Abstract
Recurrent anterior instability of the glenohumeral joint has long been an arduous problem to solve surgically, owing to its difficulty to the need to restore both osseous and dynamic constraints in the unstable shoulder. Biomechanical studies have indicated that glenoid bone loss shortens the safe arc through which the glenoid can resist axial forces; in these cases, a soft tissue repair alone may be insufficient to maintain stability. Clinical studies have confirmed that major bone loss is associated with an unfavourable outcome. The benefits of using arthroscopic procedures for surgical stabilization of the shoulder include smaller incisions and less soft tissue dissection, better access for repair and, potentially, the maximum respect for the undamaged anatomical structures. The biggest disadvantage of arthroscopic procedures until recently was the inability to successfully treat a significant bone defect. Over the last 10 years, several new arthroscopic techniques have been developed, providing new surgical options for successfully treating soft tissues and bony lesions in anterior-inferior glenohumeral instability.
Level of evidence V.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aetiology of the anteroinferior glenohumeral instability is multifactorial [8, 45]. The satisfactory treatment of this condition requires that every surgical approach is flexible enough to allow the surgeon to identify and treat any anatomical lesion that could cause instability of the shoulder [42, 52]. Over the past years, numerous techniques have been proposed for arthroscopic stabilization. Despite the improvement of the techniques, the results of arthroscopic stabilization have not yet reached those of open techniques such as the Latarjet procedure or the anterior-inferior capsular shift. The failure rate of an isolated Bankart arthroscopic repair is reported to be 15–30 % when performed in unselected patient populations and when patients are followed for more than 2 years after surgery [30]. Glenoid erosion is a quite common occurrence associated with chronic anterior instability of the shoulder, along with Hills–Sachs lesion [56]. A relationship was found between the extension of glenoid bone defect and the result of treatment for anterior instability of the shoulder [14]. Anterior shoulder instability can be classified as recurrent dislocation, recurrent subluxation or unstable painful shoulder [72]. Dislocated shoulders show the highest incidence of bone lesions, while the painful shoulder group shows the lowest incidence. The prevalence of fractures or erosion of the anterior-inferior glenoid shoulders with recurrent dislocation was reported to be from 8 to 90 % [12, 41]. Lesions are caused by impact of the upper and rear portions of the humeral head on the anteroinferior glenoid region during the episode of dislocation. Severe bone lesions are associated with failure of soft tissue techniques and, until recently, represented the maximum real limit of the arthroscopic approach [14, 22]. Appropriate imaging of the unstable shoulder shows 90 % of bony lesions [20, 27, 64]. Many authors recommend the transfer of the coracoid if the glenoid defect affects 20 % or more of the anteroposterior diameter of the glenoid [7, 17, 40, 62, 65, 67]. The relationship between the width of the glenoid defect and the clinical result continues to be investigated. So far, the exact size of the glenoid deficit that contraindicates a soft tissue technique remains unknown. In the past, more than 100 different techniques have been proposed for stabilizing the shoulder, but only some are still used. A very popular soft tissue technique still in use has been described by Bankart [5] and includes an open [35, 38, 53] and arthroscopic approach [6, 57]. In cases with bone defects either in the glenoid or in the humeral head, bony procedures are indicated [46]. These procedures, which involve the placement of a bone graft (Eden–Hybinette) or the transfer of the tip of the coracoid (Bristow) or the whole coracoid (Latarjet) on the anterior-inferior glenoid, have traditionally been performed as open procedures [2, 31, 69, 71]. Only recently new arthroscopic techniques with placement of a bone block on the anterior-inferior region of the glenoid have been described [10, 37, 65, 66].
Glenoid bone loss
In 1998, Bigliani [9] defined prognostic features associated with glenoid deficit. The author described four types of glenoid defects: type 1: non-displaced anterior glenoid fragment; type 2: small detached anterior fragment; type 3a: anterior glenoid deficits <25 %; and type 3b: defects >25 %. For type 1, 2 and 3a, the author recommends a soft tissue reconstruction, while for type 3b a glenoid augmentation is suggested. Several biomechanics studies show how glenoid shape changes when the bone loss exceeds 20–25 %. Burkhart first called this glenoid appearance “inverted pear” [16] (Fig. 1). Gerber and Nyffeler [26] demonstrated how with subsequent loss of anterior-inferior glenoid arc the resistances to dislocation decreased exponentially. Recent biomechanical studies have also introduced the concept of “glenoid track” [18, 76]. This concept has shifted the previous paradigm from engaging defects to track-off track mismatch. On 9 cadaveric shoulders, Yamamoto showed how dislocation was most likely with disruption of the medial margin of this track [76].
Clinical evaluation
Medical history
Patients with anterior glenohumeral instability with bone lesions report different traumatic mechanisms. They often include a forced external rotation with the arm abducted at 90°. A similar pattern might occur after a fall on the arm extended and abducted. The distraction forces directly on the capsule and ligaments may be the cause of the injury. In patients with history of dislocation, the force that causes the damage is generally greater and more probably produces bony lesions associated with breakage of the soft tissues. Patients who require a reduction after the first episode of dislocation are more likely to have insertional injuries of the ligaments, while the bone defects become more common as a result of subsequent episodes. Patients who spontaneously reduce the dislocation after the first episode are more prone to develop a capsular elongation, and a hyperlaxity of the rotator interval without bone lesions. Laxity must be distinguished from the instability.
