Abstract
Purpose
The river-lake ecotone in lake ecosystems can strongly influence sediment nitrate dissimilatory reduction processes. However, the mechanisms underlying these processes in river-lake ecotone ecosystems are still poorly understood. This study aims to investigate the rates of sediment nitrate dissimilatory reduction processes in Poyang Lake and clarify the mechanisms and environmental implications of these processes.
Materials and methods
Sediment samples from five river-lake ecotones and lake ecosystems were collected from Poyang Lake, China. Sediment nitrate reduction and N2O production rates were measured using 15N isotope tracing experiments. The abundance of denitrifiers, anammox 16S rRNA bacteria, and nrfA genes was quantified using the polymerase chain reaction method. Correlation analysis, redundancy analysis, and stepwise linear regression were used to evaluate the mechanisms of sediment nitrate reduction processes.
Results and discussion
Sediments in the river-lake ecotone showed significantly higher denitrification (DEN), N2O production, dissimilatory nitrate reduction to ammonium (DNRA), and anammox rates compared to lake ecosystems. DEN was the dominant process contributing to nitrate reduction, accounting for 73.36% and 74.13% in the river-lake ecotone and lake ecosystem, respectively. DEN, N2O, and DNRA rates were significantly positively correlated with TOC, sulfide, and Fe2+ contents, which control the abundance of denitrifying and nrfA genes, ultimately increasing these rates.
Conclusion
The annual input of reactive N to the lake from the watershed is almost equivalent to the annual N removal. However, higher DNRA and N2O production rates indicate that approximately 23.59% of the annual N input to the lake may be transformed to NH4+ or N2O. N retention and N2O production in the river-lake ecotone significantly exceed those in the lake ecosystem, highlighting the ecotone as a hotspot for eutrophication risk and N2O emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen (N) is an essential element for biological organisms, playing a key role in promoting primary productivity and maintaining lake ecosystem health (Saggar et al. 2013; Heffernan and Cohen 2010; Zhu et al. 2020). With the rapid development of agriculture and industry in lake watersheds, a substantial amount of reactive N is transported into lake ecosystems (Finlay et al. 2013; Zhu et al. 2020; Yang et al. 2022). The excessive input of reactive N has caused various environmental issues, particularly aquatic eutrophication and nitrous oxide (N2O) production (Jiang et al. 2020; Yang et al. 2022; Zhang et al. 2022). In lake ecosystems, sediments play an important role in nitrate removal and the mitigation of N2O emissions (Mulholland et al. 2008; Nizzoli et al. 2010; Finlay et al. 2013). However, continuous N loading and nitrate retention have converted lake sediments into significant sources of nitrate pollution and N2O emissions (Bogard et al. 2020; Kortelainen et al. 2020; Yang et al. 2022). Consequently, the problems of N pollution and N2O production in lake ecosystems have attracted global attention (Schmadel et al. 2018; Rex et al. 2021; Zhang et al. 2022). Sediment nitrate dissimilatory reduction processes, including anaerobic ammonium oxidation (anammox), denitrification (DEN), and dissimilatory nitrate reduction to ammonium (DNRA), are the primary pathways of nitrate removal or retention in aquatic environments (Stelzer et al. 2014; Reisinger et al. 2016; Xiong et al. 2017; Li et al. 2018). Understanding the mechanisms and environmental implications of these processes in lake ecosystems is necessary for controlling N pollution and reducing N2O emissions.
In lake ecosystems, sediment DEN and anammox processes lead to the reduction of NO3- to N2 gas, thus removing it from the lake ecosystem (Jiang et al. 2020; Tan et al. 2022). In the DEN process, N2O is an intermediate product that can be released into the atmosphere if the process is incomplete (Wu et al. 2021; Li et al. 2023; Xiang et al. 2023). N2O is a powerful greenhouse gas with a warming potential approximately 298 times that of carbon dioxide (Lauerwald et al. 2019; Ashiq et al. 2022; Kirkby et al. 2023). Sediment DNRA processes convert nitrate into ammonium, leading to nitrate retention in aquatic environments (Nogaro and Burgin 2014; Kim et al. 2016; Lin et al. 2017; Shelley et al. 2017). The competition among DEN, anammox, and DNRA processes in lake sediments is controlled by the high variability of sediment properties, such as C and N contents (Plummer et al. 2015; Shan et al. 2016; Gao et al. 2019; Jiang et al. 2021a). Previous studies have extensively investigated spatial and temporal distribution and environmental factors affecting sediment dissimilatory nitrate reduction processes in lake sediments. However, these processes in river-lake ecotone systems remain unclear.
The river-lake ecotone system is an important transition zone between a lake and its inflow rivers, playing a crucial role in absorbing and dissipating surface runoff pollution and being influenced by both river and lake ecosystems (Yan et al. 2022). Sediments in the river-lake ecotone reflect the environmental impacts of the river watershed on the lake ecosystem, such reactive N loading and land use changes affecting sediment N transformation. Compared with lake ecosystems, reactive N loading from the upstream river watershed is often concentrated in the river-lake ecotone, significantly regulating sediment environmental properties (such as reactive N, pH, and TOC contents) and nirS, nirK, nosZ, nrfA, and anammox 16S rRNA bacteria abundances and community structures (Wei et al. 2020; Yan et al. 2022). As a result, sediments in the river-lake ecotone become potential hotspots for N transformation and N2O emissions. Therefore, understanding the spatial distribution and mechanisms of sediment dissimilatory nitrate reduction processes in the river-lake ecotone is crucial for controlling N pollution and enhancing N removal in lake ecosystems.
With industrial and agricultural development in the Poyang Lake watershed, N inputs into the lake ecosystem have greatly increased, posing a severe eutrophication risk (Yao et al. 2016). Understanding the mechanisms and environmental implications of sediment nitrate reduction is important for maintaining the health of Poyang Lake and reducing eutrophication. Although DEN and DNRA processes in Poyang Lake have been studied (Yao et al. 2016; Zhang et al. 2016; Wang et al. 2017; Jiang et al. 2020), the nitrate dissimilatory reduction processes, including DEN, anammox, DNRA, and N2O production, as well as the differences and driven mechanisms between the river-lake ecotone system and the lake ecosystem, have not been clarified. The objectives of this study were to: (1) reveal variations in sediment dissimilatory nitrate reduction processes and N2O production rates in different river-lake ecotones; (2) identify the mechanisms of sediment nitrate reduction rates and N2O production in the river-lake ecotone; and (3) analyze nitrate fates in Poyang Lake and reveal the environmental implications of sediment nitrate reduction. These finding are of great significance for improving the understanding of nitrate removal in lake ecosystems.
2 Material and methods
2.1 Study area and sampling
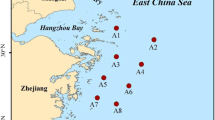
Poyang Lake is the largest freshwater lake in China, located in the northern part of Jiangxi province. The lake area covers approximately 4000 km2 during the wet season, with annual water level fluctuations of around 10 m (Wang et al. 2016; Liao et al. 2018). The Poyang Lake watershed spans 162,200 km2, of which 96.62% is within Jiangxi province (Yao et al. 2016). Five large rivers flow into the lake: Ganjiang River (GJ), Raohe River (RH), Fuhe River (FH), Xiushui River (XS), and Xinjiang River (XJ) (Fig. 1).
