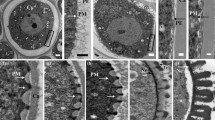
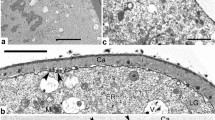
Abstract
The pollen wall is a solid and variously sculptured structure. This pattern is determined inside a tetrad. During meiosis, the callose wall is formed outside of the meiocyte/microspore to form a tetrad. Then, primexine is deposited between the callose wall and the microspore plasma membrane which will become undulated. The sporopollenin deposits on top of the undulated membrane and develops into the pollen wall pattern, while the callose wall is gradually degraded. In recent years, much progress has been made in the study of pollen wall pattern formation, at both molecular and genetic levels. In this review, we summarize these achievements mainly in Arabidopsis.

Similar content being viewed by others
References
Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62:437–460
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16(Suppl):S46–S60
Blackmore S (2007) Pollen and spores: microscopic keys to understanding the earth’s biodiversity. Plant Syst Evol 263:3–12
Lou Y, Xu XF, Zhu J et al (2014) The tapetal AHL family protein TEK determines nexine formation in the pollen wall. Nat Commun 5:3855
Jia QS, Zhu J, Xu XF et al (2015) Arabidopsis AT-hook protein TEK positively regulates the expression of arabinogalactan proteins for Nexine formation. Mol Plant 8:251–260
Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16(Suppl):S84–S97
Zhou Q, Zhu J, Cui YL et al (2015) Ultrastructure analysis reveals sporopollenin deposition and nexine formation at early stage of pollen wall development in Arabidopsis. Sci Bull 60:273–276
Kauss H (1996) Callose synthesis. In: Smallwood M, Knox P, Bowtes DJ (eds) Membranes: specialized functions in plant cells. Biso Scientific Publishers, Oxford
Rhee SY, Somerville CR (1998) Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J 15:79–88
Waterkeyn L (1962) Les parois microsporocytaires de nature callosique chez Helleborus et Tradescantia. Cellule 62:225–255
Saxena IM, Brown RM Jr (2000) Cellulose synthases and related enzymes. Curr Opin Plant Biol 3:523–531
Dong X, Hong Z, Sivaramakrishnan M et al (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Enns LC, Kanaoka MM, Torii KU et al (2005) Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol Biol 58:333–349
Huang XY, Niu J, Sun MX et al (2013) CYCLIN-DEPENDENT KINASE G1 is associated with the spliceosome to regulate CALLOSE SYNTHASE5 splicing and pollen wall formation in Arabidopsis. Plant Cell 25:637–648
Yang J, Tian L, Sun MX et al (2013) AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol 162:720–731
Hu J, Wang Z, Zhang L et al (2014) The Arabidopsis exine formation defect (EFD) gene is required for primexine patterning and is critical for pollen fertility. New Phytol 203:140–154
Mallory AC, Bartel DP, Bartel B (2005) MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant cell 17:1360–1375
Shi ZH, Zhang C, Xu XF et al (2015) Overexpression of AtTTP affects ARF17 expression and leads to male sterility in Arabidopsis. PLoS ONE 10:e0117317
Chang HS, Zhang C, Chang YH et al (2012) No primexine and plasma membrane undulation is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol 158:264–272
Heslop-Harrison J (1963) An ultrastructural study of pollen wall ontogeny in Silene pendula. Grana Palynol 4:7–24
Skvarla JJ, Larson DA (1966) Fine structure studies of Zea mays pollen. I. Cell membranes and exine ontogeny. Am J Bot 53:1112–1125
Hemsley AR, Griffith PC, Matthias R et al (2003) A model for the role of surfactants in the assembly of exine structure. Grana 42:38–42
Chen LQ, Hou BH, Lalonde S et al (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532
Guan YF, Huang XY, Zhu J et al (2008) RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol 147:852–863
Sun MX, Huang XY, Yang J et al (2013) Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod 26:83–91
Paxson-Sowders DM, Dodrill CH, Owen HA et al (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127:1739–1749
Paxson-Sowders DM, Owen HA, Makaroff CA (1997) A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 198:53–65
Ariizumi T, Hatakeyama K, Hinata K et al (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181
Fitzgerald MA, Knox RB (1995) Initiation of primexine in freeze-substituted microspores of Brassica campestris. Sex Plant Reprod 8:99–104
Southworth D, Jernstedt JA (1995) Pollen exine development precedes microtubule rearrangement in Vigna unguiculata (Fabaceae): a model for pollen wall patterning. Protoplasma 187:79–87
Brooks J, Shaw G (1968) Chemical structure of the exine of pollen walls and a new function for carotenoids in nature. Nature 219:532–533
Piffanelli P, Ross JHE, Murphy DJ (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11:65–80
Aarts MG, Hodge R, Kalantidis K et al (1997) The Arabidopsis MALE STERILITY MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J 12:615–623
Zhu J, Lou Y, Xu X et al (2011) A genetic pathway for tapetum development and function in Arabidopsis. J Integr Plant Biol 53:892–900
Zhang ZB, Zhu J, Gao JF et al (2007) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J 52:528–538
Worrall D, Hird DL, Hodge R et al (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4:759–771
Hird DL, Worrall D, Hodge R et al (1993) The anther-specific protein encoded by the Brassica napus and Arabidopsis thaliana A6 gene displays similarity to beta-1,3-glucanases. Plant J 4:1023–1033
Lu P, Chai M, Yang J et al (2014) The Arabidopsis CALLOSE DEFECTIVE MICROSPORE 1 gene is required for male fertility through regulating callose metabolism during microsporogenesis. Plant Physiol 164:1893–1904
Furness CA, Rudall PJ (2004) Pollen aperture evolution–a crucial factor for eudicot success? Trends Plant Sci 9:154–158
Dover GA (1972) The organization and polarity of pollen mother cells of Triticum aestivum. J Cell Sci 11:699–711
Magnard JL, Yang M, Chen YC et al (2001) The Arabidopsis gene tardy asynchronous meiosis is required for the normal pace and synchrony of cell division during male meiosis. Plant Physiol 127:1157-116611434-20111434-2015-1239
Albert B, Raquin C, Prigent M et al (2011) Successive microsporogenesis affects pollen aperture pattern in the tam mutant of Arabidopsis thaliana. Ann Bot 107:1421–1426
Dobritsa AA, Coerper D (2012) The novel plant protein INAPERTURATE POLLEN1 marks distinct cellular domains and controls formation of apertures in the Arabidopsis pollen exine. Plant Cell 24:4452–4464
Acknowledgments
This work was supported by the Major Research Plan from the Ministry of Science and Technology of China (2013CB945100) and the National Natural Foundation of China (31300262).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
SPECIAL TOPIC: Plant Development and Reproduction
Te Xu and Cheng Zhang contributed equally to this work.
About this article
Cite this article
Xu, T., Zhang, C., Zhou, Q. et al. Pollen wall pattern in Arabidopsis . Sci. Bull. 61, 832–837 (2016). https://doi.org/10.1007/s11434-016-1062-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-016-1062-6