Abstract
Cucurbitacin B (Cuc B), a natural compound extracted from cucurbitaceous plants, demonstrated potent anticancer activities, while the underlying mechanisms remain unclear. We investigated the anticancer effect of Cuc B on MCF-7 breast cancer cells. Cuc B drastically decreased cell viability in a concentration-dependent manner. Cuc B treatment caused DNA damage, as shown by long tails in the comet assay and increased γH2AX protein expression. Immunofluorescence staining showed that Cuc B treatment induced nuclear γH2AX foci. Cuc B activated DNA damage pathways by phosphorylation of ATM/ATR [two large phosphatidylinositol-3-kinase-like kinase family (PIKKs) members]. Furthermore, it also induced autophagy, as evidenced by monodansylcadaverine (MDC) staining and autophagic protein expression. In addition, Cuc B treatment led to increased reactive oxygen species (ROS) formation, which was inhibited by N-acetyl-l-cysteine (NAC) pretreatment. NAC pretreatment inhibited Cuc-B-induced DNA damage and autophagy. Taken together, these results suggest that ROS-mediated Cuc-B-induced DNA damage and autophagy in MCF-7 cells, which provides new insights into the anticancer molecular mechanism of Cuc B.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cucurbitacin B (Cuc B) (Fig. 1a) is a natural tetracyclic triterpene compound widely distributed in the plant kingdom originally as an antiinflammatory drug [1]. Accumulated data demonstrates that Cuc B possesses a variety of bioactivities, such as anti-inflammatory, hepatoprotective, and anticancer activities. In particular, the anticancer properties of Cuc B have drawn attention of many researchers in recent years [1, 2]. Cuc B inhibits proliferation of a series of cancer cell lines, such as laryngeal squamous cell carcinoma [3], pancreatic cancer [4], hepatocellular carcinoma [5, 6], melanoma [7], lung cancer [8], among others. Furthermore, it enhances the anticancer effects of clinical chemotherapeutic drugs: cisplatin, gemcitabine, methotrexate, docetaxel, and gemcitabine [9–12]. Documented results also demonstrate that Cuc B potently inhibits the proliferation of breast cancer cell lines both in vitro and in vivo [13–17]. Cuc B suppressed breast cancer cell growth by HER2/integrin signaling, which further led to inhibiting integrin-mediated cell survival through ILK1 and paxillin [14], by disruption of microtubule polymerization and disturbance of nucleophosmin/B23 translocation [15], and by inhibiting Wnt signaling [18]. However, clarifying the exact mechanisms needs further investigation.
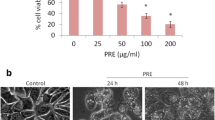
Antiproliferative activity of cucurbitacin B (Cuc B) on breast cancer MCF-7 cells. a Chemical structure of Cuc B. b Effect of Cuc B on colony formation. c Cells were treated with Cuc B (0–200 nM) for 24 h in the absence or presence of N-Acetyl-l-cysteine (NAC); cell viability was measured by MTT assay. d MCF-7 cells were treated with 200 nM Cuc B for 0–24 h; cell morphological changes were observed with an inverted-phase contrast microscope. **p < 0.05, ***p < 0.01, # p < 0.05. Cont control group
Intracellular ROS, generated mainly from mitochondrial oxidative metabolism NADPH oxidase activation in response to xenobiotics, cytokines, and bacterial invasion, have been implicated in a wide range of biological and pathological activities [19]. Cuc B induced autophagy and cell death mediated by enhanced production of ROS in HeLa cells [20]. Cuc B increased intracellular ROS levels in SW480 cells, which led to G2 cycle arrest and apoptosis [21]. High ROS levels could cause irreversible DNA damage, autophagy, and apoptosis [22]. We previously reported that Cuc B increased ROS formation, which induced DNA damage, G2/M-cell -phase arrest, and apoptosis in human lung adenocarcinoma epithelial A549 cells and K562 leukemia cells [23, 24]. In this study, we determined the role of ROS in Cuc-B-induced antiproliferative effects in MCF-7 cells from DNA damage and autophagy.
