Abstract
Plasmodium falciparum infections are the most common cause of malaria-related deaths. A significant difference between red blood cells with and without infection can be seen in their refractive indices, which can be used as a key indicator in the diagnosis of this disease. In this manuscript, a surface plasmon resonance (SPR)-based biosensor is suggested theoretically for the detection of infected red blood cells (IRBCs) with Plasmodium Falciparum. The proposed SPR sensor has the configuration: BK7 prism/Ag/BiFeO3/2D-nanomaterials/analyte. The suggested SPR sensor has a (BiFeO3) layer located between the metal (Ag) and 2D-nanomaterials to realize higher sensitivity. BiFeO3 layer exhibits outstanding characteristics, such as high index of refraction and small loss and has shown a significant shift in resonance angle within a minute variation in the analyte’s refractive index. A variety of 2D materials, including MXene, Graphene, and Black Phosphor, are used to cover the surface of the SPR structure in order to develop the efficiency of the detector. The suggested detector can demonstrate an extremely high sensitivity of 461.43 deg/RIU by adjusting the thicknesses of Ag, BiFeO3, and the quantity of 2D-nanomaterial layers. The suggested SPR-based biosensor is hopeful for use in various sectors of biosensing due to its extraordinarily high sensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Malaria is a serious illness that affects people and is one of the main causes of death in endemic nations among both adults and children. The disease poses a risk to approximately 44 percent of people on the planet (Nureye and Assefa 2020). In order to treat and control the disease and avoid complications and mortality, it must be diagnosed quickly and accurately (Bilal et al. 2015). There are five different Plasmodium classes that can cause malaria: falciparum, ovale, malariae, vivax, and knowlesi. P. falciparum infections are the most common cause of malaria-related deaths. The parasite often infects people by the Anopheles mosquito bite (Molina-Franky et al. 2020). In the form of merozoites, the parasites arrive the bloodstream after the hepatocytes are damaged and assault the red blood cells (RBCs), then they start an intraerythrocytic cycle. Red blood cells undergo structural and metabolic changes as a result of this cycle. The phase of ring, which starts this cycle, is brought on by merozoites confronting the RBCs and is pass by mononuclear trophozoites. Further, trophozoites change into cells with many nuclei known as schizonts, that can be created when hemoglobin is broken down and hemozoin is produced (Liu et al. 2016). In the intraerythrocytic series, festering erythrocytes have a varied refractive index distribution across the cell's cytoplasm, whereas healthy erythrocytes exhibit a homogenous distribution (Akpa Marcel et al. 2019). Red blood cells with and without infection have a considerable change in refractive index, which can be used as a crucial criterion in the diagnosis of malaria (Bendib and Bendib 2018). The primary approach for determining whether a patient has malaria is based on an analysis of a blood smear under an optical microscope. This procedure needs a laboratory specialist who is proficient and experienced, and the results take a while to come back. Somewhat slowly (Ragavan et al. 2018). The polymerase chain reaction (PCR) and rapid diagnostic testing (RDT) are the other two ways to diagnose malaria. These methods are less effective due to several issues with their sensitivity and specificity. Also, they are more costly and require specialized workers (Krampa et al. 2020). As a result, a more accurate and quicker device is required to identify malaria.
