Abstract
The pollen wall comprises the outer exine and the inner intine layers. It plays important roles in protecting pollen from various environmental stresses including microbial attack and in cell-cell recognition during pollination. The exine is further divided into a sexine and a nexine layer. The material for the exine is provided directly by the tapetal cells. The pollen wall of each plant has its unique pattern. After meiosis, the four microspores are enwrapped by callose to form a tetrad. The pollen-wall pattern is determined at tetrad stage. In contrast, the intine is synthesized by the microspore itself. Many genes have been identified from male-sterile mutants in Arabidopsis thaliana and rice during recent years. The majority of these genes are involved in pollen-wall formation including tapetal development, sporopollenin biosynthesis and transport, callose wall and primexine deposition. This chapter introduces the recent advance of pollen-wall formation in genetic and molecular level.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The life cycle of angiosperms can be subdivided into vegetative development culminating in the mature sporophyte and reproductive development including the formation of the gametophyte generation, pollination and fertilization. The pollen grains corresponding to the male gametophytes are formed in the anther, where the reproductive microsporocytes are neighboured to nonreproductive cell layers. During development of the pollen grain, the pollen wall forms as a robust and viscous layer covering the pollen grain. The biological function of this pollen wall is to separate the microspore from the paternal tissue during its development in the anther, to provide physical and chemical resistance against environmental stresses in the mature pollen to ensure its survival and to provide a species-specific adhesion to the stigma surface. However, the structure of the pollen wall not only mediates important biological functions but provides insight into the dynamics of plant phylogeny and reports the genetic mechanism underlying pollen-wall ontogeny. Recent research shows that most phenotypes of male sterility are connected with the abnormal development of the pollen wall. In this review, we concentrate on the genesis of each layer in pollen wall and outline the events that are essential during pollen-wall development.
1 Overview of the Angiosperm Pollen Wall
1.1 Structure of the Pollen Wall
Pattern and structure of the pollen wall represent an important feature of plant taxonomic classifications and forensic identifications and therefore have been described for many species (Cutter 1971; Stanley and Linskens 1974; Blackmore and Barnes 1990; Scott 1994). Despite the morphological diversity among taxa, the principal structure of the pollen wall shares general principles (Fig. 1). The pollen wall consists of two main layers, the outer exine and inner intine. The exine can be further divided into the sexine (a reticulate layer) and the nexine (a flat layer). The sexine consists of a so-called baculum and a tectum, sculpted in a taxon-specific manner. The exine is subtended by the nexine that acts as skeleton for the exine. In contrast to the complex exine, the intine is a relatively simple layer, which is deposited between the plasma membrane and nexine. Finally, the pollen coat or tryphine fills the spaces between the baculum to surround the sculpted pollen wall.
1.2 Development of the Pollen Wall
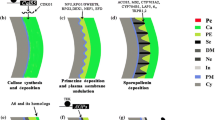
Although the development of the pollen wall varies among species, the fundamental sequence of this process has been elucidated in detail (Scott 1994; Owen and Makaroff 1995; Blackmore et al. 2007; Ariizumi and Toriyama 2011). Pollen-wall formation initiates at the late stage of meiosis. The microsporocytes secrete callose onto the plasma membrane to form a callose wall. After meiosis, the four microspores are wrapped inside this callose wall to form a tetrad. In the tetrad, the primexine is deposited between the callose and the plasma membrane. It acts as a template for the sexine-sculpting pattern. When the plasma membrane becomes undulated, the sporopollenin precursors secreted by tapetum are deposited at the peaks of undulated membrane to form probaculae and protectum. Subsequently, callose and primexine are completely degraded, and the nexine layer appears surrounding the plasma membrane in the released microspore. Upon continuous addition of material derived from the tapetum, the sexine increases in size and associates with the nexine to accomplish the exine structure. Once the nexine layer has been formed, the intine is laid down between the plasma membrane and nexine layer and extends covering the entire microspore. Finally, the intine increases in thickness, and the pollen coat (tapetal fragments) is added to the exine cavities (Fig. 2).
1.3 Constituents of the Pollen Wall
The exine is mainly made up of sporopollenin, which is highly resistant to non-oxidative physical, chemical and biological degradation. Due to the small amounts of material, the insolubility of sporopollenin and technical limitations, the details of sporopollenin components and structure are far from understood. Moreover, there is evidence for differences in chemical pathways and modifications of sporopollenin between species, adding further complexities (Edlund et al. 2004). Compared with the complex exine, the components of intine are rather similar to the primary walls of plant cells, including cellulose, hemicellulose, pectin and proteins (Brett and Waldron 1990). As third component, the pollen coat accounts for 10–15 % of total pollen mass (Piffanelli et al. 1997) and is mainly composed of nonpolar esters and very long-chain wax esters (Scott and Strohl 1962; Bianchi et al. 1990).
2 Tapetum Plays an Essential Role in Pollen Wall Formation
2.1 Tapetum Development
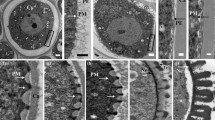
The tapetum layer occurs universally for the land plants. It is of considerable physical significance because most nutrients for the pollen development are produced by, stored in and transported from the tapetum (Dickinson 1982). When RNase is expressed in the tapetum by means of a tapetum-specific promoter, this leads to male sterility, indicating an intimate association between tapetum and microspore development (Mariani et al. 1990). There exist two major types of tapetum in angiosperms. The secretory tapetum remains in its original position with the anther wall and finally autolyses (as in Arabidopsis and Lilium longiflorum). In contrast, the amoeboid tapetum forms a periplasmodium to intrude between the developing microspores (as in rhoeo discolour and Tradescantia bracteata) (Pacini et al. 1985). Tapetum development has been traced back in Arabidopsis to the L2 layer as one of three ‘germ’ layers (L1, L2 and L3) in the stamen primordia. The L2 layer gives rise to the primary parietal cells, secondary parietal cells and the L2-derived archesporial lineage. The tapetum is specified from this archesporial lineage as the innermost one of four somatic cell layers (Sanders et al. 1999). During meiosis, the tapetum undergoes dramatic morphological changes to prepare for its nutritive function for the microspores (Echlin 1971). The cytoplasm is condensed and packed with ribosomes, mitochondria, Golgi bodies, endoplasmic reticulum and vesicles. The tapetal cells pass mitosis without cytokinesis to form binuclear daughter cells. At a late stage of meiosis, the tapetum develops into polar secretory cells lacking a primary cell wall (Stevens and Murray 1981; Bedinger 1992). Meanwhile, the hallmarks of programmed cell death (PCD) become evident in the tapetum (Varnier et al. 2005). Following meiosis, the tapetum begins to provide the precursors of sporopollenin from the inner tangential face and the intercellular tapetal space to execute the pollen exine formation (Pacini and Juniper 1979). Later, the tapetum secretes the callase complex (also termed β-1,3-glucanase) to dissolve the callose wall, releasing microspores from the tetrad (Stieglitz 1977). After completion of the first microspore mitosis, the tapetum accumulates numerous elaioplasts and cytoplasmic lipid bodies for pollen coat formation. This material is discharged into the cavities of the exine surface after the tapetum degeneration (Mascarenhas 1975; Hesse and Hess 1993). The normal developmental process of tapetum in Arabidopsis is shown in Fig. 3.