Materials and methods
Reagents and antibodies
Cuc B was purchased from ShunBo Biological Engineering Technology Co., Ltd (Shanghai, China). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH2-DA) and monodansylcadaverine (MDC) were obtained from Sigma Aldrich (St. Louis, MO, USA). Specific antibodies against GAPDH, LC3, mTOR, phospho-mTOR (Ser2448), Akt, phospho-Akt (Ser473), phospho-Akt (Thr308), ATM, phospho-ATM (Ser1981), ATR, phospho-ATR (Ser428), ULK1, phospho-ULK1 (Ser317), Beclin-1, p62, and γH2AX were purchased from Cell Signaling Technology (Danvers, USA). N-Acetyl-l-cysteine (NAC) was purchased from Beyotime (Haimen, China).
Cell lines and culture
Human breast cancer cell line MCF-7 was purchased from American Type Culture Collection (ATCC). MCF-7 cells were maintained in DMEM containing 10 % fetal bovine serum in a 5 % CO2 atmosphere at 37 °C.
MTT assay
Cells (8 × 103) were grown in 96-well plates and were added to MTT mixtures (100 μl per well, 5 mg/ml) after Cuc B (0–200 nM) treatment for 24 h. After cultured for another 4 h, the formazan was solubilized by DMSO for detection using the multilabel counter. Cells were pretreated with NAC (5 mM) for 1 h before treatment with Cuc B (200 nM) to examine the role of ROS in Cuc-B-induced antiproliferative effect.
Comet assay
Microscope slides were precoated with 0.75 % normal-melting-point agarose. MCF-7 cells were harvested after treatment with Cuc B for 4 h and mixed with 0.75 % low-melting-point agarose (104 cell/ml). After cool down in 4 °C, they were layered onto the microscope slides. The slides were submerged in prechilled lysis stock solution (1 % Triton X-100, 2.5 M NaCl and 10 mM EDTA, pH 10.5) for 1 h at 4 °C. After soaking in cold electrophoresis buffer (0.3 M NaOH and 1 mM EDTA, pH13) for 20 min, the slides were subjected to electrophoresis for 20 min at 1.5 V/cm (300 mA), removed from the electrophoresis chamber, and stained with PI for 10 min. Individual cells were viewed using an Olympus IX73 fluorescence microscope. Cells were pretreated with NAC (5 mM) for 1 h before treatment with Cuc B (200 nM).
Immunofluorescence
Cells grown on glass slides in 12-well plates were fixed with 4 % PFA at room temperature for 30 min after Cuc B treatment for 6 h. Then, the slides were permeabilized with PBS containing 0.2 % Triton at room temperature for 30 min and blocked for 30 min at 4 °C with blocking buffer containing 2.5 % bovine serum albumin and 0.2 % Triton in PBS. Cells were incubated with γH2AX antibody (1:1000) overnight and then washed with PBS twice and incubated with anti-rat IgG (1:2000) at room temperature for another 1 h. After staining with Hoechst 33342 in the dark for 30 min, cells were observed under a fluorescence inverted microscope using filter set for fluorescein isothiocyanate (FITC) and 4′-6′-diamidino-2-phenylindole (DAPI).
Colony formation assay
Cells were incubated at a density of 500 cells per well in six-well plates and treated with different concentrations of Cuc B for 2 weeks with or without NAC pretreatment (5 mM) for 1 h. After fixed with 4 % paraformaldehyde and stained with crystal violet staining solution, visible colony images were captured by an Ipad camera.
MDC staining
Cytoplasmic vacuoles were stained with MDC according to the method described previously [25]. After treatment with Cuc B, cells were incubated with MDC (50 µM) in PBS for 15 min and then washed three times with PBS and analyzed by fluorescence microscopy immediately.
