Abstract
Nerve inflammation is linked to the development of various neurological disorders. This study aimed to examine whether Glycyrrhizae Radix effectively influences the duration of the pentobarbital-induced loss of righting reflex, which may increase in a mouse model of lipopolysaccharide (LPS)-induced nerve inflammation and diazepam-induced γ-aminobutyric acid receptor hypersensitivity. Furthermore, we examined the anti-inflammatory effects of Glycyrrhizae Radix extract on LPS-stimulated BV2 microglial cells, in vitro. Treatment with Glycyrrhizae Radix significantly decreased the duration of pentobarbital-induced loss of righting reflex in the mouse model. Furthermore, treatment with Glycyrrhizae Radix significantly attenuated the LPS-induced increases in interleukin-1β, interleukin-6, and tumor necrosis factor-alpha at the mRNA level, and it significantly reduced the number of ionized calcium-binding adapter molecule-1-positive cells in the hippocampal dentate gyrus 24 h after LPS treatment. Treatment with Glycyrrhizae Radix also suppressed the release of nitric oxide, interleukin-1β, interleukin-6, and tumor necrosis factor protein in culture supernatants of LPS-stimulated BV2 cells. In addition, glycyrrhizic acid and liquiritin, active ingredients of Glycyrrhizae Radix extract, reduced the duration of pentobarbital-induced loss of righting reflex. These findings suggest that Glycyrrhizae Radix, as well as its active ingredients, glycyrrhizic acid and liquiritin, may be effective therapeutic agents for the treatment of nerve inflammation-induced neurological disorders.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nerve inflammation is linked to the development of various neurological disorders. Acute nerve inflammation is associated with the development of delirium [1, 2], and chronic nerve inflammation is involved in diseases, such as Alzheimer's disease, depression, Parkinson’s disease, and dementia [3,4,5]. To the best of our knowledge, no effective therapeutic drug targeting nerve inflammation has been developed to treat these neurological disorders. Thus, novel strategies designed to suppress nerve inflammation are an attractive avenue to treat neurological disorders.
Lipopolysaccharide (LPS), which is a component of the outer membrane of the cell wall of gram-negative bacteria, is typically used to induce nerve inflammation in rodents [6]. LPS-induced nerve inflammation enhances γ-aminobutyric acid (GABA) activity by increasing the surface expression of GABAA receptors in nerve cells and GABA-elicited chloride currents in the hippocampal neurons through the phosphatidylinositol 3-kinase/Akt pathway [7]. The amplitude of pharmacologically isolated postsynaptic GABAergic potentials significantly increased after LPS exposure [8]. Therefore, LPS-induced nerve inflammation may induce over-sedation by increasing GABA activity. Additionally, hypnotic barbiturates enhance the activation of GABAA receptors by GABA [9]. Thus, LPS-induced nerve inflammation in mice extends the length of pentobarbital-induced loss of righting reflex (LORR) [7, 10], and this is further enhanced by low-dose administration of the benzodiazepine, diazepam [7]. The development of over-sedation associated with infection and post-surgery inflammation may be attributed to GABAergic hyperactivity with neuroinflammation [10]. In clinical practice, benzodiazepines for the treatment of insomnia and sedation are frequently used to treat inflammation associated with surgery and infection [11]. Therefore, in mice, evaluating the length of LPS- and diazepam-mediated LORR induced by pentobarbital could be used to evaluate over-sedation caused by nerve inflammation postoperatively [12].
Glycyrrhizae Radix, which is commonly known as licorice, is one of the most commonly used herbal medicines in traditional drugs, foods, and cosmetics. It is used as an antitussive, expectorant, and antipyretic for its role in relieving cough, pharyngitis, bronchitis, and bronchial asthma [13, 14]. Glycyrrhizae Radix has anti-inflammatory properties and is widely prescribed clinically [15]. It has also been reported to be useful for neurological disorders, such as Parkinson’s disease [16]. Therefore, Glycyrrhizae Radix may be useful for the treatment of neurological disorders caused by nerve inflammation.
This study aimed to examine whether Glycyrrhizae Radix effectively decreases the duration of pentobarbital-induced LORR, which is extended following LPS and diazepam administration in a mouse model mimicking post-surgical nerve inflammatory conditions [12]. We further investigated whether Glycyrrhizae Radix effectively influences nerve inflammation of the hippocampus. Finally, we examined the anti-inflammatory in vitro effects of Glycyrrhizae Radix on LPS-stimulated BV2 microglial cells.