Light microscope photograph of cross sections of Arabidopsis tapetum at different stages based on the 14 stages of anther ontogeny (Sanders et al. 1999). Tapetum development with main events at different anther stages includes cell fate determination, endomitosis, apoptosis/PCD and degeneration. Genes required for the respective processes are given for the different stages
The formation of binucleate cells and large stacks of extensive endoplasmic reticulum (ER) support a nourishing function of the tapetum for microspores/pollen-grain development including pollen-wall formation. At the meiotic stage, the tapetal nucleus undergoes divisions deviating from conventional mitosis and/or nuclear fusions. In Zea mays, the division takes place in the ordinary way, but without formation of a cell plate. The two daughter nuclei remain inside the tapetal cell. For other species, various types of division peculiarities have been described (Maheshwari 1950). However, the mechanism for these specific nuclear divisions is still unclear. The tapetum shows extensive ER stacks fused with the plasma membrane (Owen and Makaroff 1995). This might facilitate protein synthesis and secretory activity to release materials into the locule. In pollen development, the ER is the major site for glycerolipid biosynthesis (Benning 2008). AtGPAT1 and AtGPAT6 are members of the glycerol-3-phosphate acyltransferase (GPAT) family, which mediate the initial synthetic step of glycerolipid biosynthesis (Zheng et al. 2003; Li-Beisson et al. 2009; Li et al. 2012). Disruption of the AtGPAT1 or AtGPAT6 genes causes defective tapetum development with reduced ER profiles, and irregular exine deposition, leading to a partial degradation of pollen grains (Zheng et al. 2003; Li et al. 2012). In the double mutant of GPAT1 and GPAT6, the defective callose dissolution affects microspore release from tetrads (Li et al. 2012).
PCD is required for development and maintenance in many multicellular organisms (Vaux and Korsmeyer 1999, see also chapter by Smertenko and Bozhkov, this volume). It has been proposed that tapetum degeneration involves programmed cell death (PCD). The cytoplasmic or structural components of the degraded tapetum serve important functions during pollen maturation (Wu and Cheung 2000). Cytological features of PCD include cell shrinkage, condensation of chromatin, swelling of ER and persistence of mitochondria (Papini et al. 1999). A proper timing of tapetum degeneration is necessary for a normal microsporogenesis. Premature or delayed tapetal PCD causes male sterility. A plant aspartic protease is associated with tapetal degeneration. The UNDEAD gene encodes an A1 aspartic protease in Arabidopsis, and silencing of UNDEAD with siRNA leads to apoptosis-like PCD in premature tapetal cells (Phan et al. 2011). Two aspartic protease-encoding genes in rice, OsAP25 and OsAP37, can promote cell death in both yeast and plant. The mutation of ETERNAL TAPETUM 1 (EAT1), an upstream regulator of OsAP25/37, delays tapetal PCD (Niu et al. 2013).
2.2 Genetic Pathway of Tapetum Development and Functions
Many genes involved in tapetum development and functions, especially those related to pollen-wall formation, have been identified in Arabidopsis (see Table 1). Among them, the EXCESS MICROSPOROCYTES1 (EMS1)/EXTRA SPOROGENOUS CELLS (EXS) and TAPETUM DETERMINANT1 (TPD1) genes trigger the signalling pathway for tapetal fate determination during early development (Ma 2005; Zhao et al. 2002; Canales et al. 2002; Yang et al. 2003). Later, several transcriptional factors regulate tapetum differentiation and pollen-wall formation. The genes DYSFUNCTIONAL TAPETUM1 (DYT1) and ABORTED MICROSPORES (AMS) encode putative basic helix-loop-helix (bHLH) transcription factors (Zhang et al. 2006; Sørensen et al. 2003), whereas DEFECTIVE in TAPETAL DEVELOPMENT and FUNCTION1 (TDF1) encodes a putative R2R3 MYB transcription factor (Zhu et al. 2008). These three genes are expressed in the tapetum and meiocytes/microspores during early development. Mutations in these genes cause tapetal hypertrophy extending into the locule and resulting in sporophytic male sterility. A further member of the R2R3 MYB family, AtMYB103 (also named MS188 or MYB80), apparently regulates the sexine formation, since in the respective mutant, the sexine layer is completely absent (Zhang et al. 2007; Zhu et al. 2010). AtMYB103 directly regulates a gene encoding an A1 aspartic protease named UNDEAD (Phan et al. 2011) leading to the model that the AtMYB103/UNDEAD system may regulate the timing of tapetal PCD, consistent with the observation that precocious PCD occurs in atmyb103 (Zhu et al. 2010). Mutation of the nuclear protein MS1, containing a leucine zipper-like and PHD-finger motives, causes tapetum vacuolation and defects in exine structure (Wilson et al. 2001; Vizcay-Barrena and Wilson 2006; Ito et al. 2007; Yang et al. 2007). Compared with the transcription factors DYT1, AMS and TDF1, the expression of the AtMYB103 and MS1 genes in tapetum and microspores occurs during a later developmental stage (Zhu et al. 2011).
Based on gene-expression profiling, several genetic networks have been proposed for tapetal development and pollen formation in Arabidopsis (Feng et al. 2012; Zhu et al. 2008; Xu et al. 2010; Phan et al. 2011; Ito et al. 2007; Yang et al. 2007; Wijeratne et al. 2007). Among these genetic networks, the transcriptional regulatory pathway involving DYT1-TDF1-AMS-MS188-MS1 could be confirmed by in situ hybridization analysis in the respective mutant background and by the phenotype of double mutants dyt1–3 tdf1, tdf1 ams-2 and ams-2 ms188–3 (Zhu et al. 2011). In this genetic pathway (Fig. 3), DYT1, TDF1 and AMS are sequentially activated to regulate early tapetum development, whereas AtMYB103 and MS1 are sequentially activated for late tapetum development and pollen-wall formation (Zhu et al. 2008, 2011).
3 Biosynthesis and Transport of Sporopollenin
Sporopollenin is assumed to consist of the heterogeneous materials derived from long-chain fatty acids, oxygenated aromatic rings and phenylpropionic acids (Guilford et al. 1988; Wehling et al. 1989; Wiermann and Gubatz 1992; Wilmesmeier et al. 1993; Piffanelli et al. 1998; Ahlers et al. 1999; Meuter-Gerhards et al. 1999). Further analyses including Fourier transform infrared spectroscopy (FT-IR), nuclear magnetic resonance spectroscopy (NMR) and X-ray photoelectron spectrometry (XPS) have elucidated that the sporopollenin polymer has a uniform composition which may be linked via ether bridges (Bubert et al. 2002). By the hydrolysis and methylation py-GC/MS method, two UV-absorbing monomers of sporopollenin have been found in pollen (Blokker et al. 2005). Other studies on the chemical similarities between the walls of spores and pollen in early land plants suggest that the first land plants may have evolved the sporopollenin polymers to protect their spores, such that sporopollenin may have arisen as the first polyester-based extracellular matrix in plant (Bowman et al. 2007; Morant et al. 2007).
The complex biochemical pathways in the tapetum leading to the sporopollenin monomers required for pollen exine formation have been well documented (Fig. 4) (Ariizumi and Toriyama 2011). First, lauric acids (C12) are esterified in the plastids of the tapetum to CoA by the fatty acyl-CoA synthetase ACOS5 (de Azevedo Souza et al. 2009). Subsequently, the resulting CoA esters enter the ER and are hydrolysed by a putative thioesterase for the regeneration of lauric acids. These lauric acids are then hydroxylated by specific members of the cytochrome P450 family in the ER, such as the Arabidopsis CYP703A2 that could be shown in vitro to efficiently catalyse the monohydroxylation at carbon atom 7 of lauric acids (Morant et al. 2007), whereas CYP704B1 preferentially catalyses end-of-chain hydroxylation of longer fatty acids (C14 to C18) (Dobritsa et al. 2009). These findings suggest that at least two types of differentially hydroxylated fatty acids may serve as monomeric building blocks during the formation of esters from hydroxylated fatty acids to export them across the membrane, providing the substrates for reduction by the gene product of Male Sterile 2 (MS2) (Schnurr et al. 2004; Morant et al. 2007). MS2 encodes a fatty acyl ACP (Acyl Carrier Protein) reductase, which can convert palmitoyl-ACP to fatty alcohols providing the monomeric constituents of sporopollenin (Chen et al. 2011).
