Abstract
This paper presents a surface plasmon resonance (SPR) sensor utilizing a prism-coupled Ag/ZnSe/BP hybrid structure with improved sensitivity for glucose detection in urine samples. In this Kretschmann configuration, multilayers are vertically stacked together to improve the optical and electronic properties of the proposed SPR sensor. The transfer matrix method (TMM) is used for the theoretical model and to analyze the performance of the sensors. The proposed SPR sensor comprises 2D materials such as black phosphorus (BP), which improve the sensitivity of the SPR-based sensor through efficient interactions with biomolecules. The resonance angle of surface plasmons shifts due to a difference in the refractive index from 1.330 to 1.337 in urine samples with various glucose levels. Initially, the study aims to compare the sensor performance parameters among different prisms (CaF2, BK7, FK51A, and SF10) coupled with a hybrid structure. The proposed sensor achieved a noticeably higher value sensitivity of 511 (deg./RIU), a quality factor of 108.377 (1/RIU), and a figure of merit of 108.374 when a CaF2 prism with an optimized thickness was used. The performance parameters, including the sensitivity, full width at half maximum (FWHM), figure of merit (FoM), and detection accuracy (DA), were measured, and the results were compared to evaluate the findings. The proposed structure can be more effective in detecting different liquid analytes in biosensing applications, including glucose detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last decade, surface plasmon resonance (SPR) sensing devices have undergone considerable development [1, 2], both from the fundamental point of view and from the design of highly sensitive devices for optical sensing of small chemical or biological entities [3].
Kretschmann [4] and Otto [5, 6] are the available configurations for SPR sensors. The prism surface is coated with a metal film in the Kretschmann configuration, whereas an air gap is maintained between the prism and the metal interface in the Otto configuration. Light bounces directly from the prism to the metal film in the Kretschmann configuration, and this property makes the Kretschmann configuration more popular than the Otto configuration [7]. Optical sensors offer novel features, such as high sensitivity, quantitative response, rapid detection, and selectivity.
Ag layered sensors are less expensive than are Au layered sensors. Moreover, Ag maintains higher detection accuracy (DA) by offering a sharper dip in the resonance curve and keeping the full-width half maximum (FWHM) narrower [8]. ZnSe is a well-known semiconductor material used in optoelectronic research, fabrication of laser diodes, field emitters, photodetectors, solar cells, photocatalysis, in vivo imaging, and sensors [9]. Here, a zinc selenide (ZnSe) layer is used to protect the silver (Ag) layer from oxidation and to increase the sensitivity. The combination of zinc selenide and silver was studied in detail in [10].
A high surface-to-volume ratio, greater biomolecule absorption, high mobility, high charge density, direct band gap, and better binding to various molecules promote the use of 2D black phosphorus (BP) thin films in biosensors [11]. In addition, BP is an excellent choice for chemical applications with high-performance potential because it exhibits appealing chemical, physical, and mechanical anisotropic properties [12]. For example, the implementation of a bilayer of BP over a conventional gaseous sensor enhances its sensitivity by 35%, and Srivastava et al. reported this fact numerically [13].
SPR-based sensors are useful in the fields of food safety [14], environmental monitoring [15], and biomedicine [16]. Recently, SPR sensors have been used to detect blood plasma concentrations [17], fat concentrations in milk [18], basal cancer cells [19], infected red blood cells (IRBCs) [20], and blood sugar levels [21]. To carry out the metabolic processes necessary for existence, energy is needed by every human cell. Glucose is an important source of energy for human cells. Unfortunately, excessive glucose intake may result in diabetes, a chronic metabolic disease. Therefore, a balanced diet of glucose should be maintained to prevent damage to the kidneys, eyes, heart, nervous system, and other organs [22]. According to a World Health Organization (WHO) report, more than 1.6 million people have died, and approximately 422 million people have been affected globally [23].
