Abstract
Monosodium urate (MSU)-mediated inflammation is closely related to gouty arthritis (GA). Dioscin, an active ingredient, has been reported to possess anti-inflammatory property. Nevertheless, the role of dioscin in GA and the underlying mechanism have not been fully understood. In the present study, we investigated the anti-inflammatory effect of dioscin on MSU-induced GA through in vivo and in vitro experiments. Histopathological analysis showed that dioscin alleviated the severity of GA concomitant with the lowered uric acid and creatinine levels. Moreover, the increasing IL-1β, IL-6, and TNF-α levels induced by MSU were decreased via administration of dioscin in mice and human synoviocytes. Western blotting results suggested that dioscin inhibited the activation of NLRP3 through down-regulating the protein expressions of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), cleaved-caspase-1, as well as IL-1β. In addition, TLR4, myeloid differentiation primary response gene 88 (MyD88), p-IKKβ, p-p65, and NF-κB p65 in nuclei levels were significantly reduced by dioscin. Importantly, dioscin remarkably lowered the NF-κB p65-DNA activity in MSU-treated mice utilizing electrophoretic mobility shift assay (EMSA) analysis. Taken together, dioscin had a protective effect against MSU-initiated inflammatory response via repressing the production of inflammatory cytokines and the activation of inflammasome NLRP3 and TLR4/NF-κB signaling pathway. The above findings revealed that dioscin could be a potential drug for the treatment of GA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gouty arthritis (GA), the most common inflammatory arthritis in adults, is characterized by elevated uric acid content in the body and the deposition of uric acid crystals mainly in and around the joint space accompanied by acute inflammation [1, 2]. Epidemiological studies reveal that gout has become an ordinary and frequently-occurring disease throughout the world, and it is a metabolic disease second only to diabetes in China [3]. In gout, monosodium urate crystals (MSU) can be phagocytosed by macrophages, thus mediating the release of pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6 and promoting the activation of inflammasome [4]. In turn, the release of these factors is capable of facilitating synovial cells to participate in inflammatory response of gout. Though anti-inflammatory therapies including NSAIDs, colchicines, as well as corticosteroids have been employed for the treatment of GA, as a result of adverse effects (significant toxicities and hyperkalaemia), their use is limited [1]. Hence, finding safe and effective drugs for preventing and curing GA is particularly necessary.
Dioscorea containing Dioscorea septemioba and Dioscorea spongiosa possesses the functions of removing dampness, clearing turbidity, and removing wind and pain. It has been intensively utilized for treating homosexual urinary leaching manifested by chyluria, joint stiffness, as well as rheumatic arthralgia [5]. The earlier studies reported that Dioscorea spongiosa had curative roles in osteoporosis through in vivo and in vitro investigations [6, 7]. The previous work indicated that dioscin extracted from Dioscorea spongiosa could reduce blood uric acid and effectively treat hyperuricemia [8]. Furthermore, Dioscorea is able to inhibit lipopolysaccharide-induced inflammation in RAW264.7 murine macrophages, and lower the level of uric acid and kidney injury in rat and mouse models of hyperuricemia [9,10,11]. Lu et al. [12] pointed out that Rhizoma Dioscorea Nipponicae might have therapeutic effects on GA via suppressing monosodium urate (MSU)-induced inflammatory response in rats and IL-1β-mediated synovial cell proliferation. It was documented that dioscin relieved arthritis triggered by collagen through inhibition of Th17 cell response [13]. What is more, dioscin exhibited a good protective effect against cartilage destruction in an osteoarthritis rat model induced by monosodium iodoacetate [14]. Therefore, it is worthy of thoroughly studying the anti-inflammatory role of dioscin in GA via in vivo and in vitro assays.
In the current study, we investigated the effect of dioscin on MSU-induced inflammation in mice (an animal model for GA) and human synoviocytes (HS). The level of inflammasome NLR family pyrin domain containing three (NLRP3) was assessed by immunohistochemistry staining and western blotting. Moreover, Toll-like receptor 4 (TLR4) and nuclear factor-κB (NF-κB) pathways tightly involved in inflammatory response were evaluated using western blotting. Our experiment results demonstrated that dioscin reduced inflammation possibly through inhibition of the activation of NLRP3 and TLR4/NF-κB signaling pathway, which was helpful to cure GA.
