Abstract
The cadmium sulfide (CdS) and lead sulfide (PbS) materials have been chosen in order to boost solar energy conversion from ultraviolet region to the near-infrared (NIR) spectral region. These materials belonging to the II–VI group have suitable direct band gaps and in solar energy conversion. CdS and PbS layers are used as window and absorber materials, respectively, in solar cells. This paper presents structural, morphological, and optical properties of PbS and CdS thin films prepared by chemical spray pyrolysis at different temperatures as well as its photovoltaic performance. The X-ray diffraction results show the PbS films have cubic structure and CdS films have hexagonal structure. FESEM measurements reveal the films have homogeneous surfaces with spherical particles. Optical analyses exhibit the optical band gap redshifts as the substrate temperature increases. Illumination current density–voltage test of ITO/CdS/PbS/Au heterojunction with different thicknesses of PbS layer has been evaluated. The optimal results are obtained in ITO/CdS/PbS/Au solar cell where thickness of PbS layer is of 0.9 μm with maximum short circuit current, open circuit voltage, and fill factor as well as highest efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ever-increasing power demand has pushed the research community to find more efficient, environmentally safely, renewable, and low-cost energy sources to replace the actual dominant oil and fossil fuel [1]. Because of the inexpensive development and the moderately high theoretical power conversion efficiency, thin-film solar cells are appearing as a highly interesting kind of renewable power sources [2]. They also have versatile features such as capability of multiple exciton generation, high stability against heat, and tunable band gap because of the quantum confinement effect [3]. PbS is a significant semiconductor compound material in the manufacturing of absorber layers for photovoltaic applications due to small band gap approximately from 0.36 to 0.42 eV, appropriate exciton Bohr radius of around 18 nm, and provides electrons and holes with efficient quantum confinement, driving to a raise in the absorption of the solar spectrum in the near infrared range [4]. These features make lead sulfide plays significant role in the photovoltaic applications [5]. CdS thin films are regarded as one of the most important substances for solar cell applications [6]. It has wide band gap (2.5 eV) with high optical transmittance and high electrical conductivity [7]. For these reasons, CdS can be used as the window layer beside with other semiconductors such as CdTe, CIGS, and ZnO [8,9,10].
There are various routes for thin-film fabrication, for instance, chemical bath deposition (CBD) [11], spin coating [12], pulsed laser deposition [13], chemical vapor deposition [14], sputtering method [15], successive ionic layer absorption and reaction (SILAR) techniques [1], and chemical spray pyrolysis (CSP) [16]. The development of low-cost polycrystalline thin-film photovoltaic devices is significant for future power production. The CSP technique has been more attractive technologies, which are well suited for large area coating, low-temperature processing, simple, economical, and energy-effective approach for fabricating thin films of semiconductors [17]. CSP is particularly a precursor undergo to chemically react by spraying a solution into a substrate heated at elevate temperature, after that the precursors react to produce the demanded material [18].
This paper reports for first time the CSP method for preparing CdS thin film as window layer and PbS thin film as absorber layer for photovoltaic applications. The impacts of substrate temperature on structural, morphological, and optical properties of the prepared films were investigated by XRD, FESEM, and UV-Vis spectroscopy, respectively. Also, photovoltaic properties of the ITO/CdS/PbS/Au solar cells were characterized by illumination with power of 50 mW/cm2. The fabricated solar cell showed a significant enhancement of efficiency from 0.07 to 0.85% by increasing absorber layer thickness from 0.3 to 0.9 μm. The photovoltaic results are discussed and compared to the results reported in the literature.
Experimental Part
Solution Preparation
A cadmium chloride (CdCl2) and thiourea (CS (NH2)2) were used as precursor materials to deposit CdS thin film. The CdCl2 and CS(NH2)2 were dissolved in deionized water in a separate manner, then mixing together at molarity of 0.02 M via magnetic stirrer for 30 min at room temperature. For PbS, lead nitrate (Pb (NO3)2) and thiourea were dissolved in deionized water at molarity of 0.05 M, then magnetically stirred for 20 min at room temperature.
Device Preparation
The ITO-coated glass substrate was used for depositing thin films. First, the slides were cut into small pieces with dimensions 1 cm × 1 cm and washed with acetone and hydrochloric acid (HCl, 20%), then placed in vacuum furnace at 50 °C to dry prior using. The PbS, CdS, and CdS/PbS thin films were grown on ITO/glass slides by utilizing the CSP route, as shown in Scheme 1. Briefly, the prepared solution was poured into the container of the nozzle and the slide was located on the heater to heat the slide to the desired temperature (200, 250, and 300 °C). The distance between the nozzle and the heated slide was maintained to be 30 cm. Then, the solution was sprayed in vertical type and the droplets of precursor were applied in good way on the slide for 30 s. After deposition, the slide was left to cool down to the room temperature [16]. To finish cell structure, a gold layer of thickness of 30 nm was performed as an Ohmic contact substance by a sputtering method, as presented in Scheme 2.