A total of 26 surface sediment samples (0–5 cm) were collected from five river-lake ecotones and lake ecosystem (DC) from February 21st to 23rd (dry season) and May 16th to 18th (wet season) in 2021 using a Van Veen grab sampler (Fig. 1). In total of 26 surface sediment samples, six samples were collected in GJ, five samples were collected in RH, three samples were collected in DC, four samples were collected in FH, XS, and XJ. For each sample, we collected five sub-samples within a 50 m transect and mix then together. Adjacent sampling sites within the same river-lake ecotone was spaced 5-10 km apart. To minimize the impact of human activities on the results, sampling points were located at least 1 km away from urban and industrial area. Samples were collected in airtight, acid-cleaned plastic bags and transferred to the laboratory within 4 hours of collection, maintained at 4 ℃. In each season, the samples are brought back to the laboratory every night to ensure the reliability of experimental analysis data. The longitude and latitude of each sample were recorded using GPS. In the laboratory, the well-mixed sediment samples were divided into three fractions: (1) frozen at -20 °C for measuring sediment physicochemical characteristics; (2) stored at 4 °C for analyzing sediment dissimilatory nitrate reduction processes rates and N2O production; and (3) frozen at −80 °C for measuring genes abundance related to nitrate reduction.
2.2 Soil physicochemical analysis
Sediment total organic carbon (TOC) content was measured using the dichromate oxidation method (Jiang et al. 2023). Before measuring TOC content, the sediment was freeze-dried using a vacuum-freeze dryer and passed through a 0.15 mm mesh sieve. Amorphous Fe oxides in the sediment were extracted using 1 M HCl, 1g of fresh sediment was placed into a 50 mL centrifuge tube, and 30 mL of 1 M HCl was added. After shaking for 16 hours, Fe2+ and Fe3+ contents in the supernatant were measured using the photometric analysis of the Ferrozine assay (Gao et al. 2019). An Orion-Sure-flow combination silver-sulfide electrode (Thermo Scientific Orion) was used to measure sediment sulfide concentration (Hou et al. 2012). Sediment NH4+, NO3−, and NO2− were extracted using a 2 M KCl solution, and their contents were determined using a continuous-flow analyzer (Yin et al. 2017). Sediment pH was measured using a pH meter at a sediment-to-water ratio of 1:2.5 (Zheng et al. 2014). Properties of Fe2+, Fe3+, sulfide, pH, NH4+, NO3−, and NO2− were measured using both fresh sediments frozen at -20 °C and fresh sediment that was converted into dry sediment based on the sediment’s water content. All sediment physicochemical properties were determined in triplicate.
2.3 Measurements of sediment DEN, anammox, and DNRA rates
Sediment potential DEN and anammox process rates were measured using the 15N isotope tracing method (Cheng et al. 2016). Fresh sediment samples stored at 4 °C were stirred into a slurry state by mixing with deionized water at a mass ratio of 1:7. Dissolved N2 and O2 in the slurry samples were removed by aeration with helium gas to ensure the dissolved N2 was not saturated. Subsequently, the slurry samples were transferred into 12.5 mL gastight borosilicate vials and pre-incubated for 48 hours at temperatures similar to the sampling site (30℃ in May and 12℃ in February) to ensure an anaerobic environment. After pre-incubation, 100 μL of 15NO3− (15N at 99%) was added to the vials containing the slurry samples. To prepare the initial samples, 200 μL of ZnCl2 solution was immediately added to half of the vials to stop microbial activity. For the final samples, microbial activity in the remaining vials were stopped after 8 hours of incubation. Dissolved 29N and 30N in the final and initial samples were measured using membrane inlet mass spectrometry (MIMS). This 15N isotope tracing method requires immediate testing of samples in an anaerobic environment with unsaturated dissolved N2. Rates of DEN and anammox in sediment were calculated by the difference in 29N2 and 30N2 between the final and initial samples. The respective contributions of DEN and anammox to total 29N2 production were quantified using the equation (Deng et al. 2015):
where P29 (nmol N g−1 h−1) denotes the total 29N2 production rate during the slurry experiments, and D29 and A29 (nmol N g−1 h−1) are the production rates of 29N2 from DEN and anammox, respectively. Given the random isotope pairing of 14N and 15N generated from 14NO3− or 15NO3−, D29 can be estimated by the equation (Gao et al. 2017):
where P30 (nmol N g−1 h−1) denotes the total 30N2 production rate and FN (%) is the fraction of 15N in NO3−, calculated from the concentrations of added 15NO3− and residual NO3− in the incubation slurries. Consequently, the potential rates of DEN and anammox were estimated using the equations (Gao et al. 2019):
where Dt and A29 (nmol N g−1 h−1) represent the potential rates of DEN and anammox, respectively.
The OX/MIMS method (15NH4+ oxidation technique combined with MIMS analysis) was used to measure the sediment DNRA rate (Yin et al. 2014). The same incubation and 15N isotope addition procedures as for DEN and anammox rates were applied. Dissolved N2 initial samples and final samples were removed using helium gas. In all vials, 200 μL of hypobromite iodine solution was injected to oxidize 15NH4+ (produced by DNRA) into 15N2, which was then measured using MIMS. Potential DNRA rates were estimated by the changes in 15NH4+ concentration during incubation, calculated using the equation (Jiang et al. 2021a):
where RDNRA (nmol N g−1 h−1) denotes the total DNRA rate, and [15NH4+]F and [15NH4+]I are the concentrations of 15NH4+ in the final and initial samples of the soil slurries, respectively. V(L) denotes the volume of the vial, W(g) denotes the dry weight of the soil, and T(h) is the incubation time.
2.4 Potential N 2 O production rate
The headspace equilibrium technique was used to measure potential N2O production (Hou et al. 2015). The incubation method was consistent with that used for the DEN rate. After incubation, 2 mL helium gas was injected into the vials to replace the water phase and create a headspace. To equilibrate the liquid and gas phases, the vials were shaken vigorously for 1 hour, and the N2O concentration of the headspace gas was measured by gas chromatography immediately. The potential N2O production rate was calculated using the following equation (Hou et al. 2015):
where P (nmol g-1 h-1) represents the potential N2O production; Oi (nmol L-1) represents the initial dissolved N2O concentrations; Of (nmol L-1) represents the final dissolved N2O concentrations; and V (h) represents the incubation times.
Sediment samples for DEN, anammox, DNRA, and N2O production rates need to be determined immediately, as prolonged storage at 4 °C can increase experimental bias. Rates of sediment DEN, anammox, DNRA, and N2O production were measured using wet sediment directly and then converted to dry sediment based on sediment water content.
2.5 DNA extraction and quantitative PCR (q-PCR) analysis
DNA from the sediments was extracted using a Powersoil™ DNA Isolation Kit according to the manufacturer’s specifications. The abundance of nirS, nirK, nosZ, anammox bacterial 16S rRNA gene, and nrfA genes were quantified through real-time q-PCR using the SYBR Green method on the ABI 7500 Detection System. Plasmid DNA containing the target gene fragment was constructed by cloning and quantified using an ultramicro spectrophotometer. A 10-fold gradient dilution series (102–109 copies) was used as a reaction template for quantitative PCR reaction with environmental sample DNA under the same conditions (Jiang et al. 2021a, 2023). The amplification efficiencies under q-PCR conditions were 92.4, 94.68%, 95.2%, 92.7%, and 93.5% for nirS, nirK, nosZ, anammox bacterial 16S rRNA, and nrfA genes, respectively. Details of the gene primers and q-PCR conditions are provided in Table S1. The abundance of these genes was calculated according to the standard curve and then converted into copies per gram of dry sediment based on sediment water content.
