescription opioid use, misuse, and diversion among street drug users in New York City. Drug and Alcohol Dependence, 92(1–3), 267–276.
Davis, C. P., Franklin, L. M., Johnson, G. S., & Schrott, L. M. (2010). Prenatal oxycodone exposure impairs spatial learning and/or memory in rats. Behavioural Brain Research, 212(1), 27–34.
Dhalla, I. A., Mamdani, M. M., Sivilotti, M. L., Kopp, A., Qureshi, O., & Juurlink, D. N. (2009). Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ, 181(12), 891–896.
Drug Enforcement Administration. (2014). Oxycodone. Sprinfield, Virginia: Drug Enforcement Administration.
Edlund, M. J., Steffick, D., Hudson, T., Harris, K. M., & Sullivan, M. (2007). Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain, 129, 355–362.
Emery, M. A., Bates, M. L. S., Wellman, P. J., & Eitan, S. (2015). Differential effects of oxycodone, hydrocodone, and morphine on the responses of D2/D3 dopamine receptors. Behavioural Brain Research, 284, 37–41.
Emery, M. A., Bates, S., Wellman, P. J., & Eitan, S. (2016). Differential effects of oxycodone, hydrocodone, and morphine on activation levels of signaling molecules. Pain Medicine, 17(5), 908–914.
Enga, R. M., Jackson, A., Damaj, M. I., & Beardsley, P. M. (2016). Oxycodone physical dependence and its oral self-administration in C57BL/6J mice. European Journal of Pharmacology, 789, 75–80.
Everitt, B. J., & Robbins, T. W. (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience, 8(11), 1481–1489.
Frank, D., Mateu-Gelabert, P., Guarino, H., Bennett, A., Wendel, T., Jessell, L., & Teper, A. (2015). High risk and little knowledge: overdose experiences and knowledge among young adult nonmedical prescription opioid users. International Journal of Drug Policy, 26(1), 84–91.
Gerak, L. R., Galici, R., & France, C. P. (2009). Self-administration of heroin and cocaine in morphine-dependent and morphine-withdrawn rhesus monkeys. Psychopharmacology, 204, 403–411.
Gibson, A. E., & Degenhardt, L. J. (2007). Mortality related to pharmacotherapies for opioid dependence: a comparative analysis of coronial records. Drug and Alcohol Review, 26(4), 405–410.
Gomes, T., & Juurlink, D. (2016). Opioid use and overdose: what we’ve learned in Ontario. Healthcare Quarterly, 18(4), 8–11.
Goodman & Gilman’s. (2001). The Pharmacological Basis of Therapeutics. In J. G. Hardman, L. E. Limbird, & A. G. Gilman (Eds.), (10th ed.). New York: McGraw-Hill.
Goodman & Gilman’s. (2011). The Pharmacological Basis of Therapeutics. In L. L. Brunton, J. S. Lazo, & K. L. Parker (Eds.), (11th ed.). New York: McGraw-Hill.
Gossop, M., Green, L., Phillips, G., & Bradley, B. (1989). Lapse, relapse and survival among opiate addicts after treatment. A prospective follow-up study. The British Journal of Psychiatry, 154, 348–353.
Gossop, M., Stewart, D., Browne, N., & Marsden, J. (2002). Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction, 97(10), 1259–1267.
Gould, T. J. (2010). Addiction and cognition. Addict SciClinPract, 5(2), 4–14.
Grau, L. E., Dasgupta, N., Harvey, A. P., Irwin, K., Givens, A., Kinzly, M. L., & Heimer, R. (2007). Illicit use of opioids: Is OxyContin® a “gateway drug”? The American Journal on Addictions, 16(3), 166–173.
Grella, S. L., Levy, A., Campbell, A., Djazayeri, S., Allen, C. P., Goddard, B., & Leri, F. (2011). Oxycodone dose-dependently imparts conditioned reinforcing properties to discrete sensory stimuli in rats. Pharmacological Research, 64(4), 364–370.
Gwira, J. A., Wiedeman, C., Dunn, J. R., et al. (2014). High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Internal Medicine, 174(5), 796–801.
Hagemeier, N. E., Gray, J. A., & Pack, R. P. (2013). Prescription drug abuse: a comparison of prescriber and pharmacist perspective. Substance Use & Misuse, 48(9), 761–768.
Harris, S. C., Perrino, P. J., Smith, I., Shram, M. J., Colucci, S. V., Bartlett, C., & Sellers, E. M. (2013). Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasallyadministered crushed oxycodone HCl abuse-deterrent controlled-release tablets in recreational opioid users. The Journal of Clinical Pharmacology, 54(4), 468–477.