Materials and methods
Animals
Male Jcl:ICR mice (9–11 weeks old; Japan CLEA, Shizuoka, Japan), with an initial weight of 26–35 g, were housed and allowed to habituate for more than 1 week prior to the start of experimentation. Animals were maintained in a temperature- (23 ± 1 °C) and humidity-controlled room (55 ± 2%); one animal was housed per cage under a constant day-night rhythm (lights were on from 07:00 to 19:00). Standard laboratory food (CE-2 from Japan CLEA) and water were available ad libitum. Animal experiments were approved by the Committee for the Care and Use of Laboratory Animals at Kochi University (approval no. O-0009; April 23, 2021).
Drugs
On the day of testing, the following drugs were used: LPS (Escherichia coli, O127:B8 L4516; Sigma-Aldrich, St. Louis, MO, USA), diazepam (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and pentobarbital sodium (Kyoritsu Seiyaku Co., Tokyo, Japan). All drugs were injected intraperitoneally (i.p.) after being dissolved in saline. Glycyrrhizic acid (GL) and liquiritin (LQ; Tokyo Chemical Industry Co., Tokyo, Japan) were suspended in water on the day of testing. GL (50 mg/kg) and LQ (25 mg/kg) were orally administered (p.o.). Glycyrrhizae Radix extract was also orally administered (500, 1000, and 2000 mg/kg p.o.). The doses of GL and LQ were determined by converting the amount contained in 1000 mg/kg of Glycyrrhizae Radix [17]. Dried roots and stolons of Glycyrrhiza uralensis Fisher (lot: R17871) were purchased from Tsumura (Tokyo, Japan). The crude drug was extracted using hot water with 20 times the volume of purified water by weight. We obtained an extract of 3.08 ± 0.08 g from 20 g of the dried roots and stolons of Glycyrrhiza uralensis Fisher. The GL and LQ contents in 1.0 g of the dried roots and stolons of Glycyrrhiza uralensis Fisher were 25.8 ± 1.8 mg and 5.9 ± 0.5 mg, respectively, procured using the following process: Glycyrrhizae Radix hot water extract (1.0 g preparation) was obtained using 20 mL of methanol with sonication for 30 min. The extract (20 µL) was injected into a Shimadzu LC-20 system (Shimadzu Corporation, Kyoto, Japan), which consisted of a Shimadzu LC-20 AR HPLC pump, Shimadzu series DGU-20A3R degasser, and Shimadzu SIL-20 A autosampler. For the three-dimensional (3D)-HPLC profile, TSK gel ODS-80TS (250 × 4.6 mm; Tosoh, Tokyo, Japan) was maintained at 40 °C with a mobile phase linear gradient from 90% A (50 mM AcOH–AcONH4 buffer) and 10% B (CH3CN) to 100% B in 60 min. The 3D-HPLC profile of the Glycyrrhizae Radix hot water extract is shown as Online Resource 1.
For GL and LQ measurements, COSMOSIL 5C18-AR-II (150 × 4.6 mm; Nakarai Tesque, Kyoto, Japan) was controlled at 40 °C. The mobile phase A solution comprised 0.2 vol% formic acid/water, and the mobile phase B solution was acetonitrile. The mobile phase comprised solution A and solution B with a solution B gradient (0–10 min, 17%; 12–18 min, 50%; 20–23 min, 95%) at a flow rate of 1.0 mL/min. GL and LQ were measured at wavelengths of 250 nm and 275 nm, respectively.
Study design
Glycyrrhizae Radix or a Glycyrrhizae Radix component (GL or LQ) was given orally on the first day [11], followed by the administration of a single dose every 24 h. The same amount of water (0.5 mL/body) was administered to control group mice. On the second day, 2 h after Glycyrrhizae Radix, GL, or LQ administration, mice were intraperitoneally injected with LPS (300 μg/kg). The same amount of saline (0.5 mL/body) was injected into the control group mice. On day 3, 2 h after Glycyrrhizae Radix, GL, or LQ administration, mice were intraperitoneally injected with diazepam (300 μg/kg), followed by an intraperitoneal injection of pentobarbital sodium (50 mg/kg) after 30 min. The LORR duration was recorded as the time between the loss and recovery of the righting movement. Mice failing to fall asleep within 15 min of pentobarbital administration were excluded [7, 12, 18, 19]. The study design is shown in Fig. 1a.
On day 2, 2 h after LPS injection, blood was removed by transcardial perfusion with ice-cold phosphate-buffered saline (PBS), followed by brain removal. mRNA expression measurements used these tissue samples from the hippocampus. On day 3 after Glycyrrhizae Radix or water administration, blood was removed by transcardial perfusion with ice-cold PBS, followed by brain removal. Immunohistochemistry used these tissue samples from the hippocampus. The study design is shown in Fig. 1b.