Surface plasmon resonance (SPR) is a potent optical technology expended in the detection of enzymes and chemicals, medical diagnostics, and safety of food (Mudgal et al. 2020; Daher et al. 2022a; Almawgani et al. 2022a; Srivastava et al. 2016). This system has been commonly used to correctly and quickly identify a range of physical and biological indicators. The majority of SPR-based sensors employ the attenuated total reflection (ATR) process, in which p-polarized (TM) incoming light generates a surface plasmon wave together with the dielectric-metal interface. In ATR configurations, an optical coupling prism's base typically has a thin metallic coating placed on it. (Daher et al. 2022b). Silver, gold, copper, aluminum, sodium and indium are the active metals that are commonly used in SPR biosensors (Maurya and Prajapati 2016; Maharana et al. 2013; Jha and Sharma 2009). Many researches have put dielectric layers over the metal layer to enhance SPR performance and provide protection against oxidation for the metal film (Yupapin et al. 2022; Huang et al. 2011). Here, we have here proposed the bismuth ferrite (BiFeO3) as a novel dielectric layer. Due to its enormous potential, high electric field absorption, and tiny gap, the BiFeO3 is a hopeful material that has received a lot of attention. It exhibits outstanding characteristics, such as high index of refraction and small loss and has shown a significant shift in resonance angle within a minute variation in the analyte's refractive index (Papich 2016). Recently, 2D-nanomaterials have become a new possibility for progressing the sensitivity of various sorts of photodetectors. Owing to its commonly adjustable direct poor gap, exceptional electrical and optical belongings, and improved carrier mobility (Li et al. 2014; Qiao et al. 2014; Rodin et al. 2014; Wang et al. 2014), black phosphorus (BP) has gained attention as an encouraging 2D nanomaterial (Churchill and Jarilloherrero 2014). BP is an allotrope of phosphorus that consists of several layers with two-dimensional structures maintained by forces of van der Waals. Additionally, the BP possesses attractive chemical, mechanical, and physical anisotropic properties that make it an excellent choice for various applications with high efficiency (Mao et al. 2016). Srivastava et al. claim that coating the SPR sensors with BP can improve the sensitivity (Srivastava and Jha 2018). On the other hand, the amazing properties of graphene have recently been identified. For instance, its great charge carrier mobility leads to significant coupling at the graphene—metal contact (Srivastava and Jha 2018). According to Wu et al., employing graphene can increase the sensitivity of SPR sensors (Elias et al. 2011). MXene has unique properties that make it a viable 2D material for biomolecule sensing, including greater binding, high carrier confinement, a small work function, a layered structure, and a high surface area (Almawgani et al. 2022b). Changing the surface terminations of MXene can change its plasmonic characteristic (Bai et al. 2016). Wu et al. (2018) have proposed an SPR detector using some layers of MXene and various metals.
An SPR-based photodetector is suggested for the detection of infected red blood cells (IRBCs) with Plasmodium Falciparum. The nanostructure employs a layer of BiFeO3 to improve the efficiency of the proposed sensor. Three 2D-nanomaterials are examined: MXene, Graphene and Black Phosphor (BP). The study examines the number of 2D-nanomaterial sheets, the thickness of the silver and BiFeO3 materials.
2 Structure consideration and theoretical method
2.1 Structure consideration
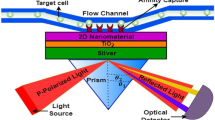
A biosensor based on SPR is suggested for the detection of different IRBCs. The suggested structure of biosensor is comprised five layers: BK7 prism, Ag, BiFeO3, 2D-nanomaterials, and analyte. The thicknesses of Ag, BiFeO3, and 2D-nanomaterial layers will be denoted it as hAg, hBF and h2D with refractive indices nAg, nBF and n2D, respectively. A schematic illustration of the suggested SPR photodetector based on BiFeO3/2D-nanomaterial layers is presented in Fig. 1.
The refractive index of BK7 glass prism can be estimated by the next equation (Lin et al. 2016)
The dielectric constant of the metal medium is acquired by Drude-Lorentz model (Avsar et al. 2015)
where \({\lambda }_{cw}=1.7614\times {10}^{-5}\) m and \({\lambda }_{pw}=1.4541\times {10}^{-7}\) m are the collision wavelength and the plasma wavelength and of silver. The refractive index of BiFeO3 is 2.9680 at a wavelength of 632.8 nm (Vasimalla and Pradhan 2021).
