Abstract
Mycobacterium tuberculosis bacteria is an illness that affects many people worldwide. Early diagnosis is crucial for patient care and can lower the death rate. As a result, sensitive and rapid detection of mycobacterium tuberculosis bacteria in the blood is crucial. In this paper, a novel surface plasmon resonance (SPRE) sensor consisting of a coupling prism, silver (Ag), barium titanate (BaTiO3) and graphene (Gr) layers is presented. The transfer matrix (TM) technique is used for the analysis of the SPRE structure. The Ag and BaTiO3 thicknesses and the number of Gr sheets are optimized to get the highest sensitivity of the proposed SPRE biosensor. The full width at half maximum (FWHM), detection accuracy (DA) and figure of merit (FOM) are investigated. The best performance has been obtained with 65 nm (Ag), and 9 nm (BaTiO3). The number of Gr layers is investigated and optimized to two layers. The highest sensitivity of 300 deg./RIU has been obtained for the proposed SPRE biosensor when the optimized thicknesses are employed. Compared with existing SPRE biosensors in the literature, the proposed sensor exhibits greater sensitivity. The excellent performance makes this SPRE-based sensor promising to be used in numerous biosensing applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is an infectious illness caused by Mycobacterium tuberculosis (MBTS). When a person coughs, for instance, MBTS can spread through the air. TB is a key cause of health worsening and mortality worldwide [1]. TB is a multisystem infectious disease because it affects various organ systems. About 25% of people are thought to have TB due to the high infection rate [2, 3]. The UN set 17 sustainable development goals in 2015, one of which is to eliminate TB by 2030. But TB research is obscured by the resource allocations such as laboratories, manpower, and clinical services [4]. Similar to other infectious illnesses, TB needs early diagnosis since treatment in the early stages can be much more effective. A microscopic examination of sputum smears, a tuberculin test, chest X-ray, tissue biopsy analysis, sputum culture, and computed tomography scan are some of the latest advancements in the diagnosis of tuberculosis. The gold standards for these are bacterial culture and acid-fast bacilli smear microscopy [5]. However, it is predicted that microscopic smear tests can identify pulmonary tuberculosis with a sensitivity of up to 70% [6]. The culture method for detecting TB mycobacterial requires 2–8 weeks and there is a limit to the minimum number of bacteria that can be detected [7]. In comparison to sputum culture, the analysis of sputum after acid-fast (ADF) staining is simpler and faster for the diagnosis of pulmonary TB. ADF staining is fast, inexpensive, and simple. Sample processing, smears thickness, conservation and preparation of the reagents, microscope quality and the proficiency of the technical team can all influence the ADF staining sensitivity and specificity [8]. In order to increase the effectiveness of the currently used techniques, additional investigations have been carried out. For instance, computer-aided tuberculosis detection using digital chest X-ray has become more prevalent in a variety of settings. However, computer-aided detection still requires improvement [9].