Materials and methods
Materials
Dioscin was purchased from Shanghai yuanye Bio-Technology Co., Ltd (B21176, China). MSU was supplied by Sigma (U2875, USA). Eight-week-old male C57BL/6 mice (20–22 g) were obtained from Liaoning Changsheng biotechnology Co., Ltd (Shenyang, China). Primary human synoviocytes (HS) were provided by Procell Life Science and Technology Co., Ltd (Wuhan, China).
Mouse models
Eight-week-old male C57BL/6 mice were randomly divided into four groups: control group, MSU group, MSU + L-Dioscin group, and MSU + H-Dioscin group. Mice were administrated by dioscin (50 or 100 mg/kg) by gavage every day, and/or MSU (25 mg/mL diluted in PBS) was injected into the right ankle of the mouse on the 8th day after intragastric administration for 1 h. Then, swollen right palm in mice was photographed at 0, 24, and 48 h. Not only were the mice euthanized with 200 mg/kg of pentobarbital, but also both blood and joint synovial tissue samples were collected for subsequent experiments. The handling of mice and experimental procedures in our study were carried out in the light of the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH, no. 85-23, 1996), and were approved by the Animal Ethics Committee of the First Affiliated Hospital, Heilongjiang University of Chinese Medicine.
Measurement of uric acid and creatinine in serum
We utilized the corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, China) to evaluate uric acid and creatinine contents in serum of mice with and without treatment in accordance with the manufacturers’ instructions.
Histopathological assessment
The fixed synovial tissues were dehydrated and embedded a mixture containing xylene as well as paraffin (60 °C, 2 h), and then cut into 5-μm-thickness slices. The slices were stained with hematoxylin–eosin (H&E) and observed under a microscope (BX53, OLUMPUS, Japan).
Cell culture and treatment
HS were cultured with dedicated medium (CM-H094, Procell) in an incubator (37 °C, 5% CO2). Cells were administrated by dioscin (15 ng/mL) or CLI-095 (5 μmol/L, TAK-242, TLR4 inhibitor, Shanghai yuanye) for 24 h, and then treated with MSU (100 μg/mL) for 12 h.
Cell viability detection
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, KGA311) was provided by KeyGEN BioTECH (Nanjing, China). Dimethyl sulfoxide (DMSO, ST038) was purchased from Beyotime Biotechnology (Shanghai, China). After treatment with dioscin or CLI-095 and/or MSU, MTT (0.5 mg/mL) was added to treated cells and reacted for 5 h under a CO2 incubator at 37 °C. The supernatant was removed from each well, and 150 μL of DMSO was added. After reaction for 10 min in the dark, the absorbance was detected at 570 nm using a microplate reader (ELX-800, Biotek, USA).
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-1β, IL-6, and TNF-α were assessed in mouse synovium tissues and cell supernatant by ELISA kits following the manufacturers’ recommendation, respectively. IL-1β kit (SEA563Mu; SEA563Hu), IL-6 kit (SEA079Mu; SEA079Hu), and TNF-α kit (SEA133Hu) were purchased from Uscn (Wuhan, China). The mouse TNF-α kit (EK0527) was supplied by Boster Biological Technology Co., Ltd (Wuhan, China).
Immunohistochemistry staining for NLRP3 in synovium tissues
The sections were deparaffinized and incubated with 3% H2O2 at room temperature (RT) for 15 min. After being washed in PBS three times, the slices were blocked with goat serum (SL038, Solarbio, China) at RT for 15 min. After being washed with PBS, the sections were cultured with rabbit NLRP3 antibody (dilution 1:100 in PBS, A12694, abclonal, China) at 4 °C overnight. Subsequently, the slices were incubated with HRP-labeled goat anti-rabbit IgG (dilution 1: 500 in PBS, #31460, Thermo Fisher Scientific, USA) (37 °C, 1 h), treated with 3,3-diaminobenzidine (DAB, DA1010, Solarbio), and counterstained with hematoxylin (H8070, Solarbio) for 3 min. Images were photographed by a microscope.
