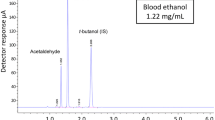
Abstract
Purpose
Concentration ratio of ethanol/n-propanol has been employed to distinguish the source of ethanol in postmortem blood, though its reliability remains controversial.
Methods
Forty-two postmortem human blood samples with ethanol levels in the range of 0.07–4.64 mg/mL were investigated. Ethanol and n-propanol were determined by head-space gas chromatography coupled with flame ionization detection, while ethyl glucuronide (EtG) and ethyl sulfate (EtS) were determined by liquid chromatography–tandem mass spectrometry.
Results
EtG and EtS were both negative in 26% of the investigated postmortem blood samples and 11% of n-propanol-negative postmortem samples, indicating that n-propanol was not a reliable marker of putrefaction. It was also found that the ratio of ethanol/n-propanol (supposed to be < 20 without antemortem ethanol consumption) was unreliable by showing great individual differences and was opposite with the result of EtG and EtS in at least 17% of n-propanol-positive postmortem blood samples. Meanwhile, 140 antemortem blood samples were investigated, as an aid to estimate the blood alcohol concentration (BAC) at the time of death for postmortem samples with ethanol both from postmortem formation and antemortem consumption. By comparing with the maximum or minimum value of EtG and EtS concentration under certain BAC in antemortem samples, the BAC range at the moment of death could be estimated in 93% of postmortem samples.
Conclusions
The present study proved that n-propanol was not a reliable marker for either putrefaction or ethanol source distinction by showing considerable false rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethanol consumption plays a significant role in traffic accidents and crimes [1,2,3,4,5,6], and the determination of blood alcohol concentration (BAC) has always been an important work in forensic labs [7]. However, in postmortem cases, it still remains a problem to determine whether ethanol comes from antemortem consumption or postmortem formation.
Several studies have demonstrated that some low-molecular-weight volatiles, such as n-propanol, isopropanol, and butanol, can be produced together with ethanol during postmortem putrefaction [8,9,10]. As a marker of putrefaction, n-propanol has received more attention [11,12,13,14]. An in vivo study using rats reported in 1982 found that the blood concentration ratio of ethanol to n-propanol was < 20 without antemortem ethanol consumption [15], which was supported by an authentic forensic case [16]. Several other studies also found that the postmortem formation of ethanol and n-propanol showed good correlation [17,18,19,20]. Thus, the ethanol/n-propanol ratio of 20 in blood has been utilized to distinguish the source of ethanol in postmortem blood and to estimate the antemortem ethanol concentration. However, these in vitro studies were performed using specific bacterial strains, which could not entirely simulate the environment of putrefaction [18, 19]. Meanwhile, some in vivo studies showed controversial results [13]. Our previous study using rats reported in 2016 found that the blood concentration ratio of ethanol/n-propanol varied from 2.1 to 205 without antemortem ethanol consumption, and from 15.2 to 115 in ethanol-administrated rats, indicating the unreliability of using ethanol/n-propanol ratio as the marker of ethanol source distinction [21]. More studies involving human samples from forensic cases are requested to verify whether the ethanol/n-propanol ratio is a reliable biomarker for the distinction of ethanol source.
As non-oxidative metabolites of ethanol, ethyl glucuronide (EtG) and ethyl sulfate (EtS) have been proved to be reliable markers for ethanol consumption in postmortem cases [22,23,24,25,26]. Simultaneous determination of these two metabolites was recommended, since EtG shows a higher sensitivity; while EtS shows a higher stability [22,23,24,25,26,27]. Negative detection results of EtG and EtS could be used to exclude antemortem ethanol consumption [14, 28]. The role played by n-propanol in distinction of ethanol source in postmortem blood could be assessed with the assistance of EtG and EtS, while no such study has been reported. Meanwhile, when there are detectable EtG and EtS in postmortem samples, it is important to estimate whether ethanol comes totally or partially from antemortem consumption. Back-calculation of antemortem BAC is in great need, since whether the decedent was under the influence of ethanol is closely related with the determination of legal responsibility. However, such estimation still remains a difficult task.