2.6 Statistical analysis
Spatio-temporal differences in the nitrate dissimilatory reduction processes of sediments were evaluated through one-way analysis of variance (ANOVA) and Dunn's test for multiple comparisons. Relationships among sediment nitrate dissimilatory reduction processes, environmental factors, and gene abundance related to nitrate reduction were analyzed using Pearson’s correlation analysis, redundancy analysis (RDA), and stepwise multiple regression analysis, and stepwise multiple regression analysis. A significance level of p < 0.05 was considered statistically significant. One-way analysis of variance, stepwise multiple regression, and Pearson’s correlation analyses were performed using SPSS 19.0. Stepwise multiple regression analysis identified the main factors influencing sediment nitrate dissimilatory reduction processes. Multivariate relationships among sediment nitrate dissimilatory reduction processes, environmental factors, and gene abundance related to nitrate reduction were examined using RDA. Pearson’s correlation analysis was used to reveal relationships between DEN, anammox, DNRA, N2O production rates, and sediment properties.
3 Results
3.1 Sediment physicochemical properties
The physicochemical properties of sediments in different river-lake ecotones and the lake ecosystem are shown in Fig. 2. Sediment Fe2+ and TOC contents were significantly higher in the wet season than in the dry season (T-test, p < 0.05). The average Fe2+ contents were 0.50 mg g-1 in the wet season and 0.42 mg g-1 in the dry season. Fe2+ contents in RH and DC were significantly lower than those in FH and XJ (one-way ANOVA, p < 0.05). NH4+ contents in RH and DC were significantly lower than those in GJ and XJ (one-way ANOVA, p<0.05), with the highest NO3- content observed in GJ. TOC contents in GJ, RH, DC, and XS were significantly lower than those in FH and XJ (one-way ANOVA, p < 0.05). NH4+ and TOC contents were 10.44 and 8.64 μg g-1, and 14.13 and 11.28 mg g-1 in the wet and dry season, respectively. Sulfide contents were 24.82 and 22.22 μg g-1 in the wet and dry season, respectively, with FH and XJ significantly higher than RH (one-way ANOVA, p < 0.05). Sediment pH in RH was significantly lower than in DC during the wet season (one-way ANOVA, p < 0.05).
Physiochemical characteristics of sediment in Poyang Lake (mean ± SE). Different letters (a, b, c, etc.) indicate significant differences (p < 0.05). GJ, RH, DC, FH, XS and XJ were represent samples in Ganjiang River, Raohe River, lake ecosystem, Fuhe River, Xiushui River and Xinjiang River, respectively
3.2 Abundance of functional genes
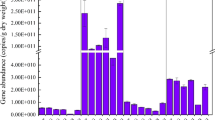
The abundance of nirS, nirK, nosZ, anammox bacterial 16S rRNA, and nrfA genes showed no significant different between the wet and dry seasons, but significant differences were observed among the river-lake ecotones (one-way ANOVA, p < 0.05) (Fig. 3). The abundance of the nirS gene ranged from 7.19 to 7.85 log10 copies g−1 in the wet season and from 7.22 to 7.84 log10 copies g−1 in the dry season. The abundance of the nirS gene in RH was significantly lower than in FH and XJ (one-way ANOVA, p < 0.05). The nirK gene abundance in FH was significantly higher than in GJ, RH, DC, XS, and GJ (one-way ANOVA, p < 0.05) with FH showing the highest abundance of nirS and nirK genes in both seasons. The nosZ gene abundance in GJ, RH, and DC was significantly lower than in FH, XS, and XJ (one-way ANOVA, p < 0.05), with abundance ranging from 7.33 to 8.60 log10 copies g−1 in the wet season and from 7.06 to 8.60 log10 copies g−1 in the dry season. The highest abundance was observed in XS (the wet season) and FH (the dry season). The abundance of anammox bacterial 16S rRNA genes in DC was significantly lower than in GJ and XJ, with abundances ranging from 6.79 to 7.98 log10 copies g−1 in the wet season and from 6.66 to 7.85 log10 copies g−1 in the dry season. The abundance of the nrfA gene in DC and RH was significantly lower than in GJ, FH, and XJ (one-way ANOVA, p < 0.05), with abundances ranging from 6.66 to 8.10 log10 copies g−1 in the wet season and from 6.66 to 8.18 log10 copies g−1 in the dry season.
3.3 Rates of dissimilatory nitrate reduction processes
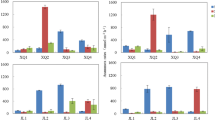
The rates of sediment dissimilatory nitrate reduction processes at different river-lake ecotones and the lake ecosystem are shown in Fig. 4. Nitrate reduction rates in Poyang Lake were significantly higher in the wet season than in the dry season (T-test, p < 0.01). The rate of DEN was significantly higher than that of anammox and DNRA in both seasons (one-way ANOVA, p < 0.01). The average rates of potential DEN in the wet and dry seasons were 6.65–30.81 and 2.28–10.65 nmol g-1 h-1, respectively. Potential DEN rates in FH and XJ were significantly higher than those in GJ, RH, DC, and XS (one-way ANOVA, p < 0.05), with the highest DEN rate observed in FH. Potential anammox rates were 0.07–1.86 nmol g-1 h-1 and 0.03–0.72 nmol g-1 h-1 in the wet and dry seasons, respectively. Anammox rates in RH and DC were significantly lower than those in GJ and FH (one-way ANOVA, p < 0.05). The potential DNRA rate in Poyang Lake was significantly higher than the anammox rate (T-test, p < 0.01), with DNRA rates of 1.52–4.52 and 0.54–1.80 nmol g-1 h-1 in the wet and dry seasons, respectively. Average DNRA rates in FH, GJ, XS, and XJ were significantly higher than those in DC and RH (one-way ANOVA, p < 0.05).
Potential dissimilatory nitrate reduction rates of sediment in Poyang Lake. Different letters (a, b, c, etc.) indicate significant differences (p < 0.05). GJ, RH, DC, FH, XS and XJ were represent samples in Ganjiang River, Raohe River, lake ecosystem, Fuhe River, Xiushui River and Xinjiang River, respectively. DEN and DNRA represent denitrification rate and dissimilatory nitrate reduction to ammonium, respectively
3.4 Potential N 2 O production rates
Potential N2O production at different river-lake ecotones and the lake ecosystem are shown in Fig. 5. Sediment N2O production was significantly higher in the wet season (0.14–11.55 nmol g-1 h-1) than in the dry season (0.09–6.47 nmol g-1 h-1) (T-test, p<0.01). Potential N2O production in GJ and RH was significantly lower than in DC, FH, XS, and XJ (one-way ANOVA, p < 0.05), with the highest N2O production observed in FH in both seasons. Sediment N2O/(N2O+N2) in FH was significantly higher than in other river-lake ecotones (one-way ANOVA, p<0.05). N2O/(N2O+N2) in the dry season (0.03–0.42) was significantly higher than in the wet season (0.02–0.32) (T-test, p < 0.05).