A subclass of surface plasmons is the surface plasmons resonance (SPRE). The SPRE sensor is used in a variety of biosensing applications, including the detection, analysis, and characterization of biomolecules and chemicals [10, 11]. Additionally, these detectors are very sensitive and enable real-time analyte measurement [12]. The SPRE sensor exhibits a sharp dip in the resonance profile at the resonance frequency/wavelength at the particular refractive index (RI) of the sensing layer [13]. The most preferred arrangement for the sensor's design is the Kretschmann configuration [14]. In this arrangement, the structure has a prism with a low RI with a metal layer is coated on its surface. The metal layer is responsible for the surface plasmons generation. A 2-dimensional (2D) material is utilized in the SPRE structures to enhance the sensor performance since the metal has low adsorbability [15,16,17,18]. To improve the biosensor performance, a Gr sheet can be used. Gr is a planar, 2D honeycomb lattice structure made of carbon atoms. It has exceptional electrical and optical properties [19, 20]. In addition to its outstanding mechanical characteristics, Gr also exhibits electrical conductivity, transparency, and biocompatibility. The strongest material is Gr, which also has a great degree of flexibility. Its tensile strength ranges from 15 to 520 MPa whereas Young's modulus is between 20 and 40 GPa [14]. This makes it the perfect choice for biomedical electronic devices. Additionally, it can accommodate fats, RNA, DNA, and biological tissue. Greater transparency and broadband absorption are characteristics of Gr [21]. Its high surface-to-volume ratio permits the adsorption of biomolecules. The carbon ring-like structure of the biomolecules makes them easily able to interact with the Gr structure. This property increases the biomolecule adsorption in the sensor [22]. An SPRE biosensor using Ag and Gr with a minimum reflectance of 0.4674 and a sensitivity of 176 deg./RIU was proposed [23]. A biosensor using an Ag layer and few layers of Franckeite was proposed with a maximum sensitivity of 188 deg./RIU [24]. A biosensor has been demonstrated which has Au, MoS2, Ni and Gr and a sensitivity of 229 deg./RIU [25]. An SPRE structure with black phosphorus as a 2D material and Au has been theoretically investigated as a sensor. A sensitivity of 245.5 deg./RIU was achieved [26]. A biosensor consisting of black phosphorus, silicon, and transition metal dichalcogenides (TMDC) has been studied and a sensitivity of 184.6 deg./RIU has been attained [27]. The plasmonic enhancing effects of black phosphorus in an SPRE structure stem from its enhanced light-matter interaction, high carrier mobility, tunable bandgap, and field confinement capabilities. These properties make black phosphorus a promising material for enhancing the sensitivity and performance of SPRE-based biosensors and other plasmonic devices. The effects of TMDC in an SPRE structure arise from their strong light-matter interaction, exciton resonances, tunable optical properties, enhanced energy transfer, and ultrathin nature. These properties make TMDs promising materials for enhancing the sensitivity, selectivity, and overall performance of SPRE-based biosensors. Recent studies show that barium titanate (BaTiO3) possesses unusual dielectric properties such as minimum loss and high RI. Moreover, it is able to create a considerable shift in the resonance SPRE dip with a smaller increment in the RI of an analyte layer [28]. BaTiO3 is a porous material with a 50–70% porosity. The average pore size is about 30 μm in diameter. It was reported that BaTiO3 does not show short-term toxicity [29]. Tetragonal crystalline BaTiO3 nanoparticles display unusual optical properties and biocompatibility. Furthermore, they do not change their morphology [30]. Most of the current SPRE structures suffer the problems of large FWHM, low-quality factor and low sensitivity. Incorporation of a BaTiO3 layer in an SPRE structure offers plasmonic enhancing capabilities through enhanced field localization, tunability of the resonance condition, improved light-matter interaction, and reduced losses. These capabilities contribute to the overall performance and sensitivity of the SPRE sensor [31,32,33]. Too much work has to be done before SPRE sensors can be commercialized.
In the current paper, a BaTiO3 layer is placed on the top of Ag thin film to improve the biosensor sensitivity, decrease the FWHM of the resonant dip, and improve the quality factor. An additional Gr layer is deposited on the top of the BaTiO3 layer to enhance the adsorption of biomolecules and hence improve the sensor sensitivity to MBTS.
Design Consideration
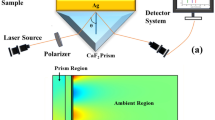
The He–Ne laser beam with a wavelength of 632.8 nm is coupled into an SPRE nanostructure using a BK7 glass prism with a RI of np. The SPRE structure has five layers: BK7 glass prism, Ag, BaTiO3, Gr and analyte medium. The thicknesses of Ag, BaTiO3 and Gr layers are symbolized as dAg, dBaTiO3 and dG and the RIs are denoted as nAg, nBaTiO3 and nG, respectively. Figure 1 shows a schematic diagram of the proposed SPRE sensor that is based on BaTiO3-Gr layers.
The BK7 glass prism has a RI given by [34]
where S1 = 1.03961212, S2 = 1.01046945, S3 = 0.231792344, U1 = 0.00600069867, U2 = 103.560653 and U3 = 0.0200179144.