Immunofluorescence assay for NF-κB p65 and NLRP3 in cells
Treated cells were fixed in 4% paraformaldehyde for 15 min and washed in PBS. Cells were incubated with 0.1% Triton X-100 (ST795, Beyotime) at RT for 0.5 h and then treated with goat serum at RT for 15 min. Samples were cultured with primary antibody including NF-κB p65 (dilution 1:100 in PBS, A2547, abclonal) and NLRP3 (dilution 1:100 in PBS, A12694, abclonal) overnight at 4 ℃ and incubated with Cy3-labeled goat anti-rabbit IgG (dilution 1:200 in PBS, A0516, Beyotime) in the dark at RT for 1 h. After being washed with PBS, cells were stained with DAPI (D106471, Aladdin, China) and visualized with a microscope.
Western blotting
Synovium tissues and treated cells were lysed in RIPA lysate (R0010, Solarbio) containing 1 mM PMSF (P0010, Solarbio) on ice for 5 min, respectively. After centrifugation (4 °C, 10,000 g, 5 min), the supernatant was used as total protein extraction. Nuclear protein was extracted from tissues or cells with the kit (R0050, Solarbio) in accordance with the manufacturer’s protocol. The protein concentration was quantified by BCA kit (PC0020, Solarbio). 20 μg of protein were separated on 5, 8, 10, and 13% gels with 5% concentration gel. After electrophoresis, the separated proteins were transferred onto PVDF membranes (IPVH00010, Millipore, USA). The membranes were blocked with 5% skimmed milk diluted in Tris-buffered saline with Tween 20 (TBST) at RT for 60 min. Then the membranes were incubated with primary antibody at 4 °C overnight and cultured with secondary antibody (37 °C, 1 h). Protein bands were observed by the gel imaging system (WD9134B, Beijing Liuyi, China) using ECL kit (PE0010, Solarbio). The information of antibodies utilized in this study was as follows: GAPDH (1:10,000, 60004-1-Ig, proteintech, China); Histone H3 (1:5000, GTX122148, Gene Tex, USA); NLRP3 (1:1000, A12694), ASC (1:1000, A1170), IL-1β (1:1000, A11369), TLR4 (1:1000, A5258), MyD88 (1:1000, A0980), and NF-κB p65 (1:1000, A19653) purchased from abclonal; caspase-1 (1:500, WL03450, wanleibio, China); p-IKKβ (Ser181, 1:1000, AF3013), IKKβ (1:1000, AF6009), and p-NF-κB p65 (Ser536, 1:1000, AF2006) provided by Affinity (China); goat anti-rabbit IgG-HRP (1:3000, SE134) and goat anti-mouse IgG-HRP (1:3000, SE131) supplied by Solarbio.
Electrophoretic mobility shift assay (EMSA)
Nuclear protein was extracted from synovium tissues via the kit (R0050, Solarbio) in the light of the manufacturer’s instructions. The protein concentration was quantified by BCA kit (PC0020, Solarbio). EMSA was performed using the kit (BITF282, Viagene, China). Briefly, the nuclear extracts were incubated with biotin-labeled NF-κB p65 probes and reacted for 20 min at RT. The reaction mixtures were separated by 6.5% non-denaturing PAGE and transferred on the membranes. The membranes were DNA cross-linked, incubated with streptavidin-HRP for 20 min at RT, and visualized with ECL kit.
Statistical analysis
Results were expressed as means ± SD. For comparing between multigroups, one-way ANOVA followed by post hoc Tukey’s test was carried out, except the statistical analysis of right palm swelling index (two-way ANOVA). P < 0.05 was considered statistically significant.
Results
Dioscin alleviated the severity of GA in mice
In this study, a GA mouse model was established. Compared to control mice, MSU stimulation increased the right palm swelling (Fig. 1a). By contrast, dioscin administration prominently improved the swelling in MSU-treated mice. Additionally, dioscin evidently lowered the increase in uric acid and creatinine contents induced by MSU (Fig. 1b). H&E staining indicated that dioscin could attenuate inflammatory symptoms in MSU-mediated GA via decreased neutrophil infiltration (Fig. 1c). Based on the above findings, dioscin alleviated the severity of GA in mice.
