Abstract
A highly efficient, visible-light-driven and hybrid-activated carbon-doped pure tungsten trioxide (WO3) composite photocatalyst, with greatest stability has been synthesized by using hydrothermal method. Carbon nanoparticles were prepared by using sugarcane bagasse, and tungsten trioxide was prepared by using sodium tungstate (Na2WO4·2H2O) as a starting material. This study considers the photo-degradation of paracetamol by photocatalysis process, under visible-light irradiation. To study the properties of the prepared materials, SEM, TEM, FTIR, PL, and UV were performed. SEM was used to find the structure and size of the prepared materials. FTIR was used to analyze the presence of functional groups in the material. PL and UV were used to analyze the optical properties of the prepared materials. The SEM images of the prepared sample of WO3 showed that it has structure-like nanocubic and rectangular rods which become sharper by addition of carbon nanoparticles. The average size of the nanorods was about 50–550 nm. Some of the nanorods have smaller sizes about 25–250 nm which became beneficial for the photo-degradation process. UV–Vis spectroscopy showed that the band gap of the prepared samples varied from 2.78 to 2.26 eV, and this variation of the band gap was due to the variation in the concentration of the carbon nanoparticles. PL-spectroscopy analyzed the excitation wavelength of the prepared sample which was 448 nm. The efficiency of the prepared samples was evaluated by using paracetamol as target pollutant; carbon-doped composites of WO3 showed a greater photocatalytic performance as compared with the pure WO3. From the results of the photocatalytic activity, it was clear that composite having 2% carbon has the highest efficiency to degrade the paracetamol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an increase in mankind’s population on earth, the problem of environmental pollution is going to increase day by day. This increasing population has caused so many environmental and pollution problems. Among all these problems, the most dangerous problem for every life is water pollution. Water pollution has direct effects on every living being because it is an important part of life. The world’s survey reports showed that almost 50% of people do not have fresh and clean water for drinking. Thus, the first priority for every human being is to save the water from all types of pollutants [18] such as dyes, heavy metals, pharmaceutical agents, rubber, leather, paper, and plastic which have become part of the drinking water and cause serious problems [11]. A type of water pollutants which are becoming more common are pharmaceuticals due to their long-lasting toxic effects on humans and animals. Paracetamol is a pharmaceutical drug and is also considered an organic pollutant. It is also one of the most three prescribed medicines in England. Frequently, the presence of paracetamol, in aquatic environment and drinking water has raised a concern due to their harmful effects on the aquatic environment, humans, and animals. The increase of its concentration in water from safe limit is affecting human health and aquatic life badly. The starting material for the commercial manufacture of paracetamol is phenol. Phenol has also been considered to be a harmful and toxic material [25]. In the present age, large-scale contributions and efforts have been made by the international community and researchers in the field to reduce the toxic effects of paracetamol. The complete degradation of paracetamol is a challenging process due to its long-lasting effects. But these effects can be reduced and converted into being less harmful by using an environment-friendly method [20]. Different methods have been used to remove organic pollutants from waste water such as radiolysis, ozonolysis, sonolysis, photo-femto reaction, and photocatalysis [15]. In this research work, photocatalysis has been chosen as a method for the degradation of paracetamol because it is green, renewable, cost effective, most suitable, and reliable. There is no difficulty in the photocatalysis method because a lot of research has been made, and bundles of the volumes are present in the literature to improve the photocatalysis process for the degradation of water pollutants. The main advantage of the photocatalysis process is that it is a renewable process [8]. In the present study, the prepared photocatalyst tungsten trioxide (WO3) was used for the degradation process, and it was prepared using the hydrothermal method, which is cost effective and easy to handle. It has indirect band gap of 2.8 eV. Since WO3 has been reported as a compound which has stable physical and chemical properties and has a greater capacity to absorb visible light, this material has been considered to be the best for the degradation of paracetamol under visible-light irradiation [12]. It has been observed that WO3 in its pure form has a low efficiency which was increased by doping carbon nanoparticles [27]. Pure WO3 has a cubic-shaped nanostructure while its composite with activated carbon has a sharper nanorod structure because the addition of activated carbon in pure WO3 affected the structure of WO3. It has been noticed that this form of carbon nanoparticles has a smaller size and high surface area for the reaction due to which the composite showed greater photocatalytic activity as compared with pure WO3 [23]. WO3 doped with carbon nanoparticles has been proven to be a good photocatalyst and has greater efficiency for the degradation of paracetamol. The composite of WO3 and activated carbon has been proven to be one of the best catalysts for the degradation of paracetamol in the presence of visible-light irradiation [17].