The RI of the Ag metal is calculated using Drude-Lorentz model [35]
where \({\lambda }_{cw}=1.7614\times {10}^{-5} m\) and \({\lambda }_{pw}=1.4541\times {10}^{-7} m\) are the collision and plasma wavelengths of Ag. The RI of Gr can be calculated as [36]
The RI of BaTiO3 is 2.0 at λ = 632.8 nm [35].
Only p-polarized light (TM-polarization) in naturally occurring materials with positive permittivity and permeability exhibits the phenomenon of SPRE. The angular modulation method can be used to study the proposed structure. With the use of the TM technique and Fresnel theory, the reflectance of waves (p-polarized) can be studied. For the surface plasmon to be stimulated, the propagation constants of the wave and the surface plasmon must be equal. In a mathematical form
where εp, ω, εAg, θi and εd are the dielectric permittivity of the prism, the incident radiation frequency, the metal dielectric permittivity, the incident angle and the dielectric permittivity, respectively.
Along the z-axis, several layers are stacked. The tangential field components at the first interface can be written in terms of those at the last interface as
where X1 (H1) and XN-1 (HN-1) are the tangential electric (magnetic) fields at the first and the last boundaries, respectively. BT is the TM that relates the field components to these boundaries. For the jth layer, the TM (Bj) can be written as
\({U}_{j}\) is the phase shift which is given by
where n1 and θ1 are the RI and the incident angle of the prism.
For TM waves, Pj is defined as
The system TM (BT) of the structure can be written as
where \({B}_{Ag}\), \({B}_{BaTiO3}\) and \({B}_{G}\) are the TMs of the Ag, BaTiO3 and Gr layers.
The reflection coefficient (r) of the structure is given in terms of Bij as
The reflectivity (R) of the proposed SPRE nanostructure is given by
Sensitivity (S), FWHM, detection accuracy (DA), and figure of merit (FOM) of SPRE sensors are usually estimated for the sensor performance. When a change in the RI (Δn) of the sensing medium occurs, the resonant angle shifts by an amount Δθres. The sensitivity depends on both Δn and Δθres according to
The FWHM is obtained from the reflectance curve as
where θ1 and θ2 are the resonance angles at 50% reflectivity.
DA is the reciprocal of the FWHM multiplied by \(\Delta {\theta }_{res}\).
FOM is the product of S and DA of the sensor
The quality factor (QF) of an SPRE can be calculated as
Results and Discussion
Fabrication Feasibility
This subsection describes the steps involved in fabricating the proposed multilayer SPRE chip. Initially, a solution containing acetone, methanol, and deionized water is applied to the BK7 prism. Subsequently, a thin silver film is coated onto the prism using a deposition technique such as thermal evaporation or physical vapor deposition. To synthesize the BaTiO3 material, a hydrothermal reaction is carried out by reacting TiO2 with an aqueous solution of Ba(OH)2. This reaction results in the formation of highly crystallized and well-dispersed perovskite-type BaTiO3. For the production of a single layer of graphene, chemical vapor deposition is considered the most promising method as it yields high-quality single-layer graphene. Subsequently, a blood sample is poured onto the sensor chip area for sensing purposes. The entire SPRE chip is then placed on a rotating table, and an optical detector positioned at the output side of the prism receives the reflected light and determines its intensity.