In urine, increased glucose concentrations cause renal glycosuria [24]. The levels of several body fluids and urine samples change with changes in glucose concentration. In the urine sample, the refractive index varies with the change in glucose concentration of the affected person from the average healthy person. Normally, the human body does not have glucose in the range of 0–15 mg/dL [25]. The unit of glucose concentration is expressed in mg per deciliter (mg/dL). Typically, the range of 0–15 mg/dL glucose concentration in the human body is absent. It is essential to supply energy to our tissues and cells and maintain optimal blood sugar levels to prevent hypoglycemia (low blood sugar) and hyperglycemia (high blood sugar). Renal glycosuria generally increases as glucose levels increase in urine samples (in the range of 165 to 180 mg/dL). Hypoglycemia indicates that the glucose concentration is less than 40 mg/dL, and hyperglycemia indicates that the glucose concentration is between 279 and 360 mg/dL [25].
The research work is divided into distinct sections. First, the structures of the four different biosensors with the necessary theories are provided. Second, the models are analyzed via different plots and charts to determine the highest sensitivity and optimum conditions at the operating wavelength of 633 nm. Third, an evaluation is developed by varying the thickness of the layers for the designed structure. Additionally, the proposed sensor characteristics and fabrication process are presented. Fourth, the results under a variety of scenarios are carried out for the detection of glucose concentration, and the SPR biosensors are compared with previously reported SPR setups. Finally, the conclusion summarizes the key insights of the paper.
Methodology
In this section, design considerations and theories for the development of four biosensors are discussed.
A. Design of the Proposed SPR Sensor Device
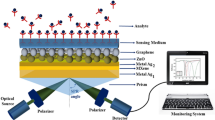
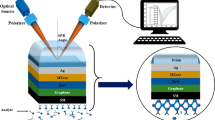
The proposed SPR sensor setup is shown in Fig. 1. It is a five-layer structure in which the sensing medium (SM) has a reflectance of 1.33 to 1.337. Here, four types of prisms are used to design four structures. In these four structures, the layers are individually varied to obtain better performance. The wavelength is taken as 633 nm for all the configurations.
Here, the four structures are named Sensor-1, Sensor-2, Sensor-3, and Sensor-4. Sensor-1 is structured as CaF2/Ag/ZnSe/BP/SM, sensor-2 is structured as BK7/Ag/ZnSe/BP/SM, sensor-3 is structured as FK51A/Ag/ZnSe/BP/SM, and sensor-4 is structured as SF10/Ag/ZnSe/BP/SM. The optimum thicknesses of the materials in the four different configurations are listed in Table 1. The thicknesses of different layers of the designed biosensors were determined by analyzing recent research on biosensors. In addition, for these thicknesses, high sensitivity and optimum results were found and checked. Moreover, for other thickness combinations, the reflectance curves were not smooth, the SPR curves obtained were flat (no significant dip), provided unexpected values, and may have resulted in practically unsuitable conditions.
Many recent studies have analyzed the design of SPR biosensors with high sensitivity and optimal results. The refractive indices (RIs) for all the materials used in this study are listed in Table 2.
B. Mathematical Expressions
The proposed sensors are designed on the basis of the Kretschmann configuration [4]. In this SPR setup, the plasmonic wave moves along the interface between the sensing medium and the metallic layer when the light beam resonantly impacts the metallic layer through the prism at a specific angle. Here, the resonance condition is de Fundam scribed by the waveforms that satisfy the condition that the wavevector of the incident p-polarized light equals the surface plasmon wave. The mathematical expression for the matching condition is [36].
where \({K}_{x}\) and \({K}_{sp}\) are the wave vectors of the incident light and the surface plasmon wave (SPW) vectors, respectively. These are defined by [36]
where \({n}_{p}\) is the RI of the prism, \(\lambda\) is the wavelength of the incident light, \({\theta }_{0}\) is the light incidence angle at the interface between the prism and the metal layer, and \({\varepsilon }_{1}, {\varepsilon }_{2}\) are the dielectric constants of the sensing medium and the metal layer, respectively.
With a change in the refractive index, the SPW propagation constant also changes directly. Therefore, the incident angle must be satisfied to meet the equality conditions given in Eq. (1). The energy of the incident light is transferred completely to the SPW with minimum reflectivity at the resonance state. The SPR sensor is intended to detect the angle at resonance (minimum reflectivity), and any change in this angle means a change in the refractive index.
