Abstract
N-(1-Phenethylpiperidin-4-yl)-N-phenylacrylamide, or acryloylfentanyl (acrylfentanyl), is a synthetic opioid and a close structural analogue of fentanyl, which is widely used in medicine as an adjunct to general anaesthesia during surgery and for pain management. Until recently, acryloylfentanyl was known only from the scientific literature, but in 2016 this non-controlled substance became available on the illicit drug market as a powder and nasal spray in Europe and the USA. By the end of 2016, detection of acryloylfentanyl in six European countries, including 47 deaths associated with the drug, had been reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) through the European Union Early Warning System, which is a part of the system designed to identify and respond to the appearance of new psychoactive substances that may pose potential public health risks similar to drugs controlled under the United Nations drug control conventions. Herein we review what is known about this potent narcotic opioid. In addition to describing its chemical properties and the synthetic routes, analytical methodologies for the identification of the substance, as well as the limited information on the biological properties, including in vitro and in vivo pharmacological studies with the substance, are summarised. Analytically confirmed acute intoxications show that the signs and symptoms of acryloylfentanyl poisoning correspond to the opioid overdose triad of decreased consciousness, miosis and respiratory depression. Importantly, naloxone works as an antidote in life-threatening poisoning. The major human urinary metabolites identified in fatal overdose cases were nor-acryloylfentanyl, as well as mono- and dihydroxylated derivatives and their conjugates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The elucidation of the chemical structure of morphine by Gulland and Robinson in 1925 [1] paved the way for the development of synthetically more tractable yet potent analgesics, cough suppressants and antidiarrheal agents, including pethidine, methadone, fentanyl, tramadol, tapentadol, ketazocine, dextromethorphan and loperamide [2]. However, the idealized goal to find potent opioid medicines devoid of serious side effects, such as abuse liability, dependence potential and respiratory depression, has so far eluded researchers.
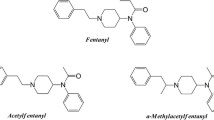
In the early 1960s, a series of structurally simple yet highly potent 4-anilinopiperidine-type analgesics were developed in the Research Laboratorium of Dr. Janssen [3, 4]. Fentanyl (Fig. 1), the first narcotic analgesic medicine belonging to this class, was followed by other structurally related substances, the fentanils [5, 6]. Due to the rapid onset and short duration of analgesic action and a relatively wide therapeutic window, a small number of this family of compounds, such as fentanyl, alfentanil, sufentanil and remifentanil, have become widely used in human medicine in surgical procedures as adjuncts to anaesthesia and for the treatment of acute and chronic pain in various formulations, while some are used in veterinary medicine as general anaesthetics, for pain management, and, in the case of carfentanil and thiafentanil, to immobilise large animals [7, 8].
Like other opioid analgesics, the analgesic activity of the fentanils is due to their activation of opioid receptors, and in particular, the μ-opioid receptor [9]. Besides their analgesic properties, a notable feature associated with μ-opioid receptor agonists is that they induce dose-dependent respiratory depression [9,10,11], which can be life-threatening. The importance of this effect is reflected in the fact that most opioid-related deaths are caused by respiratory depression [12].
Between 1979 and 1988, more than ten fentanils, including α-methylfentanyl, 3-methylfentanyl and 4-fluorofentanyl, were detected on the illicit opioid market in the United States, typically sold as heroin or “synthetic heroin”. Together, they were associated with more than 100 overdose deaths during this period [13,14,15]; later, in the mid-2000s, outbreaks of poisonings linked to clandestinely produced fentanyl also occurred [16,17,18,19]. With the exception of Estonia, which saw an epidemic mainly related to 3-methylfentanyl and fentanyl [20, 21], fentanils caused limited problems in Europe [22,23,24,25]. In the past few years, there has been a large increase in non-fatal and fatal poisonings in the United States and Canada associated with fentanils that have mostly been sold on the illicit opioid market: the fentanils are either sold as heroin to unsuspecting users, used to cut heroin, or used to make counterfeit opioid medicines [26,27,28]. They have also been sold as other drugs such as cocaine and benzodiazepines [29,30,31].
At the same time, Europe has also seen an increase in the availability of new fentanils. Twenty such substances have been identified on Europe’s drug market since 2012, with eight identified in 2016 alone. Seizures by law enforcement have also increased, as have poisonings and deaths (see, for example, [32, 33]). These developments are part of the broader changes seen on Europe’s drug market as a result of the appearance of large numbers of new psychoactive substances over the past decade [34, 35].
In Europe, a three-step legal framework of early warning, risk assessment and control measures allows the European Union (EU) to rapidly identify and react to public health threats caused by such substances. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) is responsible for the first two steps in the system, namely operating the EU Early Warning System on new psychoactive substances with Europol (the EU Police agency) and conducting risk assessments. As part of this work, the EMCDDA monitors more than 620 new psychoactive substances that have appeared on Europe’s drug market over the past 20 years. Data on these substances are collected and reported by national early warning systems of the 28 EU member states, Turkey and Norway to the EMCDDA through the EU Early Warning System as well as from other sources. Based on data reported through the EU Early Warning System on acryloylfentanyl, the EMCDDA undertook a detailed investigation on this substance as part of its early warning and risk assessment activities [33].
Herein we provide a comprehensive and up-to-date review on acryloylfentanyl, a close structural analogue of fentanyl, which was first identified on Europe’s drug market in 2016 [33] (for the methodology used for the preparation of the review, see supplementary material). Following the presentation of the physicochemical properties, the analytical methods and the known synthetic routes of this new psychoactive substance, and the in vitro and in vivo pharmacology studies, including mode of action and assumed metabolism and toxic effects, are reviewed and related to those of fentanyl. Finally, serious adverse events, including symptoms of acute intoxication and deaths reported within Europe in 2016, are described.
Acryloylfentanyl, as a new psychoactive substance
Acryloylfentanyl is a structurally simple though less studied analogue of fentanyl that was first described in the scientific literature in the 1980s [36, 37]. In early 2016, however, the substance was detected on the illicit drug market in Denmark and Sweden, and since then, has been associated with more than 40 deaths in Europe. Acryloylfentanyl, or “acryl fentanyl”, was also identified in the USA in the fourth quarter of 2016 [38].
Chemically, acryloylfentanyl is an acrylamide derivative of 4-anilinopiperidine and is an unsaturated analogue of fentanyl (Fig. 1), which is a propionamide and is included in Schedule I of the United Nations Single Convention on Narcotic Drugs of 1961, as amended by the 1972 Protocol. Neither acryloylfentanyl nor any other unsaturated amides were claimed in the original fentanyl patent by the Research Laboratorium Dr. C. Janssen [3]. While acryloylfentanyl is new on the international drug market, its side-chain methylated homologue, acryloyl-α-methylfentanyl (Fig. 1), was found in seized drugs in the USA decades ago [13, 39, 40].
Apart from acryloylfentanyl, other commonly used names are acryloyl fentanyl, acrylfentanyl, acryl fentanyl, and akrylfentanyl (in Sweden). Its systematic (IUPAC) name is N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]prop-2-enamide. Its Chemical Abstract name is N-phenyl-N-[1-(2-phenylethyl)-4-piperidinyl-2-propenamide. Acryloylfentanyl is also known by other names such as N-(1-phenethylpiperidin-4-yl)-N-acroylanilinopiperidine, N-(1-phenylethylpiperidin-4-yl)-N-phenylacrylamide, or enfentanyl. Its common street names are “acryloyl-F” and “Acr-F”. The acronym “ACF” has also been used, which should not to be confused with “AF”, which is one of the street names for acetylfentanyl [32].