Experimental Work
To synthesize the pure WO3 and carbon nanoparticles, the following materials were used, 4 g sodium tungstate dehydrate (Na2WO4·2H2O), 20 ml sulfuric acid (H2SO4), 3 g sodium chloride (NaCl), 100 ml distilled water, 20 ml hydrochloric acid (HCl), 10 ml ethanol, 50 g biomass burgesses of sugarcane, and 10 paracetamol tablets for the degradation [8].
Preparation of Carbon Nanostructure
Waste burgesses of sugarcane were collected from the sugarcane stole. First, these burgesses were washed with deionized water for three to four times and then placed independently so that these burgesses were dried naturally. Using a grinder machine, these burgesses were grinded converting them into fine powder. Sulfuric acid (10 ml) was added in the powder and after, the prepared samples were placed in an oven (20 h at 250 °C) for the drying process [19]. pH 7 was obtained after washing the samples with distilled water for several times and dried at 70 °C. The dried sample was grinded with a pestle mortar until pure carbon was obtained, as shown in Fig. 1.
Synthesis of Tungsten Trioxide
Sodium tungstate dehydrate (Na2WO4·2H2O) was used for the preparation of WO3. In 20 ml of distilled water, 0.048 g of pure sodium chloride (NaCl) and 1.27 g sodium tungstate dehydrate were added. The solution was stirred using a magnetic stirrer, and 5 ml of HCl was drop-wise added in the above solution which changed the color of the solution. Prepared sample was placed in 100 ml of autoclave for 20 h at a temperature of 250 °C [10]. After, the sample was collected in a beaker and washed with deionized water to reach its maximum pH of 7. Then the sample was dried in an oven for 2 h at 250 °C, and pure WO3 was obtained in powder form [21].
Preparation of Carbon and Tungsten Trioxide Composites
Tungsten trioxide doped with carbon nanoparticle composites was also prepared using the hydrothermal process. For the synthesis of 1% carbon-doped tungsten trioxide composite, two solutions were prepared separately; in the first solution, 0.01 g of carbon nanoparticles was added in 5 ml of ethanol and then sonicated, so all the carbon nanoparticles were suspended in the ethanol. Second, the solution was prepared by adding 1.2 g of pure tungsten trioxide in 15 ml of deionized water [7]. Then the solution was sonicated by using a sonicator for 20 to 25 min at 250 rpm in a dark room and mixed and stirred for 30 min. During the stirring process, HCl was drop wise added to the above solution, then for a complete reaction, the sample solution was placed in 100-ml autoclave for 24 h at a temperature of 250 °C [27]. After, the prepared sample was washed three to four times with deionized water and dried at 70 °C. The same procedure was repeated, and a composite having 2% carbon was formed by using 0.02 g of carbon nanoparticles in the same amount of tungsten trioxide [26]. The synthesis of composite is shown in Fig. 2.
Characterization Techniques
The synthesized photocatalysts were characterized by utilizing some characterization techniques; to understand the surface morphology of the material, scanning electron microscopy (SEM) (Hitachi TM-1000) was used. Fourier transformation infrared spectroscopy (FTIR) was used to find the functional groups of the materials, utilizing UV–Vis spectroscopy to calculate the band gap of the prepared catalysts and measuring the optical properties of the samples through photoluminescence spectroscopy (PL) [13].
Results and Discussion
SEM and TEM Analyses
SEM was used to understand the surface morphology and size of the synthesized photocatalysts. Its critical pictures showed that these prepared photocatalysts have nanocubic and rectangular-shaped rods, and their size range is from 25 to 250 nm. Their length, size, and diameter ranges were increased and decreased gradually due to the varying content ratios of carbon nanoparticles, as shown in Fig. 3.
Pure WO3 has a larger band gap and was reduced inversely as we change the concentration ratio of the carbon nanoparticles. Through the nucleus of the tungsten trioxide atom, each crystal grows randomly. Nucleation growth started when the HCl and the sodium tungstate dehydrate (Na2WO4·2H2O), reacting with each other. Ions of these materials reacted to form the nuclei of the WO3. Particle size, nucleation growth, and size of nuclei were changed by the thermodynamic parameters of the super-saturation.