Western blot assay
Cuc-B-treated cells with or without NAC (5 mM) pretreatment were harvested and total proteins were extracted. Protein contents were determined by BCA™ Protein Assay Kit. Proteins were separated on a sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5 % nonfat milk in Tris-buffered saline with 1 % Tween 20 at room temperature for 1 h, incubated with various primary antibodies overnight, and washed with Tris-buffered saline with 1 % Tween 20. Secondary antibodies labeled with horseradish peroxidase were incubated with the membrane for 2 h at room temperature. The proteins on membrane were detected by ECL advanced Western blot detection kit.
Measurement of intracellular ROS production
Production of intracellular ROS was measured by fluorescent probe DCFH2-DA. Cells were treated with Cuc B for 2 h with or without NAC pretreatment for 1 h and stained with DCFH2-DA at a final concentration of 10 μM in PBS for 30 min in the dark. Cells were collected and the fluorescence analyzed using flow cytometry.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) from at least three separate experiments. The differences between groups were analyzed using Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA) with one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls test; p < 0.05 is considered statistically significant.
Results
Cuc-B-suppressed proliferation of MCF-7 cells
The colony-formation experiment demonstrated that the colony-forming ability of MCF-7 cells was dramatically inhibited by Cuc B in a concentration-dependent manner (Fig. 1b). Meanwhile, Cuc B significantly suppressed the proliferation of MCF-7 cells in a concentration-dependent manner. NAC pretreatment was able to reverse the antiproliferative effects of Cuc B (Fig. 1c). Cuc B treatment for 6 h created no obvious morphological changes (Fig. 1d).
Cuc-B-induced DNA damage in MCF-7 cells
Comet assay showed that the comet tails were significantly prolonged in the Cuc-B-treated group compared with the control group (Fig. 2a). The formation of γH2AX histone variants is to be expected at the early stage of the occurrence of a DNA double-strand breaks (DSBs) [26]. Western blotting showed that Cuc-B-induced γH2AX expression in concentration- and time-dependent manners, which is clear after 3 h treatment (Fig. 2b). Furthermore, formation of γH2AX foci were observed in a greater proportion in Cuc-B-treated cells compared with control cells in immunofluorescence assay (Fig. 2c). ATM and ATR, two large phosphatidylinositol-3-kinase-like kinase family (PIKKs) members and upstream regulators of DNA damage, are activated in response to DNA double strand breaks [26]. Compared with the control group, phosphorylation of both ATM (Ser1981) and ATR (Ser428) were markedly increased after Cuc B treatment (Fig. 2d).
Cucurbitacin B (Cuc B)-induced DNA damage in MCF-7 cells. a Cells were treated with Cuc B (50 nM) for 3 h, and DNA damage was detected by comet assay. b Cells were treated with 50,100, and 200 nM Cuc B for 3 h and 200 nM for 1.5, 3, and 6 h, and the expression of γH2AX was detected by Western blot analysis. c Cells were treated with 200 nM Cuc B for 4 h, and translocation of γH2AX was detected using immunofluorescence assay. d Western blot analysis of ATM, ATR, and their phosphorylated forms in Cuc-B-treated MCF-7 cells
Cuc-B-induced autophagy in MCF-7 cells
Monodansylcadaverine staining showed that Cuc B treatment induced accumulation of autophagic vacuoles, as evidenced by the increased green fluorescence (Fig. 3a). Western blotting showed that Cuc B treatment led to upregulation of LC3 II expression in concentration- and time-dependent manners (Fig. 3b, c). Furthermore, Cuc B treatment inhibited protein expressions of p-mTOR, p-Akt (Ser308 and Ser473), and p62, and enhanced expressions of Beclin-1 and p-ULK1 (Ser 317) (Fig. 3c).