One of the best methods for characterizing reflectivity is the transfer matrix method (TMM). It is a precise approach because there are no approximations in this method [37].
The transmittance and reflectance calculations for the multilayer model are performed by assuming that the layers are thinly homogeneous and stacked on each other. Additionally, it is assumed that each layer is uniform, symmetric, and nonmagnetic [38].
The reflectance of incident p-polarized light is defined as [23]
where \({r}_{p}\) is the amplitude of the reflected wave that can be calculated for the N-layer model from [23]
According to the transfer matrix method, the wave propagation parameters for the N-layer model that describes the light propagation from layer (N) to layer (N + 1) can be calculated through the \({F}_{ij}\) matrix, [23]
where \({\beta }_{k}\) is the random phase constant and \({q}_{k}\) is the admittance at the kth layer, calculated by [39]
Here, \({n}_{p}\) is the RI of the prism, and \({\theta }_{0}\) is the incidence angle at the prism–metal interface. \({d}_{k}\) is the thickness of the kth layer, and \({n}_{k}\) is the reflective index.
C. Performance Parameters of the Sensor
The sensitivity (S) is defined as the ratio of the deviation in the SPR angle (\({\Delta \theta }_{res}\)) and the deviation in the sensing medium’s \(RI\) (\(\Delta n\)) [40, 41]. The expression can be found in Eq. (10):
The detection accuracy (DA) is computed as the ratio of \({\Delta \theta }_{SPR}\) to the FWHM. The expression of the DA can be found in Eq. (11):
The full width at half maximum (FWHM) provides information about the width and sharpness of the reflectance curve. The expression for the FWHM can be found in Eq. (12):
Here, \({\theta }_{2}, {\theta }_{1}\) indicate the difference between the incidence angles at half (50%) of the reflectivity.
To ascertain the sensing efficiency, another scale is the figure of merit (FoM) scale. Finding the ideal mix of components, layout, and circumstances produces a high FoM, indicating great performance, sensitivity, and efficiency. The FoM can be expressed as shown in Eq. (13):
The quality factor (QF) provides information about the resolution of the proposed SPR sensor. The expression of QF can be found in Eq. (14):
These parameters are optimized for all of the surface plasmon resonance biosensors designed in this study.
Results
This module can be divided into four sections:
A. Parameters for Optimized Thickness
The reflectance as a function of the angle of incidence plot for the four different biosensor structures (Sensor-1, Sensor-2, Sensor-3, and Sensor-4) is depicted in Figs. 2, 3, 4, and 5, respectively. Here, the SPR reflectance plots were plotted for different RIs ranging from 1.33 to 1.337, with an RI change of 0.001.
The performance parameters obtained from the different sensors (Sensor-1, Sensor-2, Sensor-3, and Sensor-4) are tabulated in Table 3, and for easy recognition of the differences in the performance parameters, bar graphs are presented in Figs. 6 and 7. These results indicate that Sensor-1 has the highest sensitivity, with a sensitivity of 511 deg/RIU and an FoM of 108.374.
Here, the unit of sensitivity is \(\text{deg}/\text{RIU},\) QF is 1/RIU, FWHM is deg, and DA is 1/deg. An analysis of the results confirmed that the combination of Sensor-1 (CaF2/Ag/ZnSe/BP/SM) results in the highest sensitivity, a perfect dip in the reflectance curve, and optimum results.
B. Thickness Optimization of the Layers for Sensor-1
Sensor-1 (CaF2/Ag/ZnSe/BP/SM) shows better sensitivity and overall performance. Therefore, with water as the sensing medium, we examined the sensor performance at different material thicknesses.