Health Canada. (2017). New measures to inform Canadians of the risks of prescription opioids out for consultation. Retrieved from https://www.canada.ca/en/health-canada/news/2017/06/new_measures_to_informcanadiansoftherisksofprescriptionopioidsou.html.
Henriksen, G., & Willoch, F. (2008). Imaging of opioid receptors in the central nervous system. Brain, 131(5), 1171–1196.
Horvitz, J. C. (2000). Mesolimbocortical and nigrostriatal dopamine responses to salient non- reward events. Neuroscience, 96(4), 651–656.
Hughes, A., Williams, M. R., Lipari, R. N., Bose, J., Copello, E. A., &Kroutil, L. A. (2015). Prescription Drug Use and Misuse in the United States: Results from the 2015 National Survey on Drug Use and Health. Retrieved from https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR2-2015/NSDUH-FFR2-2015.htm.
Hutchinson, M. R., Lewis, S. S., Coats, B. D., Skyba, D. A., Crysdale, N. Y., Berkelhammer, D. L., Brzeski, A., Northcutt, A., Vietz, C. M., Judd, C. M., et al. (2009). Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411. Brain BehavImmun, 23, 240–250.
Ikemoto, A., & Panksepp, J. (1999). The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research Reviews, 31, 6–41.
Inciardi, J. A., Surratt, H. L., Cicero, T. J., Rosenblum, A., Ahwah, C., Bailey, J. E., et al. (2010). Prescription drugs purchased through the internet: who are the end users? Drug and Alcohol Dependence, 110(1–2), 21–29.
Johnson, S. W., & North, R. A. (1992). Opioids excite dopamine neurons by hyperpolarization of local interneurons. The Journal of Neuroscience, 12(2), 483–488.
Johnston, L. D., O’Malley, P. M., Miech, R. A., Bachman, J. G., & Schulenberg, J. E. (2017). Monitoring the Future national survey results on drug use, 1975-2016: overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan.
Jones, C.M. (2013). Trends in the distribution of selected opioids by state, US, 1999–2011. Paper presented at: National Meeting Safe State Alliance, Baltimore, MD.
Jones, J. D., Vosburg, S. K., Manubay, J. M., & Comer, S. D. (2011). Oxycodone abuse in New York City: characteristics of intravenous and intranasal users. The American Journal on Addictions, 20(3), 190–195.
Jones, C. M., Paulozzi, L. J., & Mack, K. A. (2014). Sources of prescription opioid pain relievers by frequency of past-year nonmedical use. JAMA Internal Medicine, 174(5), 802.
Kahan, M., Srivastava, A., Wilson, L., Mailis-Gagnon, A., & Midmer, D. (2006). Opioids for managing chronic non-malignant pain. Canadian Family Physician, 52(9), 1091–1096.
Katz, N., & Fanciullo, G. J. (2002). Role of urine toxicology testing in the management of chronic opioid therapy. The Clinical Journal of Pain, 18, 76–82.
Katz, D. A., & Hays, L. R. (2004). Adolescent oxycontin abuse. Journal of the American Academy of Child & Adolescent Psychiatry, 43(2), 231–234.
Keller, C. E., Ashrafioun, L., Neumann, A. M., Klein, J. V., Fox, C. H., & Blondell, R. D. (2012). Practices, perceptions, and concerns of primary care physicians about opioid dependence associated with the treatment of chronic pain. Substance Abuse, 33(2), 103–113.
Kirkpatrick, S. L., & Bryant, C. D. (2015). Behavioral architecture of opioid reward and aversion in C57BL/6 substrains. Front BehavNeurosci, 8, 450.
Kokki, H., Rasanen, I., Reinikainen, M., Suhonen, P., Vanamo, K., & Ojanperä, I. (2004). Pharmacokinetics of oxycodone after intravenous, Buccal, intramuscular and gastric administration in children. Clinical Pharmacokinetics, 43(9), 613–622.
Kokki, H., Kokki, M., & Sjövall, S. (2012). Oxycodone for the treatment of postoperative pain. Expert OpinPharmacother, 13(7), 1045–1058.
Kuehn, B. M. (2013). SAMHSA: pain medication abuse a common path to heroin. JAMA, 310(14), 1433.
Lalovic, B., Kharasch, E., Hoffer, C., Risler, L., Liu-Chen, L.-Y., & Shen, D. D. (2006). Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. ClinPharmacol Ther, 79, 461–479.