MBTS Sensor
An SPRE structure-based optical sensor is investigated for sensing MBTS bacteria. The following calculations will be conducted at \(\lambda\) = 632.8 nm. The SPRE sensor has five layers: BK7 glass prism, Ag, BaTiO3, Gr and analyte medium. The RIs of “Design Consideration” to the expressions presented in Sect. 2. The RIs of the materials are given by np = 1.5151, nAg = 0.056206 + 4.2776i, nBaTiO3 = 2 and nG = 3 + 1.1487i for BK7 glass prism, Ag, BaTiO3 and Gr. Layer thicknesses are initially chosen as dAg = 40 nm, dBaTiO3 = 1 nm and dG = L × 0.34 nm where L is the number of Gr layers (initially, L = 1). Either a normal blood sample (NBSE) or a sample infected with tuberculosis bacteria (TBj), j = 1—4 makes up the analyte layer. The RIs of NBSE and TBj samples are reported in Table 1 [37]. We have used Wolfram Mathematica 11.2 software to conduct the simulation. Figure 2 shows the reflectance curves when NBSE and TBi are handled as sensing media. The figure shows a resonant dip at a resonant angle of 71.19 deg. when NBSE is used as a sensing medium. When TBi samples are used as sensing media, the resonant angle moves to a lower angle region. The dip angular position is found cell-dependent. They are at 70.79, 70.66, 70.41 and 70.16 deg. for TB1, TB2, TB3 and TB4 cells. The sensitivity is found as 133.33, 132.5, 130 and 128.75 deg./RIU for TB1, TB2, TB3 and TB4 cells. Table 1 displays the RIs of different samples, the dip position, and the sensitivity of the proposed SPRE biosensor to each cell.
Effect of Ag Layer Thickness
A proper selection of the layer thicknesses of the SPRE structure is necessary and can significantly enhance the sensor performance. This subsection and the following include an investigation of the various options for layer thicknesses. So, for a constant thickness of BaTiO3 (dBaTiO3 = 1 nm) and a monolayer of Gr (L = 1), we study the effect of the Ag thickness on the SPRE sensor performance. We consider two samples here: NBSE and TB4. The Ag thickness is varied from 40 – 65 nm in a step of 5 nm. The reflectance spectrum corresponding to each Ag thickness is studied and some of these spectra are illustrated in Fig. 3(a) and (b) for dAg = 40 nm and 60 nm, respectively. Figure 4 illustrates the sensitivity variation for different thicknesses for the Ag layer which shows an increase from 128.75 deg./RIU at dAg = 40 nm to 136.25 deg./RIU at dAg = 65 nm. The sensitivity shows a maximum at dAg = 65 nm. Table 2 reports the variation of the dip angular position, angular shift and sensitivity corresponding to different Ag layer thicknesses. Table 3 presents the variation of FWHM, DA, and FOM for different thicknesses of Ag. The FWHM decreases from 2.35 to 0.82 deg. as the Ag thickness increases from 40 to 65 nm. This decrease in the FWHM significantly improves the DA of the SPRE sensor, as shown in Table 3. It is obvious that when the Ag layer thickness increases, the DA rises as well. The maximum DA is 1.219512195 deg.−1 at dAg = 65 nm. The FOM also enhances as the Ag thickness rises because both the sensitivity and the DA are enhanced. As the thickness of the Ag layer increases, the transfer of incident wave energy to surface plasmon is dramatically enhanced. This causes an efficient excitation of surface plasmon which leads to a very high sensitivity and quality of the SPRE sensor. Consequently, the optimized Ag layer thickness is 65 nm which corresponds to the best performance. It is worth mentioning that if the Ag layer thickness is increased above 65 nm, the resonance dip disappears. So, a thickness of 65 nm can be considered as optimum.
Effect of BaTiO3 Layer Thickness
The thickness of BaTiO3 layer is varied in the range from 1 – 9 nm and the reflectance spectrum is studied for each thickness. Two of these spectra are shown in Fig. 5 for the normal blood and TB4 samples. The sensitivity is observed to enhance with the increase of BaTiO3 layer thickness (Fig. 6). It increases from 136.25 to 267.5 deg./RIU as the thickness of BaTiO3 increases from 1 to 9 nm as reported in Table 4. The sensitivity is considerably improved by 96.33% due to the increase in the BaTiO3 layer thickness. The angular position of the resonant dip shifts to higher angles as the thickness of BaTiO3 increases. On the other hand, inspection of Table 5 shows that the FWHM of the SPRE dips gets broader and broader as dBaTiO3 thickness increases from 1 to 9 nm which indicates that the energy loss of the SPRE biosensor is gradually enhanced with increasing the thickness of BaTiO3 layer. Since DA is the reciprocal of FWHM, it is reduced as dBaTiO3 increases as presented in Table 5. When dBaTiO3 changes from 1 to 9 nm, the DA decreases from 1.219512195 to 0.418410042 deg.−1 which means a decline by about 65.7% of its initial value. Moreover, Table 5 pointed out that a gradual decrease has occurred in FOM owing to the increase of BaTiO3 layer thickness. It is observed that the resonant dip disappears for dBaTiO3 > 9 nm.