The purpose of the present study was to assess the reliability of n-propanol in ethanol source distinction in postmortem blood with the assistance of EtG and EtS, and to estimate the antemortem BAC in postmortem blood when EtG and EtS were positive. The concentration of ethanol and n-propanol in 42 postmortem blood samples was measured by head-space gas chromatography coupled with flame ionization detection (HS-GC–FID), and the ethanol/n-propanol ratio was calculated. EtG and EtS were analyzed by a liquid chromatography–tandem mass spectrometry (LC–MS/MS) method to assess the reliability of n-propanol. Meanwhile, 140 antemortem blood samples were analyzed, as an aid to estimate the BAC at the time of death for postmortem samples with ethanol both from postmortem formation and antemortem consumption.
Methods
Reagents and standards
Ethanol, n-propanol, as well as their internal standard tert-butanol, were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). EtG, EtS, and their internal standards EtG-D5 and EtS-D5 were purchased from Cerilliant Co., Ltd (Round Rock, TX, USA). Ultra-pure water was prepared by a Millipore Milli-Q purification machine (Bedford, MA, USA).
Blood samples
Forty-two postmortem blood samples were collected from cardiac chambers of the deceased provided by the Department of Forensic Medicine, Fudan University. Due to restrictions on customs in China, it is a common practice to determine alcohol concentration in blood from heart chambers, which could be obtained without autopsies. Samples were stored at 4 °C after sampling and analyzed within 24 h. Neither antiseptic nor anticoagulant was added.
One hundred and forty antemortem blood samples were collected from cubital vein of people involved in driving under the influence of alcohol cases from Department of Forensic Medicine, Fudan University. Samples were anti-coagulated by sodium citrate and anti-corrupted by sodium fluoride.
Analysis of ethanol and n-propanol in blood
Concentrations of ethanol and n-propanol in blood were determined by a well-validated HS-GC–FID method. An aliquot of 100 μL of blood was transferred to a glass vial, spiked with 500 μL of tert-butanol (40 μg/mL), and immediately sealed with an air-tight cap. The samples were analyzed by an Agilent 7890 gas chromatograph coupled with a headspace sampler and a flame ionization detector (Agilent Technologies, Palo Alto, CA, USA). The head-space heater was set at 65 °C. The injection was carried out in split mode (1:5) at an injector temperature of 250 °C. The chromatographic separation was performed on DB-ALC1 capillary column (30 m × 0.32 × 1.8 μm) and DB-ALC2 capillary column (30 m × 0.32 × 1.2 μm, Agilent Technologies, Palo Alto, CA, USA). The detector temperature was set at 250 °C. Limit of detection and limit of quantification were 0.01 and 0.05 mg/mL, respectively, for ethanol, with a linearity range of 0.05–3.0 mg/mL. The LOD and LOQ were 0.0005 mg/mL and 0.001 mg/mL for n-propanol, with a linearity range of 0.001–0.2 mg/mL. Ethanol and n-propanol in gastric contents were not detected in this study, which are not required for regular forensic cases according to Chinese law.
Analysis of EtG and EtS in blood
The levels of EtG and EtS in blood were determined by a well-validated LC–MS/MS method [29].
In brief, 100 μL of methanol (containing 2 μL of IS working solution) was added to 20 μL of whole blood. After vortex and centrifugation, the resulting supernatant was vaporized to dryness and reconstituted by 50 μL of 0.1% formic acid. Five-μL aliquot was injected into the LC–MS/MS system which consisted of an Ultimate 3000 UPLC system and a TSQ Quantiva triple quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). LOD and LOQ were 2 and 5 ng/mL, respectively, for both EtG and EtS, with linearity ranges of 5–10000 ng/mL. Samples out of upper limit of quantification were diluted to 1/10 with blank human whole blood and then analyzed. Accuracy for EtG and EtS was among 87.2–106.7%. Intra-day precision was in the range of 2.1–8.2%. Inter-day precision was between 5.1 and 12.2%. Correlation coefficients of calibration curves for EtG and EtS were all higher than 0.999.
