Abstract
Purpose
Dissimilatory arsenate[As(V)]-respiring prokaryotes (DARPs) were consider to be the major player driving the reductive mobilization of As from solid phase. Mn(II) commonly coexists with DARPs in the environment. However, little is known about how Mn(II) affects the DARPs-mediated reductive mobilization of arsenic so far. This work aimed to address this issue.
Method
Three As-contaminated samples were collected from the arsenic-contaminated shallow soils. We examined the diversity and activity of the DARP population in the soils, and detected how Mn(II) affected the DARPs-mediated reductive mobilization. We also investigated how Mn(II) affected the arrA gene abundances and bacterial As(V)-respiring activities.
Results
We observed that a unique diversity of genes encoding As(V)-respiratory reductase were widely present in the shallow soils. The soils possessed high As(V)-respiring activities by using multiple electron donors. Microcosm assays indicated that compared to the microcosms without excessive Mn(II), addition of additional 10.0 mM Mn(II) to the microcosms led to 140.2%, 121.3%, and 257.8% increases of the microbial community-mediated As(III) release from the three soil samples. To further confirm this finding, a novel DARP, Bacillus sp. RM19 was ioslated from the samples. Microcosm assays with this cultivable DARP showed that the presence of additional 10.0 mM Mn(II) resulted in a 23.9% increase of the RM19-catalyzed As(III) release from the soils. Taken together, Mn(II) greatly enhances the As(V)-respiring prokaryotes-catalyzed reductive mobilization of As from soils.
Conclusion
This work provided new knowledge about the relationship between the biogeochemical cycles of As and Mn, and gained a new insight into the mechanism for the dynamic changes of As concentrations in contaminated groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As)-contaminated groundwater occurs in most countries in the World, and approximately 4.6–22.0 million people are directly or indirectly exposed on the groundwater containing As concentrations higher than the WHO standard (10 μg/L) (Rodríguez-Lado et al. 2013; Wang et al. 2019; Podgorski and Berg 2020). A question was thus raised: how was the arsenic-contaminated groundwater formed? Originally, arsenic existing as minerals is undissolved and non-toxic to organisms. However, under the biological, chemical and anthropogenic actions, the insoluble arsenic became to be weathered and dissolved, and finally recharged to the groundwater (Kudo et al. 2013; Muehe et al. 2016; Huq et al. 2020). Many investigations suggest that microorganisms, especially dissimilatory As(V)-respiring prokaryotes (DARPs), play a key role in promoting the reductive mobilization of As from solid phase (Ohtsuka et al. 2013; Cai et al. 2016; Chen et al. 2017; Kudo et al. 2013). DARPs were shown to be capable of anaerobically reducing As(V) into As(III) coupled the oxidation of multiple inorganic and organic reductants, including acetate, citrate, propionate, pyruvate, formate, succinate, glucose, lactate, hydrogen, sulfide, and yeast extract (Chen et al. 2017, 2020; Wang et al. 2014; Ghosh and Sar 2013). The functional protein is the As(V)-respiratory reductase (Arr), consisting of ArrA and ArrB subunits. The arrA gene was a genetic marker for identification of DARPs (Saltikov Newman 2003; Song et al. 2009). Some studies suggest that DARPs are able to directly reduce As(V) on the surfaces of synthetic minerals, and thus capable of mobilizing As in solid phase (Kawa et al. 2019; Guo et al. 2015; Cai et al. 2016).
In addition to microorganisms, many environmental other factors were found to play roles in the mobilization of As, contributing to dramatic fluctuations of As concentrations in groundwater (Reza et al. 2010; Chen et al. 2017; Kumarathilaka et al. 2018; Chen et al. 2020). It was shown that sulfate enhanced the DARPs-catalyzed release of As(III) from As-contaminated soils by increasing the As(V)-respiratory reductase gene abundance in the soils (Wang et al. 2017). In contrast, nitrate inhibited the DARPs-mediated release of As(III) from solid phase by decreasing the As(V)-respiratory reductase gene expression (Zhu et al. 2019). These investigations suggest that environmental inorganic substances drive the fluctuations of As concentration in groundwater.