To extract total mRNA, hippocampal samples were cut into 0.1 × 0.1 × 0.1 × cm pieces and further homogenized using an ultrasonic homogenizer (NR-50 M; Microtech, Chiba, Japan). Then, total RNA was extracted using the RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). To obtain cDNA, reverse transcription was performed using a PrimeScript RT reagent kit (Takara Bio, Otsu, Japan), and TaqMan quantitative polymerase chain reaction (PCR) was performed using a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA). The mRNA expression levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. TaqMan universal PCR master mix and PCR primers with TaqMan probes for GAPDH (Mm99999915_g1), TNF-α (Mm00443258_m1), and IL-6 (Mm00446190_m1) were purchased from Applied Biosystems.
Immunohistochemistry
Mice brains were removed after transcardiac perfusion with saline and 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The brains were post-fixed overnight in 4% paraformaldehyde and further immersed in 20% aqueous sucrose for 48 h. Then, these were frozen on powdered dry ice, and coronal sections containing the hippocampal dentate gyrus were prepared to a thickness of 30 μm using a cryostat. These sections were immersed in 10 mM PBS containing 0.2% Triton X-100 (PBST) for 30 min at 20–25 °C. Furthermore, after 2 h of incubation in 3% bovine serum albumin in PBST, the sections were immersed in rabbit anti-mouse ionized calcium-binding adapter molecule-1 (Iba1) antibody (dilution 1:500; Wako) and incubated overnight at 4 °C. The sections were then washed in PBST and incubated with Alexa Fluor 488-labeled donkey anti-rabbit immunoglobulin G antibody (diluted 1:500 in PBST; Invitrogen, Waltham, MA, USA) for 1 h at 20–25 °C. Sections were then washed in PBST and mounted using mounting media (Vectashield; Vector Laboratories, Peterborough, UK). Slides were excited with Alexa Fluor 488 dye using a mercury vapor lamp and a 470–490-nm bandpass filter, and analyzed with a fluorescence microscope (FV-1000D; Olympus, Tokyo, Japan). The light emitted from Alexa Fluor 488 was focused using a 515–550 nm bandpass filter. The stained cells were photographed at 200 × magnification. Iba1-positive cells in the bilateral hippocampal dentate gyrus (bregma − 1.5 mm to − 2.5 mm) were counted in any two hippocampal sections of each mouse, such that each set contained sections covering the entire anterior–posterior axis of the hippocampus. Iba1-positive and DAPI-positive cells in the dentate gyrus were counted by an investigator blinded to group assignment using WinRoof software (Mitani Corporation, Fukui, Japan). Iba1-positive cells were counted when DAPI-stained cells and Iba-1 antibody-stained cells overlapped.
BV2 microglial cell culture
BV2 microglial cells were purchased from AcceGen Biotechnology (ABC-TC212S; Fairfield, NJ, USA). BV2 cells were maintained at 37 °C and 5% CO2, in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 0.10 mg/mL streptomycin. BV2 cells were grown in 24-well plates at a concentration of 5.0 × 105 cells/well. BV2 cells were stimulated with 1 µg/mL LPS; cells were also simultaneously treated with Glycyrrhizae Radix extract (50, 100, 200 µg/mL) or 1.0 µM dexamethasone (Wako Pure Chemical Industries, Ltd.), as a positive control, and incubated at 37 °C for 24 h [20]. The culture supernatant was then collected and nitric oxide (NO) was measured by Griess assay, and THE IL-1β, IL-6, and TNF-α levels were measured using an ELISA kit (R&D Systems, Minnesota, MN, USA) in accordance with the manufacturer's instructions.
Statistical analysis
All statistical analyses were performed using EZR version 1.29 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [21,22,23]. Data are expressed as means ± standard deviations (SDs). One-way analysis of variance (ANOVA) was used to examine the significance of drug treatment effects, followed by Tukey's test as a multiple comparison test, to evaluate differences between groups. For comparisons between the two groups, Student’s t-test was used. Statistical significance was set at P < 0.05.
Results
Efficacy of Glycyrrhizae Radix extract for reducing pentobarbital-induced LORR duration in a mouse model of nerve inflammation
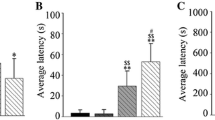
We examined the efficacy of Glycyrrhizae Radix administration for reducing the duration of pentobarbital-induced LORR in a mouse model that simulated post-surgical nerve inflammation. As shown in Fig. 2a, one-way ANOVA revealed a significant drug effect on the duration of pentobarbital-induced LORR (F(4, 45) = 10.1, P < 0.01). LPS treatment significantly increased the duration of pentobarbital-induced LORR compared to that in the control group (P < 0.01). Glycyrrhizae Radix extract at 1.0 and 2.0 g/kg body weight significantly decreased the duration of pentobarbital-induced LORR compared to that in the LPS-treated group (P < 0.05). Further, as shown in Fig. 2b, Glycyrrhizae Radix had no effect on the duration of pentobarbital-induced LORR without LPS administration in this mouse model (P = 0.40).