Initially, taking water as a sensing medium, we optimized the thickness of the Ag layer with a fixed thickness of the ZnSe layer (dZnSe = 2 nm) and BP layer (dBP = 1 nm). Figure 8, clearly shows that the reflection intensity depends on the thickness of the Ag layer, and the optimized thickness is 46 nm. Similarly, taking water as a sensing medium, the thickness of the ZnSe layer was optimized with a fixed thickness of the Ag layer (dAg = 46 nm) and BP layer (dBP = 1 nm). Figure 9 clearly shows that the reflection intensity depends on the thickness of the ZnSe layer, and the optimized thickness is 2 nm.
Finally, with water as the sensing medium, we optimized the thickness of the BP layer to a fixed thickness of the Ag layer (dAg = 46 nm) and ZnSe layer (dZnSe = 2 nm). Figure 10 clearly shows that the reflection intensity depends on the thickness of the BP layer, and the optimized thickness is 1 nm. Using this comparative analysis, the thickness of the layers for all the sensors was selected. These plots help us choose the correct combination of materials with the optimum thickness for designing SPR biosensors.
C. Analysis of Different Performance Parameters for Sensor-1 (CaF2 Prism)
For further analysis, we used Sensor-1 (CaF2/Ag/ZnSe/BP/SM) because it has better performance. For the characterization of Sensor-1, the thickness of Ag was varied to study the changes in other parameters. The thickness varied from 40 to 50 nm, and the variations in the sensitivity, FoM, and angle shift are listed in Table 4; a plot of these quantities is shown in Fig. 11.
A CaF2 glass prism/Ag/ZnSe/BP/SM nanostructure was utilized. The silver layer is employed in a variety of thicknesses between 40 and 50 nm, with steps of 1 nm. The sensitivities are calculated and found to be 393, 413, 433, 452, 473, 492, 511, 527, 540, 547, and 550 deg/RIU, corresponding to silver layer thicknesses of 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 and 50 nm, respectively. The optimal thickness of the Ag layer is 46 nm, which will be considered in the next investigation.
Similarly, the influences of certain factors on the sensitivity of the SPR photosensor to the metal thickness, number of 2D nanomaterial layers, and thickness of the ZnSe material are investigated. For all the sensors designed in this study, the BP and ZnSe layer thicknesses are varied between 1 and 10 nm, with steps of 1 nm. The thickness has been varied for all the designed structures, and the resonant dip in the reflectance spectra with optimized parameters is not available beyond these thicknesses.
The FWHM, DA, and QF are also calculated by varying the Ag thickness, as shown in Table 5. Further characterizations are performed by varying other parameters with increasing Ag thickness, as shown in Figs. 12 and 13. The results show that the QF and FOM have almost the same values because of the very low value of the minimum reflectance.
D. Mechanism of Glucose Detection in Urine Samples
Diabetism occurs due to the intake of an unbalanced diet, genetic reasons, and inactivity in performing physical activities. If a patient with diabetes has varying glucose levels in their urine, the sensor can be used to detect changes by placing urine samples. This sensor increases the refractive index, which corresponds to higher glucose concentrations, by shifting the surface plasmon resonance (SPR) angle to the right.
Here, as shown in Table 6, we collected urine samples with glucose concentrations of 0–15 mg/dL, 0.625 gm/dL, and 1.25 gm/dL, with refractive indices of 1.335, 1.336, and 1.337, respectively [42]. Similarly, samples at lower glucose concentrations were measured, and the corresponding refractive indices ranged from 1.330 to 1.334.
For an RI change of 0.001, the maximum sensitivity is 511 deg/RIU, and a minimum reflectance of 0.000024 is observed. For glucose concentration variation from 0–15 mg∕dL to 0.625 gm/dL, the angle of incidence shifts to 87.78 degrees from 87.83 degrees for 0.001 RI variation. Thus, two urine samples were tested with the proposed biosensor: a normal human urine sample (0 ~ 15 mg/dL) with an RI of 1.335, which is a reference value, and a higher glucose level (0.625 gm/dL) with an RI of 1.336. For a 0.001 increment in the RI, the minimum SPR angle difference (\(\Delta\) θSPR) becomes 0.05 degrees, and the minimum reflectance also increases to 0.3976.
