Abstract
Oleanolic acid (OA) and its derivatives such as 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO), CDDO-Me, and CDDO-Im show potent anticancer function. In this study, we elucidated the anticancer effect of SZC017, a novel OA derivative and identified the mechanisms by which SZC017 induces MCF-7 cell death. We found that SZC017 effectively decreased the cell viability of these breast cancer cells, but was less toxic to MCF10A mammary epithelial cells. Mechanisms underlying the inhibition of cell viability are apoptosis, autophagy induction, and G0/G1 phase arrest. SZC017 treatment suppressed the levels of Akt, phosphorylated-Akt (p-Akt), p-IκBα, total p65, and total p-p65, in addition to p-p65 in both the cytoplasm and nucleus. Furthermore, the inhibition of p65 nuclear translocation was confirmed by immunofluorescence staining. Cell viability was increased after pretreatment with chloroquine, an inhibitor of autophagy, whereas the level of procaspase-3 was significantly decreased. A concentration-dependent increase in the intracellular reactive oxygen species (ROS) level was observed in both MCF-7 and MDA-MB-231 cells. Additionally, pretreatment with N-acetyl-l-cysteine (NAC), a ROS scavenger, increased cell viability and the expression of Akt and procaspase-3, but decreased the ratio of LC3-II/I. These data show that SZC017 is an effectively selective anticancer agent against breast cancer cells, highlighting the potential use of this derivative as a breast cancer therapeutic agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent malignancy and the leading cause of cancer-related death in women worldwide. Approximately 1.38 million patients accounting for 23 % of the total new female cancer cases were newly diagnosed, and 458,400 accounting for 14 % of the total new female cancer deaths died in 2008. However, the 10-year relative survival rate has increased from 74.8 % in 1975 to 90.3 % in 2003 due to the improvements of treatment (i.e., chemotherapy, hormone therapy, and targeted drugs) and use of mammography screening [1, 2]. Patients diagnosed with early-stage may receive adjuvant treatments, including chemotherapy and radiation therapy, after breast-conserving surgery, while most patients with late-stage will receive chemotherapy. However, adverse effects of chemotherapy, including the possibility of cardiomyopathy, impaired fertility, premature menopausal, and congestive heart failure, will increase after treatments with HER-2 targeted drugs and anthracyclines [1]. Therefore, there is still urgent to develop novel therapeutic drugs.
Several natural products identified in plants have shown their chemopreventive and therapeutic potential on breast cancer [3]. Oleanolic acid (OA, 3β-hydroxyolean-12-en-28-oic acid), which has been isolated from more than 1600 plants and especially abundant in olive from which its name derives, is a multifunctional pentacyclic triterpenoid compound. OA exhibits many biological functions such as anti-inflammation, antivirus, anti-microbe, anti-parasitic and apoptosis induction in many cancer types [4–6]. Several OA derivatives such as CDDO, and its CDDO-Me and CDDO-Im show many biological activities, which are presently under evaluation in phase I studies. Other OA derivatives including di-CDDO (nitrile at C-17 position of CDDO) and various amides such as CDDO-MA (methyl amide), CDDO-EA (ethyl amide), and CDDO-TFEA (trifluoromethyl amide) also show potent anticancer function [5]. Thus, OA derivatives remain important fields of study in their synthesis and potential anticancer efficacy to human breast cancer.
In the present study, we aimed to explore the anticancer effect of SZC017 on human breast cancer cells in vitro and elucidate the underlying mechanisms. SZC017 effectively decreased the cell viability of both MCF-7 and MDA-MB-231 cell lines, but showed less cytotoxicity against MCF10A cells. Mechanistically, we then demonstrated that apoptosis and autophagy induction and the inhibition of the Akt/NF-κB signaling are mediated by excessive ROS in SZC017-treated MCF-7 cells. Herein, we firstly report that the potential anticancer effect of SZC017, a novel OA derivative, on human breast cancer cell line MCF-7.
Materials and methods
Chemicals
OA and SZC017 were kindly provided by Prof. Shisheng Wang (Dalian University of Technology), and 2 mg OA and SZC017 were dissolved by 186 μl dimethyl sulfoxide (DMSO) and stored at −20 °C (Fig. 1a, b), respectively. Dulbecco’s modified Eagle medium (DMEM), DMEM/F-12 medium, trypsin EDTA, horse serum, and fetal bovine serum (FBS) were purchased from GIBCO BRL (Gaithersburg, MD, USA). The Reactive Oxygen Species Assay Kit, Cell Cycle and Apoptosis Analysis Kit, and Nuclear and Cytoplasmic Extraction kit were obtained from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolinum bromide (MTT), 4′,6-diamidino-2-phenylindole (DAPI), penicillin, streptomycin, chloroquine (CQ) diphosphate salt, cholera toxin, insulin, hydrocortisone, human epidermal growth factor, and N-Acetyl-l-Cysteine (NAC) were obtained from Sigma-Aldrich (St Louis, MO, USA). The fluo-3/AM was procured from Dojindo Laboratories (Kumamoto, Japan). The Annexin V-FITC Apoptosis Detection Kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). The primary antibodies against β-actin, Histone H3, Akt, p-Akt, procaspase-9, procaspase-3, Bax, Bcl-2, Beclin 1, microtubule-associated protein 1 light chain 3 (LC3) were purchased from Proteintech (Chicago, IL, USA). Antibodies against p65, p-p65 (Ser536), p-IκBα (Ser32/Ser36) and all the secondary antibodies were obtained from Abbkine (Redlands, California, USA).