Dioscin alleviated the severity of gouty arthritis in mice. Mice were administrated by dioscin (50 or 100 mg/kg) by gavage every day, and/or MSU (25 mg/mL) was injected into the right ankle of the mouse on the 8th day after intragastric administration for 1 h. a Swollen right palm in mice was photographed at 0, 24, and 48 h. Meanwhile, the degree of right palm swelling was evaluated. b The levels of uric acid and creatinine in blood were detected with corresponding kits. Results were expressed as means ± SD (N = 6). ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01, and ***P < 0.001 compared with MSU group. c The mouse synovium tissues were stained with H&E and observed under a microscope. Scale bar: 100 μm. MSU monosodium urate, H&E hematoxylin–eosin
Dioscin repressed inflammasome NLRP3 activation in mouse synovium tissues
MSU enhanced the levels of IL-1β as well as other pro-inflammatory factors including IL-6 and TNF-α in mouse synovium tissues (Fig. 2a). However, administration of dioscin significantly reversed these levels. Furthermore, the expression of NLRP3 was measured with immunohistochemistry staining. As shown in Fig. 2b, MSU obviously elevated NLRP3 level when compared with control group, but dioscin lowered the level of NLRP3. To further demonstrate whether inflammasome NLRP3 was activated, we detected some protein expressions utilizing western blotting. Figure 2c indicated that dioscin reduced MSU-induced NLRP3, ASC, cleaved-caspase-1, and IL-1β protein expressions. In addition, there were no remarkable changes in the levels of caspase-1 and pro-IL-1β in mice with and without treatment. The above results revealed that dioscin could inhibit the activation of NLRP3.
Dioscin repressed NLRP3 activation in mouse synovium tissues. a IL-1β, IL-6, and TNF-α levels were measured in synovium tissues using ELISA. b The expression of NLRP3 in synovium tissues was assessed via immunohistochemistry assay. Scale bar: 50 μm. c NLRP3, ASC, caspase-1, c-caspase-1, pro-IL-1β, and IL-1β protein expressions in synovium tissues were detected with western blotting. GAPDH was utilized as internal reference. Data were presented as means ± SD (N = 6). ###P < 0.001 compared with control group; **P < 0.01 and ***P < 0.001 compared with MSU group. NLRP3 NLR family pyrin domain containing 3, IL-1β interleukin-1β, IL-6 interleukin-6, TNF-α tumor necrosis factor-α, ELISA enzyme-linked immunosorbent assay, ASC apoptosis-associated speck-like protein containing a caspase recruitment domain, c-caspase-1 cleaved-caspase-1
Dioscin inhibited the activation of TLR4/NF-κB pathway in mouse synovium tissues
We found that the expressions of TLR4, MyD88, p-IKKβ, p-p65, and NF-κB p65 in nuclei were increased in mice treated with MSU (Fig. 3a). In contrast, dioscin obviously decreased the increase of these protein levels induced by MSU. Moreover, the activation of NF-κB signaling pathway was further evidenced by the elevated NF-κB p65-DNA binding activity. As depicted in Fig. 3b, EMSA analysis suggested that the increase of NF-κB p65-DNA activity mediated by MSU was inhibited by dioscin. These findings indicated that dioscin suppressed TLR4/NF-κB pathway activation in MSU-treated mice.