Effect of the Number of Gr Layers
The sensor performance is examined as the number of Gr layers varies. The Ag and BaTiO3 are kept constant at 65 nm and 9 nm, respectively. Figures 7(a) and (b) show some of the spectra for Gr layers with L = 1 and L = 3, respectively. The sensitivity rises as the number of Gr sheets increases from L = 1 to L = 2 (Fig. 8). A further increase in the number of Gr sheets above L = 2 leads to a sensitivity decline as shown in Table 6. The greater absorption due to the imaginary part of the Gr dielectric constant is responsible for the sensitivity decline as more sheets of Gr are added [38, 39]. However, in a similar manner to increasing the BaTiO3 layer thickness, any growth of the number of Gr sheets above L = 1 is accompanied by a FWHM enhancement which has a negative impact on the DA and FOM of the proposed sensor (Table 7). The FWHM is directly proportional to damping in the surface plasmons which enhances with an increase in the number of Gr sheets due to the imaginary part of the Gr dielectric constant [40]. Therefore, it can be inferred that the performance of the SPRE sensor will deteriorate as the number of Gr sheets increases. A Gr bilayer may be the best choice for the proposed SPRE biosensor.
Tuberculosis Sensor Optimization
The optimal conditions are dAg = 65 nm, dBaTiO3 = 9 nm, and dG = L × 0.34 nm where L = 2. At these conditions, the reflectance is investigated in Fig. 9 for all samples which displays that the angular dip shifts to lower angles of incidence as the tuberculosis-infected samples replace the NBSE. The angular positions of the resonant dips along with sensitivity are reported in Table 8. The sensitivity of the optimized SPRE structure is found as 300, 297.5, 290 and 286.25 deg./RIU for the samples of TB1, TB2, TB3 and TB4. Additionally, Table 9 displays the FWHM, DA, and FOM which are all indicators of the sensor performance. Based on these values, the proposed BaTiO3-Gr-based SPRE sensor exhibits good performance with the optimum layer thicknesses. Table 10 also offers a comparison of the current and previously reported works. With this performance, we believe that the proposed BaTiO3-Gr-based SPRE sensor can find widespread use in the field of biosensing technologies.
Conclusion
An SPRE-based biosensor based on BaTiO3-Gr layers has been theoretically suggested and numerically investigated for sensing tuberculosis bacteria in the blood. The reflectivity of TM-polarized waves using the TM technique has been analyzed. Silver (Ag), barium titanate (BaTiO3), and Gr thicknesses of the proposed SPRE sensor have been optimized. When the thicknesses are taken as dAg = 40 nm, dBaTiO3 = 1 nm and dG = L × 0.34 nm (L = 1), a sensitivity of about 130 deg./RIU has been obtained. When the Ag layer thickness increases, all the sensor performance parameters are enhanced. This can be attributed to the following: as the Ag thickness increases, the transfer of incident light energy to surface plasmon is dramatically enhanced. This leads to an efficient excitation of surface plasmon. When the BaTiO3 thickness is increased, the sensitivity is observed to considerably improved by 96.33%. On the other hand, the FWHM of the SPRE dips gets broader and broader as dBaTiO3 thickness increases which indicates that the energy loss of the SPRE biosensor is gradually enhanced with increasing the thickness of BaTiO3 layer. When the number of Gr layers increases, the sensitivity rises as the layers of Gr sheets increase from L = 1 to L = 2. A further increase in the number of Gr sheets above L = 2 leads to a sensitivity decline. The optimal conditions of the SPRE structure are found as dAg = 65 nm, dBaTiO3 = 9 nm, and dG = L × 0.34 nm where L = 2 at which the sensitivity can reach about 300 deg./RIU. It is believed the proposed BaTiO3-Gr-based SPRE sensor can find widespread use in the field of biosensing technologies.