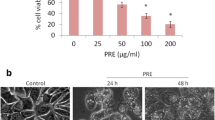
The selective inhibition effect of SZC017 on cell viability in breast cancer cells. a The chemical structure of OA. b The chemical structure of SZC017. c The inhibition effect of SZC017 on MCF10A, MCF-7, and MDA-MB-231 cell lines. Cells were treated with SZC017 at concentrations ranging from 10 to 80 µM, and cell viability was measured by the MTT assay after treatment for 24 h. The values are expressed as mean ± SD of three replicates. *Significantly different from MCF10A cell lines, *p < 0.05. d The inhibition effect of SZC017 on cell viability in MCF-7 and MDA-MB-231 cell lines, respectively. Cells were treated with OA (10, 20, 30, 40, and 80 µM) and SZC017 (10, 20, 30, 40, and 80 µM) for 24 h, and then cell viability was assessed by the MTT assay. The values are expressed as mean ± SD of three replicates. *Significantly different from OA group, *p < 0.05. e The concentration and time-dependent effect of SZC017 on MCF-7 and MDA-MB-231 cell lines. Both MCF-7 and MDA-MB-231 cell lines were treated with SZC017 at concentrations ranging from 10 to 80 µM, and cell viability was measured by the MTT assay at indicated time points, respectively. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05
Cell culture
Human breast cancer cell lines MCF-7 and MDA-MB-231, and human mammary epithelial cells MCF10A were obtained from Shanghai Institute of Biochemistry and Cell Biology. MCF-7 and MDA-MB-231 cells were maintained in high-glucose DMEM supplemented with 10 % FBS, 100 units/ml penicillin and 100 µg/ml streptomycin. MCF10A cells were maintained in DMEM/F-12 medium supplemented with 5 % horse serum, 0.1 mg/ml cholera toxin, 10 mg/ml insulin, 0.5 mg/ml hydrocortisone, 20 ng/ml human epidermal growth factor, 100 units/ml penicillin and 100 µg/ml streptomycin. Cells were incubated in a humidified atmosphere of 5 % CO2 at 37 °C.
Cell viability analysis
Cell viability was estimated using MTT assay as previously reported [7]. Briefly, cells were seeded into 96-well plates at density of 10 × 104 cells/ml and further incubated for 24 h, and then cells were treated with SZC017 with different concentrations. After an exposure to SZC017, 15 μl MTT stock solution (5 mg/ml) was added. Additional 4 h incubation later, 100 μl SDS-isobutanol-HCl solution (10 % SDS, 5 % isobutanol and 12 mM HCl) was added and the plates were further incubated at 37 °C overnight. Absorbance was measured at 570 nm with a microplate reader (Multiskan MK3; Pioneer Co; China).
Cell apoptosis analysis
The Annexin V-FITC Apoptosis Detection Kit was used to determine whether apoptosis involves in the inhibition effect of SZC017 on cell viability in MCF-7 cells. We seeded cells in 6-well plates and cultured for 24 h. The cells were then collected and washed after treatment with SZC017. After being stained with Annexin V-FITC and propidium iodide (PI) for 30 min in the dark at room temperature according to instructions of manufacturer, the samples were then analyzed using FACScan flow cytometry (BD FACSAria II; BD Co; America).
Cell cycle analysis
To determine whether the distribution of cell cycle is affected by SZC017, MCF-7 cells were cultured in 6-well plates, and then treated with SZC017 for 24 h. In order to get an accurate results of the distribution of cell cycle, suspension containing cell debris was removed [8], and we carefully collected and fixed cells in 70 % cold ethanol overnight at 4 °C. According to instructions of manufacturer, the PI staining reagent (50 mg/ml PI and 1 mg/ml RNAse in 1 ml of sodium citrate buffer, pH 7.4) was prepared and then incubated with samples in the dark at 37 °C for 30 min. Cell cycle distribution was determined by FACScan flow cytometry (BD FACSAria II; BD Co; America), and the data were analyzed using the multicycle program from Phoenix Flow Systems (San Diego, CA).
Measurement of intracellular ROS level
The level of intracellular ROS was measured by flow cytometry using Reactive Oxygen Species Assay Kit. Cells were cultured in 6-well plates, collected and washed with PBS for three times after treatment. Cells were then suspended with serum-free medium containing 10 μM DCFH-DA for 30 min in the dark at 37 °C. After being washed with serum-free medium for three times, the samples were measured using FACScan flow cytometry (BD FACSAria II; BD Co; America).
Measurement of intracellular calcium level
To assess the effect of SZC017 on intracellular calcium level in MCF-7 cells, cells were loaded with 10 μM of fluo-3/AM containing 0.05 % pluronic F-127 for 1 h at 37 °C. Then, cells were harvested by the Trypsin–EDTA and washed twice with HBSS and incubated with HBSS for 30 min at 37 °C. The intracellular calcium level was analyzed immediately by FACScan flow cytometry (BD FACSAria II; BD Co; America).
Immunofluorescence staining for p65 localization
The effect of SZC017 on the nuclear translocation of p65 was examined by means of immunofluorescence staining. Cells were cultured in chamber slides and then treated with different concentrations of SZC017 for 24 h. After then, cells were washed in phosphate-buffered saline (PBS) and fixed for 20 min at room temperature with 4 % paraformaldehyde. The samples were permeabilized with 0.4 % TritonX-100 for 10 min, and washed in PBS for three times. And then blocked with 2 % bovine serum albumin (BSA) in PBS for 1 h at 37 °C. Antibody against p65 in the 1 % blocking solution was added into the samples and incubated for overnight at 4 °C. Following three 5-min washes with PBS, fluorescein-conjugated secondary antibody were added in 1 % blocking solutions and incubated for 1 h. At last, the stained samples were mounted with DAPI (1 μg/ml for 10 min) to stain cell nuclei. After three additional 10-min washes, the samples were examined and analyzed with a fluorescence microscope (Labophot 2; Nikon, Tokyo, Japan).
Transmission electron microscopy
MCF-7 cells were seeded in 6-well plates, and treated with SZC017 for 24 h. Cells were harvested, collected and prefixed in 2.5 % glutaraldehyde overnight at 4 °C. The samples were then postfixed, dehydrated, embedded, sectioned, and stained as previously described [9]. Finally, the electron micrographs were captured using a Transmission Electron Microscope (JEM-2000EX; JEOL Co; Japan).
Western blot analysis
The whole-cell lysates were prepared in an ice-cold lysis buffer containing 150 mM NaCl, 20 mM Tris–Cl (pH 7.5), 1 % Triton X-100, 1 mM PMSF, 1 mM Na3VO4, 25 mM NaF, 1 % aprotinin, and 10 μg/ml leupeptin. Cytoplasmic and nuclear extracts were prepared using Nuclear and Cytoplasmic Extraction Kit according to the manufacturer’s instructions. The protein extracts were separated by 12 % SDS–polyacrylamide gel and then transferred to polyvinylidene difluoride (PVDF) membrane. After being blotted with TBST buffer containing 5 % nonfat dry milk, the PVDF membrane was incubated with specific primary antibody (1:1000) diluted in TBST buffer at 4 °C. The next day, membranes were probed with secondary antibody (1:1000) diluted in TBST for 1 h at room temperature. Protein bands were visualized using the enhanced Chemiluminescence reagent with LabWorks software (UVP, Upland, CA).