The cited article mainly focused on the development and validation of a developed analytical method for the determination of EtG and EtS in both postmortem blood and vitreous humor. However, n-propanol in blood was not mentioned. EtG and EtS in the two matrices from real forensic cases were proved to be reliable markers for the interpretation of postmortem ethanol source. Based on these findings in the cited article, the role played by n-propanol in distinction of ethanol source in postmortem blood was assessed in this study, with the assistance of EtG and EtS.
Statistical analysis
The statistical analysis was performed using a nonparametric test with Graphpad prism version 7.0 software (GraphPad Software Inc., San Diego, CA, USA). Correlations of BAC with EtG and EtS were assessed using Spearman’s correlation coefficient.
Results
Levels of ethanol, n-propanol, EtG and EtS in postmortem blood
The concentrations of ethanol, n-propanol, EtG and EtS in postmortem blood are listed in Table 1. BAC ranged from 0.07 to 4.64 mg/mL. Among these samples, EtG and EtS were both negative in 11 samples, accounting for 26% (11/42), with BAC ranged from 0.15 to 2.60 mg/mL. In two samples (no. 24 and 25), only EtS was detectable (< 5 ng/mL).
n-Propanol was negative in 18 samples (no. 1–18) and positive in 24 samples (no. 19–42). In n-propanol-positive samples, the ethanol/n-propanol ratio was below 20 in 11 samples (no. 19–29) and above 20 in 13 samples (no. 30–42). For cases with both negative EtG and EtS, ethanol/n-propanol ratio varied from 2.43 to 360.
Relationship of ethanol, EtG and EtS levels in antemortem blood
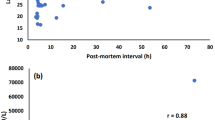
For samples where ethanol came from both antemortem consumption and postmortem formation, determination of antemortem BAC becomes an important work, which was directly related to the judgement of legal responsibility. For this purpose, 140 antemortem blood samples were analyzed to find out the relationships between antemortem BAC and concentrations of EtG and EtS. Correlation of EtG and EtS with BAC is presented in Fig. 1. EtS showed a better correlation with BAC than EtG (R = 0.884 vs 0.792). However, it was insufficient to fit a correlation curve simply by these parameters.
For the estimation of BAC at the time of death, antemortem blood samples were divided into 5 groups according to the BAC: 0, 0.01–0.19 mg/mL, 0.20–0.49 mg/mL, 0.50–0.79 mg/mL, and 0.80–3.40 mg/mL, because 0.20, 0.50 and 0.80 mg/mL were the legal limits for driving in many countries [7, 30]. In each group, minimum, maximum and median values of EtG and EtS concentrations are presented in Table 2.
Discussion
Assessment of n-propanol in distinction of ethanol source in postmortem blood with the assistance of EtG and EtS
In 18 n-propanol-negative samples (no. 1–18), if n-propanol was used as the marker for the distinction of ethanol source, it was easy to conclude that ethanol in these samples came from antemortem consumption rather than postmortem formation. However, in samples nos. 1 and 2, EtG and EtS were negative, indicating no antemortem ethanol consumption, which were on the contrary to the indication by n-propanol. Thus, the usage of n-propanol led to at least 11% (2/18) false results of ethanol source distinction among the investigated n-propanol-negative samples.
In case no. 2, BAC was quite high (2.60 mg/mL), while n-propanol, EtG and EtS were all tested negative. Although researchers concluded that lower concentrations (< 0.70 mg/mL) of ethanol were more likely to come from postmortem formation [31], in some cases, postmortem formation of ethanol could be as high as 1.2–2.2 mg/mL [13], and even up to 3.0 mg/mL in an extreme case [32]. Researchers discovered that heavy trauma might increase the risk of postmortem ethanol formation by increasing the potential of bacteria spreading [33]. In case no. 2, the decedent died from a car crash, and suffered from mechanical injury (including craniocerebral injury, lacerations on forehead and bruises all over the body surface), which might be responsible for the high concentration of postmortem ethanol formation.