Model for sporopollenin biosynthesis in the tapetum. Enzymes encoded by genes with known functions in sporopollenin biosynthesis are indicated in red. Fatty acids synthesized in plastids are esterified to CoA by ACOS5 and then hydrolyzed by a putative thioesterase in ER. The hydroxylated fatty acids produced by CYP450s are regenerated to CoA esters by ACOS5. Sporopollenin monomers, including the fatty alcohols and tetraketide products which are catalysed by MS2 and PKSs/TKPRs, respectively, are exported by ABCG26 and lipid transfer proteins (LTPs) to the locule as sporopollenin building units for pollen-wall formation
The second pathway required for sporopollenin biosynthesis is the formation of phenylpropanoids (Dobritsa et al. 2010). In Arabidopsis, POLYKETIDE SYNTHASE A (PKSA) and PKSB (also named LAP6 and LAP5) encode chalcone synthases with anther-specific expression, involved in flavonoid biosynthesis (Dobritsa et al. 2010; Kim et al. 2010). Both proteins accept fatty acyl-CoA esters as reaction substrates and condense them to malonyl-CoA, yielding triketide and tetraketide α-pyrones as reaction products (Kim et al. 2010). Sequentially, TETRAKETIDE α-PYRONE REDUCTASE1 (TKPR1) and TKPR2 (previously called DRL1 and CCRL6) reduce the carbonyl function of tetraketide α-pyrone compounds synthesized by PKSA/PKSB (Grienenberger et al. 2010). Genetic evidence suggests that the pairs of PKS and TKPR enzymes cooperating during phenylpropanoid biosynthesis also contribute to the sporopollenin precursors for exine formation (Grienenberger et al. 2010).
The sporopollenin originates from the tapetum as shown by ultrastructural evidence from different plant species (Heslop-Harrison 1968). For instance, the exine material lines the tapetal margin close to the surface of microspores in the Gramineae (Rowley et al. 1959), and the dark particles of tapetal cells observed in Silene pendula were found to be precursors presumably containing sporopollenin that eventually deliver the exine materials (Heslop-Harrison 1962). Therefore, the sporopollenin synthesized in the tapetum must be transported to the surface of the developing microspores. In Arabidopsis, ABCG26/WBC27, a member of the ATP-binding cassette (ABC) transporter superfamily, has been identified as potential transporter to export fatty alcohols and other derived monomers from tapetal cells to the surfaces of microspores during the formation of the exine layer. TEM analyses revealed a lack of sporopollenin deposition in the abcg26-1/wbc27-1 mutant, resulting in absence of probacula, bacula and tectum (Choi et al. 2011; Quilichini et al. 2010; Dou et al. 2011). Furthermore, the ABCG26/WBC27 is the direct target of transcript factor AMS, indicating that the sporopollenin translocation is under genetic control of the tapetal cells (Xu et al. 2010).
4 Pollen-Wall Pattern
The pollen-wall pattern is both under gametophytic and sporophytic control. It is determined by restriction of callose deposition, secretion of the primexine matrix, undulation of the plasma membrane and deposition of sporopollenin (Heslop-Harrison 1971; Sheldon and Dickinson 1983; Blackmore and Barnes 1987; Southworth and Jernstedt 1995). The initial pollen-wall pattern is laid down during the tetrad stage (Schmid et al. 1996). At this stage, each tetrad of microspores has already been casted by a callose wall, which is formed subsequently to meiosis. Then, the primexine is deposited between the callose wall and the plasma membrane of the microspore. Meanwhile, at the plasma membrane of the microspore, undulations appear, whose protrusions will be the sites for future probacular formation. Following the accumulation of sporopollenin, the probacula elongate, the protectum is formed adjacent to the callose, and the plasma membrane gradually returns to a smooth surface. Finally, the exine maturates by completion of the nexine and impregnation with sporopollenin, after the callose and primexine have disappeared (Paxson–Sowders et al. 1997).
4.1 Callose Wall Synthesis
Callose, a β-1,3-glucan polymer, is synthesized by callose synthase complexes which are presumed to be located in the microsporocyte membrane (Roberts 1990; Kauss 1996). After synthesis, callose surrounds the microsporocytes throughout entire meiosis. During meiosis and cytokinesis, a callosic septum grows centripetally to separate the individual microspores (Bhandari 1984; Cresti et al. 1992). Several biological functions of callosic walls have been proposed: (1) as mechanical barrier, callose fulfils an important role as a temporary wall that separates microsporocytes or the microspores themselves, such that they can disperse as single cells (Waterkeyn 1962); (2) as chemical barrier, it functions as a molecular filter isolating the developing microspores from the influence of the diploid tissue (Heslop-Harrison 1964); (3) as a source of glucose for the development of the cellulosic primexine, which provides the basic framework of the future exine (Larson and Lewis 1962); and (4) as a physical support for primexine assembly, which nucleates primexine subunits, increasing their local concentration and preventing them from diffusing into the anther locule (Nishikawa et al. 2005). However, it is uncertain whether the callose wall can directly act as a mould for the pollen-wall pattern. In Ipomoea purpurea, callose chambers mirror the imprint of the primexine matrix and were suggested to act as template for the primexine matrix and to define sculpturing patterns for the exine (Waterkeyn and Beinfait 1970). However, in some other species such as Vigna and Caesalpinia, the callose wall does not parallel the reticulate pattern of the exine (Takahashi 1989).
In Arabidopsis, 12 CALLOSE SYNTHASE (CALS) or GLUCAN SYNTHASE-LIKE (GSL) genes have been identified and classified into one gene family (Hong et al. 2001). In the tetrad, peripheral callose and interstitial callose are synthesized by different callose synthases. In the knockout mutant cals5-2, the peripheral callose of the tetrad is completely absent while the interstitial callose can still be observed. This shows that CalS5 (Gsl2) is responsible for the synthesis of peripheral callose (Dong et al. 2005). Subcellular localization showed that both Gsl1 and Gsl5 are located on the cell plate, suggesting that they may be involved in interstitial callose synthesis (Hong et al. 2001; Enns et al. 2005). Recently, it has been reported that Auxin Response Factor 17 (ARF17) can directly bind the Cals5 promoter region to regulate its expression for callose synthesis. In the arf17 mutant, callose is significantly reduced and the primexine is absent, resulting in pollen-wall patterning defects (Yang et al. 2013). Auxin plays important roles during the entire lifespan of a plant, which affects cell division, elongation and differentiation (Ljung 2013, see also chapter by Skůpa et al., this volume). ARFs are the major component of auxin signalling. It is likely that auxin may regulate the pollen-wall pattern through ARF17. Similar to auxin, CDKs as further regulator of the cell cycle were found to participate in pollen-wall patterning. CDKs have been originally identified as key regulators of cell cycle transition by binding to their regulatory cyclin partners inducing their kinase activity (Morgan 1997). However, ablation of CYCLIN-DEPENDENT KINASE G1 (CDKG1), a member of this family of cyclin-dependent protein kinases, resulted in aberrant callose deposition and defective pollen-wall formation during microspore development of Arabidopsis. The pre-mRNA splicing of the CalS5 gene was defective in cdkg1 mutant. CDKG1 is proposed to be recruited to U1 snRNP through RSZ33 to facilitate the splicing of CalS5 for callose synthesis and pollen-wall pattern (Huang et al. 2013). Thus, CDKs might also act as splicing regulators for gene expression. The expression of CalS5 is regulated by both ARF17 and CDKG1, which shows that callose synthesis for pollen-wall formation is under complicated control.
4.2 Primexine Deposition and Plasma Membrane Undulation
The primexine is mainly composed of polysaccharides, proteins and cellulose (Heslop-Harrison 1963; Rowley and Southworth 1967; Dickinson and Heslop-Harrison 1977). It is initially delivered by Golgi-derived vesicles to the space between the microspore plasma membrane and the callose layer (Fig. 5a) (Dickinson and Sheldon 1984). After the primexine is formed, the microspore plasma membrane becomes undulated (Dahl 1986; Dickinson and Sheldon 1986; Skvarla and Rowley 1987; Takahashi 1993) (Fig. 5b). Subsequently, the undulating plasma membrane produces conspicuous peaks, and the probacula are extruded onto the peaks (Fig. 5c). Finally, the protectum is formed next to the callose to complete the exine pattern (Fig. 5d).