Statistical analysis
Data are presented as mean ± standard deviation (SD) of three replicates. One-way ANOVA test and Tukey’s multiple comparison test were used to compare the statistical differences between test and treatment groups. SPSS 17.0 software was used to analyze all the data. p values <0.05 were regarded as significant.
Results
SZC017 inhibits cell viability in breast cancer cells
To determine whether SZC017 is a potential selective anticancer agent, MCF10A mammary epithelial cell line, MCF-7 and MDA-MB-231 breast cancer cell lines were treated with different concentrations of SZC017 for 24 h and cell viability was measured by MTT assay. As shown in Fig. 1c, SZC017 significantly decreased the cell viability of both MCF-7 and MDA-MB-231 breast cancer cell lines, but showed less toxic to MCF10A mammary epithelial cell line, which suggest that SZC017 was a selective anticancer agent against breast cancer cells. Moreover, SZC017 treatment could cause a more significant reduction in cell viability in breast cancer cells, as compared with OA (Fig. 1d). Furthermore, we found that MCF-7 cell line was more sensitive than MDA-MB-231 cell line in response to SZC017 treatment. Although SZC017 was able to decrease the cell viability of both breast cancer cell lines, the half maximal inhibitory concentration value (IC50) after 24 h of SZC017 treatment was 26.47 μM for MCF-7 cells and 28.09 μM for MDA-MB-231 cells, respectively. Even under the same concentration, SZC017 caused a more obvious reduction in cell viability of MCF-7 cells than of MDA-MB-231 cells with the increase of incubation time (Fig. 1e). These data suggest that SZC017 is a potential selective anticancer agent against breast cancer cells, especially MCF-7 cells.
SZC017 induces apoptosis in MCF-7 cells
To determine whether the inhibitory effect of MCF-7 cells on cell viability by SZC017 is due to the induction of apoptosis, we next performed some experiments as following. As shown in Fig. 2a, SZC017 treatment caused typical morphological changes of apoptosis such as plasma membrane blebbing, cell shrinkage and fragmentation of MCF-7 cells. A previous publication has pointed out that OA possesses the pro-apoptotic capacity against MCF-7 cells [10]. But compared with OA, the induction of apoptosis by SZC017 was more obvious in MCF-7 cells. As shown in Fig. 2b, MCF-7 cells treated with SZC017 became rounded, whereas cells treated with OA still maintained the irregular appearance. Moreover, although both SZC017 and OA were able to decrease the expression of procaspase-3, SZC017 treatment could almost completely inhibit the expression of procaspase-3 in MCF-7 cells. The ultrastructural changes were detected by TEM [11], subsequently showing that the morphological changes accompanying apoptosis were initiated by SZC017 treatment including chromatin condensation and nuclear fragmentation in MCF-7 cells. To confirm these findings associated with apoptosis, the percentage of cells undergoing apoptosis was measured by flow cytometry. We found that SZC017 induced apoptosis in MCF-7 cells in a concentration-dependent manner. For instance, after treatment with different concentrations of SZC017, the percentage of total apoptotic cells was increased from a baseline value of 1.06 % for control to 37.55 % for 20 μM group (Fig. 2d). Since procaspase-9 and procaspase-3 are essential for the intrinsic apoptotic pathway [11], we then evaluated the level of them. As shown in Fig. 2e, SZC017 significantly inhibited the expression of both procaspase-9 and procaspase-3. It was also discovered that SZC017 increased the ratio of Bax/Bcl-2 that is critical in determining the susceptibility to apoptosis via regulating mitochondrial function [12]. Taken together, our data indicate that the inhibition of cell viability of MCF-7 cells by SZC017 is due to its capacity of induction of intrinsic apoptosis.
Apoptosis induction effect of SZC017 in MCF-7 cells. a Inverted contrast phase microscopy showed morphology changes of MCF-7 cells treated with SZC017 (10, 20, and 40 µM) for 24 h. b The effect of OA and SZC017 on apoptosis in MCF-7 cells. Differences of morphology changes of MCF-7 cells were observed after treatment with OA and SZC017 for 24 h. After treatment with OA (20 µM) and SZC017 (20 µM) for 24 h, Western blot analysis of procaspase-3 was evaluated of MCF-7 cells. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05 and #significantly different from SZC017 group, #p < 0.05. c Transmission electron microscopy revealed the occurrence of apoptosis in MCF-7 cells after treatment with 20 µM SZC017 for 24 h. d Flow cytometry analysis exhibited the apoptosis induction effect of SZC017 in MCF-7 cells. After treatment with SZC017 at concentrations of 10, 20, and 40 µM for 24 h, samples were then stained with Annexin-V FITC and PI and analyzed by flow cytometry. e Western blot analysis was performed to determine apoptosis pathway induced by SZC017 in MCF-7 cells. Cells were treated with different concentrations of SZC017 (5, 10, and 20 µM) for 24 h. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05
SZC017 induces autophagy in MCF-7 cells
Autophagy can be detected through many ways such as the TEM, which is the most convincing and standard method to verify autophagy, and the level determination of LC3 and Beclin 1, which could be confirmed by Western blot assay [13]. As shown in Fig. 3a, many small vesicles (arrows) and huge vacuoles (arrowheads) were presented in cytoplasm, especially inside these compartments were cellular organelles or cytosol that was confirmed by ultrastructural morphology observations of SZC017-treated MCF-7 cells by the higher magnification. The TEM result revealed the occurrence of autophagy that these typical morphological changes clearly reflect the classical autophagy characteristics [14–16]. A previous study has demonstrated that OA can initiate autophagy in cancer cells [17]. As compared with OA, the induction of autophagy was more obvious in MCF-7 cells by SZC017. As shown in Fig. 3b, both SZC017 and OA increased the expression of LC3-II, but showed difference in the conversion of LC3-I to LC3-II which is a hallmark of autophagy [13]. After calculation, we found that the ratio of LC3-II/I of SZC017 treatment was higher than that of OA. Furthermore, our data showed that SZC017 induced autophagy in MCF-7 cells in a concentration-dependent manner. SZC017 increased the ratio of LC3-II/I in MCF-7 cells with the increase of concentration (Fig. 3c). The data showed that 5 μM SZC017 could increase the expression of Beclin 1, whereas both 10 and 20 μM SZC017 could decrease the expression of Beclin 1 in MCF-7 cells.