2.2 Theoretical modeling
All L layers are package-like configuration along the z-axis. Mathematically, tangential field of the initial limit is at z = 0 and the tangential field of the last limit is at z = zL−1 are as:
where S1 and SL−1 are the tangential electric fields at initial layer limit and Lth layer limit respectively, D1 and DL−1 are the tangential magnetic fields at initial layer limit and Lth layer limit respectively. Now, distinguishing matrix (Fj) for L-layer system can be clarified as (Daher et al. 2022c, 2022d, 2022e; Panda et al. 2022)
\({K}_{j}\) is the variation in phase experienced by the light wave as it travels through the jth layer.
where \({n}_{j}\) is the layer's refractive index and hj its thickness. \({n}_{1},{\lambda , \mathrm{and} \theta }_{1}\) are the refractive index of prism, the incident wave's wavelength and angle of incidence. Finally, \({\varepsilon }_{j}\) is the dielectric permittivity of the jth layer. For transverse magnetic (TM) wave \({Y}_{j}={({\varepsilon }_{j}-{{{(n}_{1}\mathrm{sin}\theta }_{1})}^{2})}^{1/2}/{\varepsilon }_{j}\). The full transfer matrix F of the structure can be expressed as
Finally, reflectance (R) for hybrid L-layer system can be computed by:
The sensitivity is dependent on both the resonance angle shift (Δθres) and change of RI in analytes, which is defined as (Vasimalla and Pradhan 2021)
3 Results and discussions
An SPR nanostructure based optical sensor is explored for the detection of IRBCs. The suggested structure of biosensor is comprised five layers: BK7 prism, silver, BiFeO3, 2D-nanomaterials, and analyte. According to Sect. 2, the indices of refraction of the materials are determined at λ = 632.8 nm. In the first, layers' thicknesses are chosen as hAg = 40 nm, hBF = 1 nm, and d2D (Graphene) = S \(\times \) 0.34 nm where S = 1. It is worth noting that the reflectance spectrum is not clear for hAg < 40 nm and hBF < 1 nm for the proposed structure. So we start with these values and then explore higher values in the following parts. The reflectance profiles of the suggested SPR nanostructure are studied using Mathematica software and transfer matrix approach. It is worth to mention that the least the thickness Fig. 2a, b shows the SPR reflectivity spectrums for both the normal and infected red blood cells as treated as a analytes. The suggested sensor's reflectance spectra with a 0 nm BiFeO3 layer are shown in Fig. 2a (without a BiFeO3 layer). On other hand, Fig. 2b gives the reflectivity spectra with 1 nm of BiFeO3 layer. The results indicate that as the refractive index of the analyte is decreased, the minimum reflectivity corresponding to the resonance dip gradually increases. Moreover, it is obvious that as the refractive index of the analyte decreases, the resonance angle shifts to a smaller angle. The resonance angles change from 78.16 to 73.23 and 79.67 to 74.34 for SPR sensor without the BiFeO3 layer and SPR photosensor with 1 nm of BiFeO3 layer, respectively. Figure 3 illustrates how the SPR angle varies when the analyte’s refractive index changes. The shift is fully linear across the entire range of refractive indices in linear regression analyses of the suggested SPR photosensor. As a result, for SPR photosensors based on BiFeO3, adding a 1 nm BiFeO3 layer enhances Δ\(\theta \) of SPR by at least 8% when compared to the SPR photosensor without this layer. This implies an increase in sensitivity of 8%. The sensitivity of the suggested photosensor and the photosensor without the BiFeO3 material is evaluated and then are explained in Table 1.