The primexine and the membrane undulation represent decisive factors to regulate the development of pollen-wall ornamentation. As the key organizers of pattern formation, the diverse properties of the primexine probably decide the pattern differences among taxa (Takahashi 1989; Gabarayeva and Rowley 1994; Anger and Weber 2006). The undulation of the plasma membrane contributes to the pattern in guiding probacula formation in the primexine. Different hypotheses have been proposed to explain the undulation of the membrane. In Vigna unguiculata, the primexine is secreted from microspores. The change of cytoskeletal tension in the microspore was suggested to cause the patterned undulation of the plasma membrane for the assembly of the exine layer (Southworth and Jernstedt 1995). In Brassica, fibrous materials are inserted into the invaginations in the plasma membrane. These materials will separate the peaks of the plasma membrane onto which the probacula are finally extruded (Fitzgerald and Knox 1995). In Lilium, the plasma membrane is proposed to be anchored at the callose wall at specific sites, and the areas between these sites are thought to retract from these anchor sites, finally forming the peaks and troughs of plasma membrane (Dickinson 1970; Dickinson and Sheldon 1986; Skvarla and Rowley 1987).
In Arabidopsis, several male-sterile mutants with defective primexine have been reported. Mutations of DEFECTIVE in EXINE FORMATION1 (DEX1), NO EXINE FORMATION1 (NEF1) and RUPTURED POLLEN GRAIN 1 (RPG1) genes exhibit similar phenotypes: They all show irregular deposition of the primexine and reductions in plasma-membrane undulations, followed by random deposition of sporopollenin instead of a normal exine structure. As a result, no viable pollen comes to maturity (except for rpg1 where a partial fertility is maintained). DEX1 encodes a protein that is predicted to be membrane associated and contains several potential calcium-binding domains (Paxson-Sowders et al. 1997, 2001). The phenotype of the dex1 mutant suggests that DEX1 may be a component of the primexine matrix and involved in the polymerization of the primexine. Alternatively, DEX1 could be part of the rough ER, processing and/or transporting primexine precursors to the membrane. NEF1 encodes a plastidic integral membrane protein, which may indirectly change the composition of the primexine and/or sporopollenin or cause an imbalance between synthesis and transport of fatty acids (Ariizumi et al. 2004). RPG1 encodes an MtN3/saliva family protein that is integral to the plasma membrane with seven putative transmembrane helices (Guan et al. 2008). Recently, RPG1 has been renamed to SWEET8, because its gene product was found to act as a sugar efflux transporter (Chen et al. 2010). This is consistent with the idea that primexine formation is mainly dependent on polysaccharide polymerization delivered by RPG1. Also a second member of the MtN3/saliva family, RPG2, is reported to be involved in primexine deposition. It is proposed to play a redundant function of RPG1 during later stages of pollen development (Sun et al. 2013). In the no primexine and plasma membrane undulation (npu) mutant, the primexine is completely absent and the undulation of the plasma membrane cannot be observed (Chang et al. 2012). This suggests that the primexine determines plasma membrane undulation. NPU encodes a functionally unknown protein localized to the plasma membrane with two extracellular regions. Since NPU is a transmembrane protein just like RPG1, it may also act as a sugar transporter driving the transport of polysaccharide material essential for primexine formation. Although the primexine is supposed to be secreted by microspores itself (Southworth and Jernstedt 1995), these primexine mutants show dominant-recessive Mendelian inheritance which is evidence for a contribution of the maternal sporophyte tissue (Paxson-Sowders et al. 1997; Ariizumi et al. 2004; Guan et al. 2008; Chang et al. 2012; Ariizumi and Toriyama 2011).
4.3 Dissolution of the Callose Wall
The degradation of callose around the tetrad is one of the most dramatic cytological events in microsporogenesis. After the exine pattern has been established, the callose is broken down by a callase complex secreted from the tapetum (Stieglitz 1977). The glucanase activity expressed in the anther is mainly dedicated to callose-wall dissolution and the release of young microspores into the locules (Frankel et al. 1969). This activity peaks at the time of tetrad breakdown (Stieglitz and Stern 1973), suggesting that the appropriate timing of callose wall dissolution is specific and critical for normal microspore development. Several mutants and engineered plants with alterations in timing of β-1,3-glucanase expression provide evidence that both failure in callose degradation as well as its premature onset are the primary causes of male sterilities in several species (Izhar and Frankel 1971). In transgenic tobacco with premature callose degradation, the exine is sculptured in an irregular fashion, and sporopollenin is deposited randomly, leading to variable degrees of male sterility (Worrall et al. 1992). A molecular candidate for this enzyme is the gene product of Arabidopsis A6 encoding a protein with similarity to β-1,3-glucanases. A6 promoter::GUS and RNase fusions show that the A6 gene is specifically expressed in the tapetum in a temporal pattern correlated with callase activity, suggesting that A6 is probably part of the callase enzyme complex (Hird et al. 1993). However, the molecular mechanism regulating the callose dissolution has not been identified (Scott et al. 2004).
4.4 Formation of Pollen Apertures
Pollen apertures provide the exit points for the emerging pollen tube at the time of germination and also regulate water uptake during hydration (Heslop-Harrison 1979). Size, number, shape and position of pollen apertures are specific for a species and represent one of the taxonomy-defined elements of exine patterning (Furness and Rudall 2004). It has been proposed that the aperture position is controlled by microtubules (Heslop-Harrison 1971; Dickinson and Sheldon 1986), probably by microtubule-dependent modelling of the ER which subtends the plasma membrane underneath the prospective site of the aperture, such that vesicles carrying cellulosic or sporopollenin material are shielded from these sites, only leaving the intine layer (Schmid et al. 1996). Recently, a gene involved in aperture formation has been identified in Arabidopsis thaliana. In a mutant of this gene, inaperturate pollen1 (inp1), all three apertures are lost although the pollen retains normal fertility. The INP1 gene product shows a tripartite subcellular localization in microspores when the maternal sporophyte still harbours a functional copy of this gene, demonstrating sporophytic control of aperture positioning. The aperture length is dependent on sporophytic gene dosage (Dobritsa and Coerper 2012).
5 Intine Development Is Controlled by the Gametophyte
The intine comprises cellulose, pectin and various proteins (Blackmore et al. 2007). It ensures the viability of the mature pollen grain as well as pollen-tube germination thus contributing to pollen survival and fertility (Brett and Waldron 1990; Edlund et al. 2004). Several genes involved in intine formation have been identified in Arabidopsis. AtUSP encodes a UDP-sugar pyrophosphorylase. It is the terminal enzyme in the myoinositol oxidation (MIO) pathway (Litterer et al. 2006) yielding precursors for the synthesis of glycolipids, glycoproteins and cell wall components including pectin and hemicellulose. Most pollen grains of the usp mutant lack intine layers and show a degraded cytoplasm, while there are no evident effect on the exine (Schnurr et al. 2006). AtUSP may involve in the synthesis of the matrix polysaccharides required for intine synthesis (Schnurr et al. 2006). The Arabidopsis genome contains five genes encoding reversibly glycosylated polypeptides (RGPs) (Dhugga et al. 1991,1997; Girke et al. 2004). RGP1 and RGP2 are specifically expressed in mature pollen. The rgp1 rgp2 double mutant is lethal, and the malformed pollen grains are arrested due to the poorly defined intine (Drakakaki et al. 2006). RGPs may play a role on pectin and/or polysaccharide biosynthesis required for glycoprotein glycosylation during intine development (Drakakaki et al. 2006; Li et al. 2010). CELLULOSE SYNTHASE (CESA) genes encode catalytic subunits of the cellulose synthase complexes (CSCs), responsible for the deposition of cellulose (see also chapter by Nick, this volume). Mutations of cesa1 and cesa3 show the gametophytic lethality due to the uneven intine distribution, suggesting that the cellulose microfibrils provide a framework for deposition of intine polymers (Persson et al. 2007). The fasciclin-like arabinogalactan (FLA) proteins have been known for their role in the response to abiotic stress during plant development (Johnson et al. 2003; Shi et al. 2003; MacMillan et al. 2010). In a FLA3 RNAi plant, the intine layer is absent leading to pollen abortion. Since FLA3 is distributed at the plasma membrane with a glycosylphosphatidylinositol anchor, it might modulate cellulose deposition during intine formation (Li et al. 2010). The gametophytic lethality of these mutants supports that the intine is controlled by male gametophyte.
