Abstract
Background
Bony deficiency of the anteroinferior glenoid rim as a result of a dislocation can lead to recurrent glenohumeral instability. These lesions, traditionally treated by open techniques, are increasingly being treated arthroscopically as our understanding of the pathophysiology and anatomy of the glenohumeral joint becomes clearer. Different techniques for arthroscopic management have been described and continue to evolve. While the success of the repair is surgeon dependent, the recent advances in arthroscopic shoulder surgery have contributed to the growing acceptance of arthroscopic reconstruction of glenoid bone defects to restore stability.
Questions/Purposes
The purpose of this study was to describe arthroscopic surgical management options for patients with glenohumeral osseous lesions and instability.
Methods
A comprehensive search of PubMed, Cochrane, and Medline was conducted to identify eligible studies. The reference lists of identified articles were then screened. Both technique articles and long-term outcome studies evaluating arthroscopic management of glenohumeral lesions were included.
Results
Studies included for final analysis ranged from Level II to V evidence. Technique articles include suture anchor fixation of associated glenoid rim fractures, arthroscopic reduction and percutaneous fixation of greater tuberosity fractures, arthroscopic filling (“remplissage”) of the humeral Hill-Sachs lesion, and an all-arthroscopic Latarjet procedure. The overall redislocation rate varied but was consistently <10% with a low complication rate.
Conclusion
Management of glenohumeral instability can be challenging but more recent advances in arthroscopic techniques have provided improved means of treating this diagnosis. This manuscript provides a comprehensive review of the arthroscopic treatment of osseous instability of the shoulder. It provides an in depth look at the various treatment options and describes techniques for each.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glenohumeral instability is a common orthopedic disorder most often seen in a young athletic population. Traumatic dislocations often lead to capsulolabral injury with or without osseous injury and may lead to recurrent glenohumeral dislocations. Biomechanical and cadaveric studies have elucidated the complex anatomy of the glenohumeral joint, providing further insight into the etiology of recurrent subluxation and dislocations and allowing for more successful management of this condition.
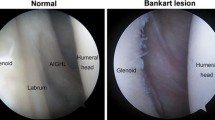
Recurrent instability of the glenohumeral joint was traditionally thought to be due to soft tissue disruption of the capsulolabral complex. More recent emphasis on restoring the osseous architecture of the glenohumeral joint, however, has led to an increase in the success rate of surgical management of this condition. The stability of the glenohumeral joint depends on a complex interplay of bony and ligamentous structures, which act as both static and dynamic stabilizers. In cases of traumatic anterior shoulder dislocation or subluxation, these stabilizing mechanisms are often disrupted leading to an increased risk for recurrent instability. Most commonly, initial shoulder dislocation results in a detachment of the anteroinferior capsulolabral complex with or without a bony avulsion [3, 19], referred to as a Bankart or bony Bankart lesion, respectively. Any fracture of the glenoid rim decreases the glenohumeral contact area and disrupts the inferior glenohumeral ligament insertion, altering the static stabilizers and predisposing patients to recurrence. Likewise, glenoid bone loss, either from an acute low-energy compression fracture or chronic attenuation secondary to recurrent shoulder dislocations, may also contribute to anterior instability by decreasing the articular arc length and reducing the surface area that resists shear and axial forces on the joint [35].
Compression fractures of the posterosuperolateral humeral head, or Hill-Sachs lesions, occur as a result of anterior dislocation, with a reported incidence between 40 and 90% [44]. These compression injuries occur along the posterosuperolateral aspect of the humeral head when it contacts the anterior glenoid. With repeat instability episodes, this region of the humeral head continues to contact the glenoid rim, which may lead to enlargement of the bony defect. In turn, as the size of this impression fracture increases, risk of recurrent instability may increase. Thus, while much of the focus of glenohumeral instability is placed on glenoid-sided lesions, optimal management requires a close evaluation of humeral bone loss as well [39].
The decision to manage these osseous injuries is dependent on both the amount of bone loss and the ability for the humeral head to actively engage the anterior glenoid during the arc of motion. The “glenoid track” concept was developed to biomechanically quantify the effects of both humeral and glenoid bone defects on shoulder instability [46]. Yamamoto et al. looked at the contact area of shoulders in various degrees of abduction in extreme external rotation in order to determine how the glenoid shifted on the humeral head. They found that in a normal shoulder, the width of the glenoid track from the rotator cuff attachment is 84% of the width of the glenoid surface [46]. When there is a bony Bankart lesion, the glenoid track is reduced by the percentage of bone loss from the bony Bankart. Patients with a Hill-Sachs lesion within the glenoid track will not engage the humerus, whereas a patient with a Hill-Sachs medial to the glenoid track, or off-track, will engage. When managing these osseous defects, it should be understood that if the medial margin of the Hill-Sachs lesion is more medial than the glenoid track, a soft tissue stabilization will unlikely create enough tension to get the lesion on-track and instability will persist [27].