Mn(II) commonly exists in the soils, aquifers, rivers, lakes, and sediments (Maity et al. 2020; Wu et al. 2010; Moskovchenko et al. 2017). It was shown that the concentrations of Mn(II) in As-contaminated groundwater are varied between 0.5 and 100 mg/L, suggesting that DARPs and Mn(II) coexist in the habitats of DARPs (Machado et al. 2019; Xie et al. 2009; de Meyer et al. 2017; Rahman et al. 2021; Dong et al. 2022). However, little is known about how Mn(II) affects the DARPs-mediated As(III) release from solid phase. This study aimed to address this issue by using microcosm assays with both microbial community and a cultivable DARP strain. The findings gained from this work would provide new knowledge on the interaction between the biogeochemical cycles of As and Mn, and help us to better understand the mechanism for the dynamic fluctuations of As concentrations in contaminated groundwater.
2 Materials and methods
2.1 Collection of As-contaminated soils
Arsenic-contaminated soils were collected from a Shimen Realgar Mine-affected vegetable field (29°38′49.95 N, 111°01′55.81E), located in the Shimen Town, Changde City of Hunan Province. The Shimen Realgar Mine had been the largest realgar mine in Asia for many years. However, because the mining activities led to severe contaminations of heavy metal in the surounding environment, it was closed in 1996. There were a few of lettuces sporadically distributed in the vegetable field, suggesting that the field has not been abandoned yet. Soil samples were collected by digging with spade from a depth of approximately 60 cm. Only intact soil blocks were collected. The samples must not be contaminated by rain, sweat, dirty hand, and surounding soils. All samples were placed into anaerobic bags immediately, and shipped to the laboratory as soon as possible.
2.2 Determination of chemical contents
Total content of arsenic in the soils was analyzed on AFS after the soils were treated with aqua regia as described previously (Chen et al. 2017). NO3− and NO2− concentrations were examined using ion chromatography. Dissolved As(III) and As(V) were examined by HPLC-AFS (High Performance Liquid Chromatography linked to Atomic Fluorescence Spectrometry). Soluble Fe(II) and Fe(III) were simultaneously determined with ferrozine reagent.
2.3 Examination of bacterial As(V)-respiring activities
Before active microcosms were prepared, soluble arsenic in the soil was washed and removed. Soil microcosm was prepared by mixing 2.5 g soils to 17.5 mL oxygen-free MM (minimal mineral) medium in an anaerobic bottle, amended with 2.0 mM As(V) and each of 10.0 mM citrate, l-malate, propionate, lactate, d-fructose, formate, succinate, pyruvate, glycerol, or butyrate as electron donor. The sterilized soil microcosms were included as controls. All of the bottles were anaerobically cultivated at 30°C until the reactions were finished. At an interval of 12 h, 0.4 mL of suspensions was collected from the bottles. The collected suspensions were centrifuged and the supernatant were taken from the bottles for measurement of the concentrations of As species.
2.4 Identification of different genes encoding As(V)-respiratory reductase
Soil total DNA was extracted and purified using Sigma GenElute™ Kit. PCR reaction was performed to amplify nearly full-length gene of As(V)-respiratory reductase with primers shown in Table S1. Produced DNA was recovered and ligated into a standard T vector for screening positive clones and sequencing. A phylogenetic tree of the obtained ArrA proteins and their homologues were generated as described previously (Chen et al. 2020).
2.5 Effects of Mn(II) on the microbial community-mediated As(III) mobilization
To conduct the microcosm assays, 2.0 g solid substance was put to 18.0 mL of oxygen-free MM medium, supplemented with 10.0 mM lactate and 10.0 mM Mn(II), or without Mn(II) in a 30-mL anaerobic bottle. The same slurries were autoclaved serving as parallel controls. All of the bottles were anaerobically cultured at 30 °C. At an appropriate interval, approximately 0.5 mL of suspensions was collected from the bottles for detecting the concentrations of soluble As, Fe, and lactate.
2.6 Isolation and identification of a new DARP
Active microcosms were prepared by mixing 2.0 g soils to 18.0 mL of the oxygen-free MM medium amended with 3.0 mM As(V), and 10.0 mM lactate in a 30-mL anaerobic bottle. The bottle was cultivated at 30°C until all As(V) was anaerobically converted to As(III). About 1.5 ml of suspended mixtures was collected from the bottle, and mixed with fresh MM medium amended with the same substances for another round of enrichment. After 2-3 rounds of enrichments, single bacterial strains were isolated and purified on agar under anaerobic conditions. To clone the 16 S rRNA gene sequence of the DARP strain, the isolate was cultivated, and the bacterial cells were collected for genomic DNA preparation by boiling method. An almost complete microbial 16 S ribosomal RNA gene was PCR-amplified with primers 27F and 1492R, and the produced DNA was shipped to Shenggong Biotechnology Corp. (Wuhan) for sequencing. To examine the As(V)-respiring activity of the isolate, bacterial cells in logarithmic phase were inoculated to MM medium with 1.0 mM As(V) and 10.0 mM lactate under anoxic conditions. The anaerobic bottles were cultivated until all As(V) was reduced to As(III). At an appropriate interval, 0.35 mL of the bacterial suspensions was collected from the bottle for analyzing the concentrations of soluble As(III).