Furthermore, for the sample variation from 0.625 gm/dL to 1.25 gm/dL, we obtain a refractive index variation of 0.001, and the SPR angle shift is from 87.78 to 87.66. Hence, when the concentration of glucose is changed from 0.625 gm/dL gm/dL, a reference value, to 1.25 gm/dL, Rmin increases from 0.3976 to 0.5427. Additionally, the \(\Delta\) θSPR increases to 0.12 degrees.
The output detector measures this shift in the SPR angle, enabling the accurate detection of glucose levels in the urine samples. A comparison with earlier research is listed in Table 7.
Fabrication
The fabrication process is explained in Fig. 14. The fabrication of Ag, ZnSe, and BP layers, which are utilized to make SPR chips, was first performed. A solution containing acetone vapor, methanol, and deionized water was applied to the glass prism. A thermal evaporator system performs physical vapor deposition (PVD) of metal layers on prism glass [49]. The BP layer can be made via a well-known chemical vapor deposition (CVD) procedure [50], which can then be chemically transferred over a ZnSe layer. After being manufactured, these chips are placed on top of the prism.
A urine sample is subsequently poured onto the sensor chip area for sensing purposes. Finally, the complete SPR chip is set over a spinning table. The reflected light is received by an optical detector on the output side of the prism, which measures its intensity.
Conclusion
This paper introduces and examines four novel, high-sensitivity, low-cost SPR biosensors for detecting glucose. The transfer matrix method (TMM) and Fresnel’s equations are applied to analyze the reflectance spectra. The optimized configurations for sensors 1 to 4 consisted of five layers: CaF2/Ag(46 nm)/ZnSe(2 nm)/BP(1 nm)/sensing medium, BK7/Ag(50 nm)/ZnSe(9 nm)/BP(1 nm)/sensing medium, FK51A/Ag(56 nm)/ZnSe(3 nm)/BP(3 nm)/sensing medium, and SF10/Ag(48 nm)/ZnSe(10 nm)/BP(6 nm)/sensing medium. Adding thin layers of ZnSe and black phosphorus to the Ag layer improved the biosensor sensitivity and resulted in a sharp dip in the resonance profile. The ZnSe layer above silver offers the necessary shielding and sensitivity increase since silver has a negative chemical interaction with the buffer solution.
After the effects of the different prisms of these sensors were analyzed, the CaF2/Ag/ZnSe/BP/SM-based sensor was characterized further, as it provided the highest sensitivity of 511 deg/RIU. The other parameters under optimum conditions obtained for this combination were a QF of 108.37751 (1/RIU), FoM of 108.374, FWHM of 4.715 (deg), and DA of 0.212089 (1/deg). This highly sensitive SPR biosensor design prevents unwanted pricking of the needle to extract blood samples from diabetic patients. Thus, the glucose level of diabetic patients can be determined through urine samples. The investigations performed in this research might also be added to future technical advances in glucose sensing, studies, and research. This design might have advantages in the fields of environmental, biomedical, and chemical studies, as well as virus detection, with new prospects.
Data Availability
No datasets were generated or analysed during the current study.