Physical examination
The diagnosis of unstable shoulder is rarely performed without a clinical history reported by the patient. The symptomatic instability is often diagnosed by history and confirmed by physical examination. It is important to distinguish between laxity and instability. Anterior hyperlaxity is defined as an external rotation with the arm adduct >85°. The lower hyperlaxity is defined when Gagey’s test is positive [23]. The test is defined as positive when the abduction of the affected limb with the scapula blocked by the examiner is >20° with respect to the other side. Instability is defined as the symptomatic expression of excessive translation of the humeral head. The presence of generalized laxity is more likely in an individual with multidirectional instability. Rarely, these patients have bone lesions associated with the instability.
Clinical tests can be divided into those that assess the laxity of the shoulder and those that assess the anterior instability of the shoulder.
Tests to evaluate shoulder laxity:
-
Drawer test [60]
-
Push–pull test
-
Sulcus sign
Tests for anterior instability:
In the literature, there is no consensus about which tests should be used in the diagnosis of unstable shoulder [58, 75], but a study by Sciascia shows that the four tests most commonly used are: apprehension, relocation, load and shift and anterior drawer.
Diagnostic value of these tests is reported in Table 1 [70].
Imaging
Plain radiographs for instability include anteroposterior view performed in three rotations (internal, neutral and external) [73]. The presence of a Hill–Sachs lesion may be noted for each rotation (absent or present). If present in external rotation, its location is higher than the humeral head. Lesions on the glenoid are distinguished in fracture avulsion and loss of anterior-inferior sclerotic outline using a Bernageau projection for the glenoid profile [47]. The interruption of the anterior bone triangle compared to the contralateral is classified into three groups. Fractures are defined as an anomaly of the anterior glenoid rim characterized by a visible bone fragment fracture. The “cliff” sign is defined as the loss of normal anterior triangle without a visible bone fragment. The sign called “blunted angle” is defined as a shape of blunt apex of the triangle [21]. If an anterior glenoid deficiency is visible with radiographs, it is important to determine the area and the percentage of bone deficit [24]. Computed tomography (CT) scanning allows for precise evaluation of anterior glenoid bone loss in multiple planes. In a sagittal view, inferior glenoid bone loss can be appreciated as a percentage of its normal area. A best-fit circle is used to approximate normal inferior glenoid surface area and observed bone loss can be calculated from this measurement [55, 63]. Glenoid bone loss will lead to an “inverted pear” appearance. The glenoid assumes an inverted pear shape when the bone deficit minimum is at least 25 %. Hill–Sachs lesions, especially those that are subtle, can also be evaluated on CT studies [39]. Armitage et al. [3] reported that Hill–Sachs lesion is oriented towards 6:46 at the top and 8:56 at the bottom of the lesion, with the average orientation towards 7:58 ± 0:48 on a clock face with 12:00 defined as the intertubercular sulcus. Magnetic resonance imaging (MRI) with or without arthrography is frequently used to evaluate the chronically unstable shoulder. In addition to providing useful information about soft tissue anatomy, including the glenoid labrum, chondral surfaces, glenohumeral capsuloligamentous structures and the rotator cuff, MRI can also demonstrate bone loss [43, 51].
Arthroscopy evaluation
Glenoid bone loss can be also qualified and quantified with arthroscopy. The bare area has been shown to reliably mark the centre of the inferior glenoid [32, 44]. Using the bare area as a landmark, a calibrated probe can used to measure the distance from the bare spot to the posterior rim and compare it to the distance from the anterior rim. Assuming that the normal inferior glenoid is shaped as a nearly perfect circle [32], anterior-inferior glenoid deficiencies can then be quantified by the following [15]: glenoid deficiency = (distance from bare spot to posterior rim − distance from bare spot to anterior rim)/(2 × distance from bare area to posterior rim). Quantification of glenoid bone loss should be routinely performed preoperatively to determine the ideal anterior stabilization procedure.
Classification
Glenohumeral instability is classified according to four factors:
-
Time/frequency
-
Degree of instability
-
Direction
-
Aetiology
Time/frequency can be defined as acute or recurrent; degree of instability ranges from subclinical forms (grade 1) to real dislocations (grade 2/3); direction of instability can be anteroinferior, anterior direct, anterosuperior, posteroinferior and multidirectional; aetiology may be atraumatic, micro-traumatic (overuse) or traumatic. In 1989, Thomas and Matsen [68] classified instabilities in two groups: TUBS and AMBRI, which must be regarded as two extremes of a wide “range”. The first group, TUBS (traumatic unidirectional Bankart surgery), is an expression of unidirectional traumatic glenohumeral instability. The second group, AMBRI (atraumatic multidirectional bilateral rehabilitation inferior shift), includes atraumatic multidirectional bilateral instability.
Decision algorithm
The algorithms of treatment depend on many factors, but the size and type (fragment or erosion) of the glenoid bone deficit is the priority. If a mobile bone fragment is associated with a labral lesion, the possibility to perform an arthroscopic repair exists, despite the size of the fragment, especially in an acute or subacute condition. If there is a bone loss, to date there are no precise guidelines. If the bone deficit is >20 % with respect to the healthy contralateral glenoid, an open or arthroscopic bone grafting procedure is recommended by most authors to fill the defect and to reconstruct the anatomic glenoid arch [22, 28, 40–42]. If the missing area of the glenoid is <10 % and there are no soft tissue alterations, an arthroscopic soft tissue reconstruction is certainly a viable treatment option to restore the stability of the joint. If the bone loss is between 10 and 20 %, other factors should be considered, such as the presence of a Hill–Sachs lesion that could represent an indication for a bony procedure.