Effect of Glycyrrhizae Radix (GR) on pentobarbital-induced loss of righting reflex duration in a mouse model. a Effect of GR on the pentobarbital-induced loss of righting reflex (LORR) duration in the mouse model. b Effect of GR (2000 mg/kg p.o.), on the pentobarbital-induced LORR duration in a mouse model without lipopolysaccharide (LPS) administration. Values are expressed as means ± SDs for groups of 10 mice. *P < 0.05, **P < 0.01, evaluated using the one-way analysis of variance followed by Tukey's tests. For the evaluation of the GR effect in a mouse model without LPS administration, Student’s t-test was used
Efficacy of Glycyrrhizae Radix extract for reducing the IL-1β, IL-6, and TNF-α mRNA levels in the hippocampus in LPS-treated mice
We examined the efficacy of Glycyrrhizae Radix administration for reducing the IL-1β, IL-6, and TNF-α mRNA levels in the hippocampus in a mouse model that simulated post-surgical nerve inflammation. As shown in Fig. 3, one-way ANOVA revealed significant drug effects on the expression of IL-1β, IL-6, and TNF-α (IL-1β: F(2, 15) = 19.3, P < 0.01; IL-6: F(2, 15) = 36.9, P < 0.01; TNF-α: F(2, 15) = 48.6, P < 0.01). LPS treatment significantly increased the mRNA expressions of IL-1β, IL-6, and TNF-α compared to those in the control group (IL-1β, P < 0.01; IL-6, P < 0.01; TNF-α, P < 0.01). Glycyrrhizae Radix extract treatment at 2.0 g/kg body weight induced a significant decrease in the mRNA expression of IL-1β, IL-6, and TNF-α compared to that in the LPS-treated group (Glycyrrhizae Radix: IL-1β, P < 0.05; IL-6, P < 0.01; TNF-α, P < 0.01).
Effect of Glycyrrhizae Radix (GR) on interleukin (IL)-1β, IL-6 and tumor necrosis factor-alpha (TNF-α) mRNA levels in the hippocampus. The IL-1β, IL-6 and TNF-α mRNA levels were measured 2 h after treatment with lipopolysaccharide (LPS; 300 µg/kg i.p.). a Effect of GR on IL-1β mRNA levels in the hippocampus. b Effect of GR on IL-6 mRNA levels in the hippocampus. c Effect of GR on Effect of GR on IL-6 mRNA levels in the hippocampus. *P < 0.05, **P < 0.01, evaluated using the one-way analysis of variance followed by Tukey’s tests
Efficacy of Glycyrrhizae Radix extract for reducing the number of Iba1-positive cells in the subgranular zone of the hippocampal dentate gyrus in LPS-treated mice
We examined the efficacy of Glycyrrhizae Radix administration for reducing the number of Iba1-positive cells in the subgranular zone of the hippocampal dentate gyrus in a mouse model that simulated post-surgical nerve inflammation. As shown in Fig. 4, one-way ANOVA revealed a significant drug effect on the number of Iba1-positive cells (F(2, 15) = 192.1, P < 0.01). The administration of LPS induced a significant increase in the number of Iba1-positive hippocampal cells compared to that in the control group (P < 0.01). Glycyrrhizae Radix administration significantly decreased Iba1-positive cells compared to that in the LPS-treated group (P < 0.01). Furthermore, under LPS administration, cell bodies became hypertrophied and amoeboid; however, Glycyrrhizae Radix administration suppressed these morphological changes (qualitative observations).
Effect of Glycyrrhizae Radix (GR) on the number of ionized calcium-binding adapter molecule-1 (Iba1)-positive cells in the subgranular zone of the hippocampal dentate gyrus in lipopolysaccharide (LPS)-treated mice. Iba1-positive cells were counted 24 h after treatment with LPS (300 µg/kg via i.p.). GR was administered orally (2000 mg/kg via p.o.) for two days, a day before and a day after LPS treatment. a Graphed the effect of GR on the number of Iba1-positive cells. b The effect of GR on tissue each samples from the hippocampus in immunostaining. **P < 0.01, evaluated using the two-way analysis of variance followed by Tukey’s tests. Scale bar = 100 µm
Efficacy of Glycyrrhizae Radix extract for reducing inflammatory factors in BV2 cells stimulated with LPS
We investigated the direct effects of Glycyrrhizae Radix extract administration on microglial BV2 cells. As shown in Fig. 5, one-way ANOVA revealed a significant drug effect on the NO level (F(5, 18) = 120.3, P < 0.01). LPS administration significantly increased the NO level compared to that in control cells (P < 0.01). Glycyrrhizae Radix extract administration significantly decreased the NO level compared to that in LPS-stimulated cells (50–200 μg/mL, P < 0.01).