References
Zayats AV, Smolyaninov II, Maradudin AA (2005) Phys Rep 408:131–314
Pitarke JM, Silkin VM, Chulkov EV, Echenique PM (2007) Rep Prog Phys 70:1–87
Abdulhalim I, Zourob M, Lakhtakia A (2008) Electromagnetics (UK) 28:214–242
Kretschmann E, Raether H (1968) Notizen: radiative decay of non radiative surface plasmons excited by light. Zeitschrift für Naturforschung A 23(12):2135–2136. https://doi.org/10.1515/zna-1968-1247.[Online].Available:doi:10.1515/zna-1968-1247
Otto A (1968) Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Zeitschrift für Physik A Hadrons nuclei 216(4):398–410
Gupta S, Ramesh K, Ahmed S, Kakkar V (2016) Lab-on-chip technology: a review on design trends and future scope in biomedical applications. Int J Bio-Sci Bio-Technol 8(5):311–322
Kumar S, Yadav A, Kumar S, Malomed BA (2024) Design and simulation of SPR sensors by employing silicon and silicon-nitride with mono and bimetal layers for sensitivity enhancement. IEEE Sens J 24(6):7671–7680. https://doi.org/10.1109/jsen.2024.3355766
Szunerits S, Castel X, Boukherroub R (2008) Surface plasmon resonance investigation of silver and gold films coated with thin indium tin oxide layers: influence on stability and sensitivity. J Phys Chem C 112(40):15813–15817
Zhang Q, Li H, Ma Y, Zhai T (2016) ZnSe nanostructures: synthesis, properties and applications. Progress in Materials Science/Progress in Materials Science 83:472–535. https://doi.org/10.1016/j.pmatsci.2016.07.005.Available:doi:10.1016/j.pmatsci.2016.07.005
Chu Q, Han B, Jin Y, Guo S, Jin S, Park E, Chen L, Mee Jung Y (2020) Surface plasmon resonance induced charge transfer effect on the Ag-ZnSe-PATP system. Spectrochimica Acta A Mol Biomol Spectrosc. Epub 2020 Nov 16. PMID: 33257248
Maheswari P, Subanya S, Ravi V, Rajesh KB, RajanJha JZ (2022) Platinum layers sandwiched between black phosphorous and graphene for enhanced SPR sensor performance. Plasmonics 17:213–222
Mao N, Tang J, Xie L, Juanxia Wu, Han B, Lin J, Deng S, Ji W, HuaXu KL, Tong L, Zhang J (2016) Optical anisotropy of black phosphorus in the visible regime. J Am ChemSoc 138:300–305
Srivastava T, Jha R (2018) Black phosphorus: a new platform for gaseous sensing based on surface plasmon resonance. IEEE 30:319–322
Neethirajan S, Ragavan V, Weng X, Chand R (2018) Biosensors for sustainable food engineering: challenges and perspectives. Biosensors 8(1):23. https://doi.org/10.3390/bios8010023
Hu C, Gan N, Chen Y, Bi L, Zhang X, Song L (2009) Detection of microcystins in environmental samples using surface plasmon resonance biosensor. Talanta 80(1):407–410. https://doi.org/10.1016/j.talanta.2009.06.044
Sathya N, Karki B, Rane K et al (2022) Tuning and sensitivity improvement of bi-metallic structure-based surface plasmon resonance biosensor with 2-D ε-tin selenide nanosheets. Plasmonics 17:1001–1008. https://doi.org/10.1007/s11468-021-01565-9
Almawgani AHM, Daher MG, Taya SA et al (2022) Detection of blood plasma concentration theoretically using SPR-based biosensor employing black phosphor layers and different metals. Plasmonics 17:1751–1764. https://doi.org/10.1007/s11468-022-01662-3
Almawgani AHM, Daher MG, Taya SA, Mashagbeh M, Colak I (2022) Optical detection of fat concentration in milk using MXene-based surface plasmon resonance structure. Biosensors 12(7):535. https://doi.org/10.3390/bios12070535.Available:doi:10.3390/bios12070535
Daher MG, Trabelsi Y, Ahmed NM et al (2022) Detection of basal cancer cells using photodetector based on a novel surface plasmon resonance nanostructure employing perovskite layer with an ultra high sensitivity. Plasmonics 17:2365–2373. https://doi.org/10.1007/s11468-022-01727-3
Daher MG, Trabelsi Y, Prajapati YK et al (2023) Highly sensitive detection of infected red blood cells (IRBCs) with plasmodium falciparum using surface plasmon resonance (SPR) nanostructure. Opt Quant Electron 55:199. https://doi.org/10.1007/s11082-022-04466-1