It is widely accepted that the management of large acute glenoid rim avulsion fractures with capsulolabral tears is managed surgically and has historically required open procedures. Recent advances in arthroscopy have broadened surgical options, as techniques for arthroscopic treatment of shoulder instability have been described. These techniques include suture anchor fixation of associated glenoid rim fractures, arthroscopic reduction and percutaneous fixation of greater tuberosity fractures, arthroscopic filling (“remplissage”) of the humeral Hill-Sachs lesion, and an all-arthroscopic Latarjet procedure (Table 1). The potential to reduce morbidity while providing equivalent biomechanical and clinical outcomes may favor arthroscopic techniques in the treatment of glenohumeral instability with associated glenoid or humeral bone loss. The purpose of this article is to debunk the traditional teaching that bony shoulder instability requires open surgical treatment. We hope to present evidence that arthroscopic techniques are appropriate and successful in appropriately selected cases of osseous instability.
Glenoid Bone Loss
Suture Anchor Fixation for Bony Bankart Lesions
It has been reported that bony defects of the glenoid rim occur in conjunction with injury to the anteroinferior capsulolabral structures in up to 70% of patients with traumatic anterior glenohumeral dislocation [38]. Bigliani classified these bony defects as type I, a displaced avulsion fracture; type II, a malunited avulsion fracture; and type III, an erosion of the glenoid rim with less than 25% (type IIIA) or more than 25% (type IIIB) deficiency [3]. In a cadaveric study by Itoi et al., they found that with a defect of 21% of the superior-inferior glenoid length would lead to persistent instability after a capsulolabral repair only [20]. When operative intervention is indicated, management of glenoid avulsion fractures has historically been performed by open reduction and internal fixation, with splitting or detachment of the subscapularis tendon. However, arthroscopic management is becoming more commonplace with increasing surgeon expertise and experience. Numerous arthroscopic techniques have been described including suture anchors placed at the glenoid rim with or without additional sutures around the fragment to increase stability, as well as the use of percutaneous cannulated screws for larger fragments [9].
Osseous glenoid defects up to 25% can be fixed arthroscopically, albeit quite challenging [42]. Smaller pieces constituting 10–15% of the glenoid surface may be better suited for this all-arthroscopic technique, as larger fragments may necessitate the need for a concomitant open procedure. The single-row technique for arthroscopic fixation of osseous Bankart lesions originally described by Porcellini et al. involves placement of a single row of suture anchors on the medial glenoid rim and then passing the sutures around the bony fragment and through the capsulolabral complex [37]. The capsulolabral complex is then secured with sliding knots. Porcellini et al. found that in 25 patients with less than 25% glenoid bone loss, the long-term outcomes were favorable for those that underwent arthroscopic fixation, especially in those that underwent surgery within 3 months of their first dislocation [37]. More recent techniques for repairing Bigliani type I fractures have detailed a double-row or suture bridge technique [1, 22, 41], initially described by Millet et al. [28]. This double-row technique has been described to restore the insertional anatomy of the capsulolabral complex by allowing for multiple points of fixation, compression across the fracture bed, and theoretically a more anatomic reduction [1, 22]. In a cadaveric study, Ahmad et al. qualitatively analyzed the glenoid capsulolabral anatomy and found that a single-row repair of the capsulolabral complex restores 42.3% of the native footprint surface area while the double-row repair restores 85.9% of the native insertional footprint [1]. Kim et al. subsequently compared the mean and peak pressure, the contact force, and pressurized contact area at the insertion of the capsulolabral complex between the two repairs and found that the double-row technique offers a greater mean interface pressure and contact area [22].
Arthroscopic Surgical Technique
This technique is performed with the patient in the beach chair position. A standard posterior portal is made followed by an anterosuperior portal in the rotator interval and an anteroinferior accessory portal just above the subscapularis. A thorough diagnostic arthroscopy can be performed through the use of twin anterior portals as described by Laurencin et al. [24]. A 70° arthroscope can be used for greater visualization of the glenoid neck, just medial to the bony Bankart. The main bony fragment is often firmly coupled with the capsulolabral complex, and thus, care must be taken not to disrupt the fragment, but the fragment must be completely elevated of the medial glenoid neck (Fig. 1c). The neck of the glenoid should then be prepared with either a rasp or shaver in order to create a bleeding surface that will allow adequate healing.
a Three-dimensional CT scan demonstrating a bony Bankart lesion involving approximately 25% of the anteroinferior glenoid. b Three-dimensional CT scan showing an accompanying depressed greater tuberosity fracture. c A left shoulder viewing from a posterior portal in beach chair position. A significant bony Bankart lesion with attached capsulolabral tissue (asterisk). Black arrow demonstrating the anterior glenoid rim. HH humeral head, G glenoid. d Medial row anchor placement through a lateral rotator interval portal utilizing a curved drill and anchor system. e Reduction and fixation of the bony fragment with a suture bridge construct to a knotless anchor on the glenoid face, with placement standard anchor placement inferior to the fracture fragment. f Final anatomic reduction with suture bridge fixation around the fragment and standard knot-tied anchors superior and inferior. g, h The concomitant greater tuberosity (GT) fracture fixed percutaneously with a 3.5-mm cannulated screw.