Characterization
Morphological features of prepared thin film were characterized by field emission scanning electron microscope (FESEM, ZEISS-SIGMA300), and the statistical distribution of the particle size was measured by using ImageJ software. UV-Vis spectroscopy (double beam, SolidSpec-3700i) was employed to determine the optical properties of prepared films. The optical absorption coefficient (α) was evaluated by utilizing following equation [19]:
where t is the film thickness and T is the transmission. The optical band gap (Eg) was determined by Tauc equation [20]:
where A, h, and ν are a constant, Plank’s constant, and the photon frequency, respectively. The X-ray diffraction technique (XRD, Philips PW 1840, λ = 1.5406°A from Cu-Kα) was accomplished to study the structural properties of prepared films and compared with JCPDS reported data. From XRD data, the interlayer spacing (d) was determined by Bragg’s law [21]:
where n, λ, and θ are the order diffraction, X-ray wavelength, and diffraction angle, respectively. The Debye–Scherer relation was accomplished to calculate the average crystallite size (L) of prepared thin films as follows [22]:
where K and B are a constant (0.9) and full width at half maximum (FWHM), respectively. The cubic lattice constant (a) of the PbS thin film was calculated by using following relation [23]:
where (hkl) are Miller indices. Also, the hexagonal lattice constants (a and c) of CdS thin film were determined by following equation [24]:
Capacitance–voltage (C-V) measurements in the reverse bias manner were evaluated with a frequency of 100 kHz via a PM 6306 Programmable LRC meter supplied by Fluke. J-V characteristics were accomplished in the forward bias manner under illumination. A DC power supply (type L, voltage range from 0.1 to 5 V), a Keithley (602) electrometer, and halogen lamp (50 mW/cm2) were used for this purpose. The thicknesses of PbS and CdS films were evaluated by laser interference and found to be 0.3, 0.6, and 0.9 μm for PbS and 150 nm for CdS.
Results and Discussion
Morphological Analysis
The surface morphology of films was characterized by utilizing FESEM technique, which generates topological images of surfaces at very high resolution. Figure 1 a, b displays the FESEM images of PbS and CdS thin films deposited on the ITO substrate at 300 °C. It is obvious that the nanoparticles have diverse size values; however, the geometry of the surface has a high degree of regularity. Figure 3 c, d displays a statistical distribution of the particle size over the surface and indicates that the value of the distribution occurs between 10 and 100 nm.
Structural Analysis
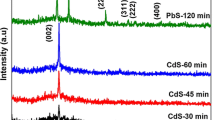
The X-ray spectra of the PbS films grown on ITO by CSP route at different temperatures are demonstrated in Fig. 2. The spectra show six peaks at 2θ = 26.1°, 30.2°, 43.1°, 51.4°, 53.2°, and 62.5°, respectively, related to the (111), (200), (220), (311), (222), and (400) diffraction planes of the polycrystalline of lead sulfide cubic phase. This observation is close to the values of the standard data of JCPDS card (05-0592) as reported in the literature [25]. All the PbS spectra exhibit that the diffraction plane intensities of major peaks increased with increment temperature of the substrate, which may due to the increasing of crystallite size and thickness. The lattice constants (a) of cubic PbS thin films are depicted in Table 1, and the estimated values were close to the standard lattice constant (ao = 0.593 nm). Figure 3 shows the X-ray spectra of CdS thin films formed on ITO by CSP method at different temperatures. The CdS thin films are appearing polycrystalline with a hexagonal phase. The intensive peaks are located at 2θ = 24°.8, 26°.52, and 28°.24, which are corresponding to (100), (002), and (101) planes, respectively. Besides, other peaks with less intensity are centered at 2θ = 36.6°, 47.8°, and 50.84° corresponding to (102), (103), and (200) planes, respectively, as reported in the previous work [26] (Table 2).