Lankenau, S. E., Teti, M., Silva, K., Bloom, J. J., Harocopos, A., & Treese, M. (2012). Patterns of prescription drug misuse among young injection drug users. Journal of Urban Health, 89(6), 1004–1016.
Le Merrer, J., Becker, J. A., Befort, K., & Kieffer, B. L. (2009). Reward processing by the opioid system in the brain. Physiological Reviews, 89(4), 1379–1412.
Leow, K. P., Wright, A. W., Cramond, T., & Smith, M. T. (1993). Determination of the serum protein binding of oxycodone and morphine using ultrafiltration. Therapeutic Drug Monitoring, 15(5), 440–447.
Leri, F., & Burns, B. H. (2005). Ultra-low-dose naltrexone reduces the rewarding potency of oxycodone and relapse vulnerability in rats. Pharmacology, Biochemistry, and Behavior, 82, 252–262.
Liu, Y. L., Liang, J. H., Yan, L. D., Su, R. B., Wu, C. F., & Gong, Z. H. (2005). Effects of l-tetrahydropalmatineon locomotor sensitization to oxycodone in mice. Acta Pharmacologica Sinica, 26, 533–538.
Mars, S. G., Bourgois, P., Karandinos, G., Montero, F., & Ciccarone, D. (2014). “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. International Journal of Drug Policy, 25(2), 257–266.
Mccabe, S. E., Cranford, J. A., Boyd, C. J., & Teter, C. J. (2007). Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addictive Behaviors, 32(3), 562–575.
Moradi, M., Esmaeili, S., Shoar, S., & Safari, S. (2012). Use of oxycodone in pain management. Anesthesiology and Pain Medicine, 1(4), 262–264.
Narita, M., Nakamura, A., Ozaki, M., Imai, S., Miyoshi, K., Suzuki, M., & Suzuki, T. (2007). Comparative pharmacological profiles of morphine and oxycodone under a neuropathic pain-like state in mice: evidence for less sensitivity morphine. Neuropsychopharmacology, 33(5), 1097–1112.
National Institute on Drug Abuse. (2016). Monitoring the future. Retrieved from https://www.drugabuse.gov/related-topics/trends-statistics/monitoring-future
Nielsen, C. K., Ross, F. B., & Smith, M. T. (2000). Incomplete, asymmetric, and route-dependent cross-tolerance between oxycodone and morphine in the dark agouti rat. The Journal of Pharmacology and Experimental Therapeutics, 295, 91–99.
Niikura, K., Ho, A., Kreek, M. J., & Zhang, Y. (2013). Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. PharmacolBiochemBehav, 110, 112–116.
Olkkola, K. T., Kontinen, V. K., Saari, T. I., & Kalso, E. A. (2013). Does the pharmacology of oxycodone justify its increasing use as an analgesic? Trends in Pharmacological Sciences, 34(4), 206–214.
Osborne, R., Joel, S., Trew, D., & Slevin, M. (1990). Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide. ClinPharmacolTher, 47, 12–19.
Osgood, E. D., Eaton, T. A., Trudeau, J. J., & Katz, N. P. (2012). A brief survey to characterize oxycodone abuse patterns in adolescents enrolled in two substance abuse recovery high schools. The American Journal of Drug and Alcohol Abuse, 38(2), 166–170.
Ostlund, S. B., & Balleine, B. W. (2008). On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Models, 5(4), 235–245.
Patrick, S. W., Schumacher, R. E., Benneyworth, B. D., Krans, E. E., McAllister, J. M., & Davis, M. M. (2012). Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA, 307(18), 1934–1940.
Peacock, A., Bruno, R., Cama, E., Kihas, I., Larance, B., Lintzeris, N., ... Degenhardt, L. (2015). Jurisdictional differences in opioid use, other licit and illicit drug use, and harms associated with substance use among people who tamper with pharmaceutical opioids. Drug and Alcohol Review, 34(6), 611–622.
Perrino, P. J., Colucci, S. V., Apseloff, G., & Harris, S. C. (2013). Pharmacokinetics, tolerability, and safety of intranasal administration of reformulated OxyContin® tablets compared with original OxyContin® tablets in healthy adults. Clinical Drug Investigation, 33(6), 441–449.
Pöyhiä, R., & Kalso, E. A. (1992). Antinociceptive effects and central nervous system depression caused by oxycodone and morphine in rats. PharmacolToxicol, 70(2), 125–130.
Rigg, K. K., Kurtz, S. P., & Surratt, H. L. (2012). Patterns of prescription medication diversion among drug dealers. Drugs: Education, Prevention and Policy, 19(2), 145–155.