To increase the SPR sensor’s sensing performance, 2D nanomaterials are frequently coated on the sensor’s surface since it has a very large specific surface area and high carrier mobility In this section, We will talk about how 2D materials affect SPR sensor performance. The performance of the photosensor is simulated when some 2D nanomaterials such that MXene, Graphene and Black Phosphor (BP) cover the surface of the nanostructure. The refractive indices of MXene, Graphene and Black Phosphor are 2.38 + 1.33i, 3 = 1.149i and 3.5 + 0.01i, respectively. Thickness of monolayer of these 2D materials are 0.993, 0.34 and 0.53 nm, respectively. Peng et al. (2016); Nurrohman and Chiu (2020); Almawgani et al. (2022c). The initial thickness of BiFeO3 is adjusted to 1 nm and that of Ag to 40 nm in order to streamline the research procedure when monolayers of various 2D nanomaterials are applied to the structure. The SPR reflectivity spectra for MXene, Graphene and Black Phosphor are shown in Fig. 4a–c, respectively as the sensing layer selected as normal and ring red blood cells (NRBC and RRBC). The first choice is as BK7 glass prism/silver/BiFeO3/MXene/analyte. The resonance angles are obtained at 80.45° and 78.92° for the NRBC and RRBC, respectively. The sensitivity is computed and recorded as 218.57 deg/RIU. Now, the second choice is selected as BK7 glass prism/silver/ BiFeO3/ Graphene /analyte and the angles of dip are located at 79.67° and 78.19° for the NRBC and RRBC, respectively. The computed sensitivity for this structure is realized as 211.43 deg/RIU. Finally, the third choice is selected as BK7 glass prism/silver/BiFeO3/BP/analyte. The angles of dip for the last structure are obtained at 80.24° and 78.67°. The sensitivity is evaluated and became as 224.28 deg/RIU. The results of these structures are tabulated in Table 2. It is obvious that the third system with BP as 2D material has higher sensitivity than other structures. Therefore, the last structure will be taken up for the upcoming investigations.
The influences of certain factors on the sensitivity of the SPR photosensor like metal\thickness, number of 2D nanomaterial layers and thickness of BiFeO3 material are inspected. The nanostructure of BK7 glass prism/silver/BiFeO3/BP/analyte is utilized. The silver layer is employed in a variety of thicknesses between 40 and 60 nm, with steps of 5 nm. The sensitivities are calculated and found as 224.28, 238.57, 251.42, 260 and 264.28 deg/RIU corresponding to silver layer thicknesses of 40, 45, 50, 55 and 60 nm, respectively. As shown from Fig. 5, the optimal thickness of the Ag layer is 60 nm which will consider in the next investigation. The calculations and results of all these thicknesses of Ag layer are clarified in Table 3. The resonant dip in the reflectance spectra is not seen for thicknesses greater than 60 nm.
On other hand, when the 2D nanomaterial covers the surface of the nanostructure, the thickness of the BiFeO3 layer needs to be modified and adjusted since the 2D nanomaterial, like BiFeO3, has a high refractive index real portion. The BiFeO3 layer is employed in a variety of thicknesses between 1 and 2.5 nm, with steps of 0.5 nm. For each thickness of the BiFeO3 layer, the sensitivity is evaluated and determined. It found as 264.28, 304.28, 357.14 and 461.42 deg/RIU for dBF = 1, 1.5, 2 and 2.5 nm, respectively. For dBF > 2.5 nm, the resonant dip vanishes. Thus, thickness of BiFeO3 layer (dBF = 2.5 nm) is the optimum. Figure 6 exhibits variation the sensitivity with thickness of BiFeO3 layer as thickness of Ag has been fixed at 60 nm with monolayer of BP. The calculations of all these thicknesses of BaTiO3 layer are listed in Table 4.
A Black Phosphor (BP) layer has a thickness of 0.53 nm. It is assumed that the BP material thickness is S × 0.34 nm, where S is the number of BP layers. The computed sensitivity is found as 461.42, 218.57 and 185.71 deg/RIU for S = 1, S = 2 and S = 3, respectively. This means sensitivity of the suggested detector shrinkages with growing the number of BP sheets as shown in Fig. 7. Therefore, monolayer is the preferred number of BP layers. The calculations of the resonant angle, shift of angle and sensitivity for all investigated values of number of BP layers are listed in Table 5.