In addition to a classification of the possible presence of bone defects preoperatively, other risk factors that may preclude arthroscopic soft tissue stabilization should be considered. If the instability severity index score (ISIS) [4] (Table 2) is greater than 6 points, a reconstruction of the isolated soft tissues may be insufficient for the stability of the shoulder, especially in the long-term follow-up [13, 50] (Table 3).
In conclusion, the preoperative evaluation of the bone loss, the clinical risk factors for recurrence of instability (ISIS scoring system), clinical examination and medical history of the patient can help the surgeon to better select patients for the appropriate surgical procedure.
Indications for surgery
Patients, who suffer a shoulder dislocation as a result of major trauma and who have no ligamentous laxity, benefit most from surgical treatment [48, 49]. Recurrence of instability is the main complication after anterior stabilization. Currently, most surgeons perform arthroscopic stabilization of the soft tissue with anchors and sutures due to more reproducible results. However, even after the recent technical developments, there is still a rate of recurrence of about 5–20 % [1, 8, 42, 59]. The best way is to identify preoperatively patients whose risk factors preclude arthroscopic soft tissue stabilization. The literature reports numerous prognostic factors. Athletes who practice contact sports have a higher incidence of recurrence after a classic Bankart stabilization. Patients with significant glenoid bone loss or with an unacceptable high clinical risk of recurrent dislocations are candidates for procedures with bone grafting, either with an open or arthroscopic approach [77].
Techniques
Arthroscopic Latarjet procedure
In this procedure, described the first time by Lafosse et al. [37], the stabilization mechanisms are as follows:
-
Increase the glenoid surface
-
Muscle-tendinous hammock effect created by the conjoint tendon passing through the subscapularis, thus creating a dynamic tension when the shoulder is in abduction and external rotation
-
Suturing the inferior glenohumeral ligament to the coracoacromial ligaments attached to the coracoid
In order to achieve a good stabilization of the joint, it is mandatory that the coracoid has a perfect alignment with the glenoid rim. A medial positioning of the graft would create unfavourable biomechanical conditions with higher recurrence rate of instability; a lateral position of the coracoid with an overlap of the graft in the joint leads to a premature osteoarthritis [37].
Surgical technique [37]
Patient is positioned in beach-chair position. A diagnostic arthroscopic evaluation is initially performed. Soft tissues between the two and five o’clock positions are resected with a radiofrequency. The coracoacromial ligament and pectoralis minor are detached from the coracoid. The coracoid is cleared of soft tissues circumferentially to its base while protecting the attachment of the conjoint tendon at the coracoid tip. Two holes are drilled and tapped over guide wires, through a portal superior to the coracoid. Then, the osteotomy of the coracoid is completed by the use of a curved osteotome. The surgeon splits the subscapularis at the junction of its inferior third and superior two-thirds. The split is completed medially via blunt dissection with a trocar and external rotation of the arm. The anterior glenoid face is then prepared with a bur used to create a flat bed of bleeding bone. The coracoid is then retrieved. The inferior surface of the coracoid is decorticated with a bur, creating a flat surface to match the anterior glenoid. The coracoid is then manipulated via a double-barrel cannula, through the subscapularis split, and onto the glenoid face between the two and five o’clock positions. Optimal positioning of the coracoid is parallel to the glenoid rim. Once graft positioning is verified, a 3.2-mm cannulated drill is used to predrill each hole before insertion of both 3.5-mm cannulated screws, beginning with the inferior screw. With the arm in abduction and external rotation, the sling effect of the conjoint tendon can be observed.
Indications
-
Anterior glenoid bone loss
-
Ligamentous tissues of poor quality
-
Revision surgery
-
Involvement in collision sports
Arthroscopic Bristow–Latarjet procedure
The arthroscopic procedure according to Bristow–Latarjet was described for the first time by Boileau et al. [10].
The effectiveness of the procedure is linked to a “triple lock”:
-
The effect of the bone block by the coracoid tip increases the extension of the glenoid surface
-
The sling effect caused by the passage of the conjoined tendon through the subscapularis
-
The reproduction of the glenoid concavity due to the labral repair
Surgical technique [10]
The patient is positioned in the beach-chair position with no traction. Shoulder arthroscopy is performed with a standard posterior and anterosuperior portal. The shoulder joint is visually assessed for lesions consistent with anterior instability. In order to harvest and prepare the tip of the coracoid, the scope is placed in the anterior subdeltoid space. An anterolateral portal located 2 cm lateral to the anterosuperior portal is established and is used as a viewing portal for this step of the procedure. The coracoid process and the conjoined tendon insertion are identified, and fibrous tissue from the neck of the scapula is removed. The coracoacromial ligament insertion and the pectoralis minor insertion are partially divided. A coracoid fragment measuring 15 mm in length is then removed. The bone fragment is then brought outside the incision for preparation. An adsorbable suture is passed through a drill hole in the coracoid fragment and through the coracobiceps tendon. Once the coracoid fragment is prepared, an arthroscopic cannula 8 mm in diameter is inserted in the anterosuperior portal, passing above the subscapularis in the rotator interval. An arthroscopic Bankart repair is then performed. Under arthroscopic vision, the glenoid neck is penetrated with a sharp-tipped awl, which prevents sliding of the guidewire along the cortical bone of the glenoid neck when drilling. A guidewire is then placed and oriented parallel to the glenoid articular surface. The guidewire is drilled until it just penetrates the posterior cortex of the glenoid. The guidewire is then overdrilled with a 10-mm cannulated reamer to a depth of 15 mm. The reamer and guidewire are then removed. A Beath pin pull-through technique is used for coracoid placement. This pin has an eyelet on its trailing end and serves as a suture passer. The Beath pin is placed into the glenoid socket and the previous hole created by the guidewire and is recovered behind the shoulder. Both ends of the suture placed previously through the coracoid fragment and the coracobiceps tendon are passed through the eyelet of the Beath pin and then recovered behind the shoulder. Progressive traction on the suture makes it possible to pull the coracoid fragment into the glenoid socket. Before the entire coracoid fragment enters the glenoid socket, a flexible guidewire for the interference screw is inserted to prevent screw divergence. The coracoid graft is then pulled inside the glenoid socket by traction on the posterior suture. The graft is then fixed in the hole by use of a 7 × 15 mm bioabsorbable interference screw, inserted over the flexible guidewire.