2.7 Effects of Mn(II) on the cultivable DARP-mediated release of As(III)
Bacterial cells in logarithmic phase were inoculated to the autoclaved As-contaminated soil-derived microcosms, which were generated by inoculating 2.0 g soils to 18.0 mL of MM medium in a 30-mL bottle under anaerobic conditions. The microcosms were further amended with 10.0 mM lactate and 10.0 mM Mn(II), or without Mn(II). All of the mixtures were cultivated at 30 °C until all As(V) was reduced to As(III). At an appropriate interval, approximately 1.5 mL of mixtures was collected from the bottles for detection of the concentrations of soluble As, Fe, and lactate.
3 Results
3.1 Geochemical features of the As-contaminated soils
Three As-contaminated soil samples were collected from R1, R2, and R3 sampling sites in the Shimen Realgar Mine-affected vegetable field. As shown in Table S2, the samples R1, R2, and R3 contained extremly high contents of total As (12,646.5, 14,502.1, and 31,990.6 mg/kg), but much lower concentration of soluble As (3.0, 194.4, and 545.8 mg/kg). The three samples also contained SO42− (60.7, 1980.9, and 58.1 mg/kg), Mn (300.3, 990.2 mg/kg, ND), Fe (38.4, 31.4 g/kg, ND), NH4+ (64.5, 57.5, and 75.9 mg/kg), and NO3− (3.0, 100.5, and 895.3 mg/kg).
3.2 As(V)-respiring activities of the soils
For the sample R1, when each of 10.0 mM citrate, l-malate, propionate, lactate, d-fructose, formate, succinate, or pyruvate was amended to the microcosms, about 7, 3, 7, 3, 3, 7, 3, and 7 days were needed to anaerobically-reduce all As(V) into As(III), respectively. This suggests that the microbial community in R1 soils had high As(V)-respiring activities by using multiple electron donors, and most preferred to use malate, lactate, d-fructose, or succinate (Fig. 1a). For the sample R2, when each of 10.0 mM citrate, l-malate, propionate, lactate, d-fructose, formate, succinate, or pyruvate was amended to the soil-derived microcosms, about 3, 10, 3, 3, 3, 3, 7, and 3 days were needed to reduce all As(V) to As(III), respectively. This suggests that the microbial community in R2 soils also possessed significant As(V)-respiring activities by using multiple electron donors, and most preferred to use citrate, propionate, lactate, d-fructose, or pyruvate (Fig. 1b). Similarly, for the sample R3, when each of 10.0 mM citrate, l-malate, propionate, lactate, d-fructose, formate, succinate, pyruvate, glycerol, or butyrate was amended to the microcosms, about 14, 3, 8, 10, 22, 7, 10, 13, 13, and 7 days were needed to reduce all As(V) to As(III), respectively. This indicates that the microbial community in R3 soils had high As(V)-respiring activities by using multiple electron donors, and most preferred to use malate (Fig. 1c).
Arsenate-respiring activities of the microbial communities from the Shimen Realgar Mine-affected vegetable field soils. Each of the active soils was mixed with the MM medium amended with 2.0 mM As(V) as the electron acceptor and 10.0 mM citrate, l-malate, propionate, lactate, d-fructose, formate, succinate, pyruvate, glycerol, or butyrate as electron donor
These data suggest that the shallow soils possessed apparent As(V)-respiring activities by using multiple electron donors, and each sample had its own unique most preferred one.