Cucurbitacin B (Cuc B)-induced autophagy in MCF-7 cells. a Cells were treated with Cuc B (0–200 nM) for 24 h and then stained with MDC and analyzed by a fluorescence microscopy. b Cells were treated with 200 nM Cuc B for 6, 12, and 24 h, and the expression level of LC3 was detected by Western blot analysis. c MCF-7 cells were treated with Cuc B (0–200 nM) for 24 h, and autophagic-related proteins were analyzed by Western blot analysis
Cuc-B-induced ROS formation in MCF-7 cells
As shown in Fig. 4a, Cuc B treatment for 2 h induced right shift of flow cytometry peaks, suggesting the production of intracellular ROS. Compared with the control group, 200 nM Cuc B treatment induced an increase of ROS by ~ 1.7 fold. Statistical analysis revealed a concentration-dependent manner (Fig. 4b). Cuc-B-induced ROS formation was completely reversed by NAC pretreatment (Fig. 4b).
Cucurbitacin B (Cuc B) induced reactive oxygen species (ROS) generation in MCF-7 cells. a Cells were treated with Cuc B (0–200 nM) for 2 h in the absence or presence of NAC (5 mM); intracellular ROS levels were detected by flow cytometry. The horizontal axis indicates 2′,7′-dichlorofluorescein (DCF) fluorescence intensity. b Statistical analysis of Cuc-B-induced intracellular ROS generation. Cont the control group. **p < 0.05, ***p<0.01, # p < 0.01
Cuc-B-induced DNA damage was mediated by ROS in MCF-7 cells
Pretreatment with NAC almost completely prevented the prolonged comet tails induced by Cuc B in comet assay (Fig. 5a). Furthermore, protein expression of γH2AX and phosphorylation of ATM and ATR was dramatically suppressed (Fig. 5b, c).
Reactive oxygen species (ROS) generation mediated DNA damage induced by Cucurbitacin B (Cuc B). a Cells were treated with Cuc B (200 nM) for 4 h in the absence or presence of NAC (5 mM), and the comet assay was performed. b γH2AX expression. c ATM, ATR, and their phosphorylated forms were detected by Western blot analysis. Cont control group
Cuc-B-induced autophagy was mediated by ROS in MCF-7 cells
In MDC staining, Cuc-B-induced green fluorescence was decreased by NAC pretreatment (Fig. 6a). Furthermore, protein expression of LC3 II was reversed by NAC (Fig. 6b). In addition, Cuc-B-induced changes of other autophgaic-related protein expressions of p-AKT (Ser308, Ser473), p-mTOR (Ser2448), p-ULK1 (Ser317), Beclin1, and p62 were significantly reversed by NAC (Fig. 6c).
Reactive oxygen species (ROS) generation mediated autophagy induced by Cucurbitacin B (Cuc B). Cells were treated with Cuc B (200 nM) for 24 h in the absence or presence of NAC (5 mM). a MDC staining, b LC3 expression, and c other autophagic proteins were detected by Western blot analysis. Cont control group
Discussion
It has been demonstrated that Cuc B suppressed proliferation of various human breast cancer cell lines such as MDA-MB-231, ZR-75-1, MCF-7, T47D, BT474, MDA-MB-453 [13], but its underlying mechanism was unclear. In our study, the antiproliferative effect of Cuc B on MCF-7 was confirmed by MTT assay and colony-forming assay. Both results showed that Cuc B was a potent cytotoxic compound to MCF-7 cells.
Our previous report found that Cuc B can induce the DNA damage that causes G2/M cell-cycle arrest in human lung adenocarcinoma epithelial A549 cells and leukemia K562 cells [23, 24]. Here, using comet assay, we found that Cuc B also induced DNA damage in MCF-7 cells. This was further confirmed by increased expression and nuclear translocation of γH2AX, a valid biomarker for DNA damage [27, 28]. ATM and ATR, two large phosphatidylinositol-3-kinase-like kinase family (PIKKs) members, are the central regulators of the DNA damage network [29]. Both are activated by DNA damage and DNA replication stress but with distinct specificities and functions, though there are cross-talks in their signaling pathways. It was found that ATM-Chk2 and ATR-Chk1 pathways were activated by DSBs and DNA single-strand breaks, respectively [29, 30]. Cuc B treatment induced phosphorylation of both ATM and ATR at Ser1981 and Ser428, respectively, suggesting that both pathways were activated. This finding was consist with findings of our previous study in K562 [24] and A549 cells [23]. Thus, induction of DNA damage might be a common mechanism of Cuc B cytotoxicity.