Availability of Data and Material
Detail about data has been provided in the article.
Code Availability
The used code can be obtained from the corresponding author upon request.
References
World Health Organization (2022) Global Tuberculosis Report 2022. WHO, Geneva, Switzerland
Houben RMGJ, Dodd PJ (2016) The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med 13:e1002152
Hrizi O, Gasmi K, Ben Ltaifa I, Alshammari H, Karamti H, Krichen M, Ben Ammar L, Mahmood MA (2022) Tuberculosis Disease Diagnosis Based on an Optimized Machine Learning Model. J Healthc Eng 8950243
Sahu S, Wandwalo E, Arinaminpathy N (2022) Exploring the Impact of the COVID-19 Pandemic on Tuberculosis Care and Prevention. J Pediatric Infect Dis Soc 11:S67–S71
Wang CH, Chang JR, Hung SC, Dou HY, Lee GB (2022) Rapid molecular diagnosis of live Mycobacterium tuberculosis on an integrated microfluidic system. Sensor Actuat B-Chem 365:131968
Azadi D, Motallebirad T, Ghaffari K, Shojaei H (2018) Mycobacteriosis and Tuberculosis: Laboratory Diagnosis. Open Microbiol J 12:41–58
Soini H, Musser JM (2001) Molecular diagnosis of mycobacteria. Clin Chem 47:809–814
Vilcheze C, Kremer L (2017) Acid-Fast Positive and Acid-Fast Negative Mycobacterium tuberculosis: The Koch Paradox. Microbiol Spectr 5:1–14
Pande T, Cohen C, Pai M, Khan FA (2016) Computer-aided detection of pulmonary tuberculosis on digital chest radiographs: A systematic review. Int J Tuberc Lung D 20:1226–1230
Mustafa F, Andreescu S (2018) Chemical and biological sensors for food-quality monitoring and smart packaging. J Food Sci Technol 49(4):383–406
Bhatia S, Jiang YC, Sun MJ, Xiong RJ (2018) Development of a surface plasmon resonance acetone sensor for noninvasive screening and monitoring of diabetes. Mater Sci Eng 383:012024
Srivastava A, Prajapati YK (2019) Performance Analysis of Silicon and Blue Phosphorene / MoS 2 Hetero-Structure Based SPRE Sensor. Photonic Sens 9(3):284–292
De Melo AA, Brito T, Fernanda M, Moreira S, Moreno R, Cruz S (2018) Theoretical analysis of sensitivity enhancement by graphene usage in optical Fiber surface plasmon resonance sensors. IEEE Trans Instrum Meas 68(5):1554–1560
Pal A, Jha A (2021) A theoretical analysis on sensitivity improvement of an SPRE refractive index sensor with graphene and barium titanate nanosheets. Optik - International Journal for Light and Electron Optics 231:166378
Bhardwaj S, Pathak NK, Ji A et al (2017) Tunable Properties of Surface Plasmon Resonance of Metal Nanospheroid: Graphene Plasmon Interaction. Plasmonics 12:193–201. https://doi.org/10.1007/s11468-016-0249-7
Arsalani S, Ghodselahi T, Neishaboorynejad T et al (2019) DNA Detection Based on Localized Surface Plasmon Resonance Spectroscopy of Ag@Au Biocomposite Nanoparticles. Plasmonics 14:1419–1426. https://doi.org/10.1007/s11468-019-00937-6
Alaguvibisha G et al (2020) Sensitivity enhancement of surface plasmon resonance sensor using hybrid configuration of 2D materials over bimetallic layer of Cu-Ni. Opt Commun 463:125337
Vahedi A, Kouhi M (2020) Liquid crystal-based surface plasmon BiosensorResonance. Plasmonics 15:61–71
Song B, Li D, Qi W, Elstner M, Fan C, Fang H (2010) Graphene on Au (111): A Highly Conductive Material with Excellent Adsorption Properties for High-Resolution Bio / Nanodetection and Identification. ChemPhysChem 19(111):585–589