Optical Analysis
The UV-Vis transmittance spectra of the PbS and CdS films deposited at different temperatures are presented in Figs. 4 and 5, respectively. We notice that the PbS films have high transmission at high wavelengths (60–70%), and reducing to (20–30%) at low wavelengths. The transmittance spectrum of the PbS minimized with increment in substrate temperature; this may be the variation in particle size distribution from one sample to another [27]. On the other hand, the CdS films have high transparency in the visible region about 80% and sharp absorption band is appearing at 470 nm. All CdS film exhibits a window layer behavior of visible light, which is useful for optoelectronic applications [28]. The increase in transmittance of the films and CdS may be attributed to the less light scattering due to the better crystal structure and surface properties. The inset of Fig. 4 demonstrates the absorption coefficient recorded with wavelength for the PbS films at different substrate temperatures. It is clear that the PbS has a high absorption coefficient at the short wavelength range (300–550 nm), then reduces at different rates to reach constant values at long wavelengths which it is above (700 nm), where the films become transparent at this wavelength. The inset of Fig. 5 shows the absorption coefficient variation with wavelength for CdS film at different temperatures. Absorption coefficient increased slightly with substrate temperature; this may be due to increasing of thickness and the degree of crystallization. The influence of the substrate temperature on the energy gap value of PbS and CdS films is shown in Figs. 6 and 7, respectively. As expected the increasing in the substrate temperature will lead to reduce in the magnitude of energy gap due to crystallization enhancement (see Table 3). These results are in good agreement with the references [29, 30]. The obtained band gap values for PbS are higher than the 0.42 eV reported for bulk PbS. In literature many authors have reported similar band gaps for films having small crystallite sizes [31]. It has been attributed to the quantum confinement. In the present study, the crystallite size is in the range of 10–14.5 nm, as determined from XRD patterns, which is smaller than the Bohr radius of PbS (18 nm). The decreasing dimension of the spherical crystallites might have influenced the density of states resulting in a red-shifting the fundamental absorption edge [32].
Electrical Measurements
Figure 8 presents the changing of capacitance with reverse bias voltage for ITO/CdS/PbS/Au heterojunction solar system produced in different thickness of the PbS film. A nonlinear reduces in capacitance with increment the reverse voltage; this resulted from alters of the width of the depletion layer [33]. From Fig. 9, it is obvious that the Vbi becomes higher as the thickness of PbS film increases (see Table 4) due to more charge carriers present in thick PbS in comparison with thin PbS. The linear relation between 1/C2 and voltage suggests the abrupt kind of the heterojunction [34]. The PbS film represents absorber sheet, and its thickness plays a significant role, which controls the electrical measurements of the solar cells. J-V behavior of the ITO/CdS/PbS/Au heterojunction with different PbS thicknesses and under illumination is presented in Fig. 10. From Table 5, we notice that the high values of JSC and VOS was observed at the large thickness of the PbS film, because of a high absorption of radiation, which led to high photocurrent produced. The fill factor (FF) for solar cells can be measured via the below formula [35].
From Table 5, we can see that the FF varies with the thickness of the PbS film. As the PbS thickness increases, the characteristics of the prepared solar cells enhanced; this can be ascribed to the decreasing of series resistance. The efficiency of the prepared solar cell was obtained by the following relation [35]:
From Fig. 10 and Table 5 it is clear that the short circuit photocurrent density (Jsc) of the cell has increased from 0.36 to 1.87 mA/cm2 by increasing of absorber layer thickness, which is an impressive 50% increase. Correspondingly, the cell efficiency has also increased. The highest value of efficiency is around 0.85% of PbS thickness of 0.9 μm. The ITO/CdS/PbS/Au solar cell fabricated with 0.9 μm showed a dense and pinhole-free highly crystalline CdS/PbS film with improved optical and electrical properties. On the other hand, the ITO/CdS/PbS/Au solar cell fabricated with 0.3 μm suffered from low efficiency and severe recombination problems at the CdS/PbS interface. The comparison between the result obtained by another researcher and this study can be seen in Table 6. An improvement in solar cell performance can be attributed to an increment in light-harvesting ability and an enhancement in charge transport ability (reduction of charge recombination) at the CdS/PbS junction.
Conclusion
In order to obtain solar cell structures of ITO/CdS/PbS/Au, window (CdS) and absorption (PbS) layers have been sequentially synthesized on ITO substrates using the CSP method. The structural and optical properties of prepared thin films were strongly depended on the changing of substrate temperature. XRD proved that all thin films are polycrystalline. Using FESEM, the present work showed that the films have a uniform surface morphology over the substrate without pinholes or cracks. It was found that the performance of fabricated solar cell depended on the thickness of the absorber sheet (PbS). The output power density and the cell efficiency have been increased with increasing the thickness of absorber layer. The photovoltaic performance of the ITO/CdS/PbS/Au solar cells showed that the solar cell fabricated with thickness 0.9 μm of the PbS film exhibited higher efficiency of 0.85% including JSC = 1.87 mA/cm2, VOC = 340 mV, and FF = 0.63 than the PbS film deposited with thickness 0.3 μm (η = 0.07%, JSC = 0.36 mA/cm2, VOC = 200 mV, FF = 0.53).