Depending on the size of the Bankart lesion, one or two medial suture anchors may be needed to secure the bony fragment. If only one is required, it should be placed in the middle of the fracture fragment, medial to the fracture bed, using a curved guide and minimize skiving off of the glenoid neck (Fig. 1d). If more than one medial anchor is needed, an additional inferior anchor should be placed prior to placing the lateral row. Once the medial anchor(s) is in place, both limbs of the suture(s) should be passed around the entire fragment and capsulolabral tissue using a suture passing device. One or two holes are then drilled on the glenoid face adjacent to the fracture line. The two limbs of each medial anchor are then loaded into a knotless anchor and seated in the preexisting drill hole while applying tension, creating a suture bridge across the fracture fragment (Fig. 1e). In the case of a 36-year-old right-hand dominant male who presented to our clinic after a traumatic shoulder dislocation, CT scan and MR imaging demonstrated a bony Bankart involving greater than 20% of glenoid, as well as a greater tuberosity fracture. A double-row suture bridge was used to capture the central portion of the bone fragment, while standard suture anchor technique was employed superior and inferior to the fragment to fixate the labrum (Fig. 1f).
Attention should then be turned to restoring the capsular anatomy. A suture anchor should be placed inferior to the fracture fragment on the medial aspect of the glenoid rim, usually at or near the 5:30 position. Standard anchor placement, suture passing, and tying techniques are performed. Additional anchors may be placed superior to the fragment in order to repair the labrum superior to the osseous Bankart lesion. It is recommended that at least one anchor be used superiorly to provide stability to the capsulolabral complex [41]. By restoring rotational control via the labral repair, this technique may provide a superior method of restoring glenoid bone loss and attaining a capsular shift than previously described single-row techniques. Additionally, there is no suture material at the bone-bone interface in the fracture bed where healing occurs as opposed to other techniques described [30, 37, 42]. By establishing points of fixation medially and laterally at the fracture site, the risk of overreduction and malreduction due to tilt is decreased. After describing the aforementioned technique, Millett et al. reported a small case series of 15 patients who underwent an arthroscopic “bony Bankart bridge.” They found that there was a significant improvement in postoperative physical outcome scores and pain relief in patients with Bigliani type I or II bony Bankart lesions. Nearly 70% of their patients returned to their sport at a level equal to or better than their preinjury level and the overall complication rate was low [29].
Arthroscopic Latarjet
In cases where significant attritional bone deficiency of the glenoid is present, arthroscopic stabilization using soft tissue procedures alone may not suffice [4]. Mologne et al. demonstrated failures of arthroscopic stabilization in all patients of their 21 patient cohort who was found to have attritional type glenoid bone loss [30]. Bony reconstruction is thus necessary in order to buttress the anterior glenoid rim and can be performed by transferring a 2–3-cm portion of the coracoid process through the rotator interval and securing it to the glenoid rim. This procedure, described by Bristow-Latarjet, has been traditionally been done open through a deltopectoral approach but has been recently described arthroscopically.
While several techniques exist for the all-arthroscopic Latarjet procedure, perhaps the most widely known is the technique developed by Lafosse et al. [23]. This approach improves visualization via a capsulolabral resection for coracoid bone graft positioning on the glenoid rim while concomitantly repairing all soft tissue lesions within the joint. Ultimately, this reconstruction produces a “triple blocking effect” as described by Boileau et al. [5]. The bone block from the coracoid acts as an extended platform off the glenoid bone defect for the humeral head to sit. This bony block serves as a static restraint. Second, the conjoint tendon lowers the inferior part of the subscapularis creating a tenodesis effect to reinforce the weakened capsule. Lastly, repair of the labrum and anterior capsule to the new glenoid rim reinforced with the coracoacromial ligament creates a bumper effect to prevent humeral head subluxation and impaction against the graft. In a cadaveric study by Yamamoto et al., the sling effect produced by the subscapularis and conjoint tendons at the end-range and mid-range of arm positions imparted the greatest stability to the shoulder during testing [47]. Dines et al. looked at the conjoint tendon in the Latarjet procedure and its effect on anterior translation of the humerus and found that the lack of the conjoint tendon sling allows for an increase in external rotation of the humerus and an associated increase in anterior translation [11]. This was further echoed by Giles et al. whose cadaveric study demonstrated that the sling effect of the conjoint tendon prevented anterior dislocation in all specimens after coracoid transfer; however, when the conjoint was unloaded, there was a 25% rate of redislocation [18].