According the previous investigations, the optimal parameters are selected as hAg = 60 nm, hBF = 2.5 nm and dBP = S × 0.53 nm, S = 1. The resonant dips for different infected red blood cells are depicted in Fig. 8. When the NRBC is taken as an analyte, the resonant angle is set up at 86.35°. It is observed when infected red blood cells (IRBCs) are used as analytes, the resonant dip changes to smaller angles. The new resonant angles for the RRBC, TRBC, and SRBC are found to be 83.12°, 79.53°, and 77.26°, respectively. The sensitivities are found as 461.43, 358.95 and 313.45 deg/RIU for the RRBC, TRBC and SRBC, respectively as exhibited in Fig. 9. The calculations of sensitivity for those IRBCs are evaluated and presented in Table 6. The highest sensitivity of the suggested photosensor is 461.43 deg/RIU for RRBC, which is an ultra-high value as compared to recently performed publications in this field as shown in Table 7.
4 Conclusion
An SPR nanostructure-based bio-photonic detector has been reported for the detection of infected red blood cells (IRBCs) with Plasmodium Falciparum. To examine the suggested structure, the TMM has been used. At first, the sensitivity are calculated without/with BiFeO3 layer. It is found the BiFeO3 layer improves the sensitivity. Three different 2D-nanomaterials have been examined which are Graphene, MXene and BP. The structure with BP as a 2D-nanomaterial has been obtained to correspond to the greatest performance Then, the sensitivity is analyzed with the thickness of Ag and BiFeO3 materials. As the thicknesses of the Ag and BiFeO3 layers are increased, the angular sensitivity grows. Also, it is deduced that the maximum sensitivity favors a lower number of BP layers. The best sensitivity is acquired at 60 nm (for Ag), 2.5 nm (for BiFeO3) and monolayer of black phosphor. The variation of angular sensitivity is then studied with infected red blood cells (IRBCs) at different stages. The greatest sensitivity is obtained as 461.43 deg/RIU corresponding to a ring phase of IRBC which is improved compared to lately published papers. As a result, we believe the suggested SPR nanostructure will provide a novel opportunity for highly efficient photodetectors.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Akpa Marcel, A., Konan, K., Tokou, Z., Kossonou, Y., Dion, S., Kaduki, K., Zoueu, J.: Malaria-infected red blood cell analysis through optical and biochemical parameters using the transport of intensity equation and the microscope’s optical properties. Sensors 19, 3045 (2019). https://doi.org/10.3390/s19143045
Almawgani, A.H.M., Taya, S.A., Daher, M.G., Colak, I., Wu, F., Patel, S.K.: Detection of glucose concentration using a surface plasmon resonance biosensor based on barium titanate layers and molybdenum disulphide sheets. Phys. Scr. 97, 065501 (2022a)
Almawgani, A.H.M., Daher, M.G., Taya, S.A., Mashagbeh, M., Colak, I.: Optical detection of fat concentration in milk using MXene-based surface plasmon resonance structure. Biosensors 12, 535 (2022b). https://doi.org/10.3390/bios12070535
Almawgani, A.H.M., Daher, M.G., Taya, S.A., Olaimat, M.M., Alhawari, A.R.H., Colak, I. Detection of blood plasma concentration theoretically using SPR-based biosensor employing black phosphor layers and different metals. Plasmonics (2022c)