3.3 Diversity of the ArrA proteins from samples
A partial gene sequence of the ArrAs from As-contaminated samples was amplified by PCR and analyzed by sequencing and bioinformatic methods. We identified 19 different ArrA proteins from the three As-contaminated soil samples, which were referred to as RAr1-19 (Fig. 2). RAr1, RAr3, RAr6, and RAr9 genes were cloned from R1 sample. RAr15 and RAr16 genes were from the R2 sample. RAr2, RAr4, RAr5, RAr7, RAr8, RAr10, RAr11, RAr12, RAr13, RAr14, RAr17, RAr18, and RAr19 genes were from R3 sample. RAr1-19 share 74.3-91.2% sequence homology to each other. A phylogenetic tree containing RAr1-19 sequences and their known homologues were constructed with an ArrA protein from Pyrobaculum arsenaticum DSM 13,514 as the outgroup. As shown in Fig. 2, RAr18 and RAr19 formed an independent branch, showing 81.8% and 81.7% sequence homology to the ArrA protein from Geobacter sp. OR-1, respectively, suggesting that RAr18 and RAr19 represent a new subfamily of ArrA proteins (Fig. 2). The sequences of RAr15, RAr16, and RAr17 show 65.5%, 78.1%, and 61.7% maximum homology with the ArrA protein from Chrysiogenes arsenatis DSM 11,915, respectively. The four ArrA proteins were affiliated to the same group. RAr11, RAr12, RAr13, and RAr14 share 83.6–89.1% homology with the ArrA from Geobacter sp. OR-1, suggesting that they belong to an ArrA family. RAr1, RAr2, RAr3, RAr4, RAr5, RAr6, RAr7, and RAr8 formed an independent branch, showing 73.9–85.3% sequence homology to the ArrA proteim from Aeromonas sp. JH155, Geobacter uraniireducens; this suggests that the eight RAr proteins constitute a novel subfamily of ArrA proteins. RAr9 and RAr10 shares 90.9% and 88.9% sequence identity to the ArrA proteins from Geobacter uranitreducen and Geobacter uranitreducen Rf4, respectively (Fig. 2).
Taken together, we showed that highly diverse DARPs were distributed in the shallow As-contaminated soils.
3.4 Mn(II) increases the microbial community-mediated reductive mobilization of arsenic
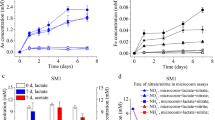
We performed microcosm assays using the R1, R2, and R3 soil samples that were amended with 10.0 mM Mn(II) or without excessive Mn(II) (Fig. 3a–f). After 21 days of anaerobic incubation, approximately 4.23, 3.14, and 2.56 mM As(III), and 0.17, 0.26, and 0.17 mM Fe(II) were released from the microcosms of R1, R2, and R3 without excessive Mn(II), respectively. In comparison, when 10 mM Mn(II) was amended to the microcosms, after 21 days of anaerobic incubation, about 10.16, 6.95, and 9.16 mM As(III), and 0.22, 0.33, and 0.28 mM Fe(II) were released from the active microcosms of the three samples, respectively. This suggests that the additions of 10.0 mM Mn(II) to the microcosms led to 140.2%, 121.3%, and 257.8% increases of the releases of As(III), and 29.4%, 26.9%, and 64.7% increases of the releases of Fe(II) from the microcosms of the three samples, respectively.
The effect of Mn(II) on the microbial community-mediated reductive mobilization of As and Fe in the soil samples from the Shimen Realgar Mine-affected vegetable field as examined using microcosm assays. a release of As(III) from R1; b release of Fe(II) from R1; c release of As(III) from R2; d release of As(III) from R3; e release of Fe(II) from R3; f changes of lactate concentration in the microcosms with or without additions of Mn(II)
We also examined the changes of lactate concentrations in the microcosms during the assays (Fig. 3g). For the microcosm assays without 10.0 mM Mn(II), after 14 days of anaerobic incubation, the concentrations of lactate in the microcosms of R1, R2, and R3 decreased from 10.0 mM to 3.7, 3.8, and 3.1 mM, respectively. In contrast, if 10.0 mM Mn(II) was added to the microcosms, after 21 days of incubation, all lactate was consumed up in all the microcosms of R1, R2, and R3.
This data suggests that the addition of 10.0 mM Mn(II) greatly promoted microbial community-mediated the reductive mobilization of As(III) from the microcosms.
3.5 Characterization of a new indigenous DARP strain from the soils
To further confirm the above findings, it is necessary to perform microcosm assays using an indigenous As(V)-respiring bacterial strain. We thus isolated a new DARP from the sample R1, which was referred to as RM19. Based on the phylogenetic tree analysis (Fig. 4a), RM19 was classified as a new member of the genus Bacillus. We thus named RM19 as Bacillus sp. RM19.