In sample no. 3, although EtG and EtS were both positive, the concentrations were < 10 ng/mL. Due to the longer detection window of EtG and EtS than ethanol [34, 35], although antemortem ethanol consumption was indicated, it was difficult to estimate whether ethanol has already been eliminated entirely at the time of death in these samples.
Besides, n-propanol has been widely viewed as a marker of putrefaction [11,12,13,14]. However, in the present study, postmortem ethanol formation was found in several cases without n-propanol formation (cases no. 1–18 in Table 1), which was also discovered by Krabseth et al. [14].
n-Propanol was positive in 24 samples (no. 19–42). Although ethanol/n-propanol ratio (20 as the cutoff value) has been used for ethanol source distinction, the present study found that for cases with both negative EtG and EtS, ethanol/n-propanol ratio varied from 2.43 to 360. This result was similar to our previous study using rats without antemortem ethanol consumption [21].
In five samples where EtG and EtS were detected 0 (no. 19–23), the ethanol/n-propanol ratios were much lower than 20. For these samples, the ethanol/n-propanol results were consistent with the result of EtG and EtS, indicating the ethanol found in these samples were due to putrefaction. In samples no. 38–42, the concentrations of EtG and EtS were quite high and the ethanol/n-propanol ratios were all above 20. For these samples, antemortem ethanol consumption could be confirmed by both ethanol/n-propanol ratio and results of non-oxidative metabolites.
In samples nos. 24–29 and 34–37, the existence of EtS or EtG indicated antemortem ethanol consumption. However, the concentrations of EtG and EtS in these samples were not very high (< 100 ng/mL). In sample no. 3, it was difficult to estimate whether the decedent was under the influence of ethanol at the moment of death.
In samples no. 30–33, if ethanol/n-propanol ratio was used to distinguish the source of ethanol, a conclusion of antemortem ethanol consumption would be drawn. However, the negative detection results of EtG and EtS indicated no postmortem ethanol consumption. It was usually difficult to know whether the decedent had consumed ethanol according to the case description. Yet there were two cases where antemortem ethanol consumption could be probably excluded. In sample no. 30, the decedent died in prison and the corpse was kept in a − 20 °C freezer for 8 months before autopsy. Considering the man was in prison before death and it was almost impossible to have alcoholic beverages in prison in China, the negative result of EtG and EtS of this sample was reliable. In sample no. 31, the decedent was in persistent vegetative state before death. This case also proved that EtG and EtS were more reliable than n-propanol, since the decedent hardly had access to alcoholic beverages during life. EtG and EtS in these cases (no. 30–33) indicated no ethanol consumption before death, which were contrary to the results of ethanol/n-propanol ratios. In conclusion, the usage of n-propanol led to at least 17% (4/24) false result of ethanol source distinction among the investigated n-propanol-positive samples.
It needed to be mentioned that, in several cases where concentrations of n-propanol were around LOQ level, ethanol/n-propanol ratio could be quite varying. Although each sample was determined in duplicate in this study, ethanol/n-propanol ratio could still show a wide range. It would lead to the limits of interpretations for the results.
Estimation of the BAC at the time of death using EtG and EtS
Although the concentrations of EtG and EtS in antemortem blood showed high individual differences, the maximum and minimum values of EtG and EtS concentrations could be used for BAC calculation. For example, the maximum concentrations of EtG and EtS in antemortem blood in ethanol negative group were 797 and 418 ng/mL, respectively. Postmortem samples with EtG and EtS blood concentrations exceeding these levels were much likely to be related to antemortem ethanol consumption. The minimum concentrations of EtG and EtS in antemortem blood in BAC 0.20–0.49 mg/mL group were 94 and 74 ng/mL, respectively. Thus, postmortem samples with EtG and EtS concentration below this value were much likely to have an antemortem BAC below 0.20 mg/mL. The maximum concentrations of EtG and EtS in antemortem blood in 0.50–0.79 mg/mL group were 2780 and 1090 ng/mL, respectively, indicating that postmortem samples with EtG and EtS concentrations above these levels were very likely to have a BAC not lower than 0.80 mg/mL.