6 Pollen Coat
The pollen coat is characterized by a complex lipid composition. Nonpolar esters form a semi-solid matrix, where proteins and other compounds are embedded. In the pollen coat, the long-chained lipids may function in cell-to-cell signalling, the lipid derivatives attract insects to facilitate pollen transmission and the carotenoids and flavonoids convey protection against UV radiation and microbial attack (Piffanelli et al. 1998; Doughty et al. 1993; Stephenson et al. 1997; Pacini and Franchi 1993; Paul et al. 1992).
Along with the sporopollenin, the tapetum also generates waxes regarded to be important for the pollen-pistil interaction (Preuss et al. 1993). As first step in wax biosynthesis, saturated C16 and C18 fatty acyl-CoAs produced in the plastid are further elongated to yield fatty acyl-CoA chains of 20–34 carbons in the ER. Then, these prolonged chains are converted to the various chemical classes of waxes by different biosynthetic pathways (Kunst and Samuels 2003). Genes acting at different steps of the wax biosynthetic pathway have been identified in Arabidopsis (Koornneef et al. 1989; McNevin et al. 1993). Mutations in ECERIFERUM (CER) affect the chemical composition of leaf and stem waxes (Jenks et al. 1995). The mutants cer1, cer3, cer6, cer8 and cer10 show male sterility. However, their fertility can be restored under conditions of high humidity (Koornneef et al. 1989; Hannoufa et al. 1996; Millar et al. 1999; Fiebig et al. 2000). CER1 encodes a nove1 protein involved in the conversion of long-chain aldehydes to alkanes. Mutation of cer1 not only alters the wax deposition on stem and fruits but also induces more numerous and smaller lipid droplets in the tryphine causing a defective exine structure (Aarts et al. 1995). The rice homologue of CER1, Wax-deficient anther1 (Wda1), shows 56 % similarity on the amino acid level. In the wda1 mutant, major components of waxes are reduced, and tapetal cells do not contain any orbicules or cytoplasmic lipid bodies causing a failure of exine formation. WDA1 is considered to mediate generation or secretion of very-long-chain aliphatic molecules, including the precursors for sporopollenin in anther walls (Jung et al. 2006).
FACELESS POLLEN-1 (FLP1) protein is also a member of the CER family (also named WAX2/CER3/YRE). It participates in the synthesis of wax in stems and siliques, components of the tryphine and the sporopollenin of exine (Ariizumi et al. 2003; Chen et al. 2003; Rowland et al. 2007; Kurata et al. 2003). In the faceless pollen-1 (flp-1) mutant, stems and siliques are reduced in wax content, and excessive tryphine fills the interstices of the exine leading to a smooth surface. This is consistent with a role for the tapetum in the control of the tryphine. The exine structure of flp-1 is sensitive to acetolysis, which may result from sporopollenin precursors in the tapetum being not properly secreted and/or transferred to the primexine (Ariizumi et al. 2003). The genetic evidence supports that sporopollenin and waxes may share partial biosynthesis pathway for pollen-wall development.
7 Summary
The pollen wall, as the most complex manifestations of plant cell walls, has the dual function to support plant gametogenesis and fertility. It consists of two main layers, the outer exine and the inner intine. The exine is controlled by the sporophyte and the intine is controlled by gametophyte. The formation of the exine comprises two developmental processes: pollen-wall pattern determination and deposition of sporopollenin precursors. Pollen-wall pattern determination is dependent on formation of a callosic wall, plasma membrane undulation and primexine deposition. The callose wall may provide a structural basis for the primexine deposition. In Arabidopsis, the gene products of CDKG1 and ARF17 regulate the transcript of CalS5, which is responsible for the synthesis of callose. Primexine formation and plasma membrane undulation successively provide the scaffold for sporopollenin deposition and polymerization. Genetic evidence shows that precise primexine formation requires the functions of the DEX1, NEF1, RPG1 and NPU proteins in Arabidopsis. Sporopollenin deposition and polymerization requires three important steps: synthesis, secretion and translocation of sporopollenin precursors. Genetic evidences have elucidated that all of these processes are controlled by tapetal cells. A transcriptional regulatory pathway involving DYT1-TDF1-AMS-MS188-MS1 has been proposed for tapetal development and pollen formation in Arabidopsis. In the metabolic pathway generating the material for pollen-wall formation, fatty acids are hydroxylated by specific members of the cytochromes P450 family; fatty acid modifications such as CoA-esterification and CoA-reduction are regulated by ACOS5 and MS2 respectively, whereas PKSA/PKSB and TKPR1/2 are required for phenylpropanoid biosynthesis. Subsequently, the sporopollenin precursors are transported from the tapetum to the surface of the microspores by the gene product of ABCG26, which is a direct target of AMS (Fig. 6).
Male sterility is an important trait in agriculture. Male-sterile varieties are valuable resources that greatly facilitate the production of hybrids via cross-pollination. In crops, heterosis can dramatically improve yield and quality, which is widely utilized in plant breeding. Because the exine is genetically controlled by the sporophyte tissue, defects in many genes essential for exine formation lead to male sterility. Therefore, manipulation of exine genes may provide novel strategies to generate male-sterile mutants which could be used for plant breeding in agriculture.