References
Dissanayake MAKL, Liyanage T, Jaseetharan T, Senadeera GKR, Dassanayake BS (2020) Effect of PbS quantum dot-doped polysulfide nanofiber gel polymer electrolyte on efficiency enhancement in CdS quantum dot-sensitized TiO2 solar cells. Electrochim Acta 347:136311. https://doi.org/10.1016/j.electacta.2020.136311
Gode F, Unlu S (2019) Synthesis and characterization of CdS window layers for PbS thin film solar cells. Mater Sci Semicond Process 90:92–100
Samadpour M, Jun HK, Parand P, Najafi MN (2019) CdS quantum dots pre-deposition for efficiency enhancement of quantum dot-sensitized solar cells. Sol Energy 188:825–830
Rohom AB, Londhe PU, Jadhav PR, Bhand GR, Chaure NB (2017) Studies on chemically synthesized PbS thin films for IR detector application. J Mater Sci Mater Electron 28:17107–17113
Rajashree C, Balu AR (2016) Tuning the physical properties of PbS thin films towards optoelectronic applications through Ni doping. Optik 127:8892–8898
Hernández-Borja J, Vorobiev YV, Ramírez-Bon R (2011) Thin film solar cells of CdS/PbS chemically deposited by an ammonia-free process. Sol Energy Mater Sol Cells 95:1882–1888
Obaid AS, Hassan Z, Mahdi MA, Bououdina M (2013) Fabrication and characterisations of n-CdS/p-PbS heterojunction solar cells using microwave-assisted chemical bath deposition. Sol Energy 89:143–151
Dharmadasa IM, Bingham PA, Echendu OK, Salim HI, Druffel T, Dharmadasa R, Sumanasekera GU, Dharmasena RR, Dergacheva MB, Mit KA, Urazov KA, Bowen L, Walls M, Abbas A (2014) Fabrication of CdS/CdTe-based thin film solar cells using an electrochemical technique. Coatings 4:380–415
Ahmed BA, Shallal IH, AL-Attar FIM (2018) Physical properties of CdS/CdTe/CIGS thin films for solar cell application. IOP Conf Series J Phys Conf Series 1032:012022
Kartopu G, Turkay D, Ozcan C, Hadibrata W, Aurang P, Yerci S, Unalan HE, Barrioz V, Qu Y, Bowen L, Gürlek AK, Maiello P, Turan R, Irvine SJC (2018) Photovoltaic performance of CdS/CdTe junctions on ZnO nanorod arrays. Sol Energy Mater Sol Cells 176:100–108
Abdallah B, Hussein R, Al-Kafri N, Zetoun W (2019) PbS thin films prepared by chemical bath deposition: effects of concentration on the morphology, structure and optical properties. Iran J Sci Technol Trans Sci 43:1371–1380
Chaki SH, Joshi HJ, Tailor JP, Deshpande MP (2014) Study of SnS2 thin film deposited by spin coating technique. Mater Res Express 4:076402
Atwa D, Azzouz IM, Badr Y (2011) Optical, structural and optoelectronic properties of pulsed laser deposition PbS thin film. Appl Phys B Lasers Opt 103:161–164
Khan MD, Hameed S, Haider N, Afzal A, Sportelli MC, Cioffi N, Malik MA, Akhtar J (2016) Deposition of morphology-tailored PbS thin films by surfactant- enhanced aerosol assisted chemical vapor deposition. Mater Sci Semicond Process 46:39–45
Silva Filho JMC, Ermakov VA, Marques FC (2018) Perovskite thin film synthesised from sputtered lead sulphide. Sci Rep 8:1563
Sarma MP, Wary G (2017) Effect of molarity on structural and optical properties of chemically deposited nanocrystalline PbS thin film. Int Lett Chem Phys Astron 74:22–35
Romero R, Lpez M, Leinen D, Martin F, Ramos-Barrado J (2004) Electrical properties of the n-ZnO/ C-Si heterojunction prepared by chemical spray pyrolysis. Mater Sci Eng B 110:78–93
Faraj MG (2015) Effect of thickness on the structural and electrical properties of spray pyrolysed lead sulfide thin films. Am J Condens Matter Phys 5:51–55
Al-Mousoi AK, Mohammed MKA, Khalaf HA (2016) Preparing and characterization of indium arsenide (InAs) thin films by chemical spray pyrolysis (CSP) technique. Optik 127:5834–5840