Dioscin inhibited the activation of TLR4/NF-κB pathway in mouse synovium tissues. a The levels of TLR4, MyD88, p-IKKβ, IKKβ, p-p65 in cytoplasm, and NF-κB p65 in nuclei were assessed in synovium tissues by western blotting and quantified. GAPDH and Histone H3 were acted as internal references, respectively. b The NF-κB p65-DNA binding activity in synovium tissues were analyzed by EMSA with a biotin-labeled probe. Results were expressed as means ± SD (N = 6). ###P < 0.001 compared with control group; **P < 0.01 and ***P < 0.001 compared with MSU group. TLR4 toll-like receptor 4, NF-κB nuclear factor-κB, MyD88 myeloid differentiation primary response gene 88, p-IKKβ phosphorylated IKKβ, EMSA electrophoretic mobility shift assay
Dioscin reduced inflammation in MSU-treated HS
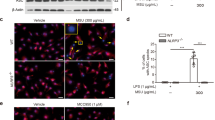
In addition to in vivo assays, we investigated the anti-inflammatory effect of dioscin on MSU-mediated inflammation through in vitro experiments. It can be seen from Fig. 4a that cell viability was increased by MSU compared to unstimulated cells. Inversely, dioscin in the presence of MSU led to a reduction in cell viability. Figure 4b showed that IL-1β, IL-6, as well as TNF-α levels were markedly lowered by dioscin. Besides, dioscin inhibited the nuclear translocation of NF-κB p65 in MSU-treated cells (Fig. 4c). Then the protein expressions of mediators in TLR4/NF-κB signaling pathway were assessed with western blotting. As displayed in Fig. 4d, dioscin significantly decreased MSU-induced up-regulation of TLR4, MyD88, p-IKKβ, p-p65, and NF-κB p65 in nuclei levels. Considering these results, dioscin effectively reduced inflammation possibly via inhibition of MSU-induced activation of TLR4/NF-κB signaling pathway in cells.
Dioscin reduced inflammation in MSU-treated primary human synoviocytes (HS). Cells were administrated by dioscin (15 ng/mL) for 24 h, and then treated with MSU (100 μg/mL) for 12 h. a After incubation, cell viability was detected via MTT assay. b The levels of IL-1β, IL-6, and TNF-α in cell supernatant were assessed by ELISA. c The level of NF-κB p65 in HS was evaluated with immunofluorescence assay. Scale bar: 50 μm. d The protein expressions of TLR4, MyD88, p-IKKβ, IKKβ, p-p65 in cytoplasm, and NF-κB p65 in nuclei were measured utilizing western blotting. GAPDH and Histone H3 were acted as internal references, respectively. Data were presented as means ± SD (N = 3). ##P < 0.01 and ###P < 0.001 compared with control group; *P < 0.05, **P < 0.01, and ***P < 0.001 compared with MSU group
Dioscin suppressed NLRP3 activation in HS induced by MSU
ELISA results indicated that dioscin repressed MSU-induced IL-1β generation (Fig. 5a). Similarly, CLI-095 (TLR4 inhibitor) also lowered the level of IL-1β. Figure 5b revealed that the level of NLRP3 was increased after stimulation with MSU, however, dioscin noticeably reversed this process. Apart from these, the protein expressions of NLRP3 and its downstream proteins were evaluated. As shown in Fig. 5c, NLRP3, ASC, cleaved-caspase-1, and IL-1β protein expressions were evidently down-regulated by dioscin in MSU-treated cells. Meanwhile, no obvious alternations in caspase-1 and pro-IL-1β levels were seen in cells with and without treatment. The above findings suggested that dioscin suppressed MSU-induced NLRP3 activation.
Dioscin suppressed NLRP3 activation in HS induced by MSU. Cells were treated with dioscin (15 ng/mL) or TLR4 inhibitor (CLI-095(TAK-242), 5 μmol/L) for 24 h, and then administrated by MSU (100 μg/mL) for 12 h. a After incubation, the level of IL-1β was determined with ELISA. b The expression of NLRP3 was detected by immunofluorescence assay. Scale bar: 50 μm. c The protein expressions of NLRP3, ASC, caspase-1, c-caspase-1, pro-IL-1β, and IL-1β were assessed via western blotting. GAPDH was used as internal reference. Results were expressed as means ± SD (N = 3). ###P < 0.001 compared with control group; **P < 0.01 and ***P < 0.001 compared with MSU group
Discussion
Emerging studies paid attention to investigating the mechanism of pain and inflammatory response of GA [12]. This study showed that the mouse right palm swelling was serious after MSU injection. At the same time, the levels of uric acid and creatinine were obviously elevated in the presence of MSU. Histopathological results demonstrated evidence for the inflammation in synovial tissues. It provided an animal model of GA for further studying the underlying mechanisms. Our researches confirmed that dioscin could attenuate MSU-induced GA in mice through lowered uric acid as well as creatinine levels and decreasing neutrophil infiltration.