When carbon nanoparticles were added in the pure WO3, it changed the orientation of WO3, as shown in Fig. 4 [4]. The micrograph of pure WO3 shows that its nanoparticles’ size ranges from 76 to 125 nm, but its composites have 2% of carbon in tungsten trioxide forming nanoparticle sizes from 50 to 102 nm with nanorods becoming more sharp [2]. Through observation of SEM micrograph, it was noticed that changed in the carbon content ratio in the WO3 semiconductor material changed its particle size, as shown in Fig. 4.
Transmission electron microscopy (TEM) was used to study the crystallinity, shape, and size of the prepared sample. Figure 5 shows the micrograph of activated carbon-modified WO3 (2%) which is showing nanoparticles with an average size of 10 nm. The activated carbon was found to be randomly distributed on the surface of WO3. The planar, flat, and transparent appearance of sheets shows the high crystallinity of the prepared sample.
Fourier Transform-Infrared Spectroscopy
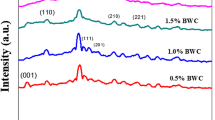
FTIR spectrometer gives the information of functional groups and wave numbers (cm−1) of the materials [25]. FTIR spectra of the pure tungsten trioxide are shown in Fig. 6a, and carbon-doped WO3 is shown in Fig. 6b. FTIR spectra of pure WO3 (Fig. 5a) show that the material has a lot of adsorption bands, due to surface symmetric vibration. Highest absorption was determined at 3512.5 cm−1 which was because of the stretching vibration of the hydroxyl group (–O–H) [3]. Adsorption peak at 804.14 cm−1 was obtained due to the stretching vibration of –CH of the phase deformation that is the lower-frequency peak of the prepared catalyst, and the adsorption band at 1632.02 cm−1 was obtained because of the amino group. At 617.88 cm−1, peak was formed because of the stretching vibration of –C=O–H group. When carbon was doped in tungsten trioxide material then its absorption rate increased gradually [9]. For 1%, the concentration ratio of the carbon nanoparticles has a small change in absorption as compared with the catalyst having 2% carbon nanoparticles, as shown in Fig. 6b.
UV–Vis Spectroscopy
Ultraviolet-visible spectrometer (UV–Vis absorption: TU-1901) was utilized to measure the band gap of the synthesized photocatalysts. Spectrum of pure tungsten trioxide and carbon-doped WO3 composites is shown in Fig. 7. Prepared samples have defects and optical band gap which were reduced due to the absorbance of the carbon nanoparticles [16]. The band gap obtained from Tauc plot of the spectrum was 2.78 eV for pure tungsten trioxide, 2.48 and 2.26 eV were the band gap of composites having 1 and 2% carbon concentration, respectively, as shown in Fig. 8. The band gap reduced due to the addition of carbon nanoparticles in WO3, and carbon particles were doped in the lattice of WO3 which changed the phases of the prepared catalysts [20]. In this semiconductor electrons, valence band moves to conduction band at its excitation level and stimulation of the electrons were also changed because of the increase in the doping concentration of the carbon material. Decrease in band gap exhibited that carbon nanoparticles pack perfectly into the interstitial space in the WO3 and electronic coupling was also impeccable [24].
Photoluminescence Measurements
The defects which were produced by the whole process had been determined by the photoluminescence spectroscopy (PL: RAMANLOG 6, UOG). Band gap and wavelength of the nanoparticles has a unique role in the photocatalytic degradation process. Formation of energy levels, their peaks, positions, and the energy intensities all depend upon the perfect doping of the materials [8]. From the PL spectrograph (Fig. 9), the measured wavelength is about 448 nm of the fabricated samples which was decreased gradually by increasing the content ratio of the carbon nanoparticles in the WO3 semiconductor photocatalyst [1]. With the help of planks, (E = hf), the band energy Eg of the synthesized semiconductor materials was also analyzed by using the first peak of the photocatalysts, and the 2nd-peak wavelength gave us information of the impurity atoms present in the catalysts. The decrease in the intensity also showed that the recombination rate of electrons and holes decreases [3].
Photocatalytic Activity for the Removal of Paracetamol
The photocatalytic performance of WO3 and carbon-doped WO3 with different concentration ratios were analyzed using the photocatalytic degradation of paracetamol. For the photocatalytic process, a photocatalytic reactor was used which has a metal halide lamp (400 W) which was used as an energy source. Without the photocatalyst, degradation of paracetamol was extremely poor, which showed that the pollutant is very stable as compared with other organic pollutants so it indicated no degradation [6]. But when 0.1 mg of WO3 was added in 1 ppm (1 mg) paracetamol powder in 100 ml deionized water and stirred in the dark room for 20 min at 250 rpm [10]. After stirring, the solution was placed in the photocatalytic reactor with 5 ml of the solution being extracted after every 30 min with the help of pipit, and UV–Vis was performed. The UV spectrograph was used to observe the degradation of the paracetamol. Similarly, the process was repeated for six times to understand the complete behavior of the photocatalyst with the solution [14].