Avsar, A., Vera-Marun, I.J., Tan, J.Y., et al.: Air-stable transport in graphene-contacted, fully encapsulated ultra-thin black phosphorus-based field-effect transistors. ACS Nano 9(4), 4138–4145 (2015)
Bai, Y., Zhou, K., Narasimalu, S., Pang, J., He, X., Wang, R.: Dependence of elastic and optical properties on surface terminated groups in two-dimensional MXene monolayers: a first-principles study. RSC Adv. 6, 35731–35739 (2016)
Bendib, S., Bendib, C.: Photonic crystals for malaria detection. J. Biosens. Bioelectron. (2018). https://doi.org/10.4172/2155-6210.1000257
Bilal, M., Saleem, M., Amanat, S., Shakoor, H., Rashid, R., Mahmood, A., Ahmed, M.: Optical diagnosis of malaria infection in human plasma using Raman spectroscopy. J. Biomed. Opt. 20(1), 17002 (2015). https://doi.org/10.1117/1.JBO.20.1.017002
Churchill, H.O., Jarilloherrero, P.: Two-dimensional crystals: phosphorus joins the family. Nat. Nanotechnol. 9(5), 330–331 (2014)
Daher, M.G., Taya, S.A., Colak, I., Patel, S.K., Olaimat, M.M., Ramahi, O.: Surface plasmon resonance biosensor based on graphene layer for the detection of waterborne bacteria. J. Biophotonics 15, e202200001 (2022a)
Daher, M.G., Trabelsi, Y., Ahmed, N.M., et al.: Detection of basal cancer cells using photodetector based on a novel surface plasmon resonance nanostructure employing perovskite layer with an ultra high sensitivity. Plasmonics 17, 2365–2373 (2022b)
Daher, M.G., Taya, S.A., Colak, I., Vigneswaran, D., Olaimat, M.M., Patel, S.K., Ramahi, O.M., Almawgani, A.H.M.: Design of a nano-sensor for cancer cell detection based on a ternary photonic crystal with high sensitivity and low detection limit. Chin. J. Phys. 77, 1168–1181 (2022c)
Daher, M.G., Taya, S.A., Colak, I., et al.: Design of a novel optical sensor for the detection of waterborne bacteria based on a photonic crystal with an ultra-high sensitivity. Opt. Quantum Electron. 54, 108 (2022d). https://doi.org/10.1007/s11082-021-03486-7
Daher, M.G., Jaroszewicz, Z., Zyoud, S.H., et al.: Design of a novel detector based on photonic crystal nanostructure for ultra-high performance detection of cells with diabetes. Opt. Quantum Electron. 54, 701 (2022e). https://doi.org/10.1007/s11082-022-04093-w
Elias, D.C., Gorbachev, R.V., Mayorov, A.S., Morozov, S.V., Zhukov, A.A., Blake, P., Ponomarenko, L.A., Grigorieva, I.V., Novoselov, K.S., Guinea, F., Geim, A.K.: Dirac cones reshaped by interaction effects in suspended graphene. Nat. Phys. 7, 701–704 (2011)
Huang, D.J., Deng, H.M., Chen, F., Deng, H., Yang, P.X., Chu, J.H.: Optical properties of BiFeO3 and Bi 0.9La0.1 FeO3 flms on silicon substrates. J. Phys. 276(1), 012168 (2011). https://doi.org/10.1088/1742-6596/276/1/012168
Jha, R., Sharma, A.K.: Chalcogenide glass prism based SPR sensor with Ag–Au bimetallic nanoparticle alloy in infrared wavelength region. J. Opt. A Pure Appl. Opt. 11, 045502 (2009)
Jia, Y., Li, Z., Wang, H., Saeed, M., Cai, H.: Sensitivity enhancement of a surface plasmon resonance sensor with platinum diselenide. Sensors 20(1), 131 (2020). https://doi.org/10.3390/s20010131
Krampa, F., Aniweh, Y., Kanyong, P., Awandare, G.: Recent advances in the development of biosensors for malaria diagnosis. Sensors 20, 799 (2020). https://doi.org/10.3390/s20030799