Chemical Abstract Service Registry Numbers (CAS RNs): 82003-75-6 for the free base, and 79279-03-1 for the hydrochloride salt. It may be noted that in the widely used “Designer Drugs Directory” [41], the CAS RN 79297-03-1 given for acryloyl-α-methylfentanyl (see Fig. 1) in fact denotes acryloylfentanyl hydrochloride. The International Chemical Identifier Key (InChI Key) for acryloylfentanyl is RFQNLMWUIJJEQF-UHFFAOYSA-N.
The molecular formula of acryloylfentanyl is C22H26N2O; the monoisotopic mass is 334.2045. Acryloylfentanyl contains one basic piperidine-nitrogen atom and thus readily forms salts with organic or inorganic acids. The reported melting point of the free base is 101–103 °C [42], while the reported melting points of its white hydrochloride salt are 259–260 °C [36], 252–258 °C (with decomposition) [37] and 191–194 °C [42, 43].
There are no solubility data on acryloylfentanyl or its salts; due to its close similarity to fentanyl, the free base is expected to be sparingly soluble in water; the hydrochloride and citrate salt are expected to have improved aqueous solubility. Relevant data for fentanyl are as follows: at ambient temperature, fentanyl base is poorly soluble in water (0.032 mg per mL at pH 5.9) [44]; the solubility of the citrate salt of fentanyl in water is ~25 mg/mL, while in ethanol it is 7.1 mg/mL [45].
Like fentanyl, acryloylfentanyl is highly lipophilic, as indicated by their comparable calculated 1-octanol/water partition coefficients (cLogP). The respective cLogP values for acryloylfentanyl and fentanyl are 4.13 and 3.89, as calculated by the ACD/ChemSketch 2015 release version (Advanced Chemistry Development Inc., Toronto, Canada). The respective cLogP values as calculated by StarDrop version 6.3.1 software (Optibrium Ltd, Cambridge, UK) for acryloylfentanyl and fentanyl are 3.61 and 3.89. The measured LogP value for fentanyl is 4.05 [46]. Due to its high lipophilicity, cross-contamination with traces of the substance during sample handling and analysis can be problematic [47].
Synthesis of acryloylfentanyl
There are two published syntheses of acryloylfentanyl, which involve the acylation of the common precursor 4-anilino-N-phenethylpiperidine (4-ANPP) with acryloyl chloride [36, 37] or 3-chloropropionyl chloride [43] (Fig. 2). The actual synthetic route used for the manufacture of acryloylfentanyl detected on the drug market is unknown, but it most likely relies on precursors and synthetic methods similar to the routes mentioned above or to those used for the manufacture of pharmaceutical fentanyl [48,49,50,51,52]. A powder sample in a capsule seized in Denmark was shown to consist of mainly acryloylfentanyl and a substantial amount of triethylamine hydrochloride (27%) and trace amounts of 4-ANPP, indicating the use of either of the routes shown in Fig. 2 for the manufacture of the drug [53].
It should be considered that, at least in theory, acryloylfentanyl may serve as a precursor to fentanyl by saturating the double bond of the acrylamide moiety using catalytic hydrogenation (see, for example, [54]).
Analysis of physical and biological samples
Acryloylfentanyl has been fully characterised by 1H and 13C nuclear magnetic resonance spectroscopy [37, 42, 53, 55], Fourier transform infrared spectroscopy (both the free base and the HCl salt) [53, 55] and gas chromatography coupled with mass spectrometry [43, 53, 55]. Quadrupole time-of-flight (QTOF) and matrix-assisted laser desorption ionization Orbitrap mass spectrometric analyses of acryloylfentanyl have also been described [53]. The ultraviolet and visible spectrum of acryloylfentanyl have not been reported. A highly sensitive capillary electrophoresis-electrospray-tandem mass spectrometry method recently developed for the trace level analysis of fentanils [56] could be applicable for acryloylfentanyl.
Similar to fentanyl, acryloylfentanyl is not expected to give a positive response to immunoassay tests developed for morphine-type opioids. An enzyme-linked immunosorbent assay kit developed for fentanyl displayed strong cross-reactivity (215%) for acryloylfentanyl [57]. Acryloylfentanyl did not show cross-reactivity with the immunoassay panel ABC-multi-10 (Simoco Diagnostic, Hillerød, Denmark), which includes MDMA [53]. It is not known whether acryloylfentanyl can be detected by any presumptive colour tests.
Recently, a liquid chromatography (LC) screening in combination with a high-resolution tandem mass spectrometry method was developed for the qualitative and quantitative forensic analysis of acryloylfentanyl and other fentanils and their human metabolites with a detection limit of <5 ng/mL [58]. A method employing LC–QTOF for the identification of in vitro and in vivo metabolites of acryloylfentanyl and three other fentanils has also been described [59].
Pharmacology: mode of action and metabolism
Antinociceptive activity and toxic effects in the mouse
There have been two studies investigating the antinociceptive activity of acryloylfentanyl in the mouse [36, 42, 43]. The first publication mentioning acryloylfentanyl describes an extensive structure-activity relationship study involving 22 fentanyl analogues, with morphine and fentanyl as comparative standards [36]. The antinociceptive activities of morphine, fentanyl and acryloylfentanyl in mice upon intraperitoneal (ip) administration are shown in Table 1. As Table 1 indicates, in this rodent model of analgesia, acryloylfentanyl is about 170 times more effective as an antinociceptive agent than morphine, though somewhat less potent than fentanyl.
Essawi [42, 43] studied five fentanyl analogues, including acryloylfentanyl, as potential receptor affinity labels and antinociceptive agents in the mouse using the hot-plate assay; morphine and fentanyl were the comparative standards. Upon ip administration at doses below 1 mg/kg, acryloylfentanyl was a more potent antinociceptive agent than fentanyl: while the effect of fentanyl at doses of 0.1, 0.2 and 0.5 mg/kg dropped considerably at 60–70 min and became insignificant at 90–100 min after treatment, “at comparable doses, acryloylfentanyl maintained considerable analgesia at 90 and 120 min after administration. In its duration, the time-response profile of acryloylfentanyl resembled more closely that of morphine (20 mg/kg) than that of fentanyl”. Remarkably, at doses of 6.8 and 17 mg/kg, the antinociceptive effect of acryloylfentanyl was sustained up to 4.5 h without signs of opioid toxicity. At the 25-mg/kg dose, motor activity was transiently inhibited for 3.5 h. However, at a dose of 50 mg/kg, convulsions developed after 1 h, and “60% lethality was observed from apparent respiratory depression”. Subcutaneous (sc) pre-administration by 30 min of 2 mg/kg naloxone blocked the antinociceptive effect of 0.85 mg/kg acryloylfentanyl for about 40 min when the antagonist effect disappeared, and analgesia and other morphine-like effects could be noted for about 50 min. A similar transient antagonist effect was observed when naloxone (2 mg/kg, sc) was administered 40 min after acryloylfentanyl-treatment (0.85 mg/kg, ip): the reversal of the antinociceptive effect lasted for 70 min, and then antinociception returned to the same level as before naloxone administration. It was concluded that acryloylfentanyl “has a mode of interaction with μ-receptors different from morphine”.