Riley, J., Eisenberg, E., Müller-Schwefe, G., Drewes, A. M., & Arendt-Nielsen, L. (2008). Oxycodone: a review of its use in the management of pain. Current Medical Research and Opinion, 24(1), 175–192.
Rosenblum, A., Parrino, M., Schnoll, S. H., Fong, C., Maxwell, C., Cleland, C. M., et al. (2007). Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug and Alcohol Dependence, 90(1), 64–71.
Ross, F. B., & Smith, M. T. (1997). The instrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated. Pain, 73, 151–157.
Ross, F. B., Wallis, S. C., & Smith, M. T. (2000). Co-administration of sub-antinociceptive doses of oxycodone and morphine produces marked antinociceptive synergy with reduced CNS side-effects in rats. Pain, 84(2), 421–428.
Rutten, K., De Vry, J., Robens, A., Tzschentke, T. M., & Van der Kam, E. L. (2011). Dissociation of rewarding, anti-aversive and anti-nociceptive effects of different classes of anti-nociceptives in the rat. European Journal of Pain, 15, 299–305.
Ruttenl, K., Vry, J., Robens, A., Tzschentke, T. M., & Kam, E. L. (2011). Dissociation of rewarding, anti-aversive and anti-nociceptive effects of different classes of anti-nociceptives in the rat. European Journal of Pain, 15(3), 299–305.
Sanchez, V., Carpenter, M. D., Yohn, N. L., & Blendy, J. A. (2016). Long-lasting effects of adolescent oxycodone exposure on reward-related behavior and gene expression in mice. Psychopharmacology, 233(23–24), 3991–4002.
Schultz, W., & Dickinson, A. (2000). Neuronal coding of prediction errors. Annu Rev Neuroscience, 23, 473–500.
Secci, M. E., Factor, J. A., Schindler, C. W., & Panlilio, L. V. (2016). Choice between delayed food and immediate oxycodone in rats. Psychopharmacology, 233(23–24), 3977–3989.
Seip-Cammack, K. M., & Shapiro, M. L. (2014). Behavioral flexibility and response selection are impaired after limited exposure to oxycodone. Learning & Memory, 21(12), 686–695.
Sellers, E. M., Schuller, R., Romach, M. K., & Horbay, G. L. (2006). Relative abuse potential of opioid formulations in Canada: a structured field study. Journal of Opioid Management, 2(4), 219–227.
Silvasti, M., Rosenberg, P., Seppälä, T., Svartling, N., & Pitkänen, M. (1998). Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. ActaAnaesthesiolScand, 42(5), 576–580.
Smith, H. S. (2009). Opioid metabolism. Mayo Clinic Proceedings, 87(7), 613–624.
Smith, M. Y., & Woody, G. (2005). Nonmedical use and abuse of scheduled medications prescribed for pain, pain-related symptoms, and psychiatric disorders: patterns, user characteristics, and management options. Curr.Psychiatry Rep., 7, 337–343.
Sng, B., Kwok, S., Mathur, D., Ithnin, F., Newton-Dunn, C., Assam, P., et al. (2016). Comparison of epidural oxycodone and epidural morphine for post-caesarean section analgesia: a randomised controlled trial. Indian Journal of Anaesthesia, 60(3), 187.
Sproule, B., Brands, B., Li, S., & Catz-Biro, L. (2009). Changing patterns in opioid addiction: characterizing users of oxycodone and other opioids. Canadian Family Physician, 55(1), 68–69.
Squeglia, L. M., Jacobus, J., & Tapert, S. F. (2009). The influence of substance use on adolescent brain development. Clinical EEG and Neuroscience, 40(1), 31–38.
Steketee, J. D., & Kalivas, P. W. (2011). FDrug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacological Reviews, 63(2), 348–365.
Stoops, W. W., Hatton, K. W., Lofwall, M. R., Nuzzo, P. A., & Walsh, S. L. (2010). Intravenous oxycodone, hydrocodone, and morphine in recreational opioid users: abuse potential and relative potencies. Psychopharmacology, 212(2), 193–203.
Substance Abuse and Mental Health Services Administration (SAMHSA). (2013). National Survey on Drug use and Health 2011 and 2012. Rockville: Center for Behavioral Health Statistics and Quality.
Substance Abuse and Mental Health Services Administration (SAMHSA). (2017). Prescription Drug Misuse and Abuse. Retrieved from https://www.samhsa.gov/topics/prescription-drug-misuse-abuse
Thompson, C. M., Wojno, H., Greiner, E., May, E. L., Rice, K. C., & Selley, D. E. (2004). Activation of G-proteins by morphine and codeine congeners: insights to the relevance of O- and N-demethylated metabolites at mu- and delta-opioid receptors. The Journal of Pharmacology and Experimental Therapeutics, 308(2), 547–554.