Autophagy induction effect of SZC017 in MCF-7 cells. a Transmission electron microscopy showed the of occurrence autophagy that was induced by SZC017 in MCF-7 cells after treatment with 20 µM SZC017 for 24 h. b The induction effect of OA and SZC017 on autophagy in MCF-7 cells. Western blot analysis of LC3 was evaluated after treatment with SZC017 (20 µM) for 24 h. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05 and # significantly different from SZC017 group, #p < 0.05. Western blot analysis was also performed to determine the expression of autophagy-related proteins (Beclin 1 and LC3) after 20 µM SZC017 treatment for 24 h in MCF-7 cells. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05
SZC017 induces G0/G1 cell cycle arrest
To determine whether cell cycle arrest is induced by SZC017, which is considered an important mechanism in inhibiting cell viability of cancer cells [18], we then performed PI staining to measure cell cycle distribution. As shown in Fig. 4, SZC017 could induce an obvious accumulation of cells in G1 phase in a concentration-dependent manner and a reduction of cell numbers in S phase. In the control group, 55.86 % of total cells were distributed in G0/G1 phase, while 36.14 % were in S phase. However, the number of cells in G0/G1 phase was increased to 70.93 %, while cells in S phase was decreased to 21.07 %, respectively. These data indicate that the inhibition of cell viability by SZC017 is associated with the cell cycle arrest at G1 phase.
The Akt is the target of SZC017 in MCF-7 cells
Akt is a major anti-apoptotic signaling pathway, and previous studies have demonstrated that chemotherapeutic agents induce cancer cell apoptosis via inhibiting the Akt pathway [18–21]. As we expected, SZC017 could significantly suppress the expression of both Akt and p-Akt which is an active form of the Akt in a concentration-dependent manner (Fig. 5a). These data suggest that Akt pathway might be an effective target for SZC017 in MCF-7 cells.
Western blot analysis of Akt and p-Akt in response to SZC017 treatment in MCF-7 cells. Treatment (24 h) of MCF-7 cells with SZC017 (5, 10, and 20 µM) resulted in suppression of both Akt and p-Akt. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05
The NF-κB signaling pathway is the target of SZC017 in MCF-7 cells
NF-κB signaling is an important downstream pathway of Akt signaling and also involved in regulating cell survival and apoptosis [22]. Therefore, we focused our attention on assessing the level of several NF-κB pathway-related proteins namely p-IκBα, total p65, total p-p65, and p-p65 in both cytoplasm and nucleus. The results showed that the NF-κB pathway was obviously suppressed by SZC017 in MCF-7 cells. As shown in Fig. 6a, SZC017 significantly suppressed the expression of p-IκBα, total p65, and total p-p65. Moreover, both p-p65 which is a critical molecule in modulating NF-κB p65 nuclear translocation and improving its DNA binding function [23, 24] in cytoplasm and nucleus were also suppressed by SZC017. To determine whether p65 nuclear translocation could be inhibited by SZC017, immunofluorescence analysis was performed. The data indicated that p65 nuclear translocation was inhibited by SZC017 with the increase of concentration (Fig. 6c). Treatment with 5 μM SZC017 exhibited little suppression on p65 nuclear translocation, but 20 μM SZC017 obviously inhibited p65 nuclear translocation. These results indicate that the NF-κB pathway is effectively suppressed by SZC017 in MCF-7 cells.
Inhibition effect of SZC017 on the NF-κB pathway. a Western blot analysis of several NF-κB pathway related proteins, including p-IκBα, total p65, total p-p65, and p-p65 in both cytoplasm and nucleus. Treatment (24 h) of MCF-7 cells with SZC017 (5, 10, and 20 µM) resulted in the expression change of these proteins. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05. b Immunofluorescence staining analysis of p65 localization. MCF-7 cells treated with SZC017 (5, 10, and 20 µM) for 24 h were labeled for p65 (red), and nuclei were stained with DAPI (blue) (Color figure online)
SZC017 inhibits cell viability and apoptosis in MCF-7 cells via the induction of autophagy
To determine whether the autophagy induced by SZC017 is associated with the inhibition of cell viability and the induction of apoptosis, the CQ, an autophagy inhibitor, was utilized owing to the inhibition of autophagy by it at a later stage [13]. Interestingly, pretreatment with CQ increased the cell viability of MCF-7 cells treated with SZC017. Except for the control and 80 μM groups, CQ caused a significant induction in the cell viability of MCF-7 cells (Fig. 7a). The pictures taken by inverted phase contrast microscopy confirmed the result of MTT assay above (Fig. 7a). Cells only treated with 20 μM SZC017 became fragmented and rounded, but cells pretreated with CQ had irregular appearance and were still alive. As shown in Fig. 7b, since pretreatment with CQ significantly increased the ratio of LC3II/I suggesting that CQ was able to effectively inhibit the autophagy induced by SZC017. To determine the relationship between the autophagy and apoptosis, we then evaluated the expression of procaspase-3 after pretreatment with CQ. Figure 7b clearly revealed that pretreatment with CQ dramatically suppressed the level of procaspase-3, as compared with only SZC017 group. These data suggest that the autophagy induced by SZC017 is a promoter of cell death and an inhibitor of the apoptosis induced by SZC017 in MCF-7 cells.