Effect of Glycyrrhizae Radix (GR) on the expression of nitric oxide (NO) in lipopolysaccharide (LPS)-stimulated BV2 cells. The expression of NO was measured 24 h after treatment with LPS (1 µg/mL) and GR (0, 50, 100, 200 µg/mL) or dexamethasone (Dex; 1 µM), as a positive control, in BV2 cells. Values are expressed as means ± standard deviations for groups of five mice. **P < 0.01 vs. the group, in which only LPS was administered, evaluated using the one-way analysis of variance followed by Tukey’s tests
As shown in Fig. 6, one-way ANOVAs revealed significant drug effects on the levels of IL-1β, IL-6, and TNF-α (IL-1β: F(5, 18) = 113, P < 0.01; TNF-α: F(5, 18) = 299, P < 0.01; IL-6: F(5, 18) = 44.8, P < 0.01). LPS stimulation also significantly increased the IL-1β, IL-6, and TNF-α levels compared to those in control cells (IL-1β, P < 0.01; IL-6, P < 0.01; TNF-α, P < 0.01). Glycyrrhizae Radix extract administration significantly decreased the concentrations of IL-1β and IL-6 in LPS-stimulated BV2 cell culture supernatants compared to those in LPS-stimulated cells (IL-1β, 100–200 μg/mL, P < 0.01; IL-6, 50–200 μg/mL, P < 0.01; TNF-α, 200 μg/mL, P < 0.01).
Effect of Glycyrrhizae Radix (GR) on the expression of interleukin (IL)-1β,IL-6 and tumor necrosis factor-alpha (TNF-α) in lipopolysaccharide (LPS)-stimulated BV2 cells. The IL-1β, IL-6, and TNF-α levels were measured at 24 h after treatment with LPS (1 µg/mL) and GR (0, 50, 100, 200 µg/mL) or dexamethasone (Dex;1 µM), as a positive control, in BV2 cells. a Effect of GR on the expression of IL-1β in LPS-stimulated BV2 cells. b Effect of GR on the expression of IL-6 in LPS-stimulated BV2 cells. c Effect of GR on the expression of TNF-α in LPS-stimulated BV2 cells. **P < 0.01 vs. the group, in which only LPS was administered, evaluated using the one-way analysis of variance followed by Tukey’s tests
Efficacy of the active ingredients in Glycyrrhizae Radix for reducing pentobarbital-induced LORR duration in a mouse model of nerve inflammation
We investigated the efficacy of GL and LQ, which are active ingredients of Glycyrrhizae Radix, at concentrations of 50 mg/kg. As shown in Fig. 7, one-way ANOVA revealed a significant drug effect on the duration of pentobarbital-induced LORR (F(4, 45) = 6.1, P < 0.01). LPS treatment significantly increased the duration of pentobarbital-induced LORR compared to that in the control group (P < 0.01). The administration of GL and LQ significantly decreased the duration of pentobarbital-induced LORR compared to that in the LPS-treated group (P < 0.01), with no difference between the GL and LQ groups (P = 0.99).
Effect of Glycyrrhizae Radix (GR; 2000 mg/kg p.o.), glycyrrhizic acid (GL; 50 mg/kg p.o.), and liquiritin (LQ; 25 mg/kg p.o.) on pentobarbital-induced loss of righting reflex duration in a mouse model. Values are expressed as means ± standard deviations for groups of 10 mice. **P < 0.01, evaluated using the one-way analysis of variance followed by Tukey's tests. LPS lipopolysaccharide
Discussion
Here, we examined the effects of Glycyrrhizae Radix extract on the duration of pentobarbital-induced LORR, as well as histological changes in the hippocampus. Our data suggest that Glycyrrhizae Radix extract reduced the LORR duration and had an anti-inflammatory efficacy on the hippocampus. Further, GL and LQ, active ingredients of Glycyrrhizae Radix, decreased the duration of pentobarbital-induced LORR in a mouse model of nerve inflammation. In addition, treatment with Glycyrrhizae Radix extract suppressed the release of NO, IL-1β, IL-6, and TNF-α from BV2 cells, which were stimulated with LPS.
Based on a previous study, effects on post-surgical neuroinflammatory conditions can be assessed by evaluating the effect on the pentobarbital-induced LORR duration enhanced by diazepam and neuroinflammation [12]. Thus, we consider this to be a suitable model to evaluate anti-neuroinflammatory drug effects. In the present study, Glycyrrhizae Radix extract reduced inflammatory responses in the hippocampus; additionally, Glycyrrhizae Radix, as well as its active ingredients, GL and LQ, decreased the duration of pentobarbital-induced LORR. Therefore, Glycyrrhizae Radix, GL, and LQ have anti-neuroinflammatory effects, suggesting an ability to inhibit excessive GABA activity associated with brain inflammation. In addition, we assessed the duration of pentobarbital-induced LORR without LPS administration, as Glycyrrhizae Radix alone may affect GABA receptors [24]. However, we found that Glycyrrhizae Radix alone had no effect on the duration of pentobarbital-induced LORR without LPS administration (Fig. 2). Therefore, the inhibitory effect of Glycyrrhizae Radix on GABA activity may be mediated by the inhibition of neuroinflammation.