MSU-mediated inflammatory reaction is specially triggered via the activation of inflammasome within monocytes/macrophages, leading to the release of inflammatory markers within the joint [15]. IL-1β is considered as one of the important pro-inflammatory mediators in GA induced by MSU [3]. In the present study, it was found that the level of IL-1β was obviously increased in model group. We also determined other indicators (IL-6 and TNF-α) elevated in MSU-treated mice and cells. On the contrary, dioscin decreased these levels. Similar findings were observed in a previous study [16]. NLRP3 inflammasome has been reported to play an essential part in the cellular defense against invading pathogens [17]. Furthermore, as evidenced by Marchetti et al. [18], in murine models of acute arthritis, NLRP3 inhibitor OLT1177 could effectively repress the release of IL-1β as well as IL-18 and subsequently mitigate the process of GA. It has been documented that MSU induces the activation of NLRP3, and then an apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and NLRP3 are gathered, which has an interaction with caspase-1 [19]. This process promotes the cleavage of caspase-1. Also, it is well recognized that the release of IL-1β can be regulated via caspase-1-containing inflammasomes such as NLRP3 [17]. Herein, the protein expressions of NLRP3, ASC, cleaved-caspase-1, and IL-1β were up-regulated by stimulation with MSU in vivo and in vitro assays. Nevertheless, these levels were reversed by dioscin, which was similar to the previous researches [19, 20].
Recently, TLRs, especially TLR4, have been acted as a family of vital proteins participating in immune response [21]. Zhou et al. [22] reported that TLR4 could be recognized as the receptor of endogenous MSU. IL-1β, IL-6, and TNF-α are very crucial components of the cascade downstream of TLRs [23]. The increased levels of these cytokines can further facilitate the production of a set of adherence factors expressed in endothelial cells to recruit more neutrophils accumulated in urate deposition site. Activated neutrophils are capable of exacerbating the inflammation through inducing autophagy and conversely secreting the majority of inflammatory mediators such as IL-1β [24]. Many studies revealed that MSU could directly bind to some cell membrane proteins (TLR2 and TLR4), thus activating NF-κB via up-regulating the protein level of myeloid differentiation factor 88 (MyD88) [25]. Besides, a previous research indicated that curcumin attenuated MSU-mediated inflammation through inhibition of TLR4 pathway activation with in vivo and in vitro experiments [20]. In this study, MSU-induced elevated protein expressions of TLR4 and MyD88 were inhibited by dioscin, demonstrating that dioscin probably suppressed the activation of TLR4 signaling pathway. The above results were consistent with Chen’s study [26] that miR-146a alleviated joint inflammation of acute arthritis rats by inhibition of TLR4 pathway.
In addition to TLR4 pathway, NF-κB signaling pathway is the key indicator in inflammatory reaction and consists of a family of transcription factors. Accumulating literatures reported that the activation of NF-κB pathway could induce the generation of inflammatory cytokines which resulted in the articular joint injury [14]. Under normal physiological conditions, NF-κB existed in the cytoplasm in an inactive form, the heterodimer of p65 and p50 subunits, which binds to IκBα protein [27]. Protein phosphorylation is an extensively modulatory way in the body, which is a critical event in cell signal transduction [12]. Once inflammatory response occurs, IKKβ controls NF-κB signaling pathway activation via phosphorylation and subsequent degradation of IκBα [28]. Then released NF-κB p65 is translocated into nucleus in which it binds to specific DNA sequences and causes transcription. As a result, a series of inflammatory indicators will be generated and GA happens. A report previously described was that morin, a bioflavonoid, inhibited MSU-induced inflammation in RAW264.7 cells through inhibition of inflammatory cytokine production and NF-κB signaling pathway activation [29]. In the current study, the levels of p-IKKβ, p-p65, and NF-κB p65 in nuclei were enhanced and the NF-κB p65-DNA activity was increased after stimulation with MSU. By contrast, dioscin reversed these changes via in vivo and in vitro assays, demonstrating that dioscin possibly inhibited the activation of NF-κB signaling pathway. Similar results were found in the earlier works previously reported [26, 30].