Effect of the autophagy on the apoptosis in SZC017-treated MCF-7 cells. a Pretreatment with CQ prevented cells from SZC017-induced inhibition of cell viability. MCF-7 cells were pretreated with CQ (20 µM) for 3 h and then treated with various concentrations of SZC017 (10, 20, 30, 40, and 80 µM) for 24 h. The cell viability was measured by MTT assay, and the morphology observations were recorded by inverted phase contrast microscopy. b Western blot analysis of the expression of LC3 and procaspase-3. MCF-7 cells were pretreated with CQ (20 µM) for 3 h and then 20 µM SZC017 was incubated with cells for further 24 h. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05 and #significantly different from SZC017 only group, #p < 0.05
Excessive ROS generation is required for SZC017-induced apoptosis, autophagy, and Akt inhibition
Mitochondrial dysfunction results in an increase in intracellular ROS generation which in turn will induce cancer cell apoptosis via mitochondrial stress signaling pathway [25]. Our result showed that SZC017 increased the intracellular ROS generation in both breast cancer cell lines in a concentration dependent manner, especially in MCF-7 cell line (Fig. 8a). Besides, the intracellular calcium is an important molecule in inducing apoptosis, therefore, we used the calcium-sensitive dye fluo-3/AM to assess whether the exposure of SZC017 could increase the calcium accumulation in MCF-7 cells. Our result showed that the fluo-3/AM fluorescence intensity was increased in response to SZC017 treatment in a concentration-dependent manner (Fig. 8b). To determine the role of ROS in SZC017-treated MCF-7 cells, NAC, an antioxidant ROS scavenger, was utilized to clean up the ROS induced by SZC017. As we expected, pretreatment with NAC prevented the intracellular ROS generation, as compared with only SZC017 group (Fig. 8c). Moreover, except the control, 40 and 80 μM groups, pretreatment with NAC could effectively increase the cell viability which was inhibited by SZC017 (Fig. 8c). Considering some reports that ROS is an important molecule in regulating apoptosis, autophagy, and the Akt pathway [13, 26, 27]. Thus, we next investigated into the effect of NAC on the changes of associated proteins with apoptosis, autophagy, and the Akt pathway. The results showed that pretreatment with NAC significantly increased the level of procaspase-3 and the Akt in SZC017-treated MCF-7 cells, but decreased the ratio of LC3-II/I (Fig. 8c). In a word, our data suggest that excessive ROS is a critical upstream factor in regulating apoptosis, autophagy, and inhibition the Akt pathway.
Effect of SZC017 on intracellular ROS generation. a SZC017 increased the intracellular ROS generation in both MCF-7 and MDA-MB-231 cells. Cells were treated with SZC017 at concentrations of 10, 20, and 30 µM for 24 h, and then the level of intracellular ROS was detected by flow cytometry. b SZC017 increased the intracellular calcium generation in MCF-7 cells. MCF-7 cells were treated with SZC017 at concentrations of 10, 20, and 30 µM for 24 h, and then the level of intracellular calcium was detected by flow cytometry. c Effect of excessive ROS generation in SZC017-treated MCF-7 cells. Cells were pretreated with 10 µg/ml NAC for 1 h and then treated with 20 µM SZC017 for further 24 h. Pretreatment with NAC (10 µg/ml) for 1 h increased the cell viability of SZC017-treated MCF-7 cells. Western blot analysis of procaspase-3, LC3, and Akt after pretreatment with 10 µg/ml NAC for 1 h. The values are expressed as mean ± SD of three replicates. *Significantly different from control, *p < 0.05 and #significantly different from SZC017 only group, #p < 0.05
Discussion
The present study demonstrated that SZC017, a novel derivative of OA, selectively killed breast cancer cells and showed more obvious pharmacological effects, including cell viability inhibition and the induction of apoptosis and autophagy, as compared with OA. Moreover, our data indicated that SZC017 caused excessive intracellular ROS generation, leading to Akt/NF-κB signaling suppression and subsequent induction of apoptosis and autophagy in MCF-7 cells. Furthermore, we proved that SZC017-induced autophagy promoted cell viability rate and inhibited apoptosis induced by SZC017.
Apoptosis is a tightly regulated process that maintains the balance of cell numbers in multicellular organisms in an orderly, non-inflammatory way, while the disruption of this balance commonly exists in human cancers [13, 28]. A previous study showed that OA exhibited an inhibitory effect on hepatocellular carcinoma by inducing apoptosis through the mitochondrial pathway [29]. Our findings also showed that SZC017 induced apoptosis in a concentration-dependent manner (Fig. 2d) and exhibited a more obvious induction of apoptosis, as compared with OA in MCF-7 cells (Fig. 2b). Apoptosis is mediated through two main pathways, namely the intrinsic pathway (characterized by mitochondrial stress and involving Bcl-2 family proteins, caspase-9, and effector caspase-3) and the extrinsic pathway (characterized by ligand-based activation of cell-surface death receptors following the activation of certain caspases). Recent studies have shown that derivatives of OA induce apoptosis in different cancer cell types mainly by the intrinsic apoptosis pathway [30–33]. Therefore, we focused on evaluating the apoptotic mechanism of several intrinsic apoptosis-related molecules. As we expected, Bcl-2, an apoptosis inhibitor, was significantly suppressed and the ratio of Bax/Bcl-2 was increased (Fig. 2e), indicating that the mitochondria was severely damaged by SZC017. As the hallmarks of intrinsic apoptosis, the activity of procaspase-9 and procaspase-3 was subsequently suppressed following the induction of mitochondrial damage by many stimulants [12, 34]. ROS can be generated in various organelles such as the mitochondria (considered the main source of intracellular oxidant production), the endoplasmic reticulum, and peroxisomes [35]. A higher level of ROS was generated by SZC017 in MCF-7 cells than in MDA-MB-231 cells (Fig. 8a), and our results confirmed that ROS was also a critical regulator of apoptosis induced by SZC017 (Fig. 8c), suggesting that ROS might be critical in regulating apoptosis in breast cancer cells. Excessive ROS generation leads to mitochondrial permeability transition pore (MPTP) formation and mitochondrial depolarization, which is dependent on a high level of intracellular calcium, and thus causes apoptotic cell death involving the inhibition of procaspase-3 in cancer cells [36].
Akt/NF-κB signaling is an important pathway that modulates multiple biological activities including cell survival, differentiation, and apoptosis induction [37, 38]. Hyperactivation of the Akt/NF-κB signaling pathway, which is considered to be an effective therapeutic target for cancers, has already been reported in a variety of human cancers such as breast cancer, non-small cell lung cancer, and hematological malignancies [20, 39, 40]. Consistent with previous reports, we also observed that the activity of both Akt and p-Akt was significantly suppressed by SZC017 (Fig. 5), suggesting that because Akt is an effective target for SZC017, its inhibition may result in cancer cell death. In recent publications, several plant-derived compounds were considered to be highly effective in suppressing the Akt pathway as a result of killing cancer cells in vitro via ROS-dependent mechanisms [41–43]. Our results show that the excessive ROS generation induced by SZC017 was effectively blocked by NAC (Fig. 8c). Subsequently, we also discovered that the level of Akt was dramatically increased after blocking ROS generation (Fig. 8c) and that a decrease in ROS could rescue the survival rate of cancer cells. These data indicate that ROS is an upstream molecule in the inhibition of Akt activity, and thus contributes to cell death in SZC017-treated MCF-7 cells.