Papageorgiou DG, Kinloch IA, Young RJ (2017) Progress in Materials Science Mechanical properties of graphene and graphene-based nanocomposites. Prog Mater Sci 90:75–127
Reina G, Gonz´ alez-Domínguez JM, Criado A, V´ azquez E, Bianco A, Prato M (2017) Promises, facts and challenges for graphene in biomedical applications. Chem Soc Rev 46(15):4400–4416
Aksimsek S, Jussila H, Sun Z (2018) Graphene – MoS 2 – metal hybrid structures for plasmonic biosensors. Opt Commun 428:233–236
Dai X, Liang Y, Zhao Y, Gan S, Jia Y, Xiang Y (2019) Sensitivity enhancement of a surface plasmon resonance with tin selenide (SnSe) allotropes. Sensors 19(1):173
Gan S, Zhao Y, Dai X, Xiang Y (2019) Sensitivity enhancement of surface plasmon resonance sensors with 2D franckeite nanosheets. Results Phys 13:102320
Nisha A, Maheswari P, Anbarasan PM, Rajesh KB, Jaroszewicz Z (2019) Sensitivity enhancement of surface plasmon resonance sensor with 2D material covered noble and magnetic material (Ni). Opt Quantum Electron 51(1)
Singh Y, Raghuwanshi SK (2019) Electromagnetic wave sensors sensitivity enhancement of the surface plasmon resonance gas sensor with black phosphorus. IEEE Sensors Lett 3(12):1–4
Pal S, Verma A, Saini JP, Prajapati YK (2019) Sensitivity enhancement using silicon-black phosphorus-TDMC coated surface plasmon resonance biosensor. IET Optoelectron 13(4):196–201
Singh Y, Paswan MK, Raghuwanshi SK (2020) Sensitivity enhancement of SPRE sensor with the black phosphorus and graphene with Bi-layer of gold for chemical sensing. Plasmonics
Ball JP, Mound BA, Nino JC, Allen JB (2014) Biocompatible evaluation of barium titanate foamed ceramic structures for orthopedic applications. J Biomed Mater Res - Part A 102(7):2089–2095
Marino A et al (2019) Piezoelectric barium titanate nanostimulators for the treatment of glioblastoma multiforme. J Colloid Interface Sci 538:449–461
Mudgal N, Saharia A, Choure KK et al (2020) Sensitivity enhancement with anti-reflection coating of silicon nitride (Si3N4) layer in silver-based Surface Plasmon Resonance (SPR) sensor for sensing of DNA hybridization. Appl Phys A 126:946. https://doi.org/10.1007/s00339-020-04126-9
Mudgal N, Yupapin P, Ali J et al (2020) BaTiO3-Graphene-Affinity Layer-Based Surface Plasmon Resonance (SPR) Biosensor for Pseudomonas Bacterial Detection. Plasmonics 15:1221–1229. https://doi.org/10.1007/s11468-020-01146-2
Wang S, Zhang J, Liu N et al (2023) Sensitivity Improvement of Bimetallic Layer-Based SPR Biosensor Using ZnO and Black Phosphorus. Plasmonics. https://doi.org/10.1007/s11468-023-01889-8
Lin Z, Jiang L, Wu L, Guo J, Dai X, Xiang Y, Fan D (2016) Tuning and sensitivity enhancement of surface plasmon resonance biosensor with graphene covered Au MoS2-Au films. IEEE Photon J 8(6):1–8
Sun P, Wang M, Liu L, Jiao L, Wei Du, Xia F, Liu M, Kong W, Dong L, Yun M (2019) Sensitivity enhancement of surface plasmon resonance biosensor based on graphene and barium titanate layers. Appl Surf Sci 475:342–347
Bruna M, Borini S (2009) Optical constants of graphene layers in the visible range. Appl Phys Lett 94(3):03190
Reddy NM, Kothandan D, Lingam SC, Ahmad A (2012) A study on refractive index of plasma of blood of patients suffering from tuberculosis. Int J Technol Eng 8:23–25