In conclusion, dioscin had a protective effect against MSU-induced inflammation through in vivo and in vitro experiments. The potential molecular mechanism was possibly accomplished by reducing the production of pro-inflammatory cytokines and inhibiting TLR4/NF-κB signaling pathway activation. Hence, dioscin could be a novel drug for the treatment of GA in future.
References
Schlesinger N (2011) Difficult-to-treat gouty arthritis: a disease warranting better management. Drugs 71:1413–1439
Schlesinger N (2012) Treatment of chronic gouty arthritis: it is not just about urate-lowering therapy. Semin Arthritis Rheu 42:155–165
Robinson PC, Horsburgh S (2014) Gout: joints and beyond, epidemiology, clinical features, treatment and co-morbidities. Maturitas 78:245–251
Zamudio-Cuevas Y, Fernandez-Torres J, Martinez-Nava GA, Martinez-Flores K, Ramirez Olvera AR, Medina-Luna D, Perez ADH, Landa-Solis C, Lopez-Reyes A (2019) Phagocytosis of monosodium urate crystals by human synoviocytes induces inflammation. Exp Biol Med 244:344–351
Chen C, Zeng CH, Zhang SQ, Wei L (2017) Research progress of Bixie. Zhongguo Zhong Yao Za Zhi 42:3488–3496
Han N, Xu J, Xu F, Liu Z, Yin J (2016) The in vivo effects of a fraction from Dioscorea spongiosa on glucocorticoid-induced osteoporosis. J Ethnopharmacol 185:53–59
Yin J, Han N, Liu Z, Xu X, Zhang B, Kadota S (2008) The in vivo anti-osteoporotic activity of some diarylheptanoids and lignans from the Rhizomes of Dioscorea spongiosa. Planta Med 74:1451–1453
Chen GL, Wei W, Xu SY (2006) Effect and mechanism of total saponin of Dioscorea on animal experimental hyperuricemia. Am J Chin Med 34:77–85
Chen Y, Chen XL, Xiang T, Sun BG, Luo HX, Liu MT, Chen ZX, Zhang SJ, Wang CJ (2016) Total saponins from dioscorea septemloba thunb reduce serum uric acid levels in rats with hyperuricemia through OATP1A1 up-regulation. J Huazhong U Sci-Med 36:237–242
Zhang Y, Jin L, Liu J, Wang W, Yu H, Li J, Chen Q, Wang T (2018) Effect and mechanism of dioscin from Dioscorea spongiosa on uric acid excretion in animal model of hyperuricemia. J Ethnopharmacol 214:29–36
Su J, Wei Y, Liu M, Liu T, Li J, Ji Y, Liang J (2014) Anti-hyperuricemic and nephroprotective effects of Rhizoma Dioscoreae septemlobae extracts and its main component dioscin via regulation of mOAT1, mURAT1 and mOCT2 in hypertensive mice. Arch Pharm Res 37:1336–1344
Lu F, Liu L, Yu DH, Li XZ, Zhou Q, Liu SM (2014) Therapeutic effect of Rhizoma Dioscoreae Nipponicae on gouty arthritis based on the SDF-1/CXCR 4 and p38 MAPK pathway: an in vivo and in vitro study. Phytother Res 28:280–288
Cao YJ, Xu Y, Liu B, Zheng X, Wu J, Zhang Y, Li XS, Qi Y, Sun YM, Wen WB, Hou L, Wan CP (2019) Dioscin, a steroidal saponin isolated from Dioscorea nipponica, attenuates collagen-induced arthritis by inhibiting Th17 cell response. Am J Chin Med 47:423–437
Lu J, Zhang T, Sun H, Wang S, Liu M (2018) Protective effects of dioscin against cartilage destruction in a monosodium iodoacetate (MIA)-indcued osteoarthritis rat model. Biomed Pharmacother 108:1029–1038
Jin HM, Kim TJ, Choi JH, Kim MJ, Cho YN, Nam KI, Kee SJ, Moon JB, Choi SY, Park DJ, Lee SS, Park YW (2014) MicroRNA-155 as a proinflammatory regulator via SHIP-1 down-regulation in acute gouty arthritis. Arthritis Res Ther 16:R88