The contributions of anammox, DEN, and DNRA to nitrate reduction are shown in Fig. 6. Potential DEN was the dominant process contributing to nitrate reduction, accounting for 83.99% and 83.67% in the wet and dry seasons, respectively. DNRA contributed 12.81% and 12.61% to nitrate reduction in the wet and dry seasons, respectively, with the largest contribution observed in RH. Sediment anammox contributed 3.20% and 3.52% to nitrate reduction in the wet and dry seasons, respectively.
Contributions of potential anammox (ANA), denitrification (DEN), dissimilatory nitrate reduction to ammonium (DNRA) and N2O production rates to nitrate reduction. a is represent Wet season and b is represent Dry season. GJ, RH, DC, FH, XS and XJ were represent samples in Ganjiang River, Raohe River, lake ecosystem, Fuhe River, Xiushui River and Xinjiang River, respectively
3.5 Influencing factors of sediment dissimilatory nitrate reduction processes and N 2 O production
The results of Pearson’s correlation analysis and RDA analysis are shown in Tables S2, S3, and Fig. 7. The anammox rate was significantly positively correlated with anammox bacterial 16S rRNA abundance and NH4+ content. Sediment DEN, DNRA, and N2O production rates were significantly positively correlated with Fe2+, TOC, and sulfide contents. The DEN rate and N2O production were significantly positively correlated with the abundance of the nirS, nirK, nosZ genes, while the DNRA rate was significantly positively correlated with the abundance of the nrfA gene. The abundance of the nirS, nirK, nosZ, and nrfA genes was significantly positively correlated with Fe2+, TOC, and sulfide contents. N2O production was positively correlated with DNF, DNRA, nirS, nirK, nosZ, Fe2+, TOC, and sulfide.
Redundancy analysis (RDA) of dissimilatory nitrate reduction processes rates, associated functional gene abundances and physicochemical characteristics. ANA represent anammox rate; AMX represent anammox bacterial 16S rRNA gene, DEN represent denitrification rate and DNRA represent dissimilatory nitrate reduction to ammonium. GJ, RH, DC, FH, XS and XJ were represent samples in Ganjiang River, Raohe River, lake ecosystem, Fuhe River, Xiushui River and Xinjiang River, respectively
Stepwise multiple regression analysis further identified the dominant factors affecting dissimilatory nitrate reduction processes. Based on the results (Table S4), the anammox rate was positively correlated with anammox bacterial 16S rRNA abundance. The DEN rate was positively correlated with nirK gene abundance and sulfide content in both the wet and dry seasons. N2O production and DNRA rates were positively correlated with the DEN rate and TOC content, respectively, in both seasons.
4 Discussion
4.1 Effects of river-lake ecotone on dissimilatory nitrate reduction processes in Poyang Lake
Compared with other aquatic ecosystems, sediment DEN and DNRA rates in Poyang Lake were similar to those in estuary, river, and lake ecosystems but significantly lower than in eutrophic and saline lakes (Table S5). The anammox rate in Poyang Lake sediments was significantly lower than in other aquatic ecosystems but similar to that in urban closed lakes. Seasonality variations in sediment potential anammox, DEN, DNRA, and N2O production rates were observed in Poyang Lake, indicating a close relationship between temperature and sediment dissimilatory nitrate reduction processes, consistent with previous studies (Korol et al. 2019; Jiang et al. 2021b; Tan et al. 2022). Temperature influences microbial metabolism, directly increasing nitrate reduction activities (Tan et al. 2022).
Nitrate reduction processes in lake sediments are regulated by differences in environmental factors (Plummer et al. 2015; Gao et al. 2019; Jiang et al. 2021a). This study observed that nitrate reduction processes in Poyang Lake sediments are significantly affected by TOC, sulfide, Fe2+, and the abundance of genes related to nitrate reduction processes. Sediment DEN, DNRA, and N2O production rates in river-lake ecotone systems were significantly higher than in the lake ecosystem. Furthermore, significantly higher TOC, sulfide, and Fe2+ contents were observed in river-lake ecotone systems, suggesting that the surrounding river watershed alters sediment environmental factors, ultimately enhancing nitrate dissimilatory reduction processes. Land use, agriculture, and urbanization in the watershed are likely to increase TOC, sulfide, Fe2+, and nitrate loading into the lake ecosystem, thereby changing sediment environmental factors and promoting nitrate dissimilatory reduction processes (Liu et al. 2016).
The sediment DEN rate was the dominant contributor to nitrate reduction, with an average contribution of 73.45%, similar to other aquatic environments (Gao et al. 2017; Yang et al. 2022). Average DEN rates in the river-lake ecotones and the lake ecosystem were 12.21 and 8.57 nmol g-1 h-1, respectively, indicating that river-lake ecotones increased DEN rates. RDA analysis showed that the sediment DEN rate in Poyang Lake is significantly affected by sediment TOC, sulfide, and Fe2+ contents, as well as the abundance of the nirS, nirK, and nosZ genes. Previous studies confirm that higher TOC content provides sufficient substrates and electron donors for denitrifying microorganisms, promoting the growth of denitrifying bacteria and increasing heterotrophic denitrification (Reisinger et al. 2016; Wang et al. 2018; Chang et al. 2021). In Poyang Lake, sediment TOC content was significantly positively correlated with the abundance of the nirS, nirK, and nosZ genes and the DEN rate, indicating that TOC content can significantly increase the abundance of these genes and ultimately promote the DEN rate. Sulfides can promote the DEN rate by serving as electron donors for denitrifier (Hou et al. 2012; Deng et al. 2015). Stepwise multiple regression analysis indicated that sulfide content and nirK gene abundance were the dominant factors affecting the DEN rate. Higher sulfide content was significantly positively correlated with the abundance of the nirK genes and the DEN rate, indicating that higher sulfide content in Poyang Lake promotes nirK gene abundance and increases the DEN rate. Changes in sediment Fe2+ contents can strongly regulate DEN rates (Shan et al. 2016; Li et al. 2022), as low-redox conditions and high substrate availability of Fe2+ favor the DEN process by promoting NO2- reduction (Picardal. 2012; Ding et al. 2014; Cheng et al. 2016). Therefore, higher Fe2+ content promotes the increase of nirS, nirK, and nosZ genes as well as the DEN rate.