There is limited information on the acute toxicity of acryloylfentanyl. In the mouse, ip injection of 25 mg/kg of the drug caused a transient suppression of motor activity; however, as mentioned above, a dose of 50 mg/kg produced convulsions 1 h after drug administration, and “60% lethality was observed from apparent respiratory depression” [43]. Based on this study, the ip LD50 value, that is the dose required to kill half of the experimental animals, of acryloylfentanyl may be estimated as between 25 and 50 mg/kg. No comparative standard was used in this particular study. Mouse ip LD50 values reported for fentanyl were 17.5 [60], 26.3 [61] and 76 mg/kg [62]; for morphine, mouse ip LD50 values of 140 [63] and 340 mg/kg [64] have been reported. These data indicate that the acute toxicity of acryloylfentanyl in the mouse is similar to that of fentanyl and is higher than that of morphine. There is no information on the chronic toxicity of acryloylfentanyl.
Interaction with opioid receptors
Maryanoff et al. [37] determined the binding affinities of a series of compounds, including acryloylfentanyl, using a rat brain receptor preparation and tritiated naloxone or naltrexone as competing receptor ligands (Table 2). The fentanyl analogues were designed as potential covalent receptor affinity labels. Morphine, fentanyl and the highly potent fentanyl analogue (+)-3-methylfentanyl were the comparative standards. The affinity of the test compounds to μ-opioid receptors was characterised by the half maximal inhibitory concentration (IC50), that is the molar concentration of the drug displacing 50% of μ-opioid receptor-preferring tritiated naloxone or naltrexone from the receptor preparation.
As seen in Table 2, the IC50 values obtained for fentanyl and acryloylfentanyl are practically identical; morphine is somewhat less effective in inhibiting the binding of radiolabelled receptor antagonists. The results of this study indicate that the opioid receptor affinity of acryloylfentanyl is similar to that of fentanyl and somewhat higher than that of morphine in this particular rat brain preparation.
Laboratory experiments failed to find evidence for irreversible binding of acryloylfentanyl to opioid receptors: after incubation, acryloylfentanyl could be completely “washed out” from the receptor preparation; the recovered receptor was able to bind radiolabelled naltrexone, which indicates reversible binding of the fentanyl analogue. Similar non-irreversible binding was observed for an acrylamide derivative of naltrexamine [65]. Thus, the sustained antinociceptive activity observed by Essawi [42, 43] is unlikely to be related to covalent binding of acryloylfentanyl to opioid receptors.
A recent study also confirmed the low reactivity of compounds containing the acrylamide “warhead”, which has often been considered as a promiscuous electrophilic moiety capable of binding to cysteine or other nucleophilic moieties present in target/off-target receptors and enzymes/other proteins [66]. Nevertheless, there has been an interest in medicinal chemistry research to develop irreversible inhibitors containing an acrylamide moiety or related electrophiles [67]; in fact, osimertinib, an N-phenylacrylamide derivative-type tyrosine kinase inhibitor, has received marketing authorisation in the USA and the EU.
Because polymerisation processes involving acrylamide or other acrylic type substances require photochemical or free radical initiation, spontaneous polymerization of acryloylfentanyl in the body can be ruled out. Whether any “biochemical” initiator could be involved in the observed long-lasting activity of acryloylfentanyl in vivo can only be speculated (see section on animal studies above). No stability studies have been carried out with acryloylfentanyl.
Other biological activity studies
A search in the PubChem Substance database for biological activity of acryloylfentanyl found 28 test results deposited, but the substance was found inactive in all assays that included a range of non-opioid-related targets [68].
Pharmacokinetics
Due to its lipophilicity, acryloylfentanyl, like fentanyl, is expected to readily cross the blood-brain barrier and also to diffuse into fat and other tissues (acryloylfentanyl is expected to have a large volume of distribution).
The pharmacokinetics and the metabolic pathway of acryloylfentanyl are expected to be similar to those of fentanyl [69,70,71,72] or acetylfentanyl [73,74,75]. A recent study determined the structures and relative abundance of the major acryloylfentanyl metabolites produced by human hepatocytes in vitro and of those detected in the urine in five cases of death related to acryloylfentanyl [59]. Using LC–QTOF analyses, these studies identified altogether 14 biotransformation products, including phase I and II metabolites. The major metabolites of acryloylfentanyl detected in human urine after hydrolysis of glucuronidated and/or sulfated phase II conjugates are depicted in Fig. 3. Similar to the well-studied metabolism of fentanyl [70, 76, 77], the biotransformation involves an oxidative N-dealkylation, presumably catalysed by cytochrome P450 (CYP450) enzymes, leading to the biologically inactive desphenethyl metabolite, that is “nor-acryloylfentanyl” (metabolite B1 in the original study). Additional oxidative metabolic processes were monohydroxylations either on the alkyl chain of the phenylethyl moiety or on the piperidine ring, affording metabolites B9 and B13 (hydroxylation site not assigned). Dihydroxylation of the aromatic ring of the phenylethyl moiety followed by O-monomethylation of the resulting catechol afforded metabolite B11 (and, to some extent, its regioisomer; not shown in Fig. 3). Monophenols (B10) and diols, such as diphenol derivatives of the aniline moiety or a species dihydroxylated at the acryl moiety (not shown in Fig. 3), were also detected. Similar to the metabolism of fentanyl, amide hydrolysis (deacylation) affording 4-ANPP (B14) was a minor pathway. Terminal monohydroxylation of the acyl chain, producing what is often called “hydroxyfentanyl”, is a biotransformation step for fentanyl in humans, but such hydroxylation cannot take place in the case of acryloylfentanyl. Intact acryloylfentanyl was also present in the urine of the decedents. The urinary concentrations of the metabolites were not quantified; the blood acryloylfentanyl concentration for these five deaths ranged from 0.05 to 1.20 ng/g.
Major human metabolites of acryloylfentanyl, with metabolite numbering according to original publication [59]. Note that due to incomplete acylation during manufacture or to product mislabelling, 4-ANPP could also be present in the consumed products; thus its detection in biological matrices might not always be indicative of metabolism
There is some information on the biological activity of two potential metabolites of acryloylfentanyl. An early study [78] assessing the opioid-like activity of several fentanyl metabolites in the guinea pig ileum assay found that 4-ANPP and 4-anilinopiperidine were less potent than either fentanyl or morphine by several orders of magnitude. The only metabolite showing significant activity in this study was a phenolic derivative hydroxylated at the 4-position of the phenylethyl moiety of fentanyl (for numbering, see Fig. 1), the activity of which was found to lie between morphine and pethidine. Therefore, the corresponding phenolic metabolite of acryloylfentanyl (not detected in the recent study [59]), if formed, may have some level of opioid activity and thus may contribute to the biological, including toxicological, properties of the parent substance.
Interindividual genetic variability in metabolising enzymes
For fentanyl, oxidative dealkylation by hepatic CYP450 3A4 and CYP450 3A5 isoenzymes has been demonstrated [71, 76, 79]. The wide variation in the expression of the genes coding for these CYP450 3A isoenzymes among populations is of clinical significance, but the toxicological consequence of such polymorphisms has not been investigated in fentanyl-overdose cases.
