Abstract
Purpose
Bacteria able to extracelluar respiration, which could be enriched in the anode of microbial fuel cells (MFCs), play important roles in dissimilatory iron reduction and arsenic (As) desorption in paddy soils. However, the response of the bacteria to As pollution is unknown.
Materials and methods
Using soil MFCs to investigate the effects of As on anode respiring bacteria (ARB) communities in paddy soils exposed to As stress. The soil MFC performances were evaluated by electrochemical methods. The bacterial community compositions on anodes were studied using Illumina sequencing.
Results and discussion
In wet 1 phase, polarization curves of MFCs showed cathode potentials were enhanced at low As exposure but inhibited at high As exposure. In the meantime, anode potentials increased with As levels. The dry-wet alternation reduced As levels in porewater and their impacts on electrodes microorganisms. Arsenic addition significantly influenced the anode microbial communities. After dry-wet cycles, Deltaproteobacteria dominated in the anode with high As.
Conclusions
The dynamic changes of the communities on cathodes and anodes of soil MFCs in paddy soils with different As addition might be explained by their different mechanisms for As detoxification. These results provide new insights into the microbial evolution in As-contaminated paddy soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As) is cancerogenic and poses health risks to humans by accumulating in water and edible parts of foods, especially in rice grain (Sun et al. 2009; Meliker et al. 2006; Zhu et al. 2008b). Mining activities and intensive use of As-tainted water for irrigation have caused an increase of As in paddy soils (Williams et al. 2006; Zhu et al. 2008a). In China, about 60 % of daily inorganic arsenic intake comes from rice growing in As-contaminated soils (Li et al. 2011).
The As bioavailability is closely related to soil redox conditions (Marin et al. 1993; Masscheleyn et al. 1991). Soil redox potentials (Eh) are measured by the redox-active chemicals, such as O2, iron (Fe) and manganese (Mn) minerals, sulfate, and nitrate (Husson 2013). The oxidizing chemicals are respired by microorganisms and reduced. When Fe and Mn oxides were reduced and dissolved, the ions pre-absorbed on their surface, e.g., arsenate (As(V)) and phosphate (PO4 3−), would release into soil pore water and become available for plant uptake (Wang et al. 2009; Gomez et al. 1999). Under flooded conditions, As concentrations in pore water could be 10 folds of which under the non-flooded conditions (Takahashi et al. 2004). Therefore, soil dry-wet alternation could significantly reduce the mobility of As in paddy soils.
Bacteria able to conduct extracellular respiration by donating electrons to an anode as terminal electron acceptor are termed anode respiring bacteria (ARB) (Miceli et al. 2012; Lovley 2008; Logan and Regan 2006). Studies have shown that ARB are responsible for the reduction of iron and manganese oxides (Lovley et al. 2004). Studies on ARB will provide new perspectives for understanding iron and arsenic biogeochemical cycling in paddy soils. Previous studies on this topic have largely focused on the effect of soil organic matter on ARB communities (Miceli et al. 2012; Dunaj et al. 2012). However, the information is scarce on the effect of arsenic on ARB communities. It is well known that As contamination could shape microbial communities in paddy soils (Edvantoro et al. 2003; Lorenz et al. 2006). However, the variation of ARB communities among paddy soils with different levels of As remains poorly understood.
The present study thus aimed to investigate whether As contamination could affect redox transformations and ARB communities of paddy soils under wet-dry cycles. In this work, soil microbial fuel cells (SMFCs) made of paddy soils with different concentrations of As was built to enrich ARB, thereby delineating the influence of As on ARB communities. Soil characteristics, the SMFC performances, and bacterial communities of electrodes were studied in this work.
2 Materials and methods
2.1 Soil sample treatment
Paddy soils were collected from Jiaxing, Zhejiang province. The soil sample was air-dried and passed through a 2-mm sieve. Every 300-g soil was mixed with 200 ml solution of As in 0, 1.0, 2.0, or 5.0 mmol L−1. The final soil As concentrations are 16, 66, 116, and 266 mg kg−1 which was designated as As0, As1, As2, or As3, respectively.