Bacillus sp. RM19 has significant As(V)-respiring activity. It was capable of anaerobically reducing all 1.0 mM As(V) to As(III) in 48 h (Fig. 4b). In addition, RM19 possesses nitrate-respiring activity, capable of anaerobically reducing 3.0 mM nitrate into nitrite in 50 days (Fig. 4c).
3.6 Mn(II) greatly enhances the Bacillus sp. RM19-mediated reductive mobilization of arsenic
Microcosm assay was used to detect how Mn(II) affect Bacillus sp. RM19-mediated reductive mobilization of As(III) from the sample R2. We found that after 21 days of anaerobic incubation, approximately 4.6 mM As(III) and 0.3 mM Fe(II) released from the microcosms without 10.0 mM Mn(II) (Fig. 5a and b). In comparison, if 10.0 mM Mn(II) was amended to the microcosms, after 21 days of incubation, approximately 5.7 mM As(III) and 0.5 mM Fe(II) were released from the microcosms (Fig. 5). This suggests that Bacillus sp. RM19 was able to significantly promote the reductive mobilization and release of As from As-contaminated soils, and the addition of 10.0 mM Mn(II) led to 23.9% and 45.1% increases in the Bacillus sp. RM19-catalyzed release of As(III) and Fe(II), respectively.
4 Discussions
Dissimilatory As(V)-respiring prokaryotes were shown to play a key role in promoting the reductive mobilization of arsenic from As-contaminated solids (Drewniak and Sklodowska 2013; Liu et al. 2022). Many other factors also are involved in controlling the transformation and mobilization/immobilization of arsenic (Wang et al. 2017; Zhu et al. 2019; Chen et al. 2020). All the biological, chemical, and anthropogenic factors work together, leading to the frequent fluctuations of arsenic concentrations in contaminated groundwater. However, it remains to be investigated for how chemicals/contaminants in the environment affect the DARPs-mediated reductive mobilization of arsenic from solid phase. Our group is interested in addressing this issue.
Mn(II) ubiquitously exists in the soils, surface water, groundwater, and sediments. Mn(II) commonly coexists with DARPs in the As-contaminated soils and aquifers (De Meyer et al. 2017; Rahman et al. 2021; Dong et al. 2022). For instance, in Bangladesh, most As-contaminated wells also contained high concentration of Mn(II) (Rahman et al. 2021). Therefore, it is interesting to investigate how Mn(II) affects the DARPs-mediated reductive mobilization and release of arsenic from solid phase. To address this issure, we collected soil samples from As-contaminated vegetable field. We found that the geochemical parameters of the three samples differed greatly from each other (Table S2); however, all of them were characterized of containing extremely high contents of solid arsenic. This provided enough substrates for detecting the activities of DARPs to catalyze the reductive mobilization and release of arsenic from solid phase. To detect the As(V)-respiring activities of microbial community in the soils, the samples were washed to remove soluble arsenic, and then mixed with MM medium for preparation of microcosm assays (Fig. 1). We added 2.0 mM soluble As(V) as substrate to each of the microcosms. Because soluble As(V) has much higher priority to be anaerobically reduced than solid As(V), before all soluble As(V) was reduced into As(III), almost no significant amount of arsenic in solid phase was reductively mobilized and released. Therefore, the As(V) in soil phase was not utilized as the substrate by the microbial communities, and can be negligible for these assays. The results suggest that all the As-contaminated soils had apparent As(V)-respiring activities using each of multiple small-molecule organic substances as an electron donor, and each sample has its own unique electron donor recipe (Fig. 1).
On the basis of the fact that diverse DARPs were distributed in the collected As-contaninated soils, we conduct performed microcosm assays with both microbial communities and cultivable As(V)-respiring bacterial cells to explore how Mn(II) affects the microorganisms-catalyzed reduction, mobilization, and release of As from soils. Because Mn(II) is an essential nutrient for bacteria, we thus used excessive Mn(II) to perform the assays. We found that Mn(II) greatly increased the microbial community and DARP-catalyzed reductive mobilization of both As and Fe. This finding provided new knowledge for the interaction between the biogeochemical processes of Mn and As.
Moreover, it was a commonly accepted view that the existence of Mn is negatively correlated with As release (Kumarathilaka et al. 2018; Keimowitz et al. 2017; Muehe et al. 2016). However, based on our finding, this description is incomplete and not accurate. Mn oxidation status generally includes Mn(II), Mn(III), Mn(IV), Mn(V), and Mn(VII). In the reducing and anaerobic environment, Mn(II) is dominant among the Mn species (Lu et al. 2021; Johnson et al. 2016). Under this circumstance, Mn would be positively correlated with the soluble As concentration. Therefore, our findings gained new knowledge on correlation between Mn and As in the environment.