Autophagy, a highly regulated process, is an important type of nonapoptotic cell death and is implicated in cancer treatment [31, 32]. Recent reports showed that several cucurbitacins induced autophagy in HeLa [33], RAW264.7 [34], Jurkat [35]. and glioblastoma [36] cells. When autophagy is induced, the cytosolic form of LC3 is processed to the lipidated and autophagosome-associated form LC3II [37, 38]. MDC, an autofluorescent probe, is used to the identify the autophagic vesicles and assess autophagy induction through measuring the accumulation of MDC-labeled vacuoles [39]. Here, we found that Cuc B induced accumulation of LC3 II and MDC fluorescence, suggesting that it induced autophagy. AKT/mTOR, ULK1, Beclin1, and p62 are among the key regulators of the autophagic process. Autophagy induction by mTOR inhibition, resulting from starvation or rapamycin treatment, is conserved from yeast to mammals and acts upstream of the ULK1 complex [40]. Beclin1 interacts with several cofactors to regulate the lipid kin mase Vps34 protein and promote formation of Beclin 1-Vps34-Vps15 core complexes, thus inducing autophagy [41]. P62/SQSTM1, as a scaffold protein, associates with proteasomes and that autophagy degrades p62/SQSTM1 [42]. AKT/mTOR and p62 inhibition and ULK1 and Beclin1 activation by Cuc B suggested that Cuc B induced autophagy by modulating these molecules. In glioblastoma, Cuc I up-regulated Beclin 1 and activated the AMP-activated protein kinase/mTOR/p70S6K pathway, but not the PI3K/AKT pathway [36]. In Jurkat cells, Cuc-B-induced autophagy was associated with G-actin reduction and persistent activation of cofilin [35]. Thus, detailing the pathways of Cuc B in autophagy need further study.
ROS has been implicated in both the induction of DNA damage and autophagy in several types of cancer cells [43–45]. Previous data suggest that Cuc B increased intracellular ROS levels in SW480, HeLa, A549, and K562 cells [23, 24, 33, 46]. In the study we report here, this effect was confirmed in MCF-7 cells. The role of ROS in Cuc-B-induced DNA damage and autophagy was examined by NAC pretreatment. NAC not only reversed Cuc-B-induced ROS formation but also reversed DNA damage and autophagy. Furthermore, it inhibited activation of ATM/ATR and reversed protein expressions of AKT/mTOR, Beclin1, p62, and ULK1. Taken together, these data indicate that Cuc-B-induced DNA damage and autophagy in MCF-7 cells is mediated by ROS generation.
In conclusion, this study showes that Cuc B induced ROS-mediated DNA damage and autophagy in breast cancer MCF-7 cells, thus providing new insight into the anticancer effect of Cuc B.