Interactions with other substances, medicines, and other forms of interactions
Should acryloylfentanyl undergo oxidative dealkylation by CYP450 3A4 and CYP450 3A5 isoenzymes, then the use of this substance with inhibitors of these isoenzymes, such as clarithromycin, indinavir, ritonavir, saquinavir, itraconazole, ketoconazole, nefazodone (all strong CYP3A inhibitors), erythromycin, fluconazole, grapefruit juice, and verapamil (all moderate CYP3A inhibitors) [80, 81], may result in prolonged high plasma concentration of acryloylfentanyl which could be toxicologically significant, for example by increasing the risk of potentially fatal respiratory depression.
The concomitant use of other central nervous system (CNS) depressants with opioid analgesics, including other opioids, sedatives/hypnotics (such as the benzodiazepines and the z-drugs), ethanol, gabapentinoids (pregabalin and gabapentin), tranquillisers, sedating anti-histamines, and skeletal muscle relaxants may produce additive depressant effects [80].
Similar to fentanyl, the use of partial opioid agonists/antagonists (such as buprenorphine, nalbuphine, pentazocine) which have high affinity to opioid receptors but relatively low intrinsic activity could partially antagonise the effects of acryloylfentanyl and may induce withdrawal symptoms in people who are opioid dependant [80]; it is unknown if such effects are possibly protective in individuals poisoned with acryloylfentanyl or other fentanils.
While data are lacking for acryloylfentanyl, the use of fentanyl with serotoninergic agents, such as selective serotonin reuptake inhibitors (the most commonly prescribed antidepressants) or serotonin-norepinephrine reuptake inhibitors or monoamine oxidase inhibitors has been associated with serotonin syndrome, which is a potentially life-threatening condition [80, 82]. This association is likely to extend to exposure to illicit drugs which act on the serotonergic system, such as MDMA and amphetamines. Unpredictable potentiation by monoamine oxidase inhibitors has been reported with some opioid analgesics [80, 82].
Human data
Routes of administration, dose regimens, and effects
Information on the routes of administration and dose regimens is limited, and originated from customs, police seizures, serious adverse event reports and Internet drug forums. Acryloylfentanyl may be taken orally as powder in capsules or as tablets; intravenous injection has also been reported. A novel and apparently popular route of intranasal administration is the use of a metered nasal spray, which delivers approximately 0.1 mL upon one actuation. Such ready-to-use nasal sprays were sold by online vendors in Sweden in 2016, and the amount of acryloylfentanyl in these sprays is typically claimed to be 20 mg as a solution in a 10-mL spray bottle capable of delivering ~100 actuations (i.e., ~0.2 mg per spray) [58]. In addition, user reports mention snorting or inhalation by smoking the free base of acryloylfentanyl; vaping of the drug from an e-cigarette containing a homemade flavoured e-liquid has also been mentioned [83]. Injecting users have used marketed nasal spray solutions or have themselves prepared a solution of the citrate salt of the free base of the drug, sometimes using alcohol as co-solvent. Preparation of a homemade transdermal patch has also been described [84].
From the available limited data, it is not possible to discern the “typical” dose or dose regimens administered by users. While a range of doses and regimens have been reported, these appear to differ depending on factors such as the route of administration, the user tolerance, the use of other drugs, and the desired effects. Given that the actual composition of the substance sold on the drug market may differ over time and among geographical areas, the dose mentioned in such reports is problematic, because the purity and/or composition of the substance ingested is typically not known by the user. In fact, it has recently been reported that some “akrylfentanyl” products sold in Sweden contained fentanyl instead of acryloylfentanyl [58]. A confusing factor regarding the dose used is the (probably) indiscriminate use of “mg” (milligram) and “μg” (microgram) weight units in Internet forums. The difference in dose by several orders of magnitude could result in a fatal overdose for an “uncut” product, if such false information is propagated on the Internet. Given the difficulties in collecting such data accurately, the information below should be used with caution.
Though human clinical studies are lacking, based on non-clinical data (see above), it may be assumed that the opioid-like effects of acryloylfentanyl manifest at doses similar to those observed for fentanyl. Those posting self-reports of use on Internet forums have mentioned that sub-milligram doses administered by nasal spray were psychoactive; doses of 0.0027–0.2 mg have been reported. In comparison, in human clinical trials, acute fentanyl doses of 0.2 mg intravenously [85], 0.015 mg/kg oral-transmucosally (lozenge) [86] or up to 1.6 mg intramuscularly [87] have been used.
Information on the psychological and behavioural effects of acryloylfentanyl is limited to serious adverse events reported to the EMCDDA [33] and self-reported experiences from Internet forums. The psychoactivity of acryloylfentanyl is reportedly similar to that of other opioids and includes relaxation and euphoria. Internet forums rarely mention side or adverse effects typical of opioids, though some discussions on these sites describe acryloylfentanyl as “longer lasting” after dosing than other new synthetic opioids.
Human poisoning cases
It is important to note that the number of serious adverse events, including deaths, involving acryloylfentanyl is likely to be underestimated, since testing for this specific substance is not commonly performed in opioid overdose cases.
Non-fatal intoxications
So far, Sweden has been the only country that has reported acryloylfentanyl-related acute intoxications. These cases relate to presentations to hospital emergency departments made between March and October 2016 [33, 58]. With one exception, the patients were men, ranging in age from 23 to 51 years; the age of the female patient was 19 years. Acryloylfentanyl was typically administered as nasal spray. There were eight cases in which acryloylfentanyl was analytically confirmed to be the sole opioid, with serum and urine acryloylfentanyl concentrations ranging from 0.5 to 2.1 and from 1.8 to 196 ng/mL (creatinine-normalized concentration: 0.2–10.5 μg/mmol creatinine), respectively.
One additional case in Sweden during this period involved a combination of acryloylfentanyl and 4-chloro-isobutyrfentanyl that was administered by injection of an extract of pills. Forensic analysis of three other intoxications that occurred in March 2016 and were believed to involve acryloylfentanyl identified fentanyl rather than acryloylfentanyl (see also the section “Routes of administration, dose regimens, and effects”). In some cases, analysis indicated the intake of alcohol or other licit or illicit substances [33, 58].
The clinical symptoms related to these acute intoxications appeared to be consistent with the use of an opioid and included decreased consciousness, respiratory depression and miosis. Other common features were tachycardia and hypertension. In six of these cases, naloxone was administered by paramedics and/or emergency department personnel [58].
Death cases
A total of 47 analytically confirmed deaths associated with acryloylfentanyl that occurred in 2016 were reported by three EU member states: Denmark (1 case), Estonia (3), and Sweden (43) [33]. Detailed information is available only for the death cases in Denmark and Sweden. The decedents were predominantly male (86%), and their age ranged from 19 to 54 years; the age of the female decedents ranged from 29 to 50 years. The deaths typically occurred in a home environment. Thirty-two of the deaths occurred between June and August 2016. In at least 40 deaths, acryloylfentanyl was either the cause of death or was likely to have contributed to death (even in the presence of other substances); in two of these deaths, acryloylfentanyl was the sole drug present.