Arthroscopic Surgical Technique
The technique for the all-arthroscopic Latarjet is performed with the patient in the semi-beach chair position with the head of the bed elevated approximately 30°. The procedure begins with the diagnostic arthroscopy where intra-articular pathology is assessed and addressed through standard posterior and anterior portals. The labrum at the 2 through 5 o’clock position is resected as is middle and inferior glenohumeral ligaments at this position. The subscapularis is split to allot for mobilization of the coracoid later in the procedure (Fig. 2a). Once the coracoid is identified, the attachments of the pectoralis minor and coracoacromial ligaments are removed, allowing visualization of the brachial plexus above the pectoralis minor and below the subcoracoid bursa. The conjoint tendon attachment to the coracoid tip is preserved. The anterior rim of the glenoid is then prepared using an arthroscopic shaver and rasp in order to create bleeding, cancellous bone for receipt of the graft.
(*All images reprinted from Journal Shoulder Elbow Surg, Vol 19/2, LaFosse L, Boyle, S., Arthroscopic Latarjet Procedure, 2-12, 2010, with permission from Elsevier.) a Subscapularis split with a switching stick elevating the muscle. b View from the anterolateral portal demonstrating the coracoid tip with a spinal needle inserted anteriorly to define the anterior tip. Two Kirschner wires are placed into the coracoid (asterisk) through a guide in a superior to inferior direction. These will be used to drill and tap the coracoid. c Coracoid osteotomy performed through the base subsequent to using a burr on the lateral, superior, and inferior edges of the coracoid. Viewing from the anterolateral portal. d Coracoid being reduced onto the coracoid positioning cannula using chia wires placed through the previously made holes. e, f View from anterolateral portal demonstrating coracoid reduction (e) with screws and final fixation on glenoid rim (f). Asterisk coracoid tip, HH humeral head, G glenoid.
A lateral portal is established to provide complete visualization anteriorly for debridement of the coracoid and exposure of the axillary nerve by dissecting the pectoralis minor from the medial and lateral border of the conjoint tendon. It should be noted that there is little distinction between the two tendons in this plane and the brachial plexus lies just behind and above the pectoralis minor. It is thus safer to detach the tendon as close to the coracoid as possible. A coracoid portal should then be created using spinal needle localization midway between the base and tip of the coracoid process. Two Kirschner wires are placed through the coracoid in an anterior to inferior direction (Fig. 2b). Using a 2.9-mm drill, two vertical drill holes approximately 8 mm apart should be created in the coracoid and suture should be passed through the holes, retrieving both ends through the coracoid portal. An osteotomy using a burr can then be performed approximately 2.5 cm above the tip of the coracoid and the bone fragment transported inferomedially (Fig. 2c). At this point, an anteroinferior portal should be created through the subscapularis tendon lateral to the axillary nerve, which will allow for improved fixation of the bone graft. Care should be taken when mobilizing the coracoid process, as the musculocutaneous nerve may be encountered. An anatomic study by Flatow et al. detailed the course of the musculocutaneous nerve and found that the frequently cited range of 5–8 cm below the coracoid cannot be relied on since 29% of the time the nerve entered the coracobrachialis proximal to 5 cm (74% if proximal branches were counted) [14].
The subscapularis is split in line with its fibers (typically horizontally) from the axillary nerve to the lesser tuberosity while avoiding the bicipital groove (Fig. 2a). To lever open this split tendon for graft passage, a blunt instrument such as a switching stick can be inserted through the posterior portal across the joint and move the superolateral portion of the subscapularis muscle. A specialized cannula in the anteroinferior portal should be used that will allow placement of two cannulated screws into the coracoid block. At this point, the arthroscope should be in the anterior portal while instrumenting through the anteroinferior portal to bring the graft through the subscapularis interval and onto the anterior rim of the glenoid. Ideal graft positioning is between 2 o’clock and 6 o’clock. Starting inferiorly, fixation of the glenoid involves placement of one or two bicortical 3.5-mm cortical screws using a 3.2-mm cannulated drill bit (Fig. 2e). The screws are placed through this anteroinferior portal using the arthroscope to visualize the glenoid rim through the anterior or posterior portal to ensure a flush reduction. A burr can then be used once fixed to smooth the graft out to match the shape of the glenoid rim (Fig. 2f).
Despite limited literature on outcomes of this technique, Boileau et al. found no incidence of neurologic injury or recurrent instability in 41 patients who underwent an all-arthroscopic Latarjet [6]. Compared to Lafosse’s technique described above which includes a capsulolabral resection, Boileau repairs the soft tissue stabilizers with suture anchors. Boileau also fixes the bone graft with sutures compared with Lafosse who uses screws. Additionally, their described complications included one bone block fracture and seven episodes of migration of the graft; however, the overall conclusion was that this is a reproducible and safe procedure [6]. Dumont et al., in the longest follow-up series of 64 patients undergoing all-arthroscopic Latarjet procedures, showed similar good results with only one patient reporting recurrent instability (1.59%) [12]. The authors did however note that 10 of their 64 patients required a return to the operating room for complications including a displaced graft, removal of prominent hardware, and one total shoulder replacement for arthritis. While technically demanding, the procedure affords a relatively safe means of achieving the triple blocking effect of stability through an all-arthroscopic means. It should be noted though, that the majority of the patients who undergo this operation in Europe and in these studies are first-time dislocators affording an easier dissection and potentially better chance at recovery. In contrast, the typical patient undergoing a Latarjet procedure for instability has often failed other forms of surgical management. Careful patient selection will be critical in deciding to proceed with an all-arthroscopic Latarjet procedure, especially if the surgeon is not entirely familiar with the open Latarjet counterpart.