It was shown that Mn(II) is an essential nutrition for the growth and proliferation of all organisms (Imlay 2008). We thus added excessive Mn(II) to the microcosms for exploration of the actual effects of Mn(II) on DARPs; this excluded the interference of Mn(II) acted as nutrition. The last question is: why does Mn(II) be able to stimulate the DARPs-mediated reductive mobilization and release of arsenic from soils? Based on the established knowledge (Mhatre et al. 2016; Hussain et al. 2018; Archibald and Fridovich 1981; Imlay 2008; Hohle and O’Brian 2012; Yin et al. 2018; Sepúlveda et al. 2010), we proposed that this observation was most likely attributed to the following mechanisms: (i) Mn(II) may promote biofilm formation, and thus increase the bacterial activity and their resistance to the arsenic toxicity; (ii) Mn(II) may help bacterial cells to scavenge free radicals, such as H·, Cl·, and NO·, and thus protected bacterial cells against damages of DNA and proteins, and saved energy; (iii) Mn(II) may increase the major carbon metabolism pathway, and thus promote bacterial growth. Taken together, it is most likely that excessive Mn(II) would confer higher resistence to the toxicity of arsenic on DARPs, and thus increased their metabolic activities. Further investigations are required to confirm this hypothesis.
5 Conclusions
Dissimilatory arsenate-respiring prokaryotes play a key role in promoting the reductive mobilization and release of arsenic. Mn(II) always coexists with DARPs in the As-contaminated environment. However, little is known about how Mn(II) affects the DARPs-mediated reductive mobilization of arsenic in the environment. We tried to address this scientific issue by working on investigation of an As-contaminated vegetable field. A unique diversity of DARPs was found to exist in the soils, which had high As(V)-respiring activities by using multiple electron donors. Microcosm assays with the soils suggest that the microbial community in the soils was capable of catalyzing the reductive mobilization of As and Fe. In comparison, if 10.0 mM Mn(II) was amended to the microcosms, in comparison to the microcosms with no additional Mn(II), the addition of 10.0 mM Mn(II) greatly increased the microbial community-catalyzed reductive mobilization of arsenic. To further investigate the microbial mechanism for this observation, a new indigenous As(V)-respiring bacterial strain was isolated from the samples. Microcosm assays also showed that the addition of 10.0 mM Mn(II) to the soils markedly enhanced the As(V)-respiring bacteria-mediated reductive mobilization of arsenic and iron. This work partially revised the common view that Mn is negatively correlated with soluble As in the environment. When Mn(II) is a dominant Mn species in the environment, Mn would be positively correlated with soluble As. The finding of this study also gained a new insight for the mechanism by which As concentrations in groundwater dynamically changed, and provided new knowledge about the interaction between the geochemical processes of Mn and As.
References
Archibald FS, Fridovich I (1981) Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol 146(3):928–936
Cai X, Zhang Z, Yin N, Du H, Li Z, Cui Y (2016) Comparison of arsenate reduction and release by three As(V)-reducing bacteria isolated from arsenic-contaminated soil of Inner Mongolia. China. Chemosphere 161:200–207
Chen X, Zeng XC, Wang J, Deng Y, Ma T, Guoji E, Mu Y, Yang Y, Li H, Wang Y (2017) Microbial communities involved in arsenic mobilization and release from the deep sediments into groundwater in Jianghan plain. Central China. Sci Total Environ 579:989–999
Chen X, Zeng XC, Kawa YK, Wu W, Zhu X, Ullah Z, Wang Y (2020) Microbial reactions and environmental factors affecting the dissolution and release of arsenic in the severely contaminated soils under anaerobic or aerobic conditions. Ecotoxicol Environ Saf 189:109946