NF-κB is considered to be a very important signaling complex that interacts with the Akt pathway, and targeting Akt/NF-κB signaling is now an attractively effective strategy for cancer therapy [44]. Alternatively, suppression of the NF-κB pathway will trigger apoptosis [45]. It also appears that the NF-κB pathway has a dual role in regulating autophagy [46]. NF-κB transcription complexes can be any of a variety of homo- and heterodimers formed by the subunits c-Rel, p65, RelB, p50/p105, and p52/p100, with the most common complex being the heterodimer p50/p65. Under unstimulated conditions, NF-κB dimers are located in the cytoplasm in an inactive form because of their association with IκBα, an inhibitor protein. In response to stimulation, IκB kinase (IKK) will be activated, leading to IκBα phosphorylation, ubiquitination, and degradation by the 26S proteasome. The released NF-κB dimer is further activated by posttranslational modifications and is then translocated to the nucleus where it binds to κB DNA elements and modulates gene expression [47]. There are two IκBα-related ways to suppress NF-κB signaling. Bortezomib, an inhibitor of proteasomes, induces cell apoptosis via the suppression of the NF-κB pathway by suppressing IκBα degradation. On the other hand, 3,5-diethyl-1,3,5-thiadiazinane-2-thione (DETT) suppresses IκBα phosphorylation, preventing IκBα from being degraded by proteasomes [48, 49]. As observed in the current study, the level of p-IκBα was significantly suppressed by SZC017 (Fig. 6a); therefore, we propose that the IκBα phosphorylation inhibition pathway is responsible for the NF-κB signaling suppression in SZC017-treated MCF-7 cells. Phosphorylation of p65 at residue Ser536 functions to facilitate p65 nuclear translocation and its DNA binding function, release p65 from HDAC1 and HDAC3, and recruit p300 to the p65 complex which in turn modulates downstream gene expression [23, 24]. A recent publication has demonstrated that p65 phosphorylation-dependent NF-κB activation plays an important role in cancer cell survival [48]. In our observations, we discovered that the level of total p65 and p-p65 were suppressed (Fig. 6b), suggesting that both p65 and p-p65 might be involved in NF-κB pathway suppression. Thus, we began to focus on the role of p-p65 in SZC017-treated MCF-7 cells. Similar to bortezomib and DETT, SZC017 was able to suppress the level of p-p65 in both the cytoplasm and nucleus (Fig. 6b), indicating that the nuclear translocation inhibition of p-p65 might be involved in NF-κB p65 suppression. Furthermore, nuclear translocation of NF-κB p65 was confirmed by immunofluorescence staining (Fig. 6c). Taken together, these data suggest that the Akt/NF-κB signaling pathway is a very effective target for SZC017 in MCF-7 cells.
Many natural products show anticancer activity in several different cancer types, mainly via autophagy-dependent mechanisms [50–52]. Autophagy is a highly conserved process with a self-protective function under particular conditions, while excessive autophagy may induce cell death and thus exhibit anticancer activity in eukaryotic cells [27]. Recent publications have demonstrated that OA treatment permits both normal and cancer cells to undergo autophagy, illustrating its anticancer functions [17, 53]. In order to compare the autophagy induction activity between OA and SZC017, we evaluated the level of LC3B expression, a hallmark of autophagy, by Western blot [13]. Our findings suggested that SZC017 showed a more obvious autophagy induction activity than OA in MCF-7 cells. Autophagy serves a dual function in regulating the fate of cancer cells; it functions as a pro-survival mechanism in response to stimuli such as nutrient deprivation, hypoxia, and metabolic-induced stress, whereas excessive autophagy may function as an inducer of non-apoptotic cell death [27]. Consistent with previous studies [17, 53], excessive autophagy induced by SZC017 was also a promoter of cell death, as evidenced by the significant increase in cell viability via the blocking of autophagy by CQ, an autophagy inhibitor [13]. Despite the complex relationship between autophagy and apoptosis [26], our observations supported that apoptosis triggered by SZC017 was significantly enhanced after blocking autophagy (Fig. 7b), suggesting that autophagy induced by SZC017 serves as an inhibitor of apoptosis in MCF-7 cells. Although apoptotic cell death could be suppressed by autophagy, autophagy induced by SZC017 still served as a promoter of cell death, suggesting that autophagic cell death could be induced by SZC017 in MCF-7 cells.
ROS is considered an important upstream molecule in starvation-induced autophagy. Atg4 being inactivated by an oxidative signal will promote lipidation of Atg8, and thus promote autophagy [54]. In our study, autophagy induction activity was suppressed after ROS generation was blocked by NAC, indicating that ROS, consistent with a previous study, could be the upstream molecule of autophagy induction in SZC017-treated MCF-7 cells. Moreover, Beclin 1 plays a critical role in modulating autophagy and would be increased in response to many stimuli [55]. However, recent publications have reported that several natural products can trigger autophagic cell death via various mechanisms through Beclin 1-dependent and Beclin 1-independent routes of autophagy [56]. Our results confirmed that excessive autophagy could be caused by SZC017 in MCF-7 cells, as evidenced by a significant increase in the LC3-II/I ratio and an accumulation of autophagosomes in SZC017-treated MCF-7 cells, while Beclin 1 expression decreased after treatment with a higher concentration of SZC017.
Mechanistically, autophagy can be induced by some stimuli via a Beclin 1 suppression-dependent mechanism. Inactivation of the NF-κB pathway is thought to make a significant contribution to Beclin 1 transcription suppression, which then induces autophagy [57]. The NF-κB pathway serves as a negative modulator of autophagy induced by ROS and starvation in many cell lines. After being stimulated, p65 will translocate to the nucleus and directly binds the Beclin 1 promoter, thereby upregulating its protein levels [58, 59]. Taken together, we propose that SZC017 induces autophagy in MCF-7 cells by an NF-κB-dependent Beclin 1 suppression mechanism.