To make photocatalyst more reactive, it was calcinated at 500 °C for 20 min before the photocatalytic activity. Kinetic theory of the degradation process was utilized to understand the quantitative relation by using this formula.
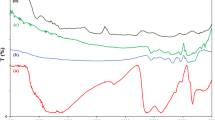
Here, C0 is initial concentration and C is final concentration of the solution after time t. UV–Vis spectrograph relation with time showed that adsorption peaks decreased as the time increases, as shown in Fig. 10, so almost 70–72% of paracetamol was degraded by pure WO3.
UV–Vis spectrograph relation with time showed that adsorption peaks decrease as the time increases so the same procedure was regained for the 1 and 2% carbon-doped WO3 nanoparticles. The degradation efficiency increased for the composite having 1% carbon nanoparticles, but the composite having 2% carbon-doped nanoparticles showed 96% degradation of paracetamol in about 180 min time duration. Figure 11 shows the degradation efficiency of the prepared nanostrcutures for the degradation of paracetamol. The photocatalytic mechanism for carbon-doped WO3 is shown in Fig. 12. The composite highly affected the photocatalytic activity treatment which was due to the addition of carbon which increased the adsorption capacity of the pollutants, and the charge separation efficiency was also increased [5, 22].
Conclusion
In this work, WO3 and carbon-doped WO3 composite were effectively synthesized using the hydrothermal method. The sharp nanocubic and rectangular rods were obtained by using simple hydrothermal method which was analyzed by SEM characterization, and this sharpness increased with the increase of the carbon ratio in WO3. Two percent carbon-doped WO3 nanoparticles gave better results as compared with 1% carbon-doped WO3 nanoparticles. Characterization techniques such as SEM, FTIR, PL, and UV–Vis spectrum indicated that doping of carbon in WO3 semiconductor material affected the size, increased the absorption area, and reduced the recombination of charge carriers and band gap, which increased the efficiency of the photocatalytic activity. WO3 photocatalyst with different concentration ratios of carbon nanoparticles showed great degradation of paracetamol in a short-time duration. Degradation of paracetamol (5 ppm) in deionized water over the different ratios of the carbon-doped WO3 prepared samples has been visualized which showed that nanocubic rod-like structure of WO3 photocatalysts showed high photocatalytic efficiency. The results of the photocatalytic activity indicated that paracetamol was highly degraded up to 85% after 180 min under visible-light irradiation inside a photocatalytic reactor by using 2% carbon-doped WO3 nanoparticles. PL and UV–Vis spectrum calculate 2.78 eV band gap for pure tungsten trioxide and 2.42–2.26 eV of the carbon-doped WO3 composites which play an important role in the degradation of paracetamol.
References
Akhi Y, Irani M, Olya ME (2016) Simultaneous degradation of phenol and paracetamol using carbon/MWCNT/Fe3O4 composite nanofibers during photo-like-Fenton process. J Taiwan Inst Chem Eng 63:327–335
Arimondo E, Inguscio M, Violino P (1977) Experimental determinations of the hyperfine structure in the alkali atoms. Rev Mod Phys 49(1):31
Garcia-Segura S, Brillas E (2017) Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J Photochem Photobiol C: Photochem Rev 31:1–35
Hart A, Orr D (1975) The degradation of paracetamol (4-hydroxyacetanilide) and other substituted acetanilides by aPenicillium species. Antonie Van Leeuwenhoek 41(1):239–247
Hepel M, Hazelton S (2005) Photoelectrocatalytic degradation of diazo dyes on nanostructured WO3 electrodes. Electrochim Acta 50(25–26):5278–5291
Kumar SG, Rao KK (2017) Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO). Appl Surf Sci 391:124–148
Lin C-F, Wu C-H, Onn Z-N (2008) Degradation of 4-chlorophenol in TiO2, WO3, SnO2, TiO2/WO3 and TiO2/SnO2 systems. J Hazard Mater 154(1–3):1033–1039