Ahmed DS, Mohammed MKA, Mohammad MR (2020) Sol-gel synthesis of Ag-doped titania-coated carbon nanotubes and study their biomedical applications. Chem Pap 74:197–208
Mohammad MR, Ahmed DS, Mohammed MKA (2019) Synthesis of Ag-doped TiO2 nanoparticles coated with carbon nanotubes by the sol–gel method and their antibacterial activities. J Sol-Gel Sci Technol 90:498–509
Mohammed MKA, Ahmed DS, Mohammad MR (2019) Studying antimicrobial activity of carbon nanotubes decorated with metal-doped ZnO hybrid materials. Mater Res Express 6:055404
Raneen IJ, Mohammed MKA (2016) Production of graphene powder by electrochemical exfoliation of graphite electrodes immersed in aqueous solution. Optik 127:6384–6389
Mohammed MKA, Ali KA, Haider AK (2016) Deposition of multi-layer graphene (MLG) film on glass slide by flame synthesis technique. Optik 127:9848–9852
Patel J, Mighri F, Ajji A, Tiwari D, Chaudhuri TK (2014) Spin-coating deposition of PbS and CdS thin films for solar cell application. Appl Phys A Mater Sci Process 117:1791–1799
Saikia D, Phukan P (2014) Fabrication and evaluation of CdS/PbS thin film solar cell by chemical bath deposition technique. Thin Solid Films 562:239–243
Khare A (2010) Effects of copper concentration on electrooptical and structural properties of chemically deposited nanosized (Zn-Cd) S:Cu films. J Lumin 130:1268–1274
Spalatu N, Hiie J, Mikli V, Krunks M, Valdna V, Maticiuc N, Raadik T, Caraman M (2015) Effect of CdCl2 annealing treatment on structural and optoelectronic properties of close spaced sublimation CdTe/CdS thin film solar cells vs. deposition conditions. Thin Solid Films 582:128–133
Kumarasinghe OK, Dissanayake A, Pemasiri BM, Dassanayake BS (2017) Thermally evaporated CdTe thin films for solar cell applications: optimization of physical properties. Mater Res Bull 96:188–195
Cruz JS, Perez RC, Delgado GT, Angel OZ (2015) CdS thin films doped with metal-organic salts using chemical bath deposition. Thin Solid Films 518:1791–1795
Droessler LM, Assender HE, Watt AAR (2012) Thermally deposited lead oxides for thin film photovoltaics. Mater Lett 71:51–53
Obaid AS, Mahdi MA, Hassan Z, Bououdina M (2012) Preparation of chemically deposited thin films of CdS/PbS solar cell. Superlattice Microst 52:816–823
Shin SW, Agawane GL, Gang MG, Moholkar AV, Moon JH, Kim JH, Lee JY (2012) Preparation and characteristics of chemical bath deposited ZnS thin films: effects of different complexing agents. J Alloys Compd 526:25–30
Altin I, Polat I, Bacaksiz E, Sokmen M (2012) ZnO and ZnS microrods coated on glass and photocatalytic activity. Appl Surf Sci 258:4861–4865
Artegiani E, Menossi D, Salavei A, di Mare S, Romeo A (2017) Analysis of the influence on the performance degradation of CdTe solar cells by the front contact. Thin Solid Films 633:101–105
Singh K (2014) PbS-CdS thin film heterojunctions for photovoltaic applications, a thesis submitted to School of Physics and Materials Science. Thapar University, Patiala
Peña-Cabrera L, Arizmendi-Morquecho A, Vorobiev P (2016) Thin film CdS/PbS solar cell by low temperature chemical bath deposition and silver doping of the window layer. J Non-Oxide Glas 8:59–66
Pérez-García CE, Meraz-Dávila S, Arreola-Jardón G, de Moure-Flores F, Ramírez-Bon R, Vorobiev YV (2020) Characterization of PbS films deposited by successive ionic layer adsorption and reaction (SILAR) for CdS/PbS solar cells application. Mater Res Express 7:015530
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammed, M.K.A. Studying the Structural, Morphological, Optical, and Electrical Properties of CdS/PbS Thin Films for Photovoltaic Applications. Plasmonics 15, 1989–1996 (2020). https://doi.org/10.1007/s11468-020-01224-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-020-01224-5