Humeral-Sided Lesions
Remplissage
Humeral-sided lesions must also be considered in cases of instability, as they are often present in cases of traumatic anterior dislocation. Determining which lesions are clinically significant is challenging and thus the decision to manage them surgically may be inconsistent for mid-sized lesions. Surgical management of these osseous lesions may include filling the humeral head defect with bone or tissue. Remplissage, a French term meaning “to fill,” involves suturing the infraspinatus tendon into the bony defect. It has been advocated in small to medium Hill-Sachs lesions (Fig. 3a, b), whereas humeral head allograft has been described for larger lesions involving >40% of the humeral head [13]. By filling the Hill-Sachs lesion with the midsubstance of the infraspinatus, one attempts to convert it to an extra-articular lesion with the goal of preventing engagement in the glenoid. This procedure has been described using either an arthroscopic or open technique, based primarily on surgeon preference [25].
a, b MRI and CT scans demonstrating a large Hill-Sachs lesion with associated soft tissue Bankart lesion. c A right shoulder in beach chair position viewed from a posterior portal. A large engaging Hill-Sachs lesion. d The Hill-Sachs lesion after bur preparation and placement of two suture anchors (Arthrex double-loaded suturetac 2.9 mm biocomposite anchors). e View from the anterior superior rotator interval portal of the suture anchors. Sutures were shuttled through the infraspinatus with a bird beak penetrator. f View again from the anterior superior portal demonstrating fill of the Hill-Sachs defect after tying of the suture anchors. The infraspinatus is to the left and humerus is to the right.
Arthroscopic Surgical Technique
The patient can be placed in either the beach chair or lateral decubitus position. The posterior portal is established in the usual fashion or shifted lateral toward the Hill-Sachs lesion, thus allowing visualization of the joint and access to the lesion (Fig. 3c). An anterior portal is established in the rotator interval using a standard outside-in technique with a spinal needle, entering the joint directly superior to the subscapularis tendon. After performing a diagnostic arthroscopy, the arthroscope is switched to the anterior portal, and the posterior portal is evaluated with respect to the Hill-Sachs lesion; it must be at a proper angle to accommodate placement of two anchors. If it is not in ideal position, a second posterior portal directly over the lesion is made. An anchor cannula is placed, and the lesion is burred approximately 2 mm in depth. Prior to placing the anchors, the subacromial space should be entered and debrided in order to facilitate easier suture management. After debriding the subacromial space, reenter the glenohumeral joint and place the first anchor at the most lateral and distal point of the lesion. A penetrating grasper is then used to pull each of the suture limbs from the joint through the infraspinatus. This process is repeated for the second anchor (Fig. 3d, e). Once the sutures are passed, it is essential to treat the Bankart lesion prior to tying the aforementioned remplissage sutures. Tying the sutures prior to fixation of the Bankart lesion limits surgical access and increases the difficulty of the capsulolabral repair. Once the Bankart lesion is fixed, the remplissage sutures are then tied down within the subacromial space, with the lateral knot first, followed by the medial knot. These sutures pull the posterior capsule and infraspinatus into the burred lesion, filling the defect (Fig. 3f).
In a series by Boileau et al. [7], 47 patients underwent arthroscopic Bankart repair combined with Hill-Sachs remplissage with the use of suture anchors. Healing of the posterior aspect of the capsule and the infraspinatus tendon into the humeral defect was observed in all 42 patients who underwent postoperative imaging. Compared with the normal (contralateral) side, the mean deficit in external rotation was 8° ± 7° with the arm at the side of the trunk and 9° ± 7° in abduction at the time of the last follow-up. Of 41 patients involved in sports, 37 (90%) were able to return postoperatively and 28 (68%) returned to the same level of sports, including those involving overhead activities. Ninety-eight percent (46) of the 47 patients had a stable shoulder at the time of the last follow-up [45]. These results have been replicated in a recent series by Wolf et al., who reported a repeat dislocation rate of 4.4% in 45 patients who were treated with remplissage for anterior shoulder instability [45]. Garcia et al. more recently described outcomes of remplissage in athletes with a 5-year follow-up and found a redislocation rate of 11.8%, with 95.5% of patients returning to sports at an average of 7 months, 81% of which returned to the same level of intensity. They noted, however, that 65% of throwing athletes reported decreased range of motion [15].