Lin C, Chen S (2019) Design of high-performance Au-Ag-dielectric-graphene based surface plasmon resonance biosensors using genetic algorithm. J Appl Phys 125:113101
Lin C, Chen S (2019) Sensitivity comparison of graphene based nearly guided-wave surface plasmon resonance biosensors with Au, Ag, Cu, and Al. J Nanophotonics 13:016006
Maharana PK, Jha R (2012) Chalcogenide prism and graphene multilayer based surface plasmon resonance affinity biosensor for high performance. Sens Actuat B Chem 169:161–166
Hossain MB, Mehedi IM, Moznuzzaman M, Abdulrazak LF, Hossain MA (2019) High performance refractive index SPRE sensor modeling employing graphene tri sheets, Vol. 15, Results in Physics, p. 102719
Pal S, Prajapati YK, Saini JP (2020) Influence of grapheme’chemical potential on SPRE biosensor using ZnO for DNA hybridization, Vol. 27, Optical Review, pp. 57–64
Singh S, Sharma AK, Lohia P, Dwivedi DK (2021) Theoritical analysis of sensitivity enhancement of surface plasmon resonance biosensor with zinc oxide and blue phosphorus/MoS2 heterostructure, Vol. 244, Optik, p. 167618
Almawgani AHM, Daher MG, Taya SA, Olaimat MM, Alhawari ARH, Colak I (2022) Detection of blood plasma concentration theoretically using SPR-based biosensor employing black phosphor layers and different metals. Plasmonics 17(4):1751–1764
Taya SA, Al-Ashi NE, Ramahi OM, Colak I, Amiri IS (2021) Surface plasmon resonance-based optical sensor using a thin layer of plasma. J Opt Soc Am B 38(8):2362–2367. https://doi.org/10.1364/JOSAB.420129
Hsu SH, Lin YY, Lu SH, Tsai IF, Lu YT, Ho HT (2013) Mycobacterium tuberculosis DNA detection using surface plasmon resonance modulated by telecommunication wavelength. Sensors (Basel) 14(1):458–467. https://doi.org/10.3390/s140100458. PMID: 24379050; PMCID: PMC3926568
Kaur B, Kumar S, Kaushik BK (2022) Antimonene, CNT and MoS2 Based SPR-Fiber-Optic Probe for Tuberculosis Detection. IEEE Sens J 22(15):14903–14910. https://doi.org/10.1109/JSEN.2022.3186995
Acknowledgements
The authors are thankful to the Deanship of Scientific Research at Najran University for funding this work under the Research Groups Funding program grant code (NU/RG/SERC/12/4).
Funding
Deanship of Scientific Research at Najran University.
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution to the paper as follows: study conception and design (Sofyan A. Taya and Malek G. Daher). Software (Abdulkarem H. M. Almawgani). Interpretation of results (Malek G. Daher and Ilhami Colak). Draft manuscript preparation (Ayman Taher Hindi and Malek G. Daher). Writing the final version (Shobhit K. Patel and Sofyan). Supervision (Sofyan A. Taya). All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study does not require ethics approval.
Consent to Participate
No consent to participate is required for this study.
Consent for Publication
No consent for publication is required for this study.
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daher, M.G., Taya, S.A., Almawgani, A.H.M. et al. Optical Biosensor Based on Surface Plasmon Resonance Nanostructure for the Detection of Mycobacterium Tuberculosis Bacteria with Ultra-High Efficiency and Detection Accuracy. Plasmonics 18, 2195–2204 (2023). https://doi.org/10.1007/s11468-023-01938-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-023-01938-2