In a clinical study, 900 mg Glycyrrhizae Radix extract was administered three times daily for 7 days to patients with acute ischemic stroke, leading to neurological improvement [25]. In the current study, 1000 mg/kg Glycyrrhizae Radix extract was administered to mice for 3 days, once daily. The human equivalent dose for mice has been reported to be 12.3 times the surface area [26]; thus, the dose of Glycyrrhizae Radix extract used in the present study was within the clinically used dose range. Future studies are required to investigate the anti-neuroinflammatory efficacy of Glycyrrhizae Radix extract in clinical practice.
LPS activates the release of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, via toll-like receptor 4 (TLR4), and leads to neuronal apoptosis [27, 28]. LPS increases TLR4 expression in microglia, suggesting that peripheral LPS can influence brain inflammation [9]. In addition, LPS administration increases Iba1 positivity, a microglial inflammation marker, in the hippocampus of mice [7, 12]. In a previous study, some ingredients of Glycyrrhizae Radix suppressed LPS-induced inflammation by preventing the inhibition of nuclear factor-kappa B degradation and inhibiting p65 translocation, which are downstream signals of TLR4 [15]. Thus, Glycyrrhizae Radix may have anti-neuroinflammatory effects on the hippocampus and reduce the LORR duration via these anti-inflammatory signals. In a previous study, LPS led to microglia activation without monocyte recruitment in the thalamus [29]. However, we did not evaluate the anti-inflammatory effects of Glycyrrhizae Radix in the thalamus and hypothalamus, which are involved in sleep and wakefulness. Future studies are required to investigate the anti-neuroinflammatory effects of Glycyrrhizae Radix in other brain regions, such as the thalamus and hypothalamus.
Glycyrrhizae Radix is a traditional medicine, and licorice is known to have anti-inflammatory, antibacterial, antioxidant, antiviral, and expectorant properties [17, 30]. GL and LQ are well-known biologically active components of Glycyrrhizae Radix, and these components also exert anti-inflammatory effects [17]. In the present study, we showed that GL and LQ partially decrease the duration of pentobarbital-induced LORR, which suggests that the Glycyrrhizae Radix ingredients, GL and LQ, impede the generation of various inflammatory mediators produced by activated macrophages/microglia. Accordingly, Glycyrrhizae Radix extract could be a good treatment option to suppress nerve inflammation in the hippocampus. Other ingredients in Glycyrrhizae Radix, such as liquiritigenin and glabridin, may also have anti-neuroinflammatory effects, as such effects have been reported for these ingredients [17]. Future studies are required to investigate the anti-neuroinflammatory effects of other Glycyrrhizae Radix ingredients.
In the present study, we evaluated the efficacy of Glycyrrhizae Radix extract for reducing microglial inflammation in LPS-stimulated BV2 microglial cells. Nerve inflammation is a typical feature of many neurodegenerative diseases, including delirium, Alzheimer’s disease, and Parkinson’s disease [3,4,5]. Inflamed microglia release inflammatory factors, such as TNF-α, IL-6, and NO, which can damage nerve cells [31]. NO is a type of free radical and is involved in microglia-mediated inflammatory processes in the central nervous system [32]. The findings of the current study showed that Glycyrrhizae Radix extract significantly reduces NO release. In addition, Glycyrrhizae Radix significantly decreased inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, in terms of both mRNA gene expression and secreted protein levels. Therefore, the findings of the present study suggest that Glycyrrhizae Radix can directly act on microglia.
In a previous study, we showed that Yokukansan and GL have anti-neuroinflammatory effects [11]. However, Yokukansan consists of seven medicinal herbs that have reported anti-inflammatory properties [33,34,35,36,37,38,39]. Further, the amount of GL administered in our previous study was higher than the amount of GL in Yokukansan [12]. Therefore, GL alone could not fully explain the effect of Yokukansan on nerve inflammation. In the present study, we investigated whether Glycyrrhizae Radix and its ingredients have anti-neuroinflammatory effects in the hippocampus because it is a component of Yokukansan and contains GL, which has been reported to reach the brain [31]. Treatment with Glycyrrhizae Radix, as well as its active ingredients, GL and LQ, significantly reduced the duration of pentobarbital-induced LORR and had anti-neuroinflammatory efficacy in the hippocampus in our mouse model of nerve inflammation. Therefore, Glycyrrhizae Radix, GL and LQ, may be effective therapeutic agents for the treatment of nerve inflammation-induced neurological disorders. Additionally, Glycyrrhizae Radix is more commonly used worldwide than Yokukansan; accordingly, Glycyrrhizae Radix may be easier to apply as a therapeutic drug for neurological disorders. However, other constituents of Glycyrrhizae Radix may also contribute to its reported anti-neuroinflammatory effects. Further studies are required to investigate the efficacy of the other active ingredients of Glycyrrhizae Radix.