Daher MG (2023) Supersensitive biosensor based on a photonic crystal nanostructure for blood sugar level monitoring with ultrahigh quality factor and low detection limit. Optik 275:170581. https://doi.org/10.1016/j.ijleo.2023.170581
Mudgal N, Saharia A, Agarwal A, Ali J, Yupapin P, Singh G (2020) Modeling of highly sensitive surface plasmon resonance (SPR) sensor for urine glucose detection. Opt Quantum Electron 52(6):1–14. https://doi.org/10.1007/s11082-020-02427-0
Mostufa S, Paul AK, Chakrabarti K (2021) Detection of hemoglobin in blood and urine glucose level samples using a graphene-coated SPR based biosensor. OSA Continuum 4(8):2164. https://doi.org/10.1364/osac.433633.[Online].Available:doi:10.1364/osac.433633
Karim MN, Anderson SR, Singh S, Ramanathan R, Bansal V (2018) Nanostructured silver fabric as a free standing NanoZyme for colorimetric detection of glucose in urine. Biosens Bioelectron 110:8–15. https://doi.org/10.1016/j.bios.2018.03.025
Sani MH, Khosroabadi S (2020) A novel design and analysis of high-sensitivity biosensor based on nanocavity for detection of blood component, diabetes, cancer and glucose concentration. IEEE Sens J 20(13):7161–7168. https://doi.org/10.1109/JSEN.2020.2964114
Singh S, Sharma AK, Lohia P et al (2022) Design and modeling of high-performance surface plasmon resonance refractive index sensor using BaTiO3. MXene and nickel hybrid nanostructure Plasmonics 17:2049–2062. https://doi.org/10.1007/s11468-022-01692-x
Singh S, Pandey S, Yadav S et al (2024) Numerical study among Au, Al, and Ag metal-based surface plasmon resonance sensor. J Opt 53:304–314. https://doi.org/10.1007/s12596-023-01107-y
Karki B, Ansari G, Uniyal A et al (2023) PtSe2 and black phosphorus employed for sensitivity improvement in the surface plasmon resonance sensor. J Comput Electron 22:106–115. https://doi.org/10.1007/s10825-022-01975-w
Srivastava A, Prajapati YK (2022) Surface plasmon resonance (SPR)-based biosensor using MXene as a BRE layer and magnesium oxide (MgO) as an adhesion layer. J Mater Sci: Mater Electron 33:8519–8528. https://doi.org/10.1007/s10854-021-06436-x
Brahmachari K, Ray M (2013) Effect of prism material on design of surface plasmon resonance sensor by admittance loci method. Front Optoelectron 6(2):185–193
El-assar M, Taha TE, El-Samie FEA et al (2023) ZnSe-based highly sensitive SPR biosensor for detection of different cancer cells and urine glucose levels. Opt Quant Electron 55:76. https://doi.org/10.1007/s11082-022-04326-y
Monzón-Hernández D, Velázquez-González JS, Luna-Moreno D, Torres-Cisneros M, Hernán dez-Roma I (2018) Prism-based surface plasmon resonance for dual-parameter sensing. IEEE Sens 18(10):4030–4037
Marple DTF (1964) Refractive index of ZnSe, ZnTe, and CdTe. J Appl Phys 35:539–542
Yesudasu V, Pradhan HS, Pandya RJ et al (2024) Numerical investigation of Ag-Franckeite-barium titanium-BP-based highly performed surface plasmon resonance sensor for virus SARS-CoV-2 detection. Plasmonics 19:167–178. https://doi.org/10.1007/s11468-023-01985-9
Karki B, Ramya KC, Sandhya Devi RS et al (2022) Titanium dioxide, black phosphorus and bimetallic layer-based surface plasmon biosensor for formalin detection: numerical analysis. Opt Quant Electron 54:451. https://doi.org/10.1007/s11082-022-03875-6
Chen WP, Chen JM (1981) Use of surface plasma waves for determination of the thickness and optical constants of thin metallic films. J Opt Soc Am 71(2):189. https://doi.org/10.1364/josa.71.000189.[Online].Available:doi:10.1364/josa.71.000189
Katsidis CC, Siapkas DI (2002) General transfer-matrix method for optical multilayer systems with coherent, partially coherent, and incoherent interference. Appl Opt 41(19):3978. https://doi.org/10.1364/ao.41.003978.[Online].Available:doi:10.1364/ao.41.003978