Nam JS, Jagga S, Sharma AR, Lee JH, Park JB, Jung JS, Lee SS (2017) Anti-inflammatory effects of traditional mixed extract of medicinal herbs (MEMH) on monosodium urate crystal-induced gouty arthritis. Chin J Nat Med 15:561–575
Zhang A, Wang P, Ma X, Yin X, Li J, Wang H, Jiang W, Jia Q, Ni L (2015) Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts. Mol Immunol 66:253–262
Marchetti C, Swartzwelter B, Koenders MI, Azam T, Tengesdal IW, Powers N, de Graaf DM, Dinarello CA, Joosten LAB (2018) NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res Ther 20:169
Li X, Xu DQ, Sun DY, Zhang T, He X, Xiao DM (2019) Curcumin ameliorates monosodium urate-induced gouty arthritis through Nod-like receptor 3 inflammasome mediation via inhibiting nuclear factor-kappa B signaling. J Cell Biochem 120:6718–6728
Chen B, Li H, Ou G, Ren L, Yang X, Zeng M (2019) Curcumin attenuates MSU crystal-induced inflammation by inhibiting the degradation of IκBα and blocking mitochondrial damage. Arthritis Res Ther 21:193
Zhang J, Mi Y, Zhou R, Liu Z, Huang B, Guo R, Wang P, Lu Y, Zhou Y, Quan S (2020) The TLR4-MyD88-NF-κB pathway is involved in sIgA-mediated IgA nephropathy. J Nephrol. https://doi.org/10.1007/s40620-020-00722-3
Zhou Q, Lin FF, Liu SM, Sui XF (2017) Influence of the total saponin fraction from Dioscorea nipponica Makino on TLR2/4-IL1R receptor singnal pathway in rats of gouty arthritis. J Ethnopharmacol 206:274–282
Zhang QB, Qing YF, Yin CC, Zhou L, Liu XS, Mi QS, Zhou JG (2018) Mice with miR-146a deficiency develop severe gouty arthritis via dysregulation of TRAF 6, IRAK 1 and NALP3 inflammasome. Arthritis Res Ther 20:45
Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K (2011) Neutrophil extracellular trap formation is associated with IL-1β and autophagy-related signaling in gout. PLoS ONE 6:e29318
Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R (2005) Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52:2936–2946
Chen X, Gao Q, Zhou L, Wang Y, Sun RR, Zhang ZY (2019) MiR-146a alleviates inflammation of acute gout arthritis rats through TLR4/MyD88 signal transduction pathway. Eur Rev Med Pharmaco 23:9230–9237
Dang Y, Li Z, Wei Q, Zhang R, Xue H, Zhang Y (2018) Protective effect of apigenin on acrylonitrile-induced inflammation and apoptosis in testicular cells via the NF-κB pathway in rats. Inflammation 41:1448–1459
Lawrence T (2009) The nuclear factor NF-κB pathway in inflammation. CSH Perspect Biol 1:a001651
Dhanasekar C, Kalaiselvan S, Rasool M (2015) Morin, a bioflavonoid suppresses monosodium urate crystal-induced inflammatory immune response in RAW 264.7 macrophages through the inhibition of inflammatory mediators, intracellular ROS levels and NF-κB activation. PLoS ONE 10:e0145093
Ma Y, Zhou LL, Yan HY, Liu M (2009) Effects of extracts from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on MSU crystal-induced rats gouty arthritis. Am J Chin Med 37:669–683
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (No. 81704055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, J., Shi, G., Li, W. et al. Preventive effect of dioscin against monosodium urate-mediated gouty arthritis through inhibiting inflammasome NLRP3 and TLR4/NF-κB signaling pathway activation: an in vivo and in vitro study. J Nat Med 75, 37–47 (2021). https://doi.org/10.1007/s11418-020-01440-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-020-01440-7