Therefore, in the present study, we demonstrated that SZC017, a novel OA derivative, is a potential selective anticancer agent against breast cancer cells in vitro. Our findings indicated that SZC017-induced excessive ROS is the upstream inhibitory molecule of Akt/NF-κB signaling and thereby triggers apoptosis and autophagy, which is not only a promoter of cell survival, but also an inhibitor of apoptosis in MCF-7 cells. However, the effect of SZC017 in other breast cancer cell lines and in vivo, as well as its role in other underlying mechanisms, remains to be further studied.
References
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A (2014) Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64(4):252–271. doi:10.3322/caac.21235
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
Preet R, Mohapatra P, Das D, Satapathy SR, Choudhuri T, Wyatt MD, Kundu CN (2013) Lycopene synergistically enhances quinacrine action to inhibit Wnt-TCF signaling in breast cancer cells through APC. Carcinogenesis 34(2):277–286. doi:10.1093/carcin/bgs351
Liby KT, Sporn MB (2012) Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev 64(4):972–1003. doi:10.1124/pr.111.004846
Shanmugam MK, Dai X, Kumar AP, Tan BK, Sethi G, Bishayee A (2014) Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: preclinical and clinical evidence. Cancer Lett 346(2):206–216. doi:10.1016/j.canlet.2014.01.016
Sheng H, Sun H (2011) Synthesis, biology and clinical significance of pentacyclic triterpenes: a multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat Prod Rep 28(3):543–593. doi:10.1039/c0np00059k
Hansen MB, Nielsen SE, Berg K (1989) Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119(2):203–210
Heinlein C, Deppert W, Braithwaite AW, Speidel D (2010) A rapid and optimization-free procedure allows the in vivo detection of subtle cell cycle and ploidy alterations in tissues by flow cytometry. Cell cycle 9(17):3584–3590
Shao Y, Gao Z, Marks PA, Jiang X (2004) Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA 101(52):18030–18035. doi:10.1073/pnas.0408345102
Allouche Y, Warleta F, Campos M, Sanchez-Quesada C, Uceda M, Beltran G, Gaforio JJ (2011) Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. J Agric Food Chem 59(1):121–130. doi:10.1021/jf102319y
Call JA, Eckhardt SG, Camidge DR (2008) Targeted manipulation of apoptosis in cancer treatment. Lancet Oncol 9(10):1002–1011. doi:10.1016/s1470-2045(08)70209-2
Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC (2001) The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol 117(2):333–340. doi:10.1046/j.0022-202x.2001.01409.x
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140(3):313–326. doi:10.1016/j.cell.2010.01.028
Bursch W (2001) The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ 8(6):569–581. doi:10.1038/sj.cdd.4400852
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Stromhaug PE, Klionsky DJ (2001) Approaching the molecular mechanism of autophagy. Traffic 2(8):524–531
Liu J, Zheng L, Zhong J, Wu N, Liu G, Lin X (2014) Oleanolic acid induces protective autophagy in cancer cells through the JNK and mTOR pathways. Oncol Rep 32(2):567–572. doi:10.3892/or.2014.3239
Qiu P, Guan H, Dong P, Li S, Ho CT, Pan MH, McClements DJ, Xiao H (2011) The p53-, Bax- and p21-dependent inhibition of colon cancer cell growth by 5-hydroxy polymethoxyflavones. Mol Nutr Food Res 55(4):613–622. doi:10.1002/mnfr.201000269
Li MH, Cha YN, Surh YJ (2006) Peroxynitrite induces HO-1 expression via PI3 K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med 41(7):1079–1091. doi:10.1016/j.freeradbiomed.2006.06.010
Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA (2012) Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog 17(1):69–95
Kim W, Yang HJ, Youn H, Yun YJ, Seong KM, Youn B (2010) Myricetin inhibits Akt survival signaling and induces Bad-mediated apoptosis in a low dose ultraviolet (UV)-B-irradiated HaCaT human immortalized keratinocytes. J Radiat Res 51(3):285–296
Prakobwong S, Gupta SC, Kim JH, Sung B, Pinlaor P, Hiraku Y, Wongkham S, Sripa B, Pinlaor S, Aggarwal BB (2011) Curcumin suppresses proliferation and induces apoptosis in human biliary cancer cells through modulation of multiple cell signaling pathways. Carcinogenesis 32(9):1372–1380. doi:10.1093/carcin/bgr032
Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M (2004) Constitutive and interleukin-1-inducible phosphorylation of p65 NF-κB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-α, IKKβ, IKKϵ, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J Biol Chem 279(53):55633–55643. doi:10.1074/jbc.M409825200
Hu J, Nakano H, Sakurai H, Colburn NH (2004) Insufficient p65 phosphorylation at S536 specifically contributes to the lack of NF-kappaB activation and transformation in resistant JB6 cells. Carcinogenesis 25(10):1991–2003. doi:10.1093/carcin/bgh198
Raimundo N, Song L, Shutt TE, McKay SE, Cotney J, Guan MX, Gilliland TC, Hohuan D, Santos-Sacchi J, Shadel GS (2012) Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell 148(4):716–726. doi:10.1016/j.cell.2011.12.027
Chen Y, Gibson SB (2008) Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy 4(2):246–248
Baehrecke EH (2005) Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol 6(6):505–510. doi:10.1038/nrm1666
Cotter TG (2009) Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer 9(7):501–507. doi:10.1038/nrc2663
Wang X, Bai H, Zhang X, Liu J, Cao P, Liao N, Zhang W, Wang Z, Hai C (2013) Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 34(6):1323–1330. doi:10.1093/carcin/bgt058
Hyer ML, Shi R, Krajewska M, Meyer C, Lebedeva IV, Fisher PB, Reed JC (2008) Apoptotic activity and mechanism of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic-acid and related synthetic triterpenoids in prostate cancer. Cancer Res 68(8):2927–2933. doi:10.1158/0008-5472.can-07-5759
Inoue S, Snowden RT, Dyer MJ, Cohen GM (2004) CDDO induces apoptosis via the intrinsic pathway in lymphoid cells. Leukemia 18(5):948–952. doi:10.1038/sj.leu.2403328