Indications
-
Anteroinferior glenoid bone loss
-
Ligamentous tissues of poor quality
-
Revision surgery
Arthroscopic bone graft procedure
The arthroscopic bone block technique was originally described by Taverna [66] (Fig. 2) and has been recently modified [65]. In the new technique, the bone graft is fixed with four endobuttons. The technique combines the Bankart repair with the transfer of the graft, harvested from the iliac crest, which is inserted through the cannula in the rotator interval and fixed on the glenoid rim. The effectiveness of this procedure is related to the effect of the bone block produced by the tricortical graft, which increases the area of the glenoid and the reproduction of the glenoid concavity produced by the insertion of the labrum on the glenoid rim and the anterior-inferior shift of the capsule and the anterior-inferior glenohumeral ligament. The purpose of this procedure is to restore the normal anatomy of the unstable shoulder.
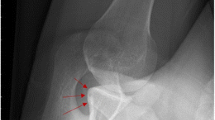
Radiographic results at 2-year follow-up of previous surgical technique for arthroscopic bone block. The two images on the left show a CT-scan 3D reconstruction of the shoulder, and the image on the right shows an anteroposterior standard radiograph. The graft is correctly positioned, and there are no signs of resorption, non-union or breaking of the fixation devices
Surgical technique [65]
Patient is placed in the beach-chair position. A standard posterior portal is created for the insertion of the arthroscope. Viewing from the posterior portal, the surgeon creates an anterior superior portal and a midglenoid portal. The labrum is detached, and all soft tissues are removed from the anterior glenoid neck. Then, the arthroscope is introduced through the anterosuperior portal, the anterior glenoid rim is further decorticated and the glenoid neck is prepared with a motorized bur to create a flat and bleeding bony surface (Fig. 3). A spinal needle is inserted from posterior to anterior along the face of the glenoid and centred on the anterior glenoid bone defect. Then, a more posteromedial portal is made to provide access to the glenoid guide. The hook end of the glenoid guide is inserted and passed along the glenoid parallel to the glenoid face to avoid damaging the articular surface, and then, it is passed over the anterior edge. Then, the guide is rotated to capture the anterior edge of the glenoid under the hook. The hook should be placed at the centre of the anterior glenoid defect, usually between the three and four o’clock positions. It is mandatory that the glenoid guide be aligned with the posterior and anterior glenoid rim.
Then, a bullet is placed in the inferior hole of the guide and is advanced until it firmly contacts the posterior aspect of the glenoid neck. The ratchet teeth of the bullet should be aligned with the screws adjacent to the guide handle. The process is repeated for the superior bullet. A 2.8-mm sleeved drill is placed in each bullet and advanced under power until exiting from the anterior aspect of the glenoid. The drills are placed 5 mm on the centre below the cortical edge of the glenoid face, parallel to one another and 10 mm apart. The inner drill is removed, leaving the cannulated outer sleeve. Once drilling is completed, the bullets can be removed. The guide can be removed at this stage. Flexible looped guidewires are then introduced into the joint by passing one wire through each sleeve posterior to anterior. Each guidewire is retrieved using a loop grasper. The wires are separated and stored. The drill sleeves should be removed after this step is completed.
The tricortical bone graft could be an autograft (harvested from the ipsilateral anterior iliac crest) or an allograft tricortical iliac crest graft. Two 2.8-mm drill holes are made 10 mm apart and 5 mm from each edge. The drill enters through the cortex and exits the cancellous side of the bone graft. The holes created correspond to the distance of the cannulated drill sleeves previously placed in the glenoid neck. With a marking pen, we colour the superior aspect of the bone graft.
Each looped guidewire is fed through the prepared bone graft and exits on the cortical side. The graft is oriented so that the cancellous surface is facing the anterior neck of the glenoid. The anterior implant is fed with the preassembled suture through the end of the looped guidewire with a classic slip knot. This can be achieved by passing the lead suture through the looped guidewire and feeding the implant through the lead suture. The graft is slid towards the end of the guidewires to lodge the implants. Anterior round endobuttons are advanced until they lie flat on the bone graft. Sutures should be taut to allow smooth movement down the cannula. The bone graft is tipped to be inserted into the 10-mm cannula, and care is taken to ensure that the superior end of the bone graft enters the cannula first. The graft is advanced by pulling the guidewires out posteriorly. Slight tension should be maintained on the sutures throughout this step. The sutures should advance the implant until the bone graft sits flush on the anterior neck of the glenoid, with each implant’s lead suture exiting the skin posteriorly. The posterior implants are placed on the transporter by advancing the instrument through the eyelet of a posterior round endobutton. The suture is passed through the transporter. The transporter is retracted to allow the suture to pass through the eyelet of the posterior round endobutton. The same steps must be performed for the second eyelet with the other side of the suture. The posterior round endobuttons are advanced until they sit flush against the posterior face of the glenoid. The knot pusher is used to secure the posterior round endobuttons. The knot pusher will provide tactile feedback when the posterior round endobuttons are properly seated.