N2O production contributed 12.43% to nitrate reduction, and stepwise multiple regression analysis results demonstrated that the DEN rate was the dominant factor regulating N2O production. Therefore, incomplete denitrification may be widespread in the sediments of Poyang Lake, and higher DEN rates significantly increase N2O production. Sediment N2O production showed a strong positive correlation with Fe2+, TOC, and sulfide contents and nirS and nirK gene abundance, indicating that these factors may promote incomplete denitrification in Poyang Lake. Higher contents of Fe2+, TOC, and sulfide have been reported to increase nirS and nirK gene abundance, ultimately promoting DEN rates and stimulating N2O production (McCrackin and Elser 2011; Gao et al. 2019; Jiang et al. 2021a). The nirS and nirK gene abundance also showed significant positive correlations with Fe2+, TOC, and sulfide contents, similar to previous studies. In the sediments of Poyang Lake, nosZ gene abundance, which enhances the conversion of N2O to N2, was influenced by sediment physicochemical properties. However, FH and XJ still showed higher N2O production, possibly due to the lower nosZ/(nirS+nirK) ratios in these locations. The ratio of nosZ/(nirS+nirK) is associated with higher N2O/(N2O+N2) ratios, and a higher ratio of nosZ/(nirS+nirK) may increase N2O reduction to N2 and decrease N2O production. (Duan et al. 2019; Zhang et al. 2021). Therefore, sediment N2O consumption is lower than N2O production under the influence of sediment physicochemical properties in Poyang Lake, indicating that higher nitrate removal may increase N2O production in Poyang Lake.
The DNRA process contributed 11.16% to nitrate reduction in Poyang Lake, representing nitrate retention in sediments. The higher DNRA rate showed significant positive correlations with TOC, sulfide, and Fe2+ contents and nrfA gene abundance (Gao et al. 2019; Jiang et al. 2020) (Fig. 7). The DNRA process is the preferred energy pathway, and higher sediment organic carbon content promotes NO3− consumption through the DNRA process (Hardison et al. 2015). Furthermore, TOC serves as the electron donor for the DNRA process, providing a favorable environment for DNRA microorganisms (nrfA gene) (Burgin and Hamilton 2007; Deng et al. 2015; Yin et al. 2017). Stepwise multiple regression analysis showed that TOC content was the dominant factor regulating the DNRA rate, indicating that TOC is crucial factor for increasing nrfA gene abundance and the DNRA rate in Poyang Lake. Sulfide content is significantly positively correlated with the DNRA rate because sulfides provide extra free energy for the nrfA gene, stimulating the DNRA rate (Simon et al. 2011; Yin et al. 2017). Under anoxic conditions, Fe2+ can be used by chemolithoautotrophic microbes, increasing the DNRA rate (Yin et al. 2017). Therefore, higher Fe2+ contents in sediments provide a favorable environment for the DNRA process (Giblin et al. 2013).
The sediment anammox rate contributed an average of 2.96% to nitrate reduction and was significantly correlated with NH4+ content and anammox bacterial 16S rRNA gene abundance, indicating that NH4+ content and bacterial activity can influence the anammox rate. NH4+ is an important substrate in the anammox process, and higher NH4+ content can increase anammox bacterial 16S rRNA gene abundance (Plummer et al. 2015; Shan et al. 2016). Therefore, higher NH4+ content promotes anammox bacterial 16S rRNA abundance and enhances the anammox rate. Sediment TOC, sulfide, Fe2+ and NH4+ contents were significantly higher in river-lake ecotones, indicating that these river-lake ecotones can alter sediment properties and ultimately increase nitrate reduction.
4.2 Environmental implications of dissimilatory nitrate reduction processes in Poyang Lake
Understanding the fate of nitrate in lake sediments is crucial for addressing lake N removal, N2O production, and N pollution problem issues. According to calculations, the average N removal from the anammox process was 8.73 g m-3 yr-1, while the DEN rate contributed 254 g m-3 yr-1 (217 g m-3 yr-1 for N2 and 37 g m-3 yr-1 for N2O) to nitrate removal. The rate of NO3- transformation to NH4+ through DNRA was 33 g m-3 yr-1, indicating that nitrate transformed to NH4+ and was retained in the sediments, potentially aggravating N pollution in Poyang Lake. Nitrate removal from DEN and anammox in river-lake ecotones and the lake ecosystem were approximately 234 g m-3 yr-1 and 157 g m-3 yr-1, respectively. Nitrate removal in river-lake ecotones significantly exceeded that in the lake ecosystem. However, sediment nitrate reduction in river-lake ecotones and the lake ecosystem were approximately 34 g m-3 yr-1 and 22 g m-3 yr-1, respectively, with N2O production rates of approximately 38 g m-3 yr-1 and 25 g m-3 yr-1, respectively. Therefore, while river-lake ecotones increased nitrate removal, they also increased nitrate reduction and N2O production, which may elevate the risk of lake eutrophication and enhance the greenhouse effect.
The fate of N in the sediments of Poyang Lake is illustrated in Fig. 8. The rate of N removal was 262 g m-3 yr-1. Over the area of 4000 km2 area of Poyang Lake, the total N removal is 1.05×106 t yr-1 (1.47×105 t for N2O and 9.02×105 t for N2). According to a previous study, the annual net anthropogenic N input into the lake was approximately 7443 kg km-2 (Chen et al. 2016). Given that the area of the Poyang Lake watershed is 163,242 km2, the total N input into the lake is 1.22×106 t yr-1, which is similar to the N removal rate. Therefore, net anthropogenic N inputs into Poyang Lake have not yet become a serious environmental problem. Approximately 11.16% of anthropogenic nitrate from the Poyang Lake watershed can be converted to NH4+ (1.32×105 t yr-1 in Poyang Lake), indicating that the DNRA process is an important pathway for N retention in Poyang Lake. Additionally, approximately 12.43% of anthropogenic nitrate from the Poyang Lake watershed can be converted to N2O, suggesting that the N2O production problem in Poyang Lake may be aggravated in the future. Nitrate fate in Poyang Lake indicates higher N2O production and nitrate retention in the river-lake ecotone, suggesting that river-lake ecotones are hot-spot regions for nitrate retention and N2O production.
Lake watershed land use changes can regulate river hydrological and biogeochemical functions, controlling seasonal N input into lake ecosystems (Basu et al. 2022; Liu et al. 2022). The discharge of anthropogenic wastewater from agriculture land or urban land into lake ecosystems is recognized as a significant factor affecting the competition among DEN, anammox, and DNRA processes (Liu et al. 2016; Yang et al. 2022). Furthermore, with increased N fertilizer input and changes in irrigation and drainage management practices, agricultural land in the watershed can alter properties such as TOC, pH, and nitrate in lake sediments, increasing N loading and regulating lake dissimilatory nitrate reduction processes (Liu et al. 2016). Therefore, lake watershed land use is a critical factor causing lake nitrate pollution and N2O production. Strengthening land use management has become crucial for controlling pollutant transfer in the watershed (Du et al. 2023). Understanding the relationships between river watershed land use and sediment N transformation may become a future research direction.
5 Conclusions
This study investigated the dissimilatory nitrate reduction processes in lake sediments across different river-lake ecotones and the lake ecosystem in Poyang Lake, China. The rates of sediment DEN, N2O production, DNRA, and anammox in the river-lake ecotone ecosystem were significantly higher than in the lake ecosystem, with these rates being higher in the wet season than in the dry season. DEN was found to be the dominant process for N removal, followed by DNRA and N2O production rates. Sediment TOC, sulfide, and Fe2+ contents are key factors controlling the abundance of denitrifying and nrfA genes, ultimately increasing the rates of DEN, DNRA processes, and N2O production. The sediment anammox rate was significantly influenced by the abundance of the anammox bacterial 16S rRNA gene and NH4+ content, but it had a minimal contribution to N removal. The fate of N in sediments indicated that the annual input of reactive N to the lake from the watershed is almost equivalent to the annual N removal. However, approximately 23.59% of the annual N input to the lake may be transformed into N2O or NH4+ through the DEN and DNRA processes, suggesting that N input to the lake increases warming potential or retention in sediments, thereby threatening water quality and ecological functions. Although nitrate removal rates were higher in river-lake ecotones, the increased N2O production and nitrate retention in these areas still pose a threat to lake N pollution and N2O emissions. Strengthening land use management has become crucial for controlling the transfer of pollutants from the river into the watershed. Understanding the relationships between river watershed land use and sediment N transformation may become a key focus of future research.