Konopleva M, Tsao T, Estrov Z, Lee RM, Wang RY, Jackson CE, McQueen T, Monaco G, Munsell M, Belmont J, Kantarjian H, Sporn MB, Andreeff M (2004) The synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res 64(21):7927–7935. doi:10.1158/0008-5472.can-03-2402
Ravanan P, Sano R, Talwar P, Ogasawara S, Matsuzawa S, Cuddy M, Singh SK, Rao GS, Kondaiah P, Reed JC (2011) Synthetic triterpenoid cyano enone of methyl boswellate activates intrinsic, extrinsic, and endoplasmic reticulum stress cell death pathways in tumor cell lines. Mol Cancer Ther 10(9):1635–1643. doi:10.1158/1535-7163.mct-10-0887
Conway EM, Pollefeyt S, Steiner-Mosonyi M, Luo W, Devriese A, Lupu F, Bono F, Leducq N, Dol F, Schaeffer P, Collen D, Herbert JM (2002) Deficiency of survivin in transgenic mice exacerbates Fas-induced apoptosis via mitochondrial pathways. Gastroenterology 123(2):619–631
Holmstrom KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15(6):411–421. doi:10.1038/nrm3801
Gerasimenko JV, Gerasimenko OV, Palejwala A, Tepikin AV, Petersen OH, Watson AJ (2002) Menadione-induced apoptosis: roles of cytosolic Ca(2+) elevations and the mitochondrial permeability transition pore. J Cell Sci 115(Pt 3):485–497
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132(3):344–362. doi:10.1016/j.cell.2008.01.020
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274. doi:10.1016/j.cell.2007.06.009
Chen Y, Scully M, Dawson G, Goodwin C, Xia M, Lu X, Kakkar A (2013) Perturbation of the heparin/heparin-sulfate interactome of human breast cancer cells modulates pro-tumourigenic effects associated with PI3K/Akt and MAPK/ERK signalling. Thromb Haemost 109(6):1148–1157. doi:10.1160/th12-12-0935
Karin M, Cao Y, Greten FR, Li ZW (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2(4):301–310. doi:10.1038/nrc780
Deeb D, Gao X, Jiang H, Janic B, Arbab AS, Rojanasakul Y, Dulchavsky SA, Gautam SC (2010) Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem Pharmacol 79(3):350–360. doi:10.1016/j.bcp.2009.09.006
Gong K, Li W (2011) Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: a potential new treatment for hepatocellular carcinoma. Free Radic Biol Med 51(12):2259–2271. doi:10.1016/j.freeradbiomed.2011.09.018
Shrimali D, Shanmugam MK, Kumar AP, Zhang J, Tan BK, Ahn KS, Sethi G (2013) Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett 341(2):139–149. doi:10.1016/j.canlet.2013.08.023
Ahmad A, Biersack B, Li Y, Kong D, Bao B, Schobert R, Padhye SB, Sarkar FH (2013) Targeted regulation of PI3 K/Akt/mTOR/NF-kappaB signaling by indole compounds and their derivatives: mechanistic details and biological implications for cancer therapy. Anti-Cancer Agents Med Chem 13(7):1002–1013
Amiri KI, Richmond A (2005) Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev 24(2):301–313. doi:10.1007/s10555-005-1579-7
Baldwin AS (2012) Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunol Rev 246(1):327–345. doi:10.1111/j.1600-065X.2012.01095.x
Gilmore TD, Herscovitch M (2006) Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene 25(51):6887–6899. doi:10.1038/sj.onc.1209982
Chen G, Han K, Xu X, Du X, Zhang Z, Tang J, Shi M, Wang M, Li J, Cao B, Mao X (2014) An anti-leishmanial thiadiazine agent induces multiple myeloma cell apoptosis by suppressing the nuclear factor kappaB signalling pathway. Br J Cancer 110(1):63–70. doi:10.1038/bjc.2013.711
Murray RZ, Norbury C (2000) Proteasome inhibitors as anti-cancer agents. Anticancer Drugs 11(6):407–417
Bredholt T, Dimba EA, Hagland HR, Wergeland L, Skavland J, Fossan KO, Tronstad KJ, Johannessen AC, Vintermyr OK, Gjertsen BT (2009) Camptothecin and khat (Catha edulis Forsk.) induced distinct cell death phenotypes involving modulation of c-FLIPL, Mcl-1, procaspase-8 and mitochondrial function in acute myeloid leukemia cell lines. Mol Cancer 8:101. doi:10.1186/1476-4598-8-101
Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A, Cao HH, Tian HY, Fung KP, Kurihara H, Pan JX, Ye WC (2013) Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis 34(6):1331–1342. doi:10.1093/carcin/bgt060
Roy R, Kumar D, Chakraborty B, Chowdhury C, Das P (2013) Apoptotic and autophagic effects of Sesbania grandiflora flowers in human leukemic cells. PLoS One 8(8):e71672. doi:10.1371/journal.pone.0071672
Liu J, Zheng L, Ma L, Wang B, Zhao Y, Wu N, Liu G, Lin X (2014) Oleanolic acid inhibits proliferation and invasiveness of Kras-transformed cells via autophagy. J Nutr Biochem 25(11):1154–1160. doi:10.1016/j.jnutbio.2014.06.006
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26(7):1749–1760. doi:10.1038/sj.emboj.7601623
Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18(4):571–580. doi:10.1038/cdd.2010.191
Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R (2008) Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ 15(8):1318–1329. doi:10.1038/cdd.2008.51
Jiang Q, Wang Y, Li T, Shi K, Li Z, Ma Y, Li F, Luo H, Yang Y, Xu C (2011) Heat shock protein 90-mediated inactivation of nuclear factor-kappaB switches autophagy to apoptosis through becn1 transcriptional inhibition in selenite-induced NB4 cells. Mol Biol Cell 22(8):1167–1180. doi:10.1091/mbc.E10-10-0860
Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C (2009) p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol 29(10):2594–2608. doi:10.1128/mcb.01396-08
Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, Codogno P (2006) NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem 281(41):30373–30382. doi:10.1074/jbc.M602097200
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 30772601) and the University Innovation Team Project Foundation of Education Department of Liaoning Province (No. LT2013019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gao, L., Wang, Y., Xu, Z. et al. SZC017, a novel oleanolic acid derivative, induces apoptosis and autophagy in human breast cancer cells. Apoptosis 20, 1636–1650 (2015). https://doi.org/10.1007/s10495-015-1179-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1179-0