Once the implant has been tensioned, the posterior knots are secured with half hitches, and the remaining sutures are cut using a blind knot cutter (Fig. 4).
The anterior labrum, capsule and ligaments are repaired to the glenoid rim with suture anchors and a standard arthroscopic soft tissue repair technique (Fig. 5a–b).
Indications (Table 4)
-
Anteroinferior glenoid bone defect
-
Revision surgery
-
Involvement in extreme sports
Complications
The three surgical techniques have some common complications, which can be divided into intra- or post-operative complications (Table 5).
Rehabilitation protocol
Rehabilitation protocol is the same in the three above-mentioned surgical procedures. After surgery, the shoulder is immobilized in a brace in abduction of 10° for 3–4 weeks. There are no limitations regarding passive movements after immobilization, and patients are subsequently allowed to regain full elevation and external rotation. After complete healing of the wound, pool exercises and return to work activities are authorized. Progressive strengthening exercises are started after 6–8 weeks. Return to contact sports and overhead mobility are generally allowed 4–6 months after surgery [25, 74].
Clinical results
Arthroscopic Latarjet procedure
In the first study in 2007, Lafosse et al. [37] reported the preliminary results in 44 patients. No complications were found, and he reported excellent clinical results. In a more recent study, the same author reported results of 100 cases [36]: 80 % of patients had excellent clinical results, 18 % good and 2 % a bad clinical outcome. Most patients returned to work at 2 months and returned to sports at 10 weeks after surgery. The graft positioning, evaluated with CT scanning, was flush with the glenoid in 80 %, medially placed in 8 %, and there was lateral overhang in 12 % of cases. On a vertical plane, the positioning was defined perfect (graft placed between three and five o’clock) in 78 % of cases, too high in 7 % and too low in 5 % of cases. Screw angle in relation to the glenoid face was on average 29°. Perioperative complications included two haematomas: one intraoperative fracture of the graft, and one transient musculocutaneous nerve palsy that fully recovered. Late complications included four cases of coracoid non-union. A further three shoulders were found to have lysis around the screws leading to prominence of the head of the screw. In total, four patients required a subsequent arthroscopic screw removal. At 26 months, an average loss of 18° of external rotation was reported. There were no cases of recurrent dislocation. In a recent study, Dumont et al. [19] evaluated patients who had undergone the arthroscopic Latarjet procedure at a minimum 5-year follow-up. Mean follow-up time was 76.4 months. One patient (1.59 %) had recurrent instability (subluxations) after the procedure. A recent prospective study evaluated with a multiplanar bidimensional computed tomography scan analysis 95 shoulders that had undergone the arthroscopic Latarjet procedure [34]. The coracoid graft resulted accurately positioned relative to the equator of the glenoid surface in 87 of 95 shoulders (91.5 %). Accurate bone block positioning on the axial view with “circle” evaluation was obtained for 77 of 95 shoulders (81 %). The mean screw angulation with the glenoid surface was 21°. One patient had transient axillary nerve palsy. Of the initial 104 patients, 3 (2.8 %) underwent revision. The author concluded that accurate positioning of the bone block onto the anterior aspect of the glenoid is possible, safe and reproducible with the arthroscopic Latarjet procedure without additional complications compared with open surgery.
Arthroscopic Bristow–Latarjet procedure
In 2010, Boileau et al. [10] treated 47 patients with glenoid bone loss and capsular deficiency with the arthroscopic Bristow–Latarjet technique. The procedure was performed entirely arthroscopically in 41 of 47 patients (88 %); a conversion to open surgery was needed in 6 patients (12 %). The axillary nerve was identified in all cases, and no neurologic injuries were observed. No patient had any recurrence of instability at the most recent follow-up (mean, 16 months). The mean Rowe score was 88, and the mean Walch–Duplay score was 87.6. The Subjective Shoulder Value was 87.5 %. The bone block was subequatorial in 98 % of the cases (46 of 47) and flush to the glenoid surface in 92 % (43 of 47); it was too lateral in 1 (2 %) and too medial (>5 mm) in 3 (6 %). There was 1 bone block fracture and 7 migrations. In a recent study, the same authors [11] evaluated 70 patients who had undergone arthroscopic Bristow–Latarjet at a mean follow-up of 35 months; 69 of 70 (98 %) patients had a stable shoulder, external rotation with arm at the side was 9° less than the non-operated side, and 58 (83 %) returned to sports at preinjury level. On the latest radiographs, 64 (91 %) had no osteoarthritis, and bone block positioning was accurate, with 63 (90 %) being below the equator, and 65 (93 %) flush to the glenoid surface. The coracoid graft healed in 51 (73 %), it failed to unite in 14 (20 %) and graft osteolysis was seen in 5 (7 %). Bone block non-union/migration did not compromise shoulder stability but was associated with persistent apprehension and less return to sports. The authors concluded that the arthroscopic Bristow–Latarjet procedure combined with Bankart repair for anterior instability with severe glenoid bone loss restored shoulder stability, maintained ROM and allowed return to sports at preinjury level. Adequate healing of the transferred coracoid process to the glenoid neck is an important factor of avoiding persistent anterior apprehension.