Data availability
Data will be made available on request.
References
Ashiq W, Vasava H, Ghimire U, Dunfield K, Daggupati P, Biswas A (2022) Seasonal agricultural wetlands act as potential source of N2O and CH4 emissions. CATENA 213:106184. https://doi.org/10.1016/j.catena.2022.106184
Basu NB, Van Meter KJ, Byrnes DK, Van Cappellen P, Brouwer R, Jacobsen BH, Jarsjö J, Rudolph DL, Cunha MC, Nelson N, Bhattacharya R, Destouni G, Olsen SB (2022) Managing nitrogen legacies to accelerate water quality improvement. Nat Geosci 15:97–105. https://doi.org/10.1038/s41561-021-00889-9
Bogard MJ, Vogt RJ, Hayes NM, Leavitt PR (2020) Unabated nitrogen pollution favors growth of toxic cyanobacteria over chlorophytes in most hypereutrophic lakes. Environ Sci Technol 54:3219–3227. https://doi.org/10.1021/acs.est.9b06299
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96. https://doi.org/10.1890/1540-9295
Chang YK, Yin GY, Hou LJ, Liu M, Zheng YL, Han P, Dong HP, Liang X, Gao DZ, Liu C (2021) Nitrogen removal processes coupled with nitrification in coastal sediments off the north east china sea. J Soils Sediments 21:3289–3299. https://doi.org/10.1007/s11368-021-02964-5
Chen F, Hou LJ, Liu M, Zheng YL, Yin GY, Lin XB, Li XF, Zong HB, Deng FY, Gao J, Jiang XF (2016) Net anthropogenic nitrogen inputs (NANI) into the Yangtze river basin and the relationship with riverine nitrogen export. J Geophys Res-Biogeo 121:451–465. https://doi.org/10.1002/2015JG003186
Cheng L, Li XF, Lin XB, Hou LJ, Liu M, Li Y, Liu S, Hu XT (2016) Dissimilatory nitrate reduction processes in sediments of urban river networks: Spatiotemporal variations and environmental implications. Environ Pollut 219:545–554. https://doi.org/10.1016/j.envpol.2016.05.093
Deng FY, Hou LJ, Liu M, Zheng YL, Yin GY, Li XF, Lin XB, Chen F, Gao J, Jiang XF (2015) Dissimilatory nitrate reduction processes and associated contribution to nitrogen removal in sediments of the Yangtze Estuary. J Geophys Res-Biogeo 120:1521–1531. https://doi.org/10.1002/2015JG003007
Ding LJ, An XL, Li S, Zhang GL, Zhu YG (2014) Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from paddy soils in a chronosequence. Environ Sci Technol 48:10641–10647. https://doi.org/10.1021/es503113s
Du MY, Yuan JY, Zhuo MC, Sadiq M, Wu JQ, Xu GR, Liu SA, Li J, Li G, Yan LJ (2023) Effects of different land use patterns on soil properties and N2O emissions on a semi-arid Loess Plateau of Central Gansu. Front Ecol Evol 11:1128236. https://doi.org/10.3389/fevo.2023.1128236
Duan P, Song Y, Li S, Xiong Z (2019) Responses of N2O production pathways and related functional microbes to temperature across greenhouse vegetable field soils. Geoderma 355:113904. https://doi.org/10.1016/j.geoderma.2019.113904
Finlay JC, Small GE, Sterner RW (2013) Human influences on nitrogen removal in lakes. Science 342:247–250. https://doi.org/10.1126/science.1242575
Gao DZ, Li XF, Lin XB, Wu DM, Jin BS, Huang YP, Hou LJ, Chen X (2017) Soil dissimilatory nitrate reduction processes in the Spartina alterniflora invasion chronosequences of a coastal wetland of southeastern China: dynamics and environmental implications. Plant Soil 421:383–399. https://doi.org/10.1007/s11104-017-3464-x
Gao DZ, Liu M, Hou LJ, Derrick YFL, Wang WQ, Li XF, Zeng AY, Zheng YL, Han P, Yang Y, Yin GY (2019) Effects of shrimp-aquaculture reclamation on sediment nitrate dissimilatory reduction processes in a coastal wetland of southeastern China. Environ Pollut 255:113219. https://doi.org/10.1016/j.envpol.2019.113219
Giblin AE, Tobias CR, Song B, Weston N, Banta GT, Rivera-Monroy VH (2013) The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26(3):124–131. https://doi.org/10.5670/oceanog.2013.54
Hardison AK, Algar CK, Giblin AE, Rich JJ (2015) Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production. Geochim Cosmochim Ac 164:146–160. https://doi.org/10.1016/j.gca.2015.04.049
Heffernan JB, Cohen MJ (2010) Direct and indirect coupling of primary production and diel nitrate dynamics in a subtropical spring-fed river. Limnol Oceanogr 55:677–688. https://doi.org/10.4319/lo.2009.55.2.0677
Hou LJ, Liu M, Carini S, Gardner W (2012) Transformation and fate of nitrate near the sediment–water interface of Copano Bay. Cont Shelf Res 35:86–94. https://doi.org/10.1016/j.csr.2012.01.004
Hou LJ, Yin GY, Liu M, Zhou JL, Zheng YL, Gao J, Zong HB, Yang Y, Gao L, Tong CF (2015) Effects of sulfamethazine on denitrification and the associated N2O release in estuarine and coastal sediments. Environ Sci Technol 49:326–333. https://doi.org/10.1021/es504433r
Jiang XY, Gao G, Zhang L, Tang XM, Shao KQ, Hu Y (2020) Denitrification and dissimilatory nitrate reduction to ammonium in freshwater lakes of the eastern plain, China: influences of organic carbon and algal bloom. Sci Total Environ 710:136303. https://doi.org/10.1016/j.scitotenv.2019.136303
Jiang YH, Yin GY, Hou LJ, Liu M, Gao DZ, Zhang ZX, Zheng YL, Han P (2021a) Variations of dissimilatory nitrate reduction processes along reclamation chronosequences in Chongming Island. China Soil till Res 206:104815. https://doi.org/10.1016/j.still.2020.104815
Jiang YH, Yin GY, Hou LJ, Liu M, Zheng YL, Han P, Zheng DS, Cheng C, Li MT (2021b) Marine aquaculture regulates dissimilatory nitrate reduction processes in a typical semi-enclosed bay of southeastern China. J Environ Sci 104:376–386. https://doi.org/10.1016/j.jes.2020.12.025
Jiang Y, Yin GY, Li Y, Hou LJ, Liu M, Chen C, Zheng DS, Wu H, Gao DZ, Zheng YL, Han P (2023) Saltwater incursion regulates N2O emission pathways and potential nitrification and denitrification in intertidal wetland. Biol Fert Soils 59:541–553. https://doi.org/10.1007/s00374-022-01625-0
Kim H, Bae HS, Reddy KR, Ogram A (2016) Distributions, abundances and activities of microbes associated with the nitrogen cycle in riparian and stream sediments of a river tributary. Water Res 106:51–61. https://doi.org/10.1016/j.watres.2016.09.048