References
Aarts MG, Keijzer CJ, Stiekema WJ, Pereira A (1995) Molecular characterization of the CE1 gene of Arabidopsis involved in epicuticular wax biosynthesis and pollen fertility. Plant Cell 7:2115–2127
Aarts MG, Hodge R, Kalantidis K, Florack D, Wilson ZA, Mulligan BJ, Stiekema WJ, Scott R, Pereira A (1997) The Arabidopsis MALE STERILITY 2 protein shares similarity with reductases in elongation/condensation complexes. Plant J 12:615–623
Ahlers H, Thom I, Lambert J, Kuckuk R, Wiermann R (1999) 1H NMR analysis of sporopollenin from Typha angustifolia. Phytochemistry 50:1095–1098
Albrecht C, Russinova E, Hecht V, Baaijens E, de Vries S (2005) The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell 17:3337–3349
Anger EM, Weber M (2006) Pollen-wall formation in Arum alpinum. Ann Bot 97:239–244
Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62:437–460
Ariizumi T, Hatakeyama K, Hinata K, Sato S, Kato T, Tabata S, Toriyama K (2003) A novel male–sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis–sensitive exine. Plant Mol Biol 53:107–116
Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39:170–181
Bedinger P (1992) The remarkable biology of pollen. Plant Cell 4:879–887
Benning C (2008) A role for lipid trafficking in chloroplast biogenesis. Prog Lipid Res 47:381–389
Bhandari NN (1984) The microsporangium. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin/Heidelberg/New York/Tokyo, pp 53–121
Bianchi G, Murelli C, Ottaviano E (1990) Maize pollen lipids. Phytochemistry 29:739–744
Blackmore S, Barnes S (1987) Pollen wall morphogenesis in Tragopogon porrifolius (Compositae: Lactuceae) and its taxonomic significance. Rev Palaeobot Palynol 52:233–246
Blackmore S, Barnes S (1990) Pollen wall development in angiosperms. In: Blackmore S, Knox RB (eds) Micro-spores: evolution and ontogeny. Academic Press, London, pp 173–192
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498
Blokker P, Yeloff D, Boelen P, Broekman RA, Rozema J (2005) Development of a proxy for past surface UV–B irradiation: a thermally assisted hydrolysis and methylation py–GC/MS method for the analysis of pollen and spores. Anal Chem 77:6026–6031
Bowman JL, Floyd SK, Sakakibara K (2007) Green genes – comparative genomics of the green branch of life. Cell 129:229–234
Brett CT, Waldron KW (1990) Physiology and biochemistry of plant cell walls. Unwin Hyman, London
Bubert H, Lambert J, Steuernagel S, Ahlers F, Wiermann R (2002) Continuous decomposition of sporopollenin from pollen of Typha angustifolia L. by acidic methanolysis. Z Naturforsch 57:1035–1041
Canales C, Bhatt AM, Scott R, Dickinson H (2002) EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol 12:1718–1727
Chang HS, Zhang C, Chang YH, Zhu J, Xu XF, Shi ZH, Zhang XL, Xu L, Huang H, Zhang S, Yang ZN (2012) No primexine and plasma membrane undulation is essential for primexine deposition and plasma membrane undulation during microsporogenesis in Arabidopsis. Plant Physiol 158:264–272
Chen X, Goodwin M, Boroff VL, Liu X, Jenks MA (2003) Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 15:1170–1185
Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, Chermak D, Antony G, White FF, Somerville SC, Mudgett MB, Frommer WB (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468:527–532
Chen W, Yu XH, Zhang K, Shi J, De Oliveira S, Schreiber L, Shanklin J, Zhang DB (2011) Male Sterile 2 encodes a plastid–localized fatty acyl-ACP reductase required for pollen exine development in Arabidopsis thaliana. Plant Physiol 157:842–853
Choi H, Jin JY, Choi S, Hwang JU, Kim YY, Suh MC, Lee Y (2011) A WBC/ABCG–type ABC transporter is essential for transport of sporopollenin precursors for exine formation in developing pollen. Plant J 65:181–193
Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI (2005) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell 17:3350–3361
Cresti M, Blackmore S, van Went JL (1992) Atlas of sexual reproduction in flowering plants. Springer, Berlin/Heidelberg/New York/Tokyo, pp 1–249
Cutter EG (1971) Plant anatomy: experiment and interpretation. Addison-Wesley, Reading
Dahl AO (1986) Observation on pollen development in Arabidopsis under gravitationally controlled environments. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 49–60
de Azevedo Souza C, Kim SS, Koch S, Kienow L, Schneider K, McKim SM, Haughn GW, Kombrink E, Douglas CJ (2009) A novel fatty Acyl-CoA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell 21:507–525
Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, Höfte H, Gonneau M, Vernhettes S (2007) Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 104:15572–15577
Dhugga KS, Ulvskov P, Gallagher SR, Ray PM (1991) Plant polypeptides reversibly glycosylated by UDP-glucose – possible components of Golgi beta-glucan synthase in pea cells. J Biol Chem 266:21977–21984
Dhugga KS, Tiwari SC, Ray PM (1997) A reversibly glycosylated polypeptide (RGP1) possibly involved in plant cell wall synthesis: purification, gene cloning, and trans-Golgi localization. Proc Natl Acad Sci U S A 94:7679–7684
Dickinson HG (1970) Ultrastructural aspects of primexine formation in the microspore tetrad of Lilium longiflorum. Cytobiologie 4:437–449
Dickinson HG (1982) The development of pollen. Rev Cytol Biol Bot 5:5–19
Dickinson HG, Heslop-Harrison J (1977) Ribosomes, membranes and organelles during meiosis in angiosperms. Philos Trans R Soc Lond Biol 277:327–342
Dickinson HG, Sheldon JM (1984) A radial system of microtubules extending between the nuclear envelope and the plasma membrane during early male haplophase in flowering plants. Planta 161:86–90
Dickinson HG, Sheldon JM (1986) The generation of patterning at the plasma membrane of the young microspore of Lilium. In: Blackmore S, Ferguson IK (eds) Pollen and spores: form and function. Academic Press, London, pp 1–17
Dobritsa AA, Coerper D (2012) The novel plant protein INAPERTURATE POLLEN1 marks distinct cellular domains and controls formation of apertures in the Arabidopsis pollen exine. Plant Cell 24:4452–4464
Dobritsa AA, Shrestha J, Morant M, Pinot F, Matsuno M, Swanson R, Møller BL, Preuss D (2009) CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol 151:574–589
Dobritsa AA, Lei Z, Nishikawa S, Urbanczyk-Wochniak E, Huhman DV, Preuss D, Sumner LW (2010) LAP5 and LAP6 encode anther–specific proteins with similarity to chalcone synthase essential for pollen exine development in Arabidopsis. Plant Physiol 153:937–955
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP (2005) Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J 42:315–328
Dou XY, Yang KZ, Zhang Y, Wang W, Liu XL, Chen LQ, Zhang XQ, Ye D (2011) WBC27, an adenosine tri-phosphate-binding cassette protein, controls pollen wall formation and patterning in Arabidopsis. J Integr Plant Biol 53:74–88
Doughty J, Hedderson F, McCubbin A, Dickinson H (1993) Interaction between a coating-borne peptide of the Brassica pollen grain and stigmatic S (self-incompatibility)-locus-specific glycoproteins. Proc Natl Acad Sci U S A 90:467–471
Drakakaki G, Zabotina O, Delgado I, Robert S, Keegstra K, Raikhel N (2006) Arabidopsis reversibly glycosylated polypeptides 1 and 2 are essential for pollen development. Plant Physiol 142:1480–1492
Echlin P (1971) The role of the tapetum during microsporogenesis of angiosperms. In: Heslop-Harrison J (ed) Pollen: development and physiology. Butterworths, London, pp 41–61
Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16(Suppl):S84–S97
Enns LC, Kanaoka MM, Torii KU, Comai L, Okada K, Cleland RE (2005) Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol Biol 58:333–349
Feng B, Lu D, Ma X, Peng Y, Sun Y, Ning G, Ma H (2012) Regulation of the Arabidopsis anther transcriptome by DYT1 for pollen development. Plant J 72:612–624
Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D (2000) Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12:2001–2008
Fitzgerald MA, Knox RB (1995) Initiation of primexine in freeze-substituted microspores of Brassica campestris. Sex Plant Reprod 8:99–104
Frankel R, Izhar S, Nitsan J (1969) Timing of callase activity and cytoplasmic male sterility in Petunia. Biochem Genet 3:451–455