References
Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX (2005) Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 22:386–399
Chen X, Bao J, Guo J, Ding Q, Lu J, Huang M, Wang Y (2012) Biological activities and potential molecular targets of cucurbitacins: a focus on cancer. Anticancer Drugs 23:777–787
Liu T, Zhang M, Zhang H, Sun C, Deng Y (2008) Inhibitory effects of cucurbitacin B on laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 265:1225–1232
Thoennissen NH, Iwanski GB, Doan NB, Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD (2009) Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res 69:5876–5884
Zhang M, Zhang H, Sun C, Shan X, Yang X, Li-Ling J, Deng Y (2009) Targeted constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B. Cancer Chemother Pharmacol 63:635–642
Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To Y, Lee WH, Li M, Chu KH, Toh M (2010) Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett 294:118–124
Zhang Y, Ouyang D, Xu L, Ji Y, Zha Q, Cai J, He X (2011) Cucurbitacin B induces rapid depletion of the G-actin pool through reactive oxygen species-dependent actin aggregation in melanoma cells. Acta Biochim Biophys Sin (Shanghai) 43:556–567
Kausar H, Munagala R, Bansal SS, Aqil F, Vadhanam MV, Gupta RC (2013) Cucurbitacin B potently suppresses non-small-cell lung cancer growth: identification of intracellular thiols as critical targets. Cancer Lett 332:35–45
Liu T, Peng H, Zhang M, Deng Y, Wu Z (2010) Cucurbitacin B, a small molecule inhibitor of the Stat3 signaling pathway, enhances the chemosensitivity of laryngeal squamous cell carcinoma cells to cisplatin. Eur J Pharmacol 641:15–22
Iwanski GB, Lee DH, En-Gal S, Doan NB, Castor B, Vogt M, Toh M, Bokemeyer C, Said JW, Thoennissen NH (2010) Cucurbitacin B, a novel in vivo potentiator of gemcitabine with low toxicity in the treatment of pancreatic cancer. Br J Pharmacol 160:998–1007
Lee DH, Thoennissen NH, Goff C, Iwanski GB, Forscher C, Doan NB, Said JW, Phillip Koeffler H (2011) Synergistic effect of low-dose cucurbitacin B and low-dose methotrexate for treatment of human osteosarcoma. Cancer Lett 306:161–170
Aribi A, Gery S, Lee DH, Thoennissen NH, Thoennissen GB, Alvarez R, Ho Q, Lee K, Doan NB, Chan KT (2013) The triterpenoid cucurbitacin B augments the antiproliferative activity of chemotherapy in human breast cancer. Int J Cancer 132:2730–2737
Wakimoto N, Yin D, O’Kelly J, Haritunians T, Karlan B, Said J, Xing H, Koeffler HP (2008) Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci 99:1793–1797
Gupta P, Srivastava SK (2014) Inhibition of Integrin-HER2 signaling by Cucurbitacin B leads to in vitro and in vivo breast tumor growth suppression. Oncotarget 5:1812–1828
Duangmano S, Sae-Lim P, Suksamrarn A, Domann FE, Patmasiriwat P (2012) Cucurbitacin B inhibits human breast cancer cell proliferation through disruption of microtubule polymerization and nucleophosmin/B23 translocation. BMC Complement Altern Med 12:185
Duangmano S, Dakeng S, Jiratchariyakul W, Suksamrarn A, Smith DR, Patmasiriwat P (2010) Antiproliferative effects of cucurbitacin B in breast cancer cells: down-regulation of the c-Myc/hTERT/telomerase pathway and obstruction of the cell cycle. Int J Mol Sci 11:5323–5338
Wakimoto N, Yin D, O’Kelly J, Haritunians T, Karlan B, Said J, Xing H, Koeffler HP (2008) Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci 99:1793–1797
Dakeng S, Duangmano S, Jiratchariyakul W, Up Y, Bogler O, Patmasiriwat P (2012) Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated beta-catenin to the nucleus. J Cell Biochem 113:49–60
Ray PD, Huang B-W, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Zhang T, Li Y, Park KA, Byun HS, Won M, Jeon J, Lee Y, Seok JH, Choi S-W, Lee S-H (2012) Cucurbitacin induces autophagy through mitochondrial ROS production which counteracts to limit caspase-dependent apoptosis. Autophagy 8:559–576
Yasuda S, Yogosawa S, Izutani Y, Nakamura Y, Watanabe H, Sakai T (2010) Cucurbitacin B induces G2 arrest and apoptosis via a reactive oxygen species-dependent mechanism in human colon adenocarcinoma SW480 cells. Mol Nutr Food Res 54:559–565