Conclusions
Anterior glenoid bone loss can be considered a difficult orthopaedic problem that leads to recurrent shoulder instability. Biomechanical studies have found an inverse relationship between the size of the glenoid defect and the stability of the shoulder: the larger the defect, the less stable the shoulder [42, 66].
Glenoid bony reconstruction is certainly needed for defects >20 %, but several other factors could indicate the need for an anterior glenoid bone grafting as a coexisting Hill–Sachs lesion or clinical conditions that increase the risk of recurrent instability (patients with an ISIS score higher than 6). To date, there is no consensus when and how bony procedures are needed to stabilize a glenohumeral joint. Further investigations are needed to determine when an isolated soft tissue repair in an unstable shoulder is not indicated. The rationale for a bony procedure is to provide an increase in the glenoid arc. Many surgical procedures exist such as coracoid transfer, tibial autograft, iliac crest autograft or allograft transfer, or osteochondral allograft.
References
Abouali JA, Hatzantoni K, Holtby R, Veillette C, Theodoropoulos J (2013) Revision arthroscopic Bankart repair. Arthroscopy 29(9):1572–1578
Allain J, Goutallier D, Glorion C (1998) Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder. J Bone Joint Surg Am 80(6):841–852
Armitage MS, Faber KJ, Drosdowech DS, Litchfield RB, Athwal GS (2010) Humeral head bone defects: remplissage, allograft, and arthroplasty. Orthop Clin North Am 41(3):417–425
Balg F, Boileau P (2007) The instability severity index score: a simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br 89(11):1470–1477
Bankart A (1938) The pathology and treatment of recurrent dislocation of the shoulder joint. Br J Surg 26:23–29
Benedetto KP, Glötzer W (1992) Arthroscopic Bankart procedure by suture technique: indications, technique, and results. Arthroscopy 8(1):111–115
Bessiere C, Trojani C, Pélégri C, Carles M, Boileau P (2013) Coracoid bone block versus arthroscopic Bankart repair: a comparative paired study with 5-year follow-up. Orthop Traumatol Surg Res 99(2):123–130
Bhatia DN, DasGupta B (2013) Surgical treatment of significant glenoid bone defects and associated humeral avulsions of glenohumeral ligament (HAGL) lesions in anterior shoulder instability. Knee Surg Sports Traumatol Arthrosc 21(7):1603–1609
Bigliani LU, Newton PM, Steinmann SP, Connor PM, Mcllveen SJ (1998) Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med 26(1):41–45
Boileau P, Mercier N, Old J (2010) Arthroscopic Bankart–Bristow–Latarjet (2B3) procedure: how to do it and tricks to make it easier and safe. Orthop Clin North Am 41(3):381–392
Boileau P, Thélu CÉ, Mercier N, Ohl X, Houghton-Clemmey R, Carles M, Trojani C (2014) Arthroscopic Bristow–Latarjet combined with bankart repair restores shoulder stability in patients with glenoid bone loss. Clin Orthop Relat Res 472(8):2413–2424
Bollier MJ, Arciero R (2010) Management of glenoid and humeral bone loss. Sports Med Arthrosc 18(3):140–148
Bouliane M, Saliken D, Beaupre LA, Silveira A, Saraswat MK, Sheps DM (2014) Evaluation of the Instability Severity Index Score and the Western Ontario Shoulder Instability Index as predictors of failure following arthroscopic Bankart repair. Bone Joint J 96-B(12):1688–1692
Burkhart SS, De Beer JF (2000) Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill–Sachs lesion. Arthroscopy 16(7):677–694
Burkhart SS, Debeer JF, Tehrany AM, Parten PM (2002) Quantifying glenoid bone loss arthroscopically in shoulder instability. Arthroscopy 18:488–491
Burkhart SS, Morgan CD, Kibler WB (2000) Shoulder injuries in overhead athletes. The “dead arm” revisited. Clin Sports Med 19:125–158
D’Ambrosi R, Perfetti C, Garavaglia G, Taverna E (2015) One step arthroscopically assisted Latarjet and posterior bone-block, for recurrent posterior instability and anterior traumatic dislocation. Int J Shoulder Surg 9:94–98
Di Giacomo G, Itoi E, Burkhart SS (2014) Evolving concept of bipolar bone loss and the Hill–Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy 30(1):90–98
Dumont GD, Fogerty S, Rosso C, Lafosse L (2014) The arthroscopic Latarjet procedure for anterior shoulder instability: 5-year minimum follow-up. Am J Sports Med 42(11):2560–2566
e Souza PM, Brandão BL, Brown E, Motta G, Monteiro M, Marchiori E (2014) Recurrent anterior glenohumeral instability: the quantification of glenoid bone loss using magnetic resonance imaging. Skeletal Radiol 43(8):1085–1092
Edwards TB, Boulahia A, Walch G (2003) Radiographic analysis of bone defects in chronic anterior shoulder instability. Arthroscopy 19:732–739
Forsythe B, Frank RM, Ahmed M, Verma NN, Cole BJ, Romeo AA, Provencher MT, Nho SJ (2015) Identification and treatment of existing copathology in anterior shoulder instability repair. Arthroscopy 31(1):154–166