Yuan Y, Ding L, Guo Z (2011) Numerical investigation for SPR-based optical fiber sensor. Sens Actuators, B Chem 157(1):240–245. https://doi.org/10.1016/j.snb.2011.03.056.[Online].Available:doi:10.1016/j.snb.2011.03.056
Fouad S, Sabri N, Poopalan P, Jamal ZAZ (2017) Surface plasmon resonance sensor sensitivity enhancement using gold- dielectric material. Int J Nanoelectron Mater 10:147–156
Mohanty G, Akhtar J, Sahoo BK (2016) Effect of semiconductor on sensitivity of a graphene-based surface plasmon resonance biosensor. Plasmonics 11(1):189–196. https://doi.org/10.1007/s11468-015-0033-0
Pandey AK, Sharma AK, Marques C (2020) On the application of SiO2/SiC grating on Ag for high-performance fiber optic plasmonic sensing of cortisol concentration. Materials 13(7):1–13
Sharma P, Sharan P (2015) Design of photonic crystal-based biosensor for detection of glucose concentration in urine. IEEE Sens J 15(2):1035–1042. https://doi.org/10.1109/JSEN.2014.2359799
Karki B, Jha A, Pal A, Srivastava V (2022) Sensitivity enhancement of refractive index-based surface plasmon resonance sensor for glucose detection. Optic Quant Electron 54(9). https://doi.org/10.1007/s11082-022-04004-z
Mudgal N, Saharia A, Agarwal A, Singh G (2020) ZnO and Bimetallic (Ag–Au) layers based surface plasmon resonance (SPR) biosensor with BaTiO3 and graphene for biosensing applications. IETE J Res. https://doi.org/10.1080/03772063.2020.1844074
Daher MG, Trabelsi Y, Ahmed NM et al (2023) Detection of Escherichia coli using highly sensitive surface plasmon resonance nanostructure (SPRN) based on MXene 2D nanomaterial. Plasmonics 18:2483–2492. https://doi.org/10.1007/s11468-023-01970-2
Kushwaha AS, Kumar A, Kumar R, Srivastava SK (2018) A study of surface plasmon resonance (SPR) based biosensor with improved sensitivity. Photonics Nanostruct-Fundam Appl 31(June):99–106. https://doi.org/10.1016/j.photonics.2018.06.003
Daher MG, Taya SA, Almawgani AHM et al (2023) Optical biosensor based on surface plasmon resonance nanostructure for the detection of mycobacterium tuberculosis bacteria with ultra-high efficiency and detection accuracy. Plasmonics 18:2195–2204. https://doi.org/10.1007/s11468-023-01938-2
Daher MG, Ahmed NM, Alsalman O et al (2023) Novel efficient surface plasmon resonance biosensor for the determination of sucrose concentration. Plasmonics 18:2069–2075. https://doi.org/10.1007/s11468-023-01928-4
Luna-Moreno D, Sánchez-álvarez A, Rodríguez-Delgado M (2020) Optical thickness monitoring as a strategic element for the development of SPR sensing applications. Sensors (switzerland). https://doi.org/10.3390/s20071807
Smith JB, Hagaman D, Ji HF (2016) Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 27(21):1–8. https://doi.org/10.1088/0957-4484/27/21/215602
Author information
Authors and Affiliations
Contributions
Kazi Mustafizur Rahman– Conceptualization, Methodology, Visualization, Validation, Formal Analysis, Software, Writing – original draft, Investigation; Md. Faysal Nayan – Visualization, Validation, Formal Analysis, Writing – review & editing; Raihan Ahmed – Visualization, Formal Analysis; MD. Mushfiqur Rahman – Visualization, Formal Analysis. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study did not require ethics approval because it did not involve human participants, animal subjects, or other sensitive data.
Consent for Publication
All the authors have given their consent to publish this work.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mustafizur Rahman, K., Nayan, F., Ahmed, R. et al. Design and Development of High Sensitive Surface Plasmon Resonance Biosensors for Glucose Detection. Plasmonics (2024). https://doi.org/10.1007/s11468-024-02463-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11468-024-02463-6