De Meyer C, Rodríguez JM, Carpio EA, García PA, Stengel C, Berg M (2017) Arsenic, manganese and aluminum contamination in groundwater resources of Western Amazonia (Peru). Sci Total Environ 607:1437–1450
Drewniak L, Sklodowska A (2013) Arsenic-transforming microbes and their role in biomining processes. Environ Sci Pollut Res Int 20(11):7728–7739
Dong L, Zhang J, Guo Z, Li M, Wu H (2022) Distributions and interactions of dissolved organic matter and heavy metals in shallow groundwater in Guanzhong basin of China. Environ Res 207:112099
Ghosh S, Sar P (2013) Identification and characterization of metabolic properties of bacterial populations recovered from arsenic contaminated ground water of North East India (Assam). Water Res 47(19):6992–7005
Guo H, Liu Z, Ding S, Hao C, Xiu W, Hou W (2015) Arsenate reduction and mobilization in the presence of indigenous aerobic bacteria obtained from high arsenic aquifers of the Hetao basin. Inner Mongolia. Environl Pollut 203:50–59
Huq ME, Fahad S, Shao Z, Sarven MS, Khan IA, Alam M, Saeed M, Ullah H, Adnan M, Saud S, Cheng Q, Ali S, Wahid F, Zamin M, Raza MA, Saeed B, Riaz M, Khan WU (2020) Arsenic in a groundwater environment in Bangladesh: Occurrence and mobilization. J Environ Manage 262:110318
Hohle TH, O’Brian MR (2012) Manganese is required for oxidative metabolism in unstressed Bradyrhizobium japonicum cells. Mol Microbiol 84(4):766–777
Hussain MS, Kwon M, Oh DH (2018) Impact of manganese and heme on biofilm formation of Bacillus cereus food isolates. PLoS ONE 13(7):e0200958
Imlay JA (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776
Johnson JE, Webb SM, Ma C, Fischer WW (2016) Manganese mineralogy and diagenesis in the sedimentary rock record. Geochim Cosmochim Acta 173:210–231
Kawa YK, Wang J, Chen X, Zhu X, Zeng XC, Wang Y (2019) Reductive dissolution and release of arsenic from arsenopyrite by a novel arsenate-respiring bacterium from the arsenic-contaminated soils. Int Bioter Biodegr 143:104712–104725
Keimowitz AR, Mailloux BJ, Wovkulich K, Harkness J, Ross JM, Chillrud SN (2017) Manganese redox buffering limits arsenic release from contaminated sediments, Union Lake, New Jersey. Appl Geochem 77:24–30
Kudo K, Yamaguchi N, Makino T, Ohtsuka T, Kimura K, Dong DT, Amachi S (2013) Release of arsenic from soil by a novel dissimilatory arsenate-reducing bacterium, Anaeromyxobacter sp. strain PSR-1. Appl Environ Microbiol 79(15):4635–4642
Kumarathilaka P, Seneweera S, Meharg A, Bundschuh J (2018) Arsenic speciation dynamics in paddy rice soil-water environment: sources, physico-chemical, and biological factors - a review. Water Res 140:403–414
Lu A, Li Y, Liu F, Liu Y, Ye H, Zhuang Z, Wang C (2021) The photogeochemical cycle of Mn oxides on the Earth’s surface. Mineral Mag 85(1):22–38
Liu EY, Xie ZM, Fang JH, Wang J, Yang Y, Chen MA (2022) Nitrate-mediated biomigration and transformation of As/Fe in arsenic-bearing ferrihydrite. Appl Geochem 138:105204
Maity S, Biswas R, Sarkar A (2020) Comparative valuation of groundwater quality parameters in Bhojpur. Bihar for Arsenic Risk Assessment. Chemosphere 259:127398
Machado I, Bühl V, Mañay N (2019) Total arsenic and inorganic arsenic speciation in groundwater intended for human consumption in Uruguay: correlation with fluoride, iron, manganese and sulfate. Sci Total Environ 681:497–502
Mhatre E, Troszok A, Gallegos-Monterrosa R, Lindstädt S, Hölscher T, Kuipers OP, Kovács ÁT (2016) The impact of manganese on biofilm development of Bacillus subtilis. Microbiology 162(8):1468–1478
Mirza BS, Sorensen DL, Dupont RR, McLean JE (2017) New arsenate reductase gene (arrA) PCR primers for diversity assessment and quantification in environmental Samples. Appl Environ Microbiol 83(4):e02725-e2816
Moskovchenko DV, Kurchatova AN, Fefilov NN, Yurtaev AA (2017) Concentrations of trace elements and iron in the Arctic soils of Belyi Island (the Kara Sea, Russia): patterns of variation across landscapes. Environ Monit Assess 189(5):210