Forensic analysis also revealed a range of other substances in the deaths, suggesting that polydrug use was common. These included a number of CNS depressants such as benzodiazepines, zopiclone, pregabalin, gabapentin, ethanol and cannabinoids (including synthetic cannabinoids), as well as synthetic cathinones, amphetamines, antidepressants and antipsychotics. However, acryloylfentanyl was the sole opioid present in 38 cases (86%). In the remaining cases, the opioids detected were buprenorphine, hydrocodone, oxycodone, 4-fluoroisobutyrfentanyl and 4-chloroisobutyrfentanyl.
Treatment of poisonings
In suspected acryloylfentanyl overdose cases, naloxone, an opioid receptor antagonist, should be administered in doses typical to rescue heroin overdoses [88]. However, given the short duration of action of naloxone and the high potency and different pharmacokinetics of acryloylfentanyl, repeated doses of the antidote may be required to prevent any reoccurrence of respiratory depression [89, 90]. In six of the acute acryloylfentanyl intoxication cases that occurred in Sweden (see above), naloxone was administered intravenously by paramedics and/or emergency department personnel at 0.1–0.4 mg, and oxygen supply was provided; one of these cases required continuous infusion for ~7 h during hospital observation; oxygen supply was also documented in seven of the cases [58].
Naloxone has also been used with success to reverse poisonings caused by other fentanils that have appeared on the drug market, including 3-methylfentanyl [91], acetylfentanyl [32], furanylfentanyl [30] and butyrylfentanyl [92].
Risks from accidental exposure
It is important to emphasize that accidental exposure, such as skin contact, inhalation or ingestion, to acryloylfentanyl and other fentanils poses a serious health risk to the public, law enforcement, medical and forensic laboratory personnel, postal services and in custodial settings. Where necessary, such risks should be assessed, and appropriate procedures, training, and environmental and personal protective measures should be provided for handling materials suspected of containing these drugs. This may include training in resuscitation and adequate provision of the opioid antagonist naloxone to reverse accidental poisoning [27, 89, 93].
Legal status
Acryloylfentanyl is not controlled under the United Nations Single Convention on Narcotic Drugs of 1961, as amended by the 1972 Protocol, or the Convention on Psychotropic Substances of 1971.
In the EU, acryloylfentanyl is controlled under drug control legislation in Cyprus, Denmark, Estonia, Finland, Ireland, Latvia, Lithuania, Sweden and the UK; in Austria and Poland the substance is controlled under specific new psychoactive substance control legislation. In Turkey, the substance is also controlled under drug control legislation. In Norway, the importation of, trade in and marketing of acryloylfentanyl is controlled by the Medicines Act [33]. “Acrylfentanyl” has been controlled in China since 1 March 2017 [94].
Conclusions
Over the past decade there has been a dramatic worldwide increase in the number and availability of new psychoactive substances. Much of this has been driven by the exploitation of globalization, economic development, and new technologies by entrepreneurs, where chemical and pharmaceutical companies based in China can produce bulk quantities of new substances and then cheaply and rapidly ship them to customers across the world. The fallout from this has been an increase in reported harms. In this paper we reviewed the case of acryloylfentanyl, which is one of 20 new fentanils that have appeared on the drug market in Europe since 2012.
Until recently, acryloylfentanyl was known only from the scientific literature, but in 2016 this non-controlled synthetic opioid became available on the illicit drug market as a powder and nasal spray in Europe and the USA. By the end of 2016, detection of acryloylfentanyl in six European countries (Denmark, Estonia, Finland, Latvia, Sweden, and SloveniaFootnote 1) had been reported to the EMCDDA through the EU Early Warning System. Chemically, acryloylfentanyl is an acrylamide derivative of 4-anilinopiperidine and is an unsaturated analogue of fentanyl, which is a controlled narcotic drug. In non-clinical laboratory studies, it has been shown to be an opioid receptor agonist and a potent and long-lasting antinociceptive agent. Typically it is sold as a legal alternative to illicit opioids.
In 2016, acryloylfentanyl was involved in 47 deaths, with at least 68% of the deaths occurring within a 3-month period. In the majority of the deaths, acryloylfentanyl was reported to be either the cause of death or to have contributed to the death. In addition, more than 20 acute intoxications suspected to be due to acryloylfentanyl were reported. The clinical features were generally consistent with opioid-like toxicity and included life-threatening effects. Naloxone was shown to be an antidote to poisoning caused by acryloylfentanyl, though in some cases repeated doses were required.
One common way of administering acryloylfentanyl appears to have been with ready-to-use nasal sprays, which were sold by online shops. These dosage forms, and others such as e-liquids, have the potential to make the use of fentanils easier (as compared to injecting) and more socially acceptable; these are developments that will require careful monitoring.
The risks from acryloylfentanyl are not limited to those who use the substance. Accidental exposure to the substance, and in fact to other fentanils, poses a serious risk to family and friends of users, as well as law enforcement, emergency personnel, medical and forensic laboratory personnel and those in custodial settings and postal services. Specific risks should be identified and appropriate risk reduction measures implemented. This may include appropriate protective equipment, training in resuscitation, and making naloxone readily available to relevant personnel in sufficient quantities in the event of exposure.
As of 1 March 2017, acryloylfentanyl has been controlled under drug control legislation in China. As a result, the open manufacture and sale of this substance may at least be deterred. Despite this development, it is important to note that since acryloylfentanyl was first detected in Europe, an additional eight new fentanils have been detected on the drug market, including 4-fluoroisobutyrfentanyl, 4-chloroisobutyrfentanyl, tetrahydrofuranylfentanyl and methoxyacetylfentanyl. Based on an analysis of the literature, there are dozens of other fentanils which could emerge as new psychoactive substances.
In recent years the emergence of hundreds of new psychoactive substances on Europe’s drug market has driven greater complexity into the drug problem. The market is highly dynamic and demonstrates increasingly innovative attempts to circumvent regulation. The recent appearance of a large number of new fentanils as part of this market is of particular concern to public health, given the serious acute risk from profound and rapid respiratory depression, which can lead to apnoea, respiratory arrest and death.
In our globalized world, early warning systems should continue to play a central role in protecting public health through the early detection and response to such threats.
Notes
Slovenia reported a sample of light green powder which was a test purchase from an Internet vendor. The sample was shipped from China and was received in May 2016.
References
Gulland JM, Robinson R (1925) The constitution of codeine and thebaine. Mem Proc Manchester Lit Phil Soc 69:79–86
Casy AF, Parfitt RT (1986) Opioid analgesics: chemistry and receptors. Plenum Press, New York
Janssen PAJ (1965) 1-Aralkyl-4-(N-aryl-carbonyl amino)piperidines and related compounds. US Patent 3,164,600, assigned to Research Laboratorium Dr. C. Janssen N.V.