Gagey OJ, Gagey N (2001) The hyperabduction test. J Bone Joint Surg Br 83(1):69–74
Garth WP, Slappey CE, Ochs CW (1984) Roentgenographic demonstration of instability of the shoulder: the apical oblique projection. A technical note. J Bone Joint Surg Am 66:1450–1453
Gaskill TR, Taylor DC, Millett PJ (2011) Management of multidirectional instability of the shoulder. J Am Acad Orthop Surg 19(12):758–767
Gerber C, Nyffeler RW (2002) Classification of glenohumeral joint instability. Clin Orthop Relat Res 400:65–76
Giles JW, Owens BD, Athwal GS (2015) Estimating glenoid width for instability-related bone loss: a CT evaluation of an MRI formula. Am J Sports Med 43(7):1726–1730
Griffin JW, Brockmeier SF (2015) Shoulder instability with concomitant bone loss in the athlete. Orthop Clin North Am 46(1):89–103
Hamner DL, Pink MM, Jobe FW (2000) A modification of the relocation test: arthroscopic findings associated with a positive test. J Shoulder Elbow Surg 9(4):263–267
Hobby J, Griffin D, Dunbar M, Boileau P (2007) Is arthroscopic surgery for stabilisation of chronic shoulder instability as effective as open surgery? A systematic review and meta-analysis of 62 studies including 3044 arthroscopic operations. J Bone Joint Surg Br 89(9):1188–1196
Hovelius L, Körner L, Lundberg B, Akermark C, Herberts P, Wredmark T, Berg E (1983) The coracoid transfer for recurrent dislocation of the shoulder. Technical aspects of the Bristow–Latarjet procedure. J Bone Joint Surg Am 65(7):926–934
Huysmans PE, Haen PS, Kidd M, Dhert WJ, Willems JW (2006) The shape of the inferior part of the glenoid: a cadaveric study. J Shoulder Elbow Surg 15:759–763
Jobe FW, Bradley JP (1989) The diagnosis and nonoperative treatment of shoulder injuries in athletes. Clin Sports Med 8(3):419–438
Kany J, Flamand O, Grimberg J, Guinand R, Croutzet P, Amaravathi R, Sekaran P (2015) Arthroscopic Latarjet procedure: is optimal positioning of the bone block and screws possible? A prospective computed tomography scan analysis. J Shoulder Elbow Surg. doi:10.1016/j.jse.2015.06.010 (epub ahead of print)
Karlsson J, Järvholm U, Swärd L, Lansing O (1995) Repair of Bankart lesions with a suture anchor in recurrent dislocation of the shoulder. Scand J Med Sci Sports 5(3):170–174
Lafosse L, Boyle S, Gutierrez-Aramberri M, Shah A, Meller R (2010) Arthroscopic Latarjet procedure. Orthop Clin North Am 41(3):393–405
Lafosse L, Lejeune E, Bouchard A, Kakuda C, Gobezie R, Kochhar T (2007) The arthroscopic Latarjet procedure for the treatment of anterior shoulder instability. Arthroscopy 23(11):1242.e1–1242.e5
Levine WN, Richmond JC, Donaldson WR (1994) Use of the suture anchor in open Bankart reconstruction. A follow-up report. Am J Sports Med 22(5):723–726
Lo IK, Parten PM, Burkhart SS (2004) The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy 20(2):169–174
Longo UG, Loppini M, Rizzello G, Ciuffreda M, Maffulli N, Denaro V (2014) Latarjet, Bristow, and Eden–Hybinette procedures for anterior shoulder dislocation: systematic review and quantitative synthesis of the literature. Arthroscopy 30(9):1184–1211
Longo UG, Loppini M, Rizzello G, Romeo G, Huijsmans PE, Denaro V (2014) Glenoid and humeral head bone loss in traumatic anterior glenohumeral instability: a systematic review. Knee Surg Sports Traumatol Arthrosc 22(2):392–414
Martetschläger F, Kraus TM, Hardy P, Millett PJ (2013) Arthroscopic management of anterior shoulder instability with glenoid bone defects. Knee Surg Sports Traumatol Arthrosc 21(12):2867–2876
Melvin JS, Mackenzie JD, Nacke E, Sennett BJ, Wells L (2008) MRI of HAGL lesions: four arthroscopically confirmed cases of false-positive diagnosis. AJR Am J Roentgenol 191(3):730–734
Montgomery WH, Wahl M, Hettrich C, Itoi E, Lippitt SB, Matsen FA (2005) Anteroinferior bone-grafting can restore stability in osseous glenoid defects. J Bone Joint Surg Am 87:1972–1977
Owens BD, Campbell SE, Cameron KL (2014) Risk factors for anterior glenohumeral instability. Am J Sports Med 42(11):2591–2596
Pagnani MJ, Warren RF, Altchek DW, Wickiewicz TL, Anderson AF (1996) Arthroscopic shoulder stabilization using transglenoid sutures. A four-year minimum followup. Am J Sports Med 24(4):459–467
Pansard E, Klouche S, Billot N, Rousselin B, Kraus TM, Bauer T, Hardy P (2013) Reliability and validity assessment of a glenoid bone loss measurement using the Bernageau profile view in chronic anterior shoulder instability. J Shoulder Elbow Surg 22(9):1193–1198
Parsons BO, Boileau P, Rhee YG, Sonnabend DA, Checchia SL, Castagna A, Flatow EL (2010) Surgical management of traumatic anterior glenohumeral instability: an international perspective. Instr Course Lect 59:245–253
Patel RM, Amin NH, Lynch TS, Miniaci A (2014) Management of bone loss in glenohumeral instability. Orthop Clin North Am 45(4):523–539
Phadnis J, Arnold C, Elmorsy A, Flannery M (2015) Utility of the Instability Severity Index Score in predicting failure after arthroscopic anterior stabilization of the shoulder. Am J Sports Med 43(8):1983–1988