Kumar, R., Pal, S., Prajapati, Y.K., Kumar, S., Saini, J.P.: Sensitivity improvement of a MXene- immobilized SPR sensor with Ga-doped-ZnO for biomolecules detection. IEEE Sensors J 22(7), 6536–6543 (2022). https://doi.org/10.1109/JSEN.2022.3154099
Li, L., Yu, Y., Ye, G.L., Ge, Q., Ou, X., Wu, H., Feng, D., Chen, X.H., Zhang, Y.: Black phosphorus field-effect transistors. Nat. Nanotechnol. 9(5), 372–377 (2014)
Lin, Z., Jiang, L., Wu, L., Guo, J., Dai, X., Xiang, Y., Fan, D.: Tuning and sensitivity enhancement of surface plasmon resonance biosensor with graphene covered Au MoS2-Au films. IEEE Photon. J. 8(6), 1–8 (2016)
Liu, P.Y., Chin, L.K., Ser, W., Chen, H., Hsieh, C.-M., Lee, C.-H., Sung, K.-B., Ayi, T., Yap, P., Liedberg, B., Wang, K., Bourouina, T., Leprince-Wang, Y.: Cell refractive index for cell biology and disease diagnosis: past, present and future. Lab Chip 16(4), 634–644 (2016). https://doi.org/10.1039/c5lc01445j
Maharana, P.K., Bhardwaj, S., Jha, R.: Electric field enhancement in surface plasmon resonance bimettalic configuration based on chalcogenide prism. J. Appl. Phys. 114, 014304–014314 (2013)
Mao, N., et al.: optical anisotropy of BP in the visible regime. J. Am. Chem. Soc. 138(1), 300–305 (2016)
Maurya, J.B., Prajapati, Y.K.: A comparative study of different metal and prism in the surface plasmon resonance biosensor having MoS2-graphene. Opt. Quantum Electron. 48, 280 (2016)
Molina-Franky, J., Cuy-Chaparro, L., Camargo, A., Reyes, C., Gómez, M., Salamanca, D., Patarroyo, M., Patarroyo, M.: Plasmodium falciparum pre-erythrocytic stage vaccine development. Malar. J. 19(1), 1–18 (2020). https://doi.org/10.1186/s12936-020-3141-z
Mudgal, N., Saharia, A., Agarwal, A., Ali, J., Yupapin, P., Singh, G.: Modeling of highly sensitive surface plasmon resonance (SPR) sensor for urine glucose detection. Opt. Quantum Electron. 52(6), 1–14 (2020)
Mudgal, N., Saharia, A., Choure, K.K., et al.: Sensitivity enhancement with anti-reflection coating of silicon nitride (Si3N4) layer in silver-based surface plasmon resonance (SPR) sensor for sensing of DNA hybridization. Appl. Phys. A 126, 946 (2020). https://doi.org/10.1007/s00339-020-04126-9
Nureye, D., Assefa, S.: Old and recent advances in life cycle, pathogenesis, diagnosis, prevention, and treatment of malaria including perspectives in ethiopia. Sci. World J. 2020, 1–17 (2020). https://doi.org/10.1155/2020/1295381
Nurrohman, D.T., Chiu, N.-F.: Surface plasmon resonance biosensor performance analysis on 2D material based on graphene and transition metal dichalcogenides. ECS J. Solid State Sci. Technol. 9, 115023 (2020)
Pal, A., Jha, A.: A theoretical analysis on sensitivity improvement of an SPR refractive index sensor with graphene and barium titanate nanosheets. Optik 231, 166378 (2021). https://doi.org/10.1016/j.ijleo.2021.166378
Pal, S., Prajapati, Y.K., Saini, J.P.: Influence of grapheme’chemical potential on SPR biosensor using ZnO for DNA hybridization. Opt. Rev. 27, 57–64 (2020)
Panda, A., Daher, M.G., Pukhrambam, P.D., et al.: Study of titanium nitride (TiN) as a novel plasmonic material for realization of Tamm-plasmon-polariton-based blood plasma sensor. Opt. Quantum Electron. 54, 796 (2022). https://doi.org/10.1007/s11082-022-04191-9