Percutaneous Arthroscopic Greater Tuberosity Fracture Fixation
Isolated greater tuberosity fractures are often the result of an anterior glenohumeral dislocation from a fall on an outstretched hand [2, 26, 40]. These fractures may represent avulsion of the attached rotator cuff or propagation of a Hill-Sachs lesion. Recognizing the injury and quantifying the displacement can be difficult on standard radiographs because of the osseous overlap [32]. Advanced imaging, in the form of commuted tomography, is sometimes necessary to objectively determine the degree of displacement, which in large part will determine the appropriate treatment strategy. Generally, isolated greater tuberosity fractures that are either nondisplaced or have less than 5 mm of displacement can be treated successfully nonoperatively. Those with greater than 10 mm displacement are largely treated operatively. The controversy lies in how to treat fractures displaced between 5 and 10 mm. Initially, these were recommended to be treated nonoperatively [31]; however, current literature favors operative treatment in fractures of 5 mm or more of displacement in the general population and more than 3 mm in active patients, such as heavy laborers and athletes [17]. In nonoperative treatment, young age and female sex were found to be important predictors of outcome as well [34, 36].
Treating these fractures arthroscopically has recently become a more appealing option in carefully selected patients. General treatment principles, regardless of open or arthroscopic technique, include fracture mobilization, preparation of fracture bed, and, with small avulsion fractures, excision of the fragment with rotator cuff repair. Relative contraindications to arthroscopic treatment are those fractures with significant displacement, fixed fragment retraction, large fracture fragments, and patients with poor bone stock.
Arthroscopic Surgical Technique
There are two general techniques that can be employed arthroscopically to treat a greater tuberosity fracture. One uses a cannulated screw over Kirschner wires [16, 43] with or without a washer [10]. A second technique uses a suture bridge, in a fashion similar to a double-row rotator cuff repair [21]. Regardless of planned technique, the patient is placed in the beach chair position. The arthroscope is initially introduced through a standard posterior portal, although the procedure and visualization can be easier with this portal favoring a slightly more superolateral position. After a diagnostic examination of both the joint and subacromial space to identify any concomitant injuries, a lateral portal is created with the assistance of a spinal needle to ensure proper placement. The fracture fragment is identified, debrided, and mobilized.
When a percutaneous screw is the chosen fixation method, the fracture is first reduced under fluoroscopy, with the assistance of a grasper and blunt trocar. Usually, two Kirschner wires are placed percutaneously and are sufficient to stabilize the tuberosity fragment at a 45° angle to the humeral diaphysis (Fig. 4a–d). Fluoroscopy is used again to ensure reduction and appropriate wire position. Next, a partially threaded, cannulated screw is introduced through a small incision over the Kirschner wire. The size of the screw varies according to the size of the fragment, the size of the humeral head, and the bone integrity. A washer can be used if the tissue is friable or of poor quality. Adequate fixation is confirmed with rotation of the shoulder and a repeat glenohumeral exam should be conducted in order to ensure there is no intra-articular penetration.
a Injury x-ray showing a shoulder fracture-dislocation with a displaced greater tuberosity fragment. b A left shoulder in beach chair position, viewing the glenohumeral joint from a posterior portal, demonstrating the displaced greater tuberosity (GT) fragment. Asterisk designates the fracture bed. c The same fragment after reduction and fixation. d Postoperative x-rays demonstrating fixation of the fracture fragment with cannulated screws.
If a suture bridge technique is chosen, after fracture preparation, two suture anchors are placed medial to the fragment. An 18-gauge needle is placed through the intact cuff medially and no. 2 polydioxanone (PDS) sutures are passed through the needle. The PDS is used to shuttle the sutures through the cuff. Alternatively, a standard suture passing device can be used to penetrate the rotator cuff just medial to the fragment. The medial row sutures are then tied sliding knots or alternatively a knotless repair is performed. Next, pilot holes are created for knotless lateral row anchors approximately 5 mm distal to the lateral edge of the fragment. A suture strand from each knot is threaded through each lateral anchor and advanced through the pilot holes (Fig. 5a–d). Fluoroscopy should be used to confirm fracture reduction at the end of the procedure. Using this type of fixation may aid in treatment of osteopenic bone or fracture comminution in order to improve strength of fixation.
a Pre-operative x-ray demonstrating a displaced greater tuberosity fracture after a recent dislocation event. b A right shoulder in beach chair position, viewing the glenohumeral joint from a posterior portal, demonstrating the displaced greater tuberosity (GT) fragment. (Arrow pointing to GT avulsion with attached rotator cuff. Asterisk at fracture bed.) c The subacromial space viewed from the lateral portal demonstrating suture bridge repair of the fracture fragment. d Postoperative x-ray demonstrating reduction of the greater tuberosity fracture.
Surgical management of glenohumeral instability encompasses a large range of procedures. The diagnosis and optimal treatment for such instability associated with bony defects is complex and challenging. Recent literature regarding the pathoanatomy and biomechanics of glenohumeral instability has expanded our understanding of this condition and has improved the way in which associated lesions are managed. While the results of open repair are consistent and well documented in the literature, advances in arthroscopy have allowed us to manage patients with glenoid and humeral-sided defects with less morbidity [8]. Analyses of recent trends in surgical stabilization show some shift in the management of these lesions, with a trend toward arthroscopic management for appropriately selected cases [33].