Muehe EM, Morin G, Scheer L, Le PP, Esteve I, Daus B (2016) Arsenic(V) incorporation in vivianite during microbial reduction of arsenic(V)-bearing biogenic Fe(III) (oxyhydr)oxides. Environ Sci Technol 50:2281–2291
Ohtsuka T, Yamaguchi N, Makino T, Sakura K, Kimura K, Kudo K, Homma E, Dong DT, Amachi S (2013) Arsenic dissolution from Japanese paddy soil by a dissimilatory arsenate-reducing bacterium Geobacter sp. OR-1. Environ Sci Technol 47(12):6263–6271
Podgorski J, Berg M (2020) Global threat of arsenic in groundwater. Science 368(6493):845–850
Rahman M, Tushar M, Zahid A, Mustafa MG, Siddique M, Ahmed KM (2021) Spatial distribution of manganese in groundwater and associated human health risk in the southern part of the Bengal Basin. Environ Sci Pollut Res Int 28(30):41061–41070
Reza AH, Jean JS, Lee MK, Liu CC, Bundschuh J, Yang HJ, Lee JF, Lee YC (2010) Implications of organic matter on arsenic mobilization into groundwater: evidence from northwestern (Chapai-Nawabganj), central (Manikganj) and southeastern (Chandpur) Bangladesh. Water Res 44(19):5556–5574
Rodríguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q, Johnson CA (2013) Groundwater arsenic contamination throughout China. Science 341(6148):866–868
Song B, Chyun E, Jaffé PR, Ward BB (2009) Molecular methods to detect and monitor dissimilatory arsenate-respiring bacteria (DARB) in sediments. FEMS Microbiol Ecol 68(1):108–117
Saltikov CW, Newman DK (2003) Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci U S A 100(19):10983–10988
Sepúlveda C, Poch A, Espinoza R, Cardemil E (2010) Electrostatic interactions play a significant role in the affinity of Saccharomyces cerevisiae phosphoenolpyruvate carboxykinase for Mn2+. Biochimie 92(7):814–819
Wang P, Sun G, Jia Y, Meharg AA, Zhu Y (2014) A review on completing arsenic biogeochemical cycle: microbial volatilization of arsines in environment. J Environ Sci 26(2):371–381
Wang J, Zeng XC, Zhu X, Chen X, Zeng X, Mu Y, Yang Y, Wang Y (2017) Sulfate enhances the dissimilatory arsenate-respiring prokaryotes-mediated mobilization, reduction and release of insoluble arsenic and iron from the arsenic-rich sediments into groundwater. J Hazard Mater 339:409–417
Wang YX, Pi KF, Fendorf S, Deng YM, Xie XJ (2019) Sedimentogenesis and hydrobiogeochemistry of high arsenic Late Pleistocene-Holocene aquifer systems. Earth-Sci Rev 189:79–98
Wu S, Xia X, Lin C, Chen X, Zhou C (2010) Levels of arsenic and heavy metals in the rural soils of Beijing and their changes over the last two decades (1985–2008). J Hazard Mater 179(3):860–868
Xie X, Ellis A, Wang Y, Xie Z, Duan M, Su C (2009) Geochemistry of redox-sensitive elements and sulfur isotopes in the high arsenic groundwater system of Datong Basin. China. Sci Total Environ 407(12):3823–3835
Yin Y, Gao Y, Li S, Jiang G, Wei Q (2018) Studies on the activation of isocitrate dehydrogenase kinase/phosphatase (AceK) by Mn2+ and Mg2+. Biometals 31(6):991–1002
Zeng XC, E, G.J., Wang, J.N., Wang, N., Chen, X.M., Mu, Y., et al (2016) Functions and unique diversity of genes and microorganisms involved in arsenite oxidation from the tailings of a realgar mine. Appl Environ Microbiol 82(24):7019–7029
Zhu X, Zeng XC, Chen X, Wu W, Wang Y (2019) Inhibitory effect of nitrate/nitrite on the microbial reductive dissolution of arsenic and iron from soils into pore water. Ecotoxicology 28(5):528–538
Funding
This study was funded by the General Programs (No. 41472219) and the Foundations for Innovative Research Groups (No. 41521001) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible editor: Yuan Ge
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, Y., Shi, W., Wu, W. et al. Excessive bivalent manganese promotes the arsenate-respiring bacteria-mediated reduction and mobilization of arsenic from contaminated vegetable field soils. J Soils Sediments 22, 3166–3175 (2022). https://doi.org/10.1007/s11368-022-03275-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-022-03275-z