Papich, M.G.: Kanamycin Sulfatein: Saunders Handbook of Veterinary Drug. Elsevier, London (2016)
Peng, Q., Wang, Z., Sa, B., Wu, B., Sun, Z.: Electronic structures and enhanced optical properties of blue phosphorene/transition metal dichalcogenides van der Waals heterostructures. Sci. Rep. 6, 1–10 (2016)
Qiao, J., Kong, X., Hu, Z.X., Yang, F., Ji, W.: High-mobility transport anisotropy and linear dichroism in few-layerblack phosphorus. Nat. Commun. 5, 4475 (2014)
Ragavan, K.V., Kumar, S., Swaraj, S., Neethirajan, S.: Advances in biosensors and optical assays for diagnosis and detection of malaria. Biosens. Bioelectron. 105, 188–210 (2018). https://doi.org/10.1016/j.bios.2018.01.037
Rodin, A.S., Carvalho, A., Castro Neto, A.H.: Strain-induced gap modification in black phosphorus. Phys. Rev. Lett. 112(17), 176801–176801 (2014)
Srivastava, T., Jha, R.: Black phosphorus: A new platform for gaseous sensing based on surface plasmon resonance. IEEE 99, 1–1 (2018)
Srivastava, T., Purkayastha, A., Jha, R.: Graphene based surface plasmon resonance gas sensor for terahertz. Opt. Quantum Electron. 48(6), 334 (2016)
Taya, S.A., Al-Ashi, N.E., Ramahi, O.M., Colak, I., Amiri, I.S.: Surface plasmon resonance-based optical sensor using a thin layer of plasma. J. Opt. Soc. Am. B 38, 2362–2337 (2021)
Uniyal, A., Chauhan, B., Pal, A.: Bi-metallic films of gold, MXene, and graphene nano film-based surface plasmon resonance sensor for malaria detection: a numerical analysis (2022). https://doi.org/10.21203/rs.3.rs-1766754/v1
Vasimalla, Y., Pradhan, H.S.: A highly performed SPR biosensor based on bismuth ferrite-bromide materials-BP/graphene hybrid structure. Opt. Quantum Electron. 53, 695 (2021). https://doi.org/10.1007/s11082-021-03347-3
Wang, X., Jones, A.M., Seyler, K.L., Tran, V., Jia, Y., Zhao, H., Wang, H., Yang, L., Xu, X., Xia, F.: Highly anisotropic and robust excitons in monolayer black phosphorus. Nat. Nanotechnol. 10(6), 517–521 (2014)
Wu, L., You, Q., Shan, Y., Gan, S., Zhao, Y., Dai, X., Xiang, Y.: Few layer Ti3C2Tx MXene: a promising surface plasmon resonance biosensing material to enhance sensitivity. Sens. Actuators B Chem. 277, 210–215 (2018)
Yupapin, P., Trabelsi, Y., Vigneswaran, D., Taya, S.A., Daher, M.G., Colak, I.: Ultra-high-sensitive sensor based on surface plasmon resonance structure having Si and graphene layers for the detection of chikungunya virus. Plasmonics 17, 1315–1321 (2022)
Acknowledgements
Not applicable.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, MGD; Data curation, Formal analysis, Investigation, MGD, YT, and YKP; Methodology, MGD, YT, and AP; Resources, Software, MGD,YT, and AP; Supervision, Validation, NMA and ANZR; Visualization, Writing-original draft, MGD and ANZR; Writing—review editing,
Corresponding author
Ethics declarations
Conflict of interest
No competing interests.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daher, M.G., Trabelsi, Y., Prajapati, Y.K. et al. Highly sensitive detection of infected red blood cells (IRBCs) with plasmodium falciparum using surface plasmon resonance (SPR) nanostructure. Opt Quant Electron 55, 199 (2023). https://doi.org/10.1007/s11082-022-04466-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-022-04466-1