Richards RD, Sartoris DJ, Pathria MN, Resnick D (1994) Hill–Sachs lesion and normal humeral groove: MR imaging features allowing their differentiation. Radiology 190:665–668
Rossy WH, Cieslak K, Uquillas CA, Rokito A (2014) Current trends in the management of recurrent anterior shoulder instability. Bull Hosp Jt Dis (2013) 72(3):210–216
Rowe CR, Patel D, Southmayd WW (1978) The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am 60(1):1–16
Rowe CR, Zarins B (1981) Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am 63(6):863–872
Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y (2005) Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med 33(6):889–893
Saliken DJ, Bornes TD, Bouliane MJ, Sheps DM, Beaupre LA (2015) Imaging methods for quantifying glenoid and Hill–Sachs bone loss in traumatic instability of the shoulder: a scoping review. BMC Musculoskelet Disord 18(16):164
Savoie FH 3rd, Miller CD, Field LD (1997) Arthroscopic reconstruction of traumatic anterior instability of the shoulder: the Caspari technique. Arthroscopy 13(2):201–209
Sciascia AD, Spigelman T, Kibler WB, Uhl TL (2012) Frequency of use of clinical shoulder examination tests by experienced shoulder surgeons. J Athl Train 47(4):457–466
Shibata H, Gotoh M, Mitsui Y, Kai Y, Nakamura H, Kanazawa T, Okawa T, Higuchi F, Shirahama M, Shiba N (2014) Risk factors for shoulder re-dislocation after arthroscopic Bankart repair. J Orthop Surg Res 4(9):53
Silliman JF, Hawkins RJ (1993) Classification and physical diagnosis of instability of the shoulder. Clin Orthop Relat Res 291:7–19
Speer KP, Hannafin JA, Altchek DW, Warren RF (1994) An evaluation of the shoulder relocation test. Am J Sports Med 22(2):177–183
Streubel PN, Krych AJ, Simone JP, Dahm DL, Sperling JW, Steinmann SP, O’Driscoll SW, Sanchez-Sotelo J (2014) Anterior glenohumeral instability: a pathology-based surgical treatment strategy. J Am Acad Orthop Surg 22(5):283–294
Sugaya H, Moriishi J, Dohi M, Kon Y, Tsuchiya A (2003) Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am 85(5):878–884
Sugaya H (2014) Techniques to evaluate glenoid bone loss. Curr Rev Musculoskelet Med 7(1):1–5
Taverna E, D’Ambrosi R, Perfetti C, Garavaglia G (2014) Arthroscopic bone graft procedure for anterior inferior glenohumeral instability. Arthrosc Tech 3(6):e653–e660
Taverna E, Golanò P, Pascale V, Battistella F (2008) An arthroscopic bone graft procedure for treating anterior-inferior glenohumeral instability. Knee Surg Sports Traumatol Arthrosc 16(9):872–875
Taverna E (2010) The treatment of anterior glenoid bone loss. In: Taverna E, Gleyze P, Randelli P (eds) Shoulder instability: 2010. TIMEO Editore, Bologna, pp 61–72
Thomas SC, Matsen FA III (1989) An approach to the repair of avulsion of the glenohumeral ligaments in the management of traumatic anterior glenohumeral instability. J Bone Joint Surg Am 71(4):506–513
Torg JS, Balduini FC, Bonci C, Lehman RC, Gregg JR, Esterhai JL, Hensal FJ (1987) A modified Bristow–Helfet–May procedure for recurrent dislocation and subluxation of the shoulder. Report of two hundred and twelve cases. J Bone Joint Surg Am 69(6):904–913
van Kampen DA, van den Berg T, van der Woude HJ, Castelein RM, Terwee CB, Willems WJ (2013) Diagnostic value of patient characteristics, history, and six clinical tests for traumatic anterior shoulder instability. J Shoulder Elbow Surg 22(10):1310–1319
Vander Maren C, Geulette B, Lewalle J, Mullier J, Autrique JC, Thiery J, Deneufbourg J (1993) Coracoid process abutment according to Latarjet versus the Bankart operation. A comparative study of the results in 50 cases. Acta Orthop Belg 59(2):147–155
Walch G (1996) Chronic anterior glenohumeral instability. J Bone Joint Surg Br 78(4):670–677
Walz DM, Burge AJ, Steinbach L (2015) Imaging of shoulder instability. Semin Musculoskelet Radiol 19(3):254–268
Wilk KE, Macrina LC (2013) Nonoperative and postoperative rehabilitation for glenohumeral instability. Clin Sports Med 32(4):865–914
Wright AA, Wassinger CA, Frank M, Michener LA, Hegedus EJ (2013) Diagnostic accuracy of scapular physical examination tests for shoulder disorders: a systematic review. Br J Sports Med 47:886–892
Yamamoto N, Itoi E, Abe H, Minagawa H, Seki N, Shimada Y, Okada K (2007) Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg 16:649–656
Yamamoto N, Kijima H, Nagamoto H, Kurokawa D, Takahashi H, Sano H, Itoi E (2015) Outcome of Bankart repair in contact versus non-contact athletes. Orthop Traumatol Surg Res 101(4):415–419
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Taverna, E., Garavaglia, G., Ufenast, H. et al. Arthroscopic treatment of glenoid bone loss. Knee Surg Sports Traumatol Arthrosc 24, 546–556 (2016). https://doi.org/10.1007/s00167-015-3893-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3893-0