Based on the relationship of ethanol, EtG and EtS levels in antemortem blood samples, the BAC range at the moment of death could be estimated in 93% postmortem samples. It could be inferred that the antemortem BAC of postmortem blood samples no. 3, 24–29 and 34–36 in Table 1 were not higher than 0.20 mg/mL. One-fifth mg/mL was the legal limit for driving under the influence of alcohol in most countries, and the ethanol detected in sample no. 3, 24–28, 34 and 35 partially resulted from postmortem formation. As for samples no. 39–42, the concentrations EtG and EtS both exceeded the maximum values in 0.50–0.79 mg/mL BAC group. Thus, antemortem BAC in these cases could be inferred to be not lower than 0.80 mg/mL, which was consistent to the detected postmortem BAC. However, it was still impossible to estimate the BAC range when samples showed an EtG level of 118–797 ng/mL or an EtS level of 168–418 ng/mL, which accounted for 7% (3/42) in the present study.
Comparison of EtG and EtS in the distinction of ethanol source in postmortem blood samples
When comparing EtG and EtS, it was discovered that in most cases, concentration of EtG was higher than that of EtS, which was in accordance with many other researches [11, 12, 14, 36]. Thus, EtG was believed to show a higher sensitivity of antemortem ethanol consumption. Besides, EtS could only be determined by LC–MS/MS. EtG could also be determined after derivatization by gas chromatography–mass spectrometry (GC–MS) [37], which was more commonly used in most forensic labs. However, EtG was unstable especially under high temperatures or heavy putrefaction [24, 25, 38]. In comparison, EtS showed higher stability in postmortem samples [11, 14, 27, 38, 39]. Negative results of EtS have been utilized for exclusion of antemortem ethanol consumption [14, 28]. The higher stability of EtS may also explain the better correlation of EtS (R = 0.884) with BAC than EtG (R = 0.792) in the present study. Besides, some individuals were unable to form EtG due to reduced activity of related enzyme [40]. Under such circumstances, EtS became an essential marker to determine ethanol source. Thus, simultaneous application of EtG and EtS provided a both sensitive and specific result of ethanol source distinction, while EtS showed a higher reliability when the results of these two metabolites were on the contrary [14]. In the present study, the ratio of EtG/EtS was found to be 0.34–3.93 in antemortem blood samples. Whether the formation or degradation of EtG should be considered when the ratio of EtG/EtS exceeds this range in postmortem cases needs further study.
Conclusions
Although n-propanol has been widely employed in ethanol source distinction in postmortem samples, the present study proved that n-propanol led to at least 11% false result of ethanol source distinction in n-propanol-negative postmortem samples. The concentration ratio of ethanol/n-propanol was proved not be a reliable biomarker due to great individual differences and opposite result with that of EtG and EtS in at least 17% of the investigated postmortem cases. Also, n-propanol was found not to be a reliable marker of putrefaction. The present study also summarized the concentration range of EtG and EtS in antemortem samples under certain BAC, and the BAC range at the time of death could be estimated in 93% of postmortem samples. Even so, further studies were required to achieve more accurate BAC estimation in postmortem samples when ethanol came from both postmortem formation and antemortem consumption. Estimation should be extremely prudent when the concentrations of EtG and EtS were near the critical value of each BAC group.