Filomeni G, De Zio D, Cecconi F (2014) Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ 22:377–388
Guo J, Wu G, Bao J, Hao W, Lu J, Chen X (2014) Cucurbitacin B induced ATM-mediated DNA damage causes G2/M cell cycle arrest in a ROS-dependent manner. PLoS One 9:e88140
Guo J, Zhao W, Hao W, Ren G, Lu J, Chen X (2014) Cucurbitacin B induces DNA damage, G2/M phase arrest, and apoptosis mediated by reactive oxygen species (ROS) in leukemia K562 cells. Anticancer Agents Med Chem 14:1146–1153
Munafo DB, Colombo MI (2001) A novel assay to study autophagy: regulation of autophagosome vacuole size by amino acid deprivation. J Cell Sci 114:3619–3629
Lord CJ, Ashworth A (2012) The DNA damage response and cancer therapy. Nature 481:287–294
Garcia-Canton C, Anadon A, Meredith C (2012) gammaH2AX as a novel endpoint to detect DNA damage: applications for the assessment of the in vitro genotoxicity of cigarette smoke. Toxicol In Vitro 26:1075–1086
Mah LJ, El-Osta A, Karagiannis TC (2010) gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 24:679–686
Maréchal A, Zou L (2013) DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 5(9)
Smith J, Tho LM, Xu N, Gillespie DA (2010) The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res 108:73–112
Nagelkerke A, Bussink J, Geurts-Moespot A, Sweep FC, Span PN (2014) Therapeutic targeting of autophagy in cancer. Part II: pharmacological modulation of treatment-induced autophagy. Semin Cancer Biol 31:99–105
Thorburn A, Thamm DH, Gustafson DL (2014) Autophagy and cancer therapy. Mol Pharmacol 85:830–838
Zhang T, Li Y, Park KA, Byun HS, Won M, Jeon J, Lee Y, Seok JH, Choi SW, Lee SH, Man Kim J, Lee JH, Son CG, Lee ZW, Shen HM, Hur GM (2012) Cucurbitacin induces autophagy through mitochondrial ROS production which counteracts to limit caspase-dependent apoptosis. Autophagy 8:559–576
He J, Wang Y, Xu LH, Qiao J, Ouyang DY, He XH (2013) Cucurbitacin IIa induces caspase-3-dependent apoptosis and enhances autophagy in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int Immunopharmacol 16:27–34
Zhu JS, Ouyang DY, Shi ZJ, Xu LH, Zhang YT, He XH (2012) Cucurbitacin B induces cell cycle arrest, apoptosis and autophagy associated with G actin reduction and persistent activation of cofilin in Jurkat cells. Pharmacology 89:348–356
Yuan G, Yan SF, Xue H, Zhang P, Sun JT, Li G (2014) Cucurbitacin I induces protective autophagy in glioblastoma in vitro and in vivo. J Biol Chem 289:10607–10619
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326
Vázquez CL, Colombo MI. (2009) Chapter 6 assays to assess autophagy induction and fusion of autophagic vacuoles with a degradative compartment, using monodansylcadaverine (MDC) and DQ‐BSA. In: Daniel JK (ed) Methods in enzymology. Academic Press, pp 85–95
Rubinsztein DC, Codogno P, Levine B (2012) Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11:709–730
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18:571–580
Myeku N, Figueiredo-Pereira ME (2011) Dynamics of the degradation of ubiquitinated proteins by proteasomes and autophagy: association with sequestosome 1/p62. J Biol Chem 286:22426–22440
Scherz-Shouval R, Elazar Z (2007) ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 17:422–427
Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11:777–790
Kurz EU, Lees-Miller SP (2004) DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair 3:889–900
Yasuda S, Yogosawa S, Izutani Y, Nakamura Y, Watanabe H, Sakai T (2010) Cucurbitacin B induces G2 arrest and apoptosis via a reactive oxygen species-dependent mechanism in human colon adenocarcinoma SW480 cells. Mol Nutr Food Res 54:559–565
Acknowledgments
This study was supported by the Science and Technology Development Fund, Macao S.A.R. (FDCT) (No. 039/2014/A1) and the Research Fund of the University of Macau (No. MYRG118(Y2-L4)-ICMS13-CXP).
Conflict of interest
The authors declare that there are no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, G., Sha, T., Guo, J. et al. Cucurbitacin B induces DNA damage and autophagy mediated by reactive oxygen species (ROS) in MCF-7 breast cancer cells. J Nat Med 69, 522–530 (2015). https://doi.org/10.1007/s11418-015-0918-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-015-0918-4