Kirkby R, Friedl J, Takeda N, Rosa DD, Rowlings DW, Grace PR (2023) Nonlinear response of N2O and N2 emissions to increasing soil nitrate availability in a tropical sugarcane soil. J Soils Sediments 23:2065–2071. https://doi.org/10.1007/s11368-023-03482-2
Korol AR, Noe GB, Ahn C (2019) Controls of the spatial variability of denitrification potential in nontidal floodplains of the Chesapeake Bay watershed, USA. Geoderma 338:14–29. https://doi.org/10.1016/j.geoderma.2018.11.015
Kortelainen P, Larmola T, Rantakari M, Juutinen S, Alm J, Martikainen PJ (2020) Lakes as nitrous oxide sources in the boreal landscape. Glob Chang Biol 26:1432–1445. https://doi.org/10.1111/gcb.14928
Lauerwald R, Regnier P, Figucircdo V, Enrich-Prast A, Bastviken D, Lehner B, Maavara T, Raymond P (2019) Natural lakes are a minor global source of N2O to the atmosphere. Global Biogeochem Cycl 33:1564–1581. https://doi.org/10.1029/2019GB006261
Li SJ, Luo ZX, Ji GD (2018) Seasonal function succession and biogeographic zonation of assimilatory and dissimilatory nitrate-reducing bacterioplankton. Sci Total Environ 637–638:1518–1525. https://doi.org/10.1016/j.scitotenv.2018.05.020
Li SJ, Liao YH, Pang YM, Dong XL, Strous M, Ji GD (2022) Denitrification and dissimilatory nitrate reduction to ammonia in long-term lake sediment microcosms with iron (II). Sci Total Environ 807:150835. https://doi.org/10.1016/j.scitotenv.2021.150835
Li LX, Chen ML, Liu SS, Bao HY, Yang DX, Qu H, Chen Y (2023) Does the aging behavior of microplastics affect the process of denitrification by the difference of copper ion adsorption? J Hazard Mater 452:131276. https://doi.org/10.1016/j.jhazmat.2023.131276
Liao F, Wang G, Shi Z, Cheng G, Kong Q, Mu W, Guo L (2018) Estimation of groundwater discharge and associated chemical fluxes into Poyang Lake, China: approaches using stable isotopes (δD and δ18O) and radon. Hydrogeol J 26:1625–1638. https://doi.org/10.1007/s10040-018-1793-3
Lin XB, Li XF, Gao DZ, Liu M, Cheng L (2017) Ammonium production and removal in the sediments of Shanghai river networks: Spatiotemporal variations, controlling factors, and environmental implications. J Geophys Res-Biogeo 122:2461–2478. https://doi.org/10.1002/2017JG003769
Liu WZ, Xiong ZQ, Liu H, Zhang QF, Liu GH (2016) Catchment agriculture and local environment affecting the soil denitrification potential and nitrous oxide production of riparian zones in the Han River Basin, China. Agr Ecosyst Environ 216:147–154. https://doi.org/10.1016/j.agee.2015.10.002
Liu J, Liu XL, Wang Y, Li Y, Li YY, Yuan HZ, Fang LC, Wu JS (2022) Upstream 2000 ha is the boundary of the stream water nitrogen and phosphorus saturation concentration threshold in the subtropical agricultural catchment. CATENA 211:105960. https://doi.org/10.1016/j.catena.2021.105960
McCrackin ML, Elser JJ (2011) Greenhouse gas dynamics in lakes receiving atmospheric nitrogen deposition. Glob Biogeochem Cycl 25:1–12. https://doi.org/10.1029/2010GB003897
Mulholland PJ, Helton AM, Poole GC, Hall RO, Hamilton SK, Peterson BJ, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Findlay SEG, Gregory SV, Grimm NB, Johnson SL, McDowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Beaulieu JJ, Bernot MJ, Burgin AJ, Crenshaw CL, Johnson LT, Niederlehner BR, O’Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452:202-U246. https://doi.org/10.1038/nature06686
Nizzoli D, Carraro E, Nigro V, Viaroli P (2010) Effect of organic enrichment and thermal regime on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in hypolimnetic sediments of two lowland lakes. Water Res 44:2715–2724. https://doi.org/10.1016/j.watres.2010.02.002
Nogaro G, Burgin AJ (2014) Influence of bioturbation on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in freshwater sediments. Biogeochemistry 120:279–294. https://doi.org/10.1007/s10533-014-9995-9
Picardal FW (2012) Abiotic and microbial interactions during anaerobic transformations of Fe (II) and NOX-. Front Microbiol 3:1–7. https://doi.org/10.3389/fmicb.2012.00112
Plummer P, Tobias C, Cady D (2015) Nitrogen reduction pathways in estuarine sediments: Influences of organic carbon and sulfide. J Geophys Res-Biogeo 120:1958–1972. https://doi.org/10.1002/2015JG003057
Reisinger AJ, Tank JL, Hoellein TJ, Hall RO (2016) Sediment, water column, and open-channel denitrification in rivers measured using membrane-inlet mass spectrometry. J Geophys Res-Biogeo 121:1258–1274. https://doi.org/10.1002/2015JG003261
Rex D, Clough TJ, Lanigan GJ, Jansen-Willems AB, Condron LM, Richards KG, Müller C (2021) Gross N transformations vary with soil moisture and time following urea deposition to a pasture soil. Geoderma 386:114904. https://doi.org/10.1016/j.geoderma.2020.114904
Saggar S, Jha N, Deslippe J, Bolan NS, Luo J, Giltrap DL, Kim DG, Zaman M, Tillman RW (2013) Denitrification and N2O:N2 production in temperate grasslands: processes, measurements, modelling and mitigating negative impacts. Sci Total Environ 465:173–195. https://doi.org/10.1016/j.scitotenv.2012.11.050
Schmadel NM, Harvey JW, Alexander RB, Schwarz GE, Moore RB, Eng K, Gomez-Velez JD, Boyer EW, Scott D (2018) Thresholds of lake and reservoir connectivity in river networks control nitrogen removal. Nat Commun 9:2779. https://doi.org/10.1038/s41467-018-05156-x
Shan J, Zhao X, Sheng R, Xia YQ, Ti CP, Quan XF, Wang SW, Wei WX, Yan XY (2016) Dissimilatory nitrate reduction processes in typical Chinese Paddy soils: rates, relative contributions, and influencing factors. Environ Sci Technol 50:9972–9980. https://doi.org/10.1021/acs.est.6b01765
Shelley F, Klaar M, Krause S, Trimmer M (2017) Enhanced hyporheic exchange flow around woody debris does not increase nitrate reduction in a sandy streambed. Biogeochemistry 136:353–372. https://doi.org/10.1007/s10533-017-0401-2
Simon J, Kern M, Hermann B, Einsle O, Butt JN (2011) Physiological function and catalytic versatility of bacterial multihaem cytochromes c involved in nitrogen and sulfur cycling. Biochem Soc Trans 39:1864–1870. https://doi.org/10.1042/BST20110713