United Nations. (2016). World drug report on international narcotics control board for 2016. Vienna: United Nations.
Upadhyay, J., et al. (2010). Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain, 133, 2098–2114.
U.S. Cong., Senate Caucus on International Narcotics Control. (2014, May 14). National Institute on Drug Abuse. (ND. Volkow, Author) [Cong. Rept.]. Retrieved from https://www.drugabuse.gov/about-nida/legislative-activities/testimony-to-congress/2016/americas-addiction-to-opioids-heroin-prescription-drug-abuse.
Vilà-Balló, A., Mas-Herrero, E., Ripollés, P., Simó, M., Miró, J., Cucurell, D., et al. (2017). Unraveling the role of the hippocampus in reversal learning. The Journal of Neuroscience, 37(28), 6686–6697.
Volkow, N. D., & Mclellan, A. T. (2016). Opioid abuse in chronic pain—misconceptions and mitigation strategies. New England Journal of Medicine, 374(13), 1253–1263.
Vosburg, S. K., Eaton, T. A., Sokolowska, M., Osgood, E. D., Ashworth, J. B., Trudeau, J. J., et al. (2016). Prescription opioid abuse, prescription opioid addiction, and heroin abuse among adolescents in a recovery high school: a pilot study. Journal of Child & Adolescent Substance Abuse, 25(2), 105–112.
Wade, C. L., Vendruscolo, L. F., Scholosburg, J. E., Hernandez, D. O., & Koob, G. F. (2015). Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology, 40(2), 421–428.
Walsh, S. L., Nuzzo, P. A., Lofwall, M. R., & Holtman, J. R. (2008). The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug and Alcohol Dependence, 98(3), 191–202.
Webster, L. R., Bath, B., Medve, R. A., Marmon, T., & Stoddard, G. J. (2012). Randomized, double-blind, placebo-controlled study of the abuse potential of different formulations of oral oxycodone. Pain Medicine, 13(6), 790–801.
Weele, C. M., Porter-Stransky, K. A., Mabrouk, O. S., Lovic, V., Singer, B. F., Kennedy, R. T., & Aragona, B. J. (2014). Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. European Journal of Neuroscience, 40(7), 3041–3054.
Wiebelhaus, J. M., Walentiny, D. M., & Beardsley, P. M. (2016). Effects of acute and repeated administration of oxycodone and naloxone-precipitated withdrawal on intracranial self-stimulation in rats. Behavioral Pharmacology, 356(1), 43–52.
Wise, R. A., & Koob, G. F. (2014). The development and maintenance of drug addiction. Neuropsychopharmacology, 39(2), 254–262.
Wisniewski, A. M., Purdy, C. H., & Blondell, R. D. (2008). The epidemiologic association between opioid prescribing, non-medical use, and emergency department visits. Journal of Addictive Diseases, 27(1), 1–11.
Wong, A., Macleod, D., Robinson, J., Koutsogiannis, Z., Graudins, A., & Greene, S. L. (2015). Oxycodone/naloxone preparation can cause acute withdrawal symptoms when misused parenterally or taken orally. ClinToxicol (Phila), 53(8), 815–818.
Wu, L., Pilowsky, D. J., & Patkar, A. A. (2008). Non-prescribed use of pain relievers among adolescents in the United States. Drug and Alcohol Dependence, 94, 1–11.
Yanagidate, F. (2004). Epidural oxycodone or morphine following gynaecologicalsurgery. British Journal of Anaesthesia, 93(3), 362–367.
Zacny, J. P., & Drum, M. (2010). Psychopharmacological effects of oxycodone in healthy volunteers: roles of alcohol-drinking status and sex. Drug and Alcohol Dependence, 107, 209–214.
Zhang, Y., Picetti, R., Butelman, E. R., Schlussman, S. D., Ho, A., & Kreek, M. J. (2009). Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology, 34, 912–922.
Zhang, Y., Brownstein, A., Buonora, M., Niikura, K., Ho, A., Rosa, J. C., et al. (2015). Self-administration of oxycodone alters synaptic plasticity gene expression in the hippocampus differentially in male adolescent and adult mice. Neuroscience, 285, 34–46.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Meenu Minhas and Dr. Francesco Leri declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Minhas, M., Leri, F. A Multifaceted Analysis of Oxycodone Addiction. Int J Ment Health Addiction 16, 1016–1032 (2018). https://doi.org/10.1007/s11469-017-9827-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11469-017-9827-y