References
Girasek DC, Gielen AC, Smith GS (2002) Alcohol’s contribution to fatal injuries: a report on public perceptions. Ann Emerg Med 39:622–630
Snijder M, Calabria B, Dobbins T, Knight A, Shakeshaft A (2018) A need for tailored programs and policies to reduce rates of alcohol-related crimes for vulnerable communities and young people: an analysis of routinely collected police data. Alcohol Alcohol 53:578–585
Tindall J, Groombridge D, Wiggers J, Gillham K, Palmer D, Clinton-McHarg T, Lecathelinais C, Miller P (2016) Alcohol-related crime in city entertainment precincts: public perception and experience of alcohol-related crime and support for strategies to reduce such crime. Drug Alcohol Rev 35:263–272
Carfora A, Campobasso CP, Cassandro P, Petrella R, Borriello R (2018) Alcohol and drugs use among drivers injured in road accidents in Campania (Italy): a 8-year retrospective analysis. Forensic Sci Int 288:291–296
Gomez-Restrepo C, Gomez-Garcia MJ, Naranjo S, Rondon MA, Acosta-Hernandez AL (2014) Alcohol consumption as an incremental factor in health care costs for traffic accident victims: evidence in a medium sized Colombian city. Accid Anal Prev 73:269–273
Kelly AT, Mozayani A (2012) An overview of alcohol testing and interpretation in the 21st century. J Pharm Pract 25:30–36
Kugelberg FC, Jones AW (2007) Interpreting results of ethanol analysis in postmortem specimens: a review of the literature. Forensic Sci Int 165:10–29
Flanagan RJ, Connally G (2005) Interpretation of analytical toxicology results in life and at postmortem. Toxicol Rev 24:51–62
Leikin JB, Watson WA (2003) Post-mortem toxicology: what the dead can and cannot tell us. J Toxicol Clin Toxicol 41:47–56
Moriya F, Ishizu H (1994) Can microorganisms produce alcohol in body cavities of a living person?: a case report. J Forensic Sci 39:883–888
Hoiseth G, Karinen R, Christophersen A, Morland J (2010) Practical use of ethyl glucuronide and ethyl sulfate in postmortem cases as markers of antemortem alcohol ingestion. Int J Legal Med 124:143–148
Vezzoli S, Bernini M, De Ferrari F (2015) Ethyl glucuronide in vitreous humor and blood postmortem specimens: analysis by liquid chromatography–electrospray tandem mass spectrometry and interpreting results of neo-formation of ethanol. Ann Ist Super Sanita 51:19–27
Ziavrou K, Boumba VA, Vougiouklakis TG (2005) Insights into the origin of postmortem ethanol. Int J Toxicol 24:69–77
Krabseth H, Morland J, Hoiseth G (2014) Assistance of ethyl glucuronide and ethyl sulfate in the interpretation of postmortem ethanol findings. Int J Legal Med 128:765–770
Nanikawa R, Ameno K, Hashimoto Y, Hamada K (1982) Medicolegal studies on alcohol detected in dead bodies—alcohol levels in skeletal muscle. Forensic Sci Int 20:133–140
Nanikawa R, Kotoku S (1974) Medicolegal observations on a dead body drawn up from the sea bed, with special reference to ethanol and diatoms. Forensic Sci 3:225–232
Takayasu T, Ohshima T, Tanaka N, Maeda H, Kondo T, Nishigami J, Nagano T (1995) Postmortem degradation of administered ethanol-d6 and production of endogenous ethanol: experimental studies using rats and rabbits. Forensic Sci Int 76:129–140
Boumba VA, Economou V, Kourkoumelis N, Gousia P, Papadopoulou C, Vougiouklakis T (2012) Microbial ethanol production: experimental study and multivariate evaluation. Forensic Sci Int 215:189–198
Boumba VA, Kourkoumelis N, Gousia P, Economou V, Papadopoulou C, Vougiouklakis T (2013) Modeling microbial ethanol production by E. coli under aerobic/anaerobic conditions: applicability to real postmortem cases and to postmortem blood derived microbial cultures. Forensic Sci Int 232:191–198
Felby S, Nielsen E (1993) Postmortem blood alcohol concentration. Blutalkohol 30:244–250
Liang H, Kuang S, Guo L, Yu T, Rao Y (2016) Assessment of the role played by n-propanol found in postmortem blood in the discrimination between antemortem consumption and postmortem formation of ethanol using rats. J Forensic Sci 61:122–126
Hoiseth G, Karinen R, Christophersen AS, Olsen L, Normann PT, Morland J (2007) A study of ethyl glucuronide in post-mortem blood as a marker of ante-mortem ingestion of alcohol. Forensic Sci Int 165:41–45