Stelzer RS, Scott JT, Bartsch LA, Parr TB (2014) Particulate organic matter quality influences nitrate retention and denitrification in stream sediments: Evidence from a carbon burial experiment. Biogeochemistry 119:387–402. https://doi.org/10.1007/s10533-014-9975-0
Tan EH, Hsu TC, Zou WB, Yan XL, Huang ZX, Chen B, Chang YK, Zheng ZZ, Zheng LW, Xu M, Tan L, Kao SJ (2022) Quantitatively deciphering the roles of sediment nitrogen removal in environmental and climatic feedbacks in two subtropical estuaries. Water Res 224:119121. https://doi.org/10.1016/j.watres.2022.119121
Wang XL, Xu LG, Wan RR, Chen YW (2016) Seasonal variations of soil microbial biomass within two typical wetland areas along the vegetation gradient of Poyang Lake, China. CATENA 137:483–493. https://doi.org/10.1016/j.catena.2015.10.020
Wang HX, Zhang L, Yao XL, Xue B, Yan WJ (2017) Dissolved nitrous oxide and emission relating to denitrification across the Poyang Lake aquatic continuum. J Environ Sci 52:130–140. https://doi.org/10.1016/j.jes.2016.03.021
Wang SY, Wang WD, Liu L, Zhuang LJ, Zhao SY, Su Y, Li YX, Wang MZ, Wang C, Xu LY, Zhu GB (2018) Microbial nitrogen cycle hotspots in the plant-bed/ditch system of a constructed wetland with N2O mitigation. Environ Sci Technol 52:6226–6236. https://doi.org/10.1021/acs.est.7b04925
Wei HC, Gao DZ, Liu Y, Lin XB (2020) Sediment nitrate reduction processes in response to environmental gradients along an urban river-estuary-sea continuum. Sci Total Environ 718:137185. https://doi.org/10.1016/j.scitotenv.2020.137185
Wu X, Probst A, Barret M, Payre-Suc V, Camboulive T, Granouillac F (2021) Spatial variation of denitrification and key controlling factors in streams and ponds sediments from a critical zone (southwestern France). Appl Geochem 131:105009. https://doi.org/10.1016/j.apgeochem.2021.105009
Xiang H, Hong YG, Wu JP, Wang Y, Ye F, Hu Z, Qu ZM, Long AM (2023) NosZ–II–type N2O-reducing bacteria play dominant roles in determining the release potential of N2O from sediments in the Pearl River Estuary. China Environ Pollut 329:121732. https://doi.org/10.1016/j.envpol.2023.121732
Xiong ZQ, Guo LD, Zhang QF, Liu GH, Liu WZ (2017) Edaphic conditions regulate denitrification directly and indirectly by altering denitrifier abundance in wetlands along the Han River, China. Environ Sci Technol 51:5483–5491. https://doi.org/10.1021/acs.est.6b06521
Yan ZW, Wu L, Lv T, Tong C, Gao ZY, Liu Y, Xing B, Chao CX, Li Y, Wang LG, Liu CH, Yu D (2022) Response of spatio-temporal changes in sediment phosphorus fractions to vegetation restoration in the degraded river-lake ecotone. Environ Pollut 308:119650. https://doi.org/10.1016/j.envpol.2022.119650
Yang ZB, Lu L, Cheng Z, Xian JR, Yang YX, Liu LX, Xu XX (2022) Dissimilatory nitrate reduction in urban lake ecosystems: A comparison study between closed and open lakes in Chengdu. China Water Res 214:118218. https://doi.org/10.1016/j.watres.2022.118218
Yao XL, Zhang L, Zhang YL, Xu H, Jiang XY (2016) Denitrification occurring on suspended sediment in a large, shallow, subtropical lake (Poyang Lake, China). Environ Pollut 219:501–511. https://doi.org/10.1016/j.envpol.2016.05.073
Yin GY, Hou LJ, Liu M, Liu ZF, Gardner WS (2014) A novel membrane inlet mass spectrometer method to measure 15NH4+ for isotope-enrichment experiments in aquatic ecosystems. Environ Sci Technol 48:9555–9562. https://doi.org/10.1021/es501261s
Yin GY, Hou LJ, Liu M, Li XF, Zheng YL, Gao J, Jiang XF, Wang R, Yu CD, Lin XB (2017) DNRA in intertidal sediments of the Yangtze Estuary. J Geophys Res-Biogeo 122:1988–1998. https://doi.org/10.1002/2017JG003766
Zhang L, Yao X, Tang C, Xu H, Jiang X, Zhang Y (2016) Influence of long-term inundation and nutrient addition on denitrification in sandy wetland sediments from Poyang lake, a large shallow subtropical lake in China. Environ Pollut 219:440. https://doi.org/10.1016/j.envpol.2016.05.046
Zhang QQ, Zhang X, Duan PP, Jiang XY, Shen HJ, Yan XY, Xiong ZQ (2021) The effect of long-term biochar amendment on N2O emissions: Experiments with N15–O18 isotopes combined with specific inhibition approaches. Sci Total Environ 769:144533. https://doi.org/10.1016/j.scitotenv.2020.144533
Zhang DD, Li MY, Yang YC, Yu H, Xiao FS, Mao CZ, Huang J, Yu YH, Wang YF, Wu B, Wang C, Shu LF, He ZL, Yan QY (2022) Nitrite and nitrate reduction drive sediment microbial nitrogen cycling in a eutrophic lake. Water Res 220:118637. https://doi.org/10.1016/j.watres.2022.118637
Zheng YL, Hou LJ, Newell S, Liu M, Zhou JL, Zhao H, You LL, Cheng XL (2014) Community dynamics and activity of ammonia-oxidizing prokaryotes in intertidal sediments of the Yangtze Estuary. Appl Environ Microbiol 80:408–419. https://doi.org/10.1128/AEM.03035-13
Zhu L, Shi WQ, Dam BV, Kon LW, Yu JH, Qin BQ (2020) Algal accumulation decreases sediment nitrogen removal by uncoupling nitrification-denitrification in shallow eutrophic lakes. Environ Sci Techno 54:6194–6201. https://doi.org/10.1021/acs.est.9b05549
Acknowledgements
This work was funded by the Science and Technology Research Project of Jiangxi Provincial Education Department (No. GJJ2200373), Jiangxi Natural Science Foundation (20232BAB213057), National Natural Science Foundations of China (No. 42161021 and 42061018) and interdisciplinary project (No. 164320H1847).
Funding
Science and Technology Research Project of Jiangxi Provincial Education Department, GJJ2200373, Yinghui Jiang, Jiangxi Natural Science Foundation, 20232BAB213057, Yinghui Jiang, National Natural Science Foundations of China, 42161021, Mingjun Ding, 42061018, Hua Zhang, interdisciplinary project, 164320H1847, Zhenglei Xie.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Shiming Ding
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Y., Xie, Z., Ding, M. et al. Sediment nitrate dissimilatory reduction processes in the river-lake ecotone of Poyang Lake, China: Mechanisms and environmental implications. J Soils Sediments (2024). https://doi.org/10.1007/s11368-024-03890-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11368-024-03890-y