In conclusion, our results suggest that Glycyrrhizae Radix administration inhibits inflammation in the hippocampus and can be used as a therapeutic drug for the treatment of nerve inflammation-induced neurological disorders. However, further clinical trials are required to confirm these findings in humans, as the present study utilized a mouse model.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Material availability
Not applicable.
References
Alam A, Hana Z, Jin Z, Suen KC, Ma D (2018) Surgery, neuroinflammation and cognitive impairment. EBioMedicine 37:547–556
Yang T, Velagapudi R, Terrando N (2020) Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol 21:1319–1326
Minter MR, Taylor JM, Crack PJ (2016) The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J Neurochem 136:457–474
Bright F, Werry EL, Dobson-Stone C, Piguet O, Ittner LM, Halliday GM, Hodges JR, Kiernan MC, Loy CT, Kassiou M, Kril JJ (2019) Neuroinflammation in frontotemporal dementia. Nat Rev Neurol 15:540–555
Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, Brizard B, El Hage W, Surget A, Belzung C, Camus V (2021) Neuroinflammation and depression: a review. Eur J Neurosci 53:151–171
Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP (2008) Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 5:15
Kitamura Y, Hongo S, Yamashita Y, Yagi S, Otsuki K, Miki A, Okada A, Ushio S, Esumi S, Sendo T (2019) Influence of lipopolysaccharide on diazepam-modified loss of righting reflex duration by pentobarbital treatment in mice. Eur J Pharmacol 842:231–238
Hellstrom IC, Danik M, Luheshi GN, Williams S (2005) Chronic LPS exposure produces changes in intrinsic membrane properties and a sustained IL-beta-dependent increase in GABAergic inhibition in hippocampal CA1 pyramidal neurons. Hippocampus 15:656–664
Cazareth J, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A (2014) Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: importance of CCR2/CCL2 signaling. J Neuroinflammation 11:132
Maldonado JR (2013) Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 21:1190–1222
Olkkola KT, Ahonen J (2008) Midazolam and other benzodiazepines. Handb Exp Pharmacol 182:335–360
Kawada K, Ishida T, Jobu K, Morisawa S, Kawazoe T, Nishida M, Nishimura S, Tamura N, Yoshioka S, Miyamura M (2022) Yokukansan suppresses neuroinflammation in the hippocampus of mice and decreases the duration of lipopolysaccharide- and diazepam-mediated loss of righting reflex induced by pentobarbital. J Nat Med 76:634–644
Cao Y, Wang Y, Ji C, Ye J (2004) Determination of liquiritigenin and isoliquiritigenin in Glycyrrhiza uralensis and its medicinal preparations by capillary electrophoresis with electrochemical detection. J Chromatogr A 1042:203–209
Kao TC, Wu CH, Yen GC (2014) Bioactivity and potential health benefits of licorice. J Agric Food Chem 62:542–553
Yang R, Yuan BC, Ma YS, Zhou S, Liu Y (2017) The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm Biol 55:5–18
Petramfar P, Hajari F, Yousefi G, Azadi S, Hamedi A (2020) Efficacy of oral administration of licorice as an adjunct therapy on improving the symptoms of patients with Parkinson’s disease, a randomized double blinded clinical trial. J Ethnopharmacol 247:112226
Yu JY, Ha JY, Kim KM, Jung YS, Jung JC, Oh S (2015) Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 20:13041–13054
Darias V, Abdala S, Martin-Herrera D, Tello ML, Vega S (1998) CNS effects of a series of 1,2,4-triazolyl heterocarboxylic derivatives. Pharmazie 53:477–481
Wolfman C, Viola H, Marder M, Wasowski C, Ardenghi P, Izquierdo I, Paladini AC, Medina JH (1996) Anxioselective properties of 6,3’-dinitroflavone, a high-affinity benzodiazepine receptor ligand. Eur J Pharmacol 318:23–30
Hui B, Yao X, Zhang L, Zhou Q (2020) Dexamethasone sodium phosphate attenuates lipopolysaccharide-induced neuroinfammation in microglia BV2 cells. Naunyn Schmiedebergs Arch Pharmacol 393:1761–1768
Kawada K, Ohta T, Tanaka K, Miyamura M, Tanaka S (2019) Addition of suvorexant to ramelteon therapy for improved sleep quality with reduced delirium risk in acute stroke patients. J Stroke Cerebrovasc Dis 28:142–148
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48:452–458