References
Ahmad CS, Galano GJ, Vorys GC, Covey AS, Gardner TR, Levine WN. Evaluation of glenoid capsulolabral complex insertional anatomy and restoration with single- and double-row capsulolabral repairs. J Shoulder Elbow Surg. 2009;18(6):948–54. doi:10.1016/j.jse.2009.03.022.
Bahrs C, Lingenfelter E, Fischer F, Walters EM, Schnabel M. Mechanism of injury and morphology of the greater tuberosity fracture. J Shoulder Elbow Surg. 2006;15(2):140–7. doi:10.1016/j.jse.2005.07.004.
Bigliani LU, Newton PM, Steinmann SP, Connor PM, McLlveen SJ. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. The American journal of sports medicine. 1998;26(1):41–5.
Boileau P, Villalba M, Hery JY, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755–63. doi:10.2106/JBJS.E.00817.
Boileau P, Mercier N, Old J. Arthroscopic Bankart-Bristow-Latarjet (2B3) procedure: how to do it and tricks to make it easier and safe. Orthop Clin North Am. 2010;41(3):381–92. doi:10.1016/j.ocl.2010.03.005.
Boileau P, Mercier N, Roussanne Y, Thelu CE, Old J. Arthroscopic Bankart-Bristow-Latarjet procedure: the development and early results of a safe and reproducible technique. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2010;26(11):1434–50. doi:10.1016/j.arthro.2010.07.011.
Boileau P, O'Shea K, Vargas P, Pinedo M, Old J, Zumstein M. Anatomical and functional results after arthroscopic Hill-Sachs remplissage. J Bone Joint Surg Am. 2012;94(7):618–26. doi:10.2106/JBJS.K.00101.
Bottoni CR, Smith EL, Berkowitz MJ, Towle RB, Moore JH. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized clinical trial. The American journal of sports medicine. 2006;34(11):1730–7. doi:10.1177/0363546506288239.
Cameron SE. Arthroscopic reduction and internal fixation of an anterior glenoid fracture. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 1998;14(7):743–6.
Carrera EF, Matsumoto MH, Netto NA, Faloppa F. Fixation of greater tuberosity fractures. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2004;20(8):e109–11. doi:10.1016/j.arthro.2004.07.017.
Dines JS, Dodson CC, McGarry MH, Oh JH, Altchek DW, Lee TQ. Contribution of osseous and muscular stabilizing effects with the Latarjet procedure for anterior instability without glenoid bone loss. J Shoulder Elbow Surg. 2013;22(12):1689–94. doi:10.1016/j.jse.2013.02.014.
Dumont GD, Fogerty S, Rosso C, Lafosse L. The arthroscopic latarjet procedure for anterior shoulder instability: 5-year minimum follow-up. The American journal of sports medicine. 2014;42(11):2560–6. doi:10.1177/0363546514544682.
Flatow EL, Warner JI. Instability of the shoulder: complex problems and failed repairs: part I. Relevant biomechanics, multidirectional instability, and severe glenoid loss. Instr Course Lect. 1998;47:97–112.
Flatow EL, Bigliani LU, April EW. An anatomic study of the musculocutaneous nerve and its relationship to the coracoid process. Clin Orthop Relat Res. 1989(244):166–71.
Garcia GH, Wu HH, Liu JN, Huffman GR, Kelly JD. Outcomes of the Remplissage procedure and its effects on return to sports: average 5-year follow-up. The American journal of sports medicine. 2016;44(5):1124–30. doi:10.1177/0363546515626199.
Gartsman GM, Taverna E, Hammerman SM. Arthroscopic treatment of acute traumatic anterior glenohumeral dislocation and greater tuberosity fracture. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 1999;15(6):648–50. doi:10.1053/ar.1999.v15.0150642.
George MS. Fractures of the greater tuberosity of the humerus. J Am Acad Orthop Surg. 2007;15(10):607–13.
Giles JW, Boons HW, Elkinson I, Faber KJ, Ferreira LM, Johnson JA et al. Does the dynamic sling effect of the Latarjet procedure improve shoulder stability? A biomechanical evaluation. J Shoulder Elbow Surg. 2013;22(6):821–7. doi:10.1016/j.jse.2012.08.002.
Hintermann B, Gachter A. Arthroscopic findings after shoulder dislocation. The American journal of sports medicine. 1995;23(5):545–51.
Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000;82(1):35–46.
Kim KC, Rhee KJ, Shin HD, Kim YM. Arthroscopic fixation for displaced greater tuberosity fracture using the suture-bridge technique. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2008;24(1):120 e1–3. doi:10.1016/j.arthro.2007.05.003.
Kim DS, Yoon YS, Chung HJ. Single-row versus double-row capsulolabral repair: a comparative evaluation of contact pressure and surface area in the capsulolabral complex-glenoid bone interface. The American journal of sports medicine. 2011;39(7):1500–6. doi:10.1177/0363546511399863.