Janssen PAJ, Van der Eycken CAM (1968) The chemical anatomy of potent morphine-like analgesics. In: Burger A (ed) Drugs affecting the central nervous system. Marcel Dekker, NewYork, pp 25–60
Van Bever WFM, Niemegeers CJE, Schellekens KHL, Janssen PAJ (1976) N-4-Substituted 1-(2-arylethyl)-4-piperidinyl-N-phenylpropanamides, a novel series of extremely potent analgesics with unusually high safety margin. Arzneimittelforschung 26:1548–1551
Van Daele PGH, De Bruyn MFL, Boey JM, Sanczuk S, Agten JTM, Janssen PAJ (1976) Synthetic analgesics: N-(1-[2-arylethyl]-4-substituted 4-piperidinyl) N-arylalkanamides. Arzneimittelforschung 26:1521–1531
KuKanich B, Papich MG (2009) Opioid analgesic drugs. In: Riviere JE, Papich HR (eds) Veterinary pharmacology and therapeutics. Wiley-Blackwell, Ames, pp 301–335
Lance RW, Kenny DE (2012) Thiafentanil oxalate (A3080) in nondomestic ungulate species. In: Muller RE, Fowler M (eds) Fowler’s zoo and wild animal medicine: current therapy. Saunders, St. Louis, pp 589–595
Kieffer BL (1999) Opioids: first lessons from knockout mice. Trends Pharmacol Sci 20:19–26. doi:10.1016/S0165-6147(98)01279-6
Pattinson KT (2008) Opioids and the control of respiration. Br J Anaesth 100:747–758. doi:10.1093/bja/aen094
Romberg R, Sarton E, Teppema L, Matthes HWD, Kieffer BL, Dahan A (2003) Comparison of morphine-6-glucuronide and morphine on respiratory depressant and antinociceptive responses in wild type and μ-opioid receptor deficient mice. Br J Anaesth 91:862–870. doi:10.1093/bja/aeg279
White JM, Irvine RJ (1999) Mechanisms of fatal opioid overdose. Addiction 94:961–972. doi:10.1046/j.1360-0443.1999.9479612.x
Anonymous (1983) New fentanyl compound. Microgram 16:147
Henderson GL (1988) Designer drugs: past history and future prospects. J Forensic Sci 33:569–575
Henderson GL (1991) Fentanyl-related deaths: demographics, circumstances, and toxicology of 112 cases. J Forensic Sci 36:422–433
U.S. Drug Enforcement Administration (2007) Control of a chemical precursor used in the illicit manufacture of fentanyl as a List I chemical. Fed Regist 72:20039–20047
Schumann H, Erickson T, Thompson TM, Zautcke JL, Denton JS (2008) Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol 46:501–506. doi:10.1080/15563650701877374
U.S. Drug Enforcement Administration (2017) NFLIS brief: fentanyl, 2001–2015. https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLISFentanylBrief2017.pdf. Accessed 17 April 2017
U.S. Drug Enforcement Administration (2017) Control of immediate precursor used in the illicit manufacture of fentanyl as a schedule II controlled substance. Fed Regist 75:37295–37299
Ojanperä I, Gergov M, Liiv M, Riikoja A, Vuori E (2008) An epidemic of fatal 3-methylfentanyl poisoning in Estonia. Int J Legal Med 122:395–400. doi:10.1007/s00414-008-0230-x
Tuusov J, Vals K, Tõnisson M, Riikoja A, Denissov G, Väli M (2013) Fatal poisoning in Estonia 2000–2009. Trends in illegal drug-related deaths. J Forensic Legal Med 20:51–56. doi:10.1016/j.jflm.2012.04.023
Berens AIL, Voets AJ, Demedts P (1996) Illicit fentanyl in Europe. Lancet 347(9011):1334–1335. doi:10.1016/S0140-6736(96)90981-2
European Monitoring Centre for Drugs and Drug Addiction (2012) Fentanyl in Europe: EMCDDA trendspotter study. Publications Office of the European Union, Luxembourg. http://www.emcdda.europa.eu/attachements.cfm/att_191974_EN_TD3112230ENN_Fentanyl.pdf. Accessed 22 April 2017
Mounteney J, Giraudon I, Denissov G, Griffiths P (2015) Fentanyls: are we missing the signs? Highly potent and on the rise in Europe. Int J Drug Policy 26:626–631. doi:10.1016/j.drugpo.2015.04.003
Kronstrand R, Druid H, Holmgren P, Rajs J (1997) A cluster of fentanyl-related deaths among drug addicts in Sweden. Forensic Sci Int 88:185–195. doi:10.1016/S0379-0738(97)00068-6
Centers for Disease Control and Prevention (2015) Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. CDCHAN-00384. CDC Health Alert Advisory, October 26, 2015. https://emergency.cdc.gov/han/han00384.asp. Accessed 22 April 2017
Centers for Disease Control and Prevention (2016) Fentanyl: preventing occupational exposure to emergency responders. November 28, 2016. https://www.cdc.gov/niosh/topics/fentanyl/default.html. Accessed 22 April 2017
Sutter ME, Gerona RR, Davis MT, Roche BM, Colby DK, Chenoweth JA, Adams AJ, Owen KP, Ford JB, Black HB, Albertson TE (2017) Fatal fentanyl: one pill can kill. Acad Emerg Med 24:106–113. doi:10.1111/acem.13034
San Francisco Department of Public Health (2015) Severe opioid overdoses in San Francisco caused by fentanyl-containing “Xanax” pill. 10-22-2015. http://www.sfcdcp.org/document.html?id=1005. Accessed 22 April 2017
Klar SA, Brodkin E, Gibson E, Padhi S, Predy C, Green C, Lee V (2016) Notes from the field: furanyl-fentanyl overdose events caused by smoking contaminated crack cocaine—British Columbia, Canada, July 15–18, 2016. MMWR Morb Mortal Wkly Rep 65:1015–1016. http://dx.doi.org/10.15585/mmwr.mm6537a6. Accessed 22 April 2017
Tomassoni AJ, Hawk KF, Jubanyik K, Nogee DP, Durant T, Lynch KL, Patel R, Dinh D, Ulrich A, D’Onofrio G (2017) Multiple fentanyl overdoses—New Haven, Connecticut, June 23, 2016. MMWR Morb Mortal Wkly Rep 66:107–111. http://dx.doi.org/10.15585/mm6604a4. Accessed 22 April 2017
European Monitoring Centre for Drugs and Drug Addiction (2016) EMCDDA–Europol Joint Report on a new psychoactive substance: N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl] acetamide (acetylfentanyl). Publications Office of the European Union, Luxembourg. http://www.emcdda.europa.eu/system/files/publications/2693/TDAS16001ENN.PDF. Accessed 22 April 2017. doi: 10.2810/890694
European Monitoring Centre for Drugs and Drug Addiction (2017) EMCDDA–Europol Joint Report on a new psychoactive substance: N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide (acryloylfentanyl). Publications Office of the European Union, Luxembourg. http://www.emcdda.europa.eu/system/files/publications/3873/TI_PUBPDF_TDAS17001ENN_PDFWEB_20170221105322.pdf. Accessed 22 April 2017. doi:10.2810/87713
Evans-Brown M, Sedefov R (2017) New psychoactive substances: driving greater complexity into the drug problem. Addiction 112:36–38. doi:10.1111/add.13528
European Monitoring Centre for Drugs and Drug Addiction (2016) EU drug markets report. In depth analysis. Publications Office of the European Union, Luxembourg. http://www.emcdda.europa.eu/publications/eu-drug-markets/2016/in-depth-analysis. Accessed 22 April 2017. doi: 10.2810/219411
Zhu Y, Ge B, Fang S, Zhu Y, Dai Q, Tan Z, Huang Z, Ghen X (1981) Studies on potent analgesics. I. Synthesis and analgesic activity of derivatives of fentanyl (in Chinese). Yaoxue Xuebao [Acta Pharmaceutica Sinica] 16:199–210
Maryanoff BE, Simon EJ, Gioannini T, Gorissen H (1982) Potential affinity labels for the opiate receptor based on fentanyl and related compounds. J Med Chem 25:903–909. doi:10.1021/jm00350a006
U.S. Drug Enforcement Administration (2017) Emerging threat report: fourth quarter 2016. https://ndews.umd.edu/resources/dea-emerging-threat-reports. Accessed 10 April 2017
Clark AB, Nelson JD (1984) China White—a California phenomenon. Abstract. J Forensic Sci Soc 24:284
Cooper D, Jacob M, Allen A (1986) Identification of fentanyl derivatives. J Forensic Sci 31:511–528
Valter K, Arrizabalaga P (1998) Designer drugs directory. Elsevier Science SA, Amsterdam, p 166
Essawi MYH (1998) Synthesis of fentanyl analogues as nonequilibrium irreversible ligands for opioid receptors. Bull Fac Pharmacy (Cairo Univ) 36(3):39–45
Essawi MYH (1999) Fentanyl analogues with a modified propanamido group as potential affinity labels: synthesis and in vivo activity. Pharmazie 54:307–308
Prodduturi S, Smith GJ, Wokowich AM, Doub WH, Westenberger BJ, Buhse L (2009) Reservoir based fentanyl transdermal drug delivery systems: effect of patch age on drug release and skin permeation. Pharm Res 26:1344–1352. doi:10.1007/s11095-009-9843-0
Moffat AC, Osselton MD, Widdop B (2011) Clarke’s analysis of drugs and poisons. Pharmaceutical Press, London, pp 1400–1402
Hansch C, Leo A, Hoekman D (1995) Exploring QSAR. Hydrophobic, electronic, and steric constants. American Chemical Society, Washington, DC, p 348
Degg B (2014) LC-MS-MS method developed to detect synthetic opioid. The Column 10(3):9. http://www.chromatographyonline.com/lc%E2%80%93ms%E2%80%93ms-method-developed-detect-synthetic-opioid. Accessed 17 April 2017
Janssen PAJ, Gardocki JF (1964) Method for producing analgesia. US Patent 3,141,823, assigned to Research Laboratorium Dr. C. Janssen N.V.