Kawada K, Ohta T, Tanaka K, Miyamoto N (2018) Reduction of nicardipine-related phlebitis in patients with acute stroke by diluting its concentration. J Stroke Cerebrovasc Dis 27:1783–1788
Jang EY, Choe ES, Hwang M, Kim SC, Lee JR, Kim SG, Jeon JP, Buono RJ, Yang CH (2008) Isoliquiritigenin suppresses cocaine-induced extracellular dopamine release in rat brain through GABA(B) receptor. Eur J Pharmacol 587:124–128
Ravanfar P, Namazi G, Atigh M, Zafarmand S, Hamedi A, Salehi A, Izadi S, Borhani-Haghighi A (2016) Efficacy of whole extract of licorice in neurological improvement of patients after acute ischemic stroke. J Herb Med 6:12–17
Nair AB, Jacob S (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7:27–31
Dehkordi NG, Noorbakhshnia M, Ghaedi K, Esmaeili A, Dabaghi M (2015) Omega-3 fatty acids prevent LPS-induced passive avoidance learning and memory and CaMKII-α gene expression impairments in hippocampus of rat. Pharmacol Rep 67:370–375
Mirahmadi SM, Shahmohammadi A, Rousta AM, Azadi MR, Fahanik-Babaei J, Baluchnejadmojarad T, Roghani M (2018) Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine 104:151–159
Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A (2003) Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA 100:9614–9619
Gumpricht E, Dahl R, Devereaux MW, Sokol RJ (2005) Licorice compounds glycyrrhizin and 18β-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem 280:10556–10563
Smith JA, Das A, Ray SK, Banik NL (2012) Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull 87:10–20
Subedi L, Gaire BP, Kim S-Y, Parveen A (2021) Nitric oxide as a target for phytochemicals in anti-neuroinflammatory prevention therapy. Int J Mol Sci 22:4771
Ikarashi Y, Mizoguchi K (2016) Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol Ther 166:84–95
Furuya M, Miyaoka T, Tsumori T, Liaury K, Hashioka S, Wake R, Tsuchie K, Fukushima M, Ezoe S, Horiguchi J (2013) Yokukansan promotes hippocampal neurogenesis associated with the suppression of activated microglia in Gunn rat. J Neuroinflammation 10:145
Cheng CY, Ho TY, Lee EJ, Su SY, Tang NY, Hsieh CL (2008) Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med 36:1105–1119
Khaksa G, Zolfaghari ME, Dehpour AR, Samadian T (1996) Anti-inflammatory and anti-nociceptive activity of disodium glycyrrhetinic acid hemiphthalate. Planta Med 62:326–328
Nukaya H, Yamashiro H, Fukazawa H, Ishida H, Tsuji K (1996) Isolation of inhibitors of TPA-induced mouse ear edema from Hoelen, Poria cocos. Chem Pharm Bull (Tokyo) 44:847–849
Seo MJ, Kim SJ, Kang TH, Rim HK, Jeong HJ, Um JY, Hong SH, Kim HM (2011) The regulatory mechanism of β-eudesmol is through the suppression of caspase-1 activation in mast cell-mediated inflammatory response. Immunopharmacol Immunotoxicol 33:178–185
Yuan D, Ma B, Yang JY, Xie YY, Wang L, Zhang LJ, Kano Y, Wu CF (2009) Anti-inflammatory effects of rhynchophylline and isorhynchophylline in mouse N9 microglial cells and the molecular mechanism. Int Immunopharmacol 9:1549–2155
Funding
This work was supported by JSPS KAKENHI Grant Number 20H01008.
Author information
Authors and Affiliations
Contributions
KK: conceptualization, methodology, Investigation, writing—original draft, project administration, funding acquisition. TI: conceptualization, methodology, formal analysis, investigation, writing—review and editing. KJ: validation, investigation, resources, writing—review and editing. SM: investigation. TK: investigation. MN: investigation. SN: investigation. NT: investigation. SY: investigation, data curation. MM: resources, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Animal experiments were approved by the Committee for the Care and Use of Laboratory Animals at Kochi University (approval no. O-0009, April 23, 2021).
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawada, K., Ishida, T., Jobu, K. et al. Glycyrrhizae Radix suppresses lipopolysaccharide- and diazepam-induced nerve inflammation in the hippocampus, and contracts the duration of pentobarbital- induced loss of righting reflex in a mouse model. J Nat Med 77, 561–571 (2023). https://doi.org/10.1007/s11418-023-01700-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-023-01700-2