Lafosse L, Lejeune E, Bouchard A, Kakuda C, Gobezie R, Kochhar T. The arthroscopic Latarjet procedure for the treatment of anterior shoulder instability. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2007;23(11):1242 e1–5. doi:10.1016/j.arthro.2007.06.008.
Laurencin CT, Deutsch A, O'Brien SJ, Altchek DW. The superolateral portal for arthroscopy of the shoulder. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 1994;10(3):255–8.
Lynch JR, Clinton JM, Dewing CB, Warme WJ, Matsen FA, 3rd Treatment of osseous defects associated with anterior shoulder instability. J Shoulder Elbow Surg. 2009;18(2):317–28. doi:10.1016/j.jse.2008.10.013.
McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615–20.
Metzger PD, Barlow B, Leonardelli D, Peace W, Solomon DJ, Provencher MT. Clinical application of the “glenoid track” concept for defining humeral head engagement in anterior shoulder instability: a preliminary report. Orthop J Sports Med. 2013;1(2):2325967113496213. doi:10.1177/2325967113496213.
Millett PJ, Braun S. The “bony Bankart bridge” procedure: a new arthroscopic technique for reduction and internal fixation of a bony Bankart lesion. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2009;25(1):102–5. doi:10.1016/j.arthro.2008.07.005.
Millett PJ, Horan MP, Martetschlager F. The “bony Bankart bridge” technique for restoration of anterior shoulder stability. The American journal of sports medicine. 2013;41(3):608–14. doi:10.1177/0363546512472880.
Mologne TS, Provencher MT, Menzel KA, Vachon TA, Dewing CB. Arthroscopic stabilization in patients with an inverted pear glenoid: results in patients with bone loss of the anterior glenoid. The American journal of sports medicine. 2007;35(8):1276–83. doi:10.1177/0363546507300262.
Neer CS, 2nd Displaced proximal humeral fractures: part I. Classification and evaluation. 1970. Clin Orthop Relat Res. 2006;442:77–82.
Ogawa K, Yoshida A, Ikegami H. Isolated fractures of the greater tuberosity of the humerus: solutions to recognizing a frequently overlooked fracture. J Trauma. 2003;54(4):713–7. doi:10.1097/01.TA.0000057230.30979.49.
Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in Bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. The American journal of sports medicine. 2011;39(9):1865–9. doi:10.1177/0363546511406869.
Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bulletin (Hospital for Joint Diseases (New York, NY)). 1997;56(3):171–6.
Piasecki DP, Verma NN, Romeo AA, Levine WN, Bach BR, Jr, Provencher MT. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg. 2009;17(8):482–93.
Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185–9. doi:10.1016/j.injury.2005.02.018.
Porcellini G, Campi F, Paladini P. Arthroscopic approach to acute bony Bankart lesion. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2002;18(7):764–9.
Porcellini G, Paladini P, Campi F, Paganelli M. Long-term outcome of acute versus chronic bony Bankart lesions managed arthroscopically. The American journal of sports medicine. 2007;35(12):2067–72. doi:10.1177/0363546507305011.
Provencher MT, Bhatia S, Ghodadra NS, Grumet RC, Bach BR, Jr, Dewing CB et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am. 2010;92 Suppl 2:133–51. doi:10.2106/JBJS.J.00906.
Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am. 1956;38-A(5):957–77.
Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am. 1978;60(1):1–16.
Sugaya H, Moriishi J, Kanisawa I, Tsuchiya A. Arthroscopic osseous Bankart repair for chronic recurrent traumatic anterior glenohumeral instability. J Bone Joint Surg Am. 2005;87(8):1752–60. doi:10.2106/JBJS.D.02204.
Taverna E, Sansone V, Battistella F. Arthroscopic treatment for greater tuberosity fractures: rationale and surgical technique. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2004;20(6):e53–7.
Taylor DC, Arciero RA. Pathologic changes associated with shoulder dislocations. Arthroscopic and physical examination findings in first-time, traumatic anterior dislocations. The American journal of sports medicine. 1997;25(3):306–11.
Wolf EM, Arianjam A. Hill-Sachs remplissage, an arthroscopic solution for the engaging Hill-Sachs lesion: 2- to 10-year follow-up and incidence of recurrence. J Shoulder Elbow Surg. 2014;23(6):814–20. doi:10.1016/j.jse.2013.09.009.
Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg. 2007;16(5):649–56. doi:10.1016/j.jse.2006.12.012.
Yamamoto N, Muraki T, An KN, Sperling JW, Cofield RH, Itoi E et al. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am. 2013;95(15):1390–7. doi:10.2106/JBJS.L.00777.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David A Porter, MD; Michael Birns, MD; Sarah J Hobart, MD; Marc Kowalsky, MD; and Gregory J. Galano, MD, have declared that they have no conflict of interest.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
N/A.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Rights and permissions
About this article
Cite this article
Porter, D.A., Birns, M., Hobart, S.J. et al. Arthroscopic Treatment of Osseous Instability of the Shoulder. HSS Jrnl 13, 292–301 (2017). https://doi.org/10.1007/s11420-017-9553-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-017-9553-9