Lin CJ, Liao S-J, Kao L-C, Liou SYH (2015) Photoelectrocatalytic activity of a hydrothermally grown branched Zno nanorod-array electrode for paracetamol degradation. J Hazard Mater 291:9–17
Luo J, Hepel M (2001) Photoelectrochemical degradation of naphthol blue black diazo dye on WO3 film electrode. Electrochim Acta 46(19):2913–2922
Lv K, Li J, Qing X, Li W, Chen Q (2011) Synthesis and photo-degradation application of WO3/TiO2 hollow spheres. J Hazard Mater 189(1–2):329–335
Ma S, Frederick B (2003) Reactions of aliphatic alcohols on WO3 (001) surfaces. J Phys Chem B 107(43):11960–11969
Monser L, Darghouth F (2002) Simultaneous LC determination of paracetamol and related compounds in pharmaceutical formulations using a carbon-based column. J Pharm Biomed Anal 27(6):851–860
Prescott LF (2000) Paracetamol, alcohol and the liver. Br J Clin Pharmacol 49(4):291–301
Ramos-Delgado N, Gracia-Pinilla M, Maya-Trevino L, Hinojosa-Reyes L, Guzman-Mar J, Hernández-Ramírez A (2013) Solar photocatalytic activity of TiO2 modified with WO3 on the degradation of an organophosphorus pesticide. J Hazard Mater 263:36–44
Safaei J, Ullah H, Mohamed NA, Noh MFM, Soh MF, Tahir AA et al (2018) Enhanced photoelectrochemical performance of Z-scheme g-C3N4/BiVO4 photocatalyst. Appl Catal B Environ 234:296–310
Sellers PJ (1985) Canopy reflectance, photosynthesis and transpiration. Int J Remote Sens 6(8):1335–1372
Sirés I, Garrido JA, Rodríguez RM, Centellas F, Arias C, Brillas E (2006) Electrochemical degradation of paracetamol from water by catalytic action of Fe2+, Cu2+, and UVA light on electrogenerated hydrogen peroxide. J Electrochem Soc 153(1):D1–D9
Tahir MB, Ashraf M, Rafique M, Ijaz M, Farman S, Iqra Mubeen I (2019) Activated carbon doped WO3 for photocatalytic degradation of rhodamine-B. Appl Nanosci. https://doi.org/10.1007/s13204-019-01141-y
Tanyildizi A (2016) Tekstil boyar maddelerinin fotokatalitik degradasyon ile giderimi/the removal of textile dye by photocatalytic degradation. Available from http://hdl.handle.net/11508/17968
Trovó AG, Nogueira RFP, Agüera A, Fernandez-Alba AR, Malato S (2012) Paracetamol degradation intermediates and toxicity during photo-Fenton treatment using different iron species. Water Res 46(16):5374–5380
Valdez HA, Jiménez GG, Granados SG, de León CP (2012) Degradation of paracetamol by advance oxidation processes using modified reticulated vitreous carbon electrodes with TiO2 and CuO/TiO2/Al2O3. Chemosphere 89(10):1195–1201
Widiyandari H, Purwanto A, Balgis R, Ogi T, Okuyama K (2012) CuO/WO3 and Pt/WO3 nanocatalysts for efficient pollutant degradation using visible light irradiation. Chem Eng J 180:323–329
Xie S, Ouyang K (2017) Degradation of refractory organic compounds by photocatalytic fuel cell with solar responsive WO3/FTO photoanode and air-breathing cathode. J Colloid Interface Sci 500:220–227
Xie G, Chang X, Adhikari BR, Thind SS, Chen A (2016) Photoelectrochemical degradation of acetaminophen and valacyclovir using nanoporous titanium dioxide. Chin J Catal 37(7):1062–1069
Yang L, Liya EY, Ray MB (2008) Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res 42(13):3480–3488
Yumin C (2006) Photocatalytic degradation of MO by complex nanometer particles WO3/TiO2. Rare Metals 25(6):649–653
Zhang L, Yin X, Li SFY (2015) Bio-electrochemical degradation of paracetamol in a microbial fuel cell-Fenton system. Chem Eng J 276:185–192
Funding
This work was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under Grant No. D-188-297-1441. The authors, therefore, acknowledge with thanks DSR technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahzad, K., Tahir, M.B., Ashraf, M. et al. Interfacial Growth of Activated Carbon on WO3 Nanoplates for Enhanced Photocatalytic Activity by Surface Plasmon Resonance. Plasmonics 15, 1205–1212 (2020). https://doi.org/10.1007/s11468-020-01135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-020-01135-5