Zee S-H, Wang W-K (1980) A new process for the synthesis of fentanyl. J Chin Chem Soc 27:147–149
Casy AF, Huckstep MR (1988) Structure-activity studies of fentanyl. J Pharm Pharmacol 40:605–608. doi:10.1111/j.2042-7158.1988.tb05318.x
Gupta PK, Ganesan K, Pande A, Malhotra RC (2005) A convenient one pot synthesis of fentanyl. J Chem Res 2005:452–453. doi:10.3184/030823405774309078
Vardanyan RS, Hruby VJ (2015) Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications. Future Med Chem 6:385–412. doi:10.4155/fmc.13.215
Breindhal T, Kimergård A, Andreasen MF, Pedersen DS (2016) Identification of a new psychoactive substance in seized material: the synthetic opioid N-phenyl-N-[1-(2-phenethyl)piperidin-4-yl]propen-2-amide (Acrylfentanyl). Drug Test Anal 9:415–422. doi:10.1002/dta.2046
Huckle D, Lockhart IM, Wright M (1972) 4,5-Dihydro-1-benzoxepin-3(2H)-one, N-substituted 2,3-dihydro-1,5-benzoxazepin-4(5H)-ones, and related compounds. J Chem Soc Perkin Trans 1 1972:2425–2428. doi:10.1039/P19720002425
Slovenian National Forensic Laboratory (2016) Analytical report. Acryloyl-F (C22H26N2O). N-(1-Phenethylpiperidin-4-yl)-N-phenylacrylamide. European Project RESPONSE to challenges in forensic drug analyses. http://www.policija.si/apps/nfl_response_web/0_Analytical_Reports_final/Acryloyl-F-ID-1565-16_report010816.pdf. Accessed 17 April 2017
Rittgen J, Pütz M, Zimmerman R (2012) Identification of fentanyl derivatives at trace levels with nonaqueous capillary electrophoresis-electrospray-tandem mass spectrometry (MSn, n = 2, 3): analytical method and forensic applications. Electrophoresis 33:1595–1605. doi:10.1002/elps.201100655
Neogen (2016) Fentanyl analog ELISA kit for forensic drug detection. http://toxicology.neogen.com/pdf/TechSheet/Fentanyl.pdf. Accessed 10 April 2017
Helander A, Bäckberg M, Signell P, Beck O (2017) Intoxications involving acrylfentanyl and other novel designer fentanyls—results from the Swedish STRIDA project. Clin Toxicol. doi:10.1080/15563650.2017.1303141
Watanabe S, Vikingsson S, Roman M, Green H, Kronstrand R, Wohlfarth A (2017) In vitro and in vivo metabolite identification for the new synthetic opioids acetylfentanyl, acrylfentanyl, furanylfentanyl, and 4-fluoroisobutyrylfentanyl. AAPS J. doi:10.1208/s12248-017-0070-z
Gupta PK, Yadav SK, Bhutia YD, Singh P, Rao P, Gujar NL, Ganesan K, Bhattacharya R (2013) Synthesis and comparative bioefficacy of N-(1-phenethyl-4-piperidinyl)propionanilide (fentanyl) and its 1-substituted analogs in Swiss albino mice. Med Chem Res 22:3888–3896. doi:10.1007/s00044-012-0390-6
Vuckovic S, Prostran M, Ivanovic M, Dosen-Micovic L, Savic Vujovic K, Vucetic C, Kadija M, Mikovic Z (2011) Pharmacological evaluation of 3-carbomethoxy fentanyl in mice. Pharmaceuticals 4:233–243. doi:10.3390/ph4020233
Vartanyan RS, Martirosyan VO, Vartanyan SA, Vlasenko ÉV, Durgaryan LK, Azlivyan AS (1989) Synthesis and analgesic activity of 4-anilides of 1-substituted-2,5-dimethylpiperidines. Pharm Chem J 23:383–387. doi:10.1007/BF00758289
TOXNET (2016) Morphine. https://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+2134. Accessed 10 April 2017
Bianchi C, Franceschini J (1954) Experimental observations on Haffner’s method for testing analgesic drugs. Br J Pharmacol 9:280–284. doi:10.1111/j.1476-5381.1954.tb01681.x
Archer S, Michael J, Michael M, Simon EJ, Abdelhamid EME, Nelson WL, Koolpe GA (1985) Chloroacryloyl amides and alpha-methylenelactones from naltrexone, oxymorphone and fentanyl. Neuropeptides 5:395–398
Jöst C, Nitsche C, Scholz T, Roux L, Klein CD (2014) Promiscuity and selectivity in covalent enzyme inhibition: a systematic study of electrophilic fragments. J Med Chem 57:7590–7599. doi:10.1021/jm5006918
Liu C, Sabnis Y, Zhao Z, Zhang T, Buhrlage SJ, Jones LH, Gray NS (2013) Developing irreversible inhibitors of the protein kinase cysteinome. Chem Biol 20:146–159. doi:10.1016/j.chembiol.2012.12.006
National Center for Biotechnology Information. PubChem Substance database (2012) MLS003960120. 5.1 Bioassay results. Deposit date: 2012-09-15. https://pubchem.ncbi.nlm.nih.gov/substance/144091883. Accessed 10 April 2017
McClain DA, Hug CC (1980) Intravenous fentanyl kinetics. Clin Pharmacol Ther 28:106–114. doi:10.1038/clpt.1980.138
Goromaru T, Matsuura H, Yoshimura N, Miyawaki T, Sameshima T, Miyao J, Furuta T, Baba S (1984) Identification and quantitative determination of fentanyl metabolites in patients by gas chromatography–mass spectrometry. Anesthesiology 61:73–77
Guitton J, Désage M, Alamercery S, Dutruch L, Dautraix S, Perdrix JP, Brazier JL (1997) Gas chromatographic–mass spectrometry and gas chromatographic–Fourier transform infrared spectroscopy assay for the simultaneous identification of fentanyl metabolites. J Chromatogr B 59:59–70. doi:10.1016/S0378-4347(97)00050-9
DePriest AZ, Puet BL, Holt AC, Roberts A, Cone EJ (2015) Metabolism and disposition of prescription opioids: a review. Forensic Sci Rev 27:115–145
Patton AL, Seely KA, Pulla S, Rusch NJ, Moran CL, Fantegrossi WE, Knight LD, Marraffa JM, Kennedy PD, James LP, Endres GW, Moran JH (2014) Quantitative measurement of acetyl fentanyl and acetyl norfentanyl in human urine by LC-MS/MS. Anal Chem 86:1760–1766. doi:10.1021/ac4036197
Melen’tev AB, Kataev SS, Dvorskaya ON (2015) Identification and analytical properties of acetyl fentanyl metabolites. J Anal Chem 70:240–248. doi:10.1134/S1061934815020124