Furness CA, Rudall PJ (2004) Pollen aperture evolution – a crucial factor for eudicot success? Trends Plant Sci 9:154–158
Gabarayeva NI, Rowley JR (1994) Exine development in Nymphaea colorata (Nymphaeaceae). Nord J Bot 14:671–691
Girke T, Lauricha J, Tran H, Keegstra K, Raikhel N (2004) The cell wall navigator database: a systems–based approach to organism-unrestricted mining of protein families involved in cell wall metabolism. Plant Physiol 136:3003–3008
Grienenberger E, Kim SS, Lallemand B, Geoffroy P, Heintz D, Souza Cde A, Heitz T, Douglas CJ, Legrand M (2010) Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell 22:4067–4083
Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN (2008) RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol 147:852–863
Guilford WJ, Schneider DM, Labovitz J, Opella SJ (1988) High resolution solid state 13C NMR spectroscopy of sporopollenins from different plant taxa. Plant Physiol 86:134–136
Hannoufa A, Negruk V, Eisner G, Lemieux B (1996) The CER3 gene of Arabidopsis thaliana is expressed in leaves, stems, roots, flowers and apical meristems. Plant J 10:459–467
Heslop-Harrison J (1962) Origin of exine. Nature 195:1069–1071
Heslop-Harrison J (1963) An ultrastructural study of pollen wall ontogeny in Silene pendula. Grana Palynol 4:7–24
Heslop-Harrison J (1964) Cell walls, cell membranes, and protoplasmic connections during meiosis and pollen development. In: Linskens HF (ed) Pollen physiology and fertilisation. North Holland Publishing Company, Amsterdam, pp 39–47
Heslop-Harrison J (1968) Tapetal origin of pollen-coat substances in Lilium. New Phytol 67:779–786
Heslop-Harrison J (1971) The pollen wall: structure and development. In: Heslop-Harrison J (ed) Pollen: development and physiology. Butterworth, London, pp 75–98
Heslop-Harrison J (1979) An interpretation of the hydrodynamics of pollen. Am J Bot 66:737–743
Hesse M, Hess MW (1993) Recent trends in tapetum research. A cytological and methodological review. Plant Syst Evol 7:127–145
Hird DL, Worrall D, Hodge R, Smartt S, Paul W, Scott R (1993) The anther-specific protein encoded by the Brassica napus and Arabidopsis thaliana A6 gene displays similarity to beta-1,3-glucanases. Plant J 4:1023–1033
Hong Z, Delauney AJ, Verma DPS (2001) A cell-plate-specific callose synthase and its interaction with phragmoplastin. Plant Cell 13:755–768
Huang XY, Niu J, Sun MX, Zhu J, Gao JF, Yang J, Zhou Q, Yang ZN (2013) CDKG1 is associated with spliceosome to regulate CalS5 splicing and pollen wall formation in Arabidopsis. Plant Cell 25:637–648
Ito T, Nagata N, Yoshiba Y, Ohme–Takagi M, Ma H, Shinozaki K (2007) Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 19:3549–3562
Izhar S, Frankel R (1971) Mechanism of male sterility in Petunia: the relationship between pH, callase activity in the anthers, and the breakdown of the microsporogenesis. Theor Appl Genet 41:104–108
Jenks MA, Tuttle HA, Eigenbrode SD, Feldmann KA (1995) Leaf epicuticular waxes of the eceriferum mutants in Arabidopsis. Plant Physiol 108:369–377
Johnson KL, Jones BJ, Bacic A, Schultz CJ (2003) The fasciclin–like arabinogalactan proteins of Arabidopsis. A multigene family of putative cell adhesion molecules. Plant Physiol 133:1911–1925
Jung KH, Han MJ, Lee DY, Lee YS, Schreiber L, Franke R, Faust A, Yephremov A, Saedler H, Kim YW, Hwang I, An G (2006) Wax-deficient anther 1 is involved in cuticle and wax production in rice anther walls and is required for pollen development. Plant Cell 18:3015–3032
Kauss H (1996) Callose synthesis. In: Smallwood M, Knox P, Bowtes DJ (eds) Membranes: specialized functions in plant cells. Bios Scientific Publishers, Oxford
Kim SS, Grienenberger E, Lallemand B, Colpitts CC, Kim SY, Souza Cde A, Geoffroy P, Heintz D, Krahn D, Kaiser M, Kombrink E, Heitz T, Suh DY, Legrand M, Douglas CJ (2010) LAP6/POLYKETIDE SYNTHASE A and LAP5/POLYKETIDE SYNTHASE B encode hydroxyalkyl α-pyrone synthases required for pollen development and sporopollenin biosynthesis in Arabidopsis thaliana. Plant Cell 22:4045–4066
Koornneef M, Hanhart CJ, Thiel F (1989) A genetic and phenotypic description of eceriferum (cer) mutants in Arabidopsis thaliana. J Hered 80:118–122
Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42:51–80
Kurata T, Kawabata-Awai C, Sakuradani E, Shimizu S, Okada K, Wada T (2003) The YORE-YORE gene regulates multiple aspects of epidermal cell differentiation in Arabidopsis. Plant J 36:55–66
Larson A, Lewis CW (1962) Pollen wall development in Parkinsonia aculeata. Grana Palynol 3:21–27
Li J, Yu M, Geng LL, Zhao J (2010) The fasciclin-like arabinogalactan protein gene, FLA3, is involved in microspore development of Arabidopsis. Plant J 64:482–497
Li XC, Zhu J, Yang J, Zhang GR, Xing WF, Zhang S, Yang ZN (2012) Glycerol-3-phosphate acyltransferase 6 (GPAT6) is important for tapetum development in Arabidopsis and plays multiple roles in plant fertility. Mol Plant 5:131–142
Li-Beisson Y, Pollard M, Sauveplane V, Pinot F, Ohlrogge J, Beisson F (2009) Nanoridges that characterize the surface morphology of flowers require the synthesis of cutin polyester. Proc Natl Acad Sci U S A 106:22008–22013
Litterer LA, Schnurr JA, Plaisance KL, Storey KK, Gronwald JW, Somers DA (2006) Characterization and expression of Arabidopsis UDP-sugar pyrophosphorylase. Plant Physiol Biochem 44:171–180
Ljung (2013) Auxin metabolism and homeostasis during plant development. Development 140:943–950
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56:393–434
MacMillan CP, Mansfield SD, Stachurski ZH, Evans R, Southerton SG (2010) Fasciclin-like arabinogalactan proteins: specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. Plant J 62:689–703
Mariani C, De Beuckeleer M, Truettner J, Leemans J, Goldberg RB (1990) Induction of male sterility in plants by a chimeric ribonuclease gene. Nature 347:737–741
Mascarenhas JP (1975) The biochemistry of angiosperm pollen development. Bot Rev 41:259–314
Maheshwari P (1950) An introduction to the embryology of angiosperms. McGraw-Hill, New York
McNevin JP, Woodward W, Hannoufa A, Feldmann KA, Lemieux B (1993) Isolation and characterization of eceriferum (cer) mutants induced by T–DNA insertions in Arabidopsis thaliana. Genome 36:610–618
Meuter–Gerhards A, Riegart S, Wiermann R (1999) Studies on sporopollenin biosynthesis in Cucurbita maxima (DUCH)-II: the involvement of aliphatic metabolism. J Plant Physiol 154:431–436
Millar AA, Clemens S, Zachgo S, Giblin ME, Taylor DC, Kunst L (1999) CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. Plant Cell 11:825–838
Morant M, Jorgensen K, Schaller H, Pinot F, Møller BL, Werck–Reichhart D, Bak S (2007) CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell 19:1473–1487
Morgan DO (1997) Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol 13:261–291
Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Preuss D (2005) Callose (β-1,3 glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol 5:22–30
Niu N, Liang W, Yang X, Jin W, Wilson ZA, Hu J, Zhang D (2013) EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat Commun. doi:10.1038/ncomms2396
Owen HA, Makaroff CA (1995) Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae). Protoplasma 185:7–21
Pacini E, Franchi GG (1993) Role of the tapetum in pollen and spore dispersal. Plant Syst Evol 7:1–11
Pacini E, Juniper BE (1979) The ultrastructure of pollen grain development in the olive (Olea europaea). II. Secretion by the tapetal cells. New Phytol 83:165–174
Pacini E, Franchi GG, Hesse M (1985) The tapetum: its form, function, and possible phylogeny in Embryophyta. Plant Syst Evol 149:155–185
Papini A, Mosti S, Brighigna L (1999) Programmed-cell-death events during tapetum development of angiosperms. Protoplasma 207:213–221
Paul W, Hodge R, Smartt S, Draper J, Scott R (1992) The isolation and characterisation of the tapetum-specific Arabidopsis thaliana A9 gene. Plant Mol Biol 19:611–622
Paxson–Sowders DM, Owen HA, Makaroff CA (1997) A comparative ultrastructural analysis of exine pattern development in wild-type Arabidopsis and a mutant defective in pattern formation. Protoplasma 198:53–65
Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA (2001) DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol 127:1739–1749
Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, Khitrov N, Auer M, Somerville CR (2007) Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci U S A 104:15566–15571
Phan HA, Iacuone S, Li SF, Parish RW (2011) The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell 23:2209–2224
Piffanelli P, Ross JHE, Murphy DJ (1997) Intra and extracellular lipid composition and associated gene expression patterns during pollen development in Brassica napus. Plant J 11:549–652
Piffanelli P, Ross JHE, Murphy DJ (1998) Biogenesis and function of the lipidic structures of pollen grains. Sex Plant Reprod 11:65–80
Preuss D, Lemieux B, Yen G, Davis RW (1993) A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev 7:974–985
Quilichini TD, Friedmann MC, Samuels AL, Douglas CJ (2010) ATP-binding cassette transporter G26 is required for male fertility and pollen exine formation in Arabidopsis. Plant Physiol 154:678–690
Roberts K (1990) Structures at the plant cell surface. Curr Opin Cell Biol 2:920–928
Rowland O, Lee R, Franke R, Schreiber L, Kunst L (2007) The CER3 wax biosynthetic gene from Arabidopsis thaliana is allelic to WAX2/YRE/FLP1. FEBS Lett 581:3538–3544
Rowley JR, Southworth D (1967) Deposition of sporopollenin on lamellae of unit membrane dimensions. Nature 213:703–704
Rowley JR, Muechlethaler K, Frey-Wyssling A (1959) A route for the transfer of materials through the pollen grain wall. J Biophys Biochem Cytol 6:537–538
Sanders PM, Bui AQ, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex Plant Reprod 11:297–322
Schmid MA, Eberwein RK, Hesse M (1996) Pattern morphogenesis in cell walls of diatoms and pollen grains: a comparison. Protoplasma 193:144–173
Schnurr J, Shockey J, Browse J (2004) The acyl–CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16:629–642
Schnurr JA, Storey KK, Jung HJ, Somers DA, Gronwald JW (2006) UDP-sugar pyrophosphorylase is essential for pollen development in Arabidopsis. Planta 224:520–532
Scott RJ (1994) Pollen exine: the sporopollenin enigma and the physics of pattern. In: Scott RJ, Stead MA (eds) Molecular and cellular aspects of plant reproduction. University Press, Cambridge, pp 49–81
Scott RW, Strohl MJ (1962) Extraction and identification of lipids from loblolly pine pollen. Phytochemistry 1:189–193
Scott RJ, Spielmana M, Dickinsonb HG (2004) Stamen structure and function. Plant Cell 16:46–60
Sheldon JM, Dickinson HG (1983) Determination of patterning in the pollen wall of Lilium henryi. J Cell Sci 63:191–208
Shi HZ, Kim YS, Guo Y, Stevenson B, Zhu JK (2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 15:19–32
Skvarla JJ, Rowley JR (1987) Ontogeny of pollen in Poinciana (Leguminoseae). I. Development of exine template. Rev Palaeobot Palynol 50:293–311
Sørensen A, Krober S, Unte US, Huijser P, Dekker K, Saedler H (2003) The Arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J 33:413–423
Southworth D, Jernstedt JA (1995) Pollen exine development precedes microtubule rearrangement in Vigna unguiculata (Fabaceae): a model for pollen wall patterning. Protoplasma 187:79–87
Stanley RG, Linskens HF (1974) Pollen biology, biochemistry and management. Springer, New York/Berlin/Heidelberg
Stephenson AG, Doughty J, Dixon S, Elleman C, Hiscock S, Dickinson HG (1997) The male determinant of self-incompatibility in Brassica oleracea is located in the pollen coating. Plant J 12:1351–1359
Stevens VA, Murray BG (1981) Studies on heteromorphic self-incompatibility systems: the cytochemistry and ultrastructure of the tapetum of Primula obconica. J Cell Sci 50:419–431
Stieglitz H (1977) Role of β-1,3-glucanasein postmeiotic microspore release. Dev Biol 57:87–97
Stieglitz H, Stern H (1973) Regulation of/3-1,3 glucanase activity in developing anthers of Lilium. Dev Biol 34:169–173
Sun MX, Huang XY, Yang J, Guan YF, Yang ZN (2013) Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Sex Plant Reprod 26:83–91
Takahashi M (1989) Pattern determination of the exine in Caesalpinia japonica (Leguminosae: Caesalpinioideae). Am J Bot 75:1615–1626
Takahashi M (1993) Exine initiation and substructure in pollen of Caesalpinia japonica (Leguminoseae: Caesalpinioideae). Am J Bot 80:192–197
Varnier AL, Mazeyrat-Gourbeyre F, Sangwan RS, Clement C (2005) Programmed cell death progressively models the development of anther sporophytic tissues from the tapetum and is triggered in pollen grains during maturation. J Struct Biol 152:118–128
Vaux DL, Korsmeyer SJ (1999) Cell death in development. Cell 96:245–254
Vizcay-Barrena G, Wilson ZA (2006) Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J Exp Bot 57:2709–2717
Waterkeyn L (1962) Les parois microsporocytaires de nature callosique chez Helleborus et Tradescantia. Cellule 62:225–255
Waterkeyn L, Beinfait A (1970) On a possible function of the callosic special wall in Ipomoea purpuea (L.) roth. Grana 10:13–20
Wehling K, Niester C, Boon JJ, Willemse MTM, Wiermann R (1989) p-Coumaric acid-a monomer in the sporopollenin skeleton. Planta 179:376–380
Wiermann R, Gubatz S (1992) Pollen wall and sporopollenin. Int Rev Cytol 140:35–72
Wijeratne AJ, Zhang W, Sun Y, Liu W, Albert R, Zheng Z, Oppenheimer DG, Zhao D, Ma H (2007) Differential gene expression in Arabidopsis wild-type and mutant anthers: insights into anther cell differentiation and regulatory networks. Plant J 52:14–29
Wilmesmeier S, Steuernagel S, Wiermann R (1993) Comparative FTIR and 13C CP/MAS NMR spectroscopic investigations on sporopollenin of different systematic origins. Z Naturforsch 48c:697–701
Wilson ZA, Morroll SM, Dawson J, Swarup R, Tighe PJ (2001) The Arabidopsis MALE STERILITY 1 (MS1) gene is a transcriptional regulator of male gametogenesis, with homology to the PHD–finger family of transcription factors. Plant J 28:27–39
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4:759–771
Wu H, Cheung AY (2000) Programmed cell death in plant reproduction. Plant Mol Biol 44:267–281
Xu J, Yang C, Yuan Z, Zhang D, Gondwe MY, Ding Z, Liang W, Zhang D, Wilson ZA (2010) The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell 22:91–107
Yang SL, Xie LF, Mao HZ, Puah CS, Yang WC, Jiang L, Sundaresan V, Ye D (2003) TAPETUM DETERMINANT1 is required for cell specialization in the Arabidopsis anther. Plant Cell 15:2792–2804
Yang C, Vizcay–Barrena G, Conner K, Wilson ZA (2007) MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 19:3530–3548
Yang J, Tian L, Sun MX, Huang XY, Zhu J, Guan YF, Jia QS, Yang ZN (2013) AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol 162:720–731
Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H (2006) Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 133:3085–3095
Zhang ZB, Zhu J, Gao JF, Wang C, Li H, Li H, Zhang HQ, Zhang S, Wang DM, Wang QX, Huang H, Xia HJ, Yang ZN (2007) Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J 52:528–538
Zhao DZ, Wang GF, Speal B, Ma H (2002) The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev 16:2021–2031
Zheng Z, Xia Q, Dauk M, Shen W, Selvaraj G, Zou J (2003) Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 15:1872–1887
Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN (2008) Defective in Tapetal development and function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J 55:266–277
Zhu J, Zhang GQ, Chang YH, Li XC, Yang J, Huang XY, Yu QB, Chen H, Wu TL, Yang ZN (2010) AtMYB103 is a crucial regulator of several pathways affecting Arabidopsis anther development. Sci China Life Sci 53:1112–1122
Zhu J, Lou Y, Xu XF, Yang ZN (2011) A genetic pathway for tapetum development and function in Arabidopsis. J Integr Plant Biol 53:892–900
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Lou, Y., Zhu, J., Yang, Z. (2014). Molecular Cell Biology of Pollen Walls. In: Nick, P., Opatrny, Z. (eds) Applied Plant Cell Biology. Plant Cell Monographs, vol 22. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-41787-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-642-41787-0_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-41786-3
Online ISBN: 978-3-642-41787-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)