Schloegl H, Rost T, Schmidt W, Wurst FM, Weinmann W (2006) Distribution of ethyl glucuronide in rib bone marrow, other tissues and body liquids as proof of alcohol consumption before death. Forensic Sci Int 156:213–218
Baranowski S, Serr A, Thierauf A, Weinmann W, Grosse PM, Wurst FM, Halter CC (2008) In vitro study of bacterial degradation of ethyl glucuronide and ethyl sulphate. Int J Leg Med 122:389–393
Hoiseth G, Karinen R, Johnsen L, Normann PT, Christophersen AS, Morland J (2008) Disappearance of ethyl glucuronide during heavy putrefaction. Forensic Sci Int 176:147–151
Hoiseth G, Kristoffersen L, Larssen B, Arnestad M, Hermansen NO, Morland J (2008) In vitro formation of ethanol in autopsy samples containing fluoride ions. Int J Leg Med 122:63–66
Halter CC, Laengin A, Al-Ahmad A, Wurst FM, Weinmann W, Kuemmerer K (2009) Assessment of the stability of the ethanol metabolite ethyl sulfate in standardised degradation tests. Forensic Sci Int 186:52–55
Hegstad S, Kristoffersen L, Liane VH, Spigset O (2017) EtG and EtS in autopsy blood samples with and without putrefaction using UPLC–MS-MS. J Anal Toxicol 41:107–113
Wang H, Li BX, Wang FL, Chang J, Zhang YF, Rao YL (2019) Determination of ethyl glucuronide and ethyl sulfate in human whole blood and vitreous humor by LC–MS/MS and applications to the interpretation of postmortem ethanol findings. J Anal Toxicol. https://doi.org/10.1093/jat/bkz082
Jones AW (2000) Medicolegal alcohol determination—blood- or breath-alcohol concentration? Forensic Sci Rev 12:23–47
O’Neal CL, Poklis A (1996) Postmortem production of ethanol and factors that influence interpretation: a critical review. Am J Forensic Med Pathol 17:8–20
Canfield DV, Kupiec T, Huffine E (1993) Postmortem alcohol production in fatal aircraft accidents. J Forensic Sci 38:914–917
Winek CL, Winek CL, Wahaba WW (1995) The role of trauma in postmortem blood alcohol determination. Forensic Sci Int 71:1–8
Cabarcos P, Alvarez I, Tabernero MJ, Bermejo AM (2015) Determination of direct alcohol markers: a review. Anal Bioanal Chem 407:4907–4925
Hoiseth G, Morini L, Polettini A, Christophersen A, Morland J (2009) Blood kinetics of ethyl glucuronide and ethyl sulphate in heavy drinkers during alcohol detoxification. Forensic Sci Int 188:52–56
Zhang X, Zheng F, Lin Z, Johansen SS, Yu T, Liu Y, Huang Z, Li J, Yan J, Rao Y (2017) Simultaneous determination of ethanol’s four types of non-oxidative metabolites in human whole blood by liquid chromatography tandem mass spectrometry. Anal Chim Acta 963:68–75
Sharma P, Bharat V, Murthy P (2015) Quantitation of ethyl glucuronide in serum and urine by gas chromatography–mass spectrometry. Indian J Med Res 141:75–80
Liu Y, Zhang X, Li J, Huang Z, Lin Z, Wang J, Zhang C, Rao Y (2018) Stability of ethyl glucuronide, ethyl sulfate, phosphatidylethanols and fatty acid ethyl esters in postmortem human blood. J Anal Toxicol 42:346–352
Helander A, Dahl H (2005) Urinary tract infection: a risk factor for false-negative urinary ethyl glucuronide but not ethyl sulfate in the detection of recent alcohol consumption. Clin Chem 51:1728–1730
Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP (1995) The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Eng J Med 333:1171–1175
Acknowledgements
This research was supported by Science and Technology Commission of Shanghai Municipality Technical Standards Special Projects (no. 17DZ22204100) and National Key R&D Program of China (no. 2017YFC0803504-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Use of blood samples in this study was approved by Ethics Committee of School of Basic Medical Sciences, Fudan University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, H., Li, J., Huang, Z. et al. Assessment of the role played by n-propanol in distinction of ethanol source in postmortem blood with the assistance of ethyl glucuronide and ethyl sulfate. Forensic Toxicol 38, 195–202 (2020). https://doi.org/10.1007/s11419-019-00507-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-019-00507-9