Poklis J, Poklis A, Wolf C, Mainland M, Hair L, Devers K, Chrostowski L, Arbefeville E, Merves M, Pearson J (2015) Postmortem tissue distribution of acetyl fentanyl, fentanyl and their respective nor-metabolites analyzed by ultrahigh performance liquid chromatography with tandem mass spectrometry. Forensic Sci Int 257:435–441. doi:10.1016/j.forsciint.2015.10.021
Labroo RB, Paine MF, Thummel KE, Kharasch ED (1997) Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos 25:1072–1080
Higashikawa Y, Suzuki S (2008) Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds: novel metabolites in rat urine following injection of α-methylfentanyl, one of the most abused typical designer drug. J Health Sci 54:629–637. doi:10.1248/jhs.54.629
Schneider E, Brune K (1986) Opioid activity and distribution of fentanyl metabolites. Naunyn Schmiedebergs Arch Pharmacol 334:267–274. doi:10.1007/BF00508781
Jin M, Gock SB, Jannetto PJ, Jentzen JM, Wong SH (2005) Pharmacogenomics as molecular autopsy for forensic toxicology: genotyping cytochrome P450 3A4*1B and 3A5*3 for 25 fentanyl cases. J Anal Toxicol 29:590–598. doi:10.1093/jat/29.7.590
European Medicines Agency (EMA) (2016) Instanyl. Annex 1. Summary of product characteristics. 07/03/2017 Instanyl -EMEA/H/C/000959-IA/0041/G. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000959/WC500033141.pdf. Accessed 23 April 2017
McCance-Katz EF, Sullivan LE, Nallani S (2010) Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed opioids. Am J Addict 19:4–16. doi:10.1111/j.1521-0391.2009.00005.x
Gillman PK (2005) Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth 95:434–441. doi:10.1093/bja/aei210
Reddit (2017) Acrylfentanyl: observations and data. https://www.reddit.com/r/researchchemicals/comments/4y4imi/acrylfentanyl_observations_and_data. Accessed 23 April 2017
Reddit (2017) Dosing acrylfentanyl. https://www.reddit.com/r/researchchemicals/comments/5pk6s0/dosing_acrylfentanyl/?st=izb144sc&sh=49140898. Accessed 23 April 2017
Hess R, Stiebler G, Herz A (1972) Pharmacokinetics of fentanyl in man and the rabbit. Eur J Clin Pharmacol 4:137–141. doi:10.1007/BF00561135
Streisand JB, Varvel JR, Stanski DR, Le Maire L, Ashburn MA, Hague BI, Tarver SD, Stanley TH (1991) Absorption and bioavailability of oral transmucosal fentanyl citrate. Anesthesiology 75:223–229
Gorodetzky CW, Martin WR (1965) A comparison of fentanyl, droperidol, and morphine. Clin Pharmacol Ther 6:731–739. doi:10.1002/cpt196566731
European Monitoring Centre for Drugs and Drug Addiction (2015) Preventing fatal overdoses: a systematic review of the effectiveness of take-home naloxone, EMCDDA Papers. Publications Office of the European Union, Luxembourg. doi:10.2810/396726
Centers for Disease Control and Prevention (2013) Recommendations for laboratory testing for acetyl fentanyl and patient evaluation and treatment for overdose with synthetic opioid. CDC Health Alert Advisory, June 20, 2013. http://emergency.cdc.gov/han/han00350.asp. Accessed 22 April 2017
Food and Drug Administration (2016) FDA Advisory Committee on the most appropriate dose or doses of naloxone to reverse the effects of life-threatening opioid overdose in the community settings. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndAnalgesicDrugProductsAdvisoryCommittee/UCM522688.pdf. Accessed 17 April 2017
Martin M, Hecker J, Clark R, Frye J, Jehle D, Lucid EJ, Harchelroad F (1991) China White epidemic: an eastern United States emergency department experience. Ann Emerg Med 20:158–164. doi:10.1016/S0196-0644(05)81216-8
Bäckberg M, Beck O, Jönsson K-H, Helander A (2015) Opioid intoxications involving butyrfentanyl, 4-fluorobutyrfentanyl, and fentanyl from the Swedish STRIDA project. Clin Toxicol 53:609–617. doi:10.3109/15563650.2015.1054505
U.S. Drug Enforcement Administration (2016) News release; DEA issues carfentanil warning to police and public. September 22, 2016. https://www.dea.gov/divisions/hq/2016/hq092216.shtml. Accessed 17 April 2017
United Nations Office on Drugs and Crime (2017) March 2017—China: carfentanil, furanylfentanyl, acrylfentanyl and valerylfentanyl placed under national control. https://www.unodc.org/LSS/Announcement/Details/c514525c-afb0-430f-8321-7e63d40b53aa. Accessed 17 April 2017
Acknowledgements
The preparation of this publication was supported, in part, by a contract from the European Monitoring Centre for Drugs and Drug Addiction (contract code CT.16.SAT.0099.1.0) to István Ujváry. We thank Ms. Veronika Mikes for interpreting Chinese articles. The authors would also like to extend their sincere thanks and appreciation to the Early Warning System correspondents of the Reitox national focal points and experts from their national early warning system networks; the Europol national units and Europol Project Synergy; Dr. Anders Helander, Department of Laboratory Medicine and Department of Clinical Pharmacology, Karolinska Institutet, Stockholm; Dr. Torben Breindahl, Department of Clinical Biochemistry, North Denmark Regional Hospital, Aalborg University, Hjørring; and Ms. Anabela Almeida, Mr. Andrew Cunningham and Ms. Paulete Duque at the EMCDDA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of article, formal informed consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ujváry, I., Jorge, R., Christie, R. et al. Acryloylfentanyl, a recently emerged new psychoactive substance: a comprehensive review. Forensic Toxicol 35, 232–243 (2017). https://doi.org/10.1007/s11419-017-0367-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-017-0367-8