2.2 Soil MFC configuration and operation
A SMFC consisted of the following: a polypropylene pot with an inner diameter of 65 mm and a height of 110 mm, circular carbon felt electrodes (both anode and cathode) with a diameter of 60 mm and a thickness of 5 mm, a soil moisture sampler (10 Rhizon MOM, Rhizosphere Research Products, Netherlands) adjacent to the anode, and a 2000-Ω external resistor. The anode and cathode were connected by PTFE-wrapped titanium wires. The SMFC consisted of four layers as shown in Fig. S4 (Electronic Supplementary Material), which were a 20-mm soil layer from the bottom, anode carbon felt layer, another 60-mm soil layer, and cathode carbon felt layer in surface water. There were three replicates for each treatment.
The SMFC experienced two wet phases and one dry phase during the experiment. When the SMFCs were flooded, deionized water was daily added to maintain liquid level constant for 15 days. After the 15-day wet phase, no water was added and the dry phase started. When the SMFC voltages dropped to zero, the operation was moved to the second wet phase for another 20 days by adding deionized water to similar level at first phase. All of the assembled MFCs were incubated at 28 °C in the dark.
2.3 MFC performance analysis
Cell voltages were monitored by a data acquisition board (USB6225, National Instruments Corporation, USA) and recorded measurements daily for 40 days. Current density was calculated according to formula J = U / (RA), where U is the recorded voltage, R stands for the external resistance, and A stands for the electrode surface area. At the end of each wet phase, anode and cathode potentials were measured by an electrochemical workstation (CHI1040B, Chenhua, China) with a Ag/AgCl (+198 mV vs standard hydrogen electrode SHE) reference electrode. The SMFCs run for 1 h at open circuit before the measurement. Polarization curves were obtained by linear sweep voltammetry (LSV). The electrochemical workstation imposed a linear voltage that decreased from the OCV to a cell potential of 0 mV at a rate of 10 mV s−1. The currents were recorded by the electrochemical workstation, and the anode potentials versus Ag/AgCl were recorded by the data acquisition board. The cathode potentials were calculated by the imposed cell voltage and anode potentials. The power outputs were calculated using P = IU, where I is the recorded current, U the imposed voltage.
2.4 Fe(II) and total As in pore water
After each wet phase, the soil pore water was sampled and analyzed for Fe(II) and total As. One-milliliter sample was pre-acidified for 10 min with 0.2 mL 0.75 mol L−1 HCl for Fe(II) analysis by the colorimetric reagent 1, 10-phenanthroline. Total As in pore water was measured by an inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 2000 DV, Perkin Elmer, USA).
2.5 Scanning electron microscopy
Subsamples of carbon felt were cut from the SMFC anodes and slowly rinsed by sterilized ultrapure water. Before scanning electron microscopy (SEM) observation, samples were fixed in 2.5 % glutaraldehyde for 1 h at room temperature and then for 12 h at 4 °C. Subsequently, the samples were dehydrated through 30, 50, 75, 90, 95, and 100 % (v/v) ethanol, 10 min for each level, and then dried for another 12 h. Finally, the samples were mounted onto a copper sample holder, coated with gold, and imaged at 3 kV on SEM (S-3000 N, Hitachi, Japan).
2.6 Illumina sequencing and processing of sequencing data
Total DNA from biofilm samples on the carbon felts after the second wet phase was extracted with the FastDNA SPIN Kit for Soil (MP Biomedicals, USA) and subjected to Illumina sequencing. The V4-V5 regions of bacterial 16S ribosomal RNA genes were amplified using primers 515F (5′-barcode-GTGCCAGCMGCCGCGG-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′). Amplifications were started with an initial denaturation step at 95 °C for 2 min, followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s and ended with an extension step at 72 °C for 5 min. After purification, equal amounts of the PCR products with different barcodes were mixed and submitted to the Research and Testing Laboratory (Majorbio, Shanghai) for sequencing on an Illumina MiSeq platform.
Raw fastq files were demultiplexed, quality-filtered using the Quantitative Insights Into Microbial Ecology toolkit-version 1.7 (Caporaso et al. 2010). A randomly selected subset of 30,000 sequences per sample was used for subsequent community analyses. Operational taxonomic units (OTUs) were clustered with 97 % similarity cutoff using UPARSE-version 7.1 (http://drive5.com/uparse/), and chimeric sequences were identified and removed using UCHIME. The phylogenetic affiliation of each 16S ribosomal RNA (rRNA) gene sequence was analyzed by RDP Classifier (http://rdp.cme.msu.edu/) against the SILVA (SSU117/119) 16S rRNA database using a confidence threshold of 70 %.
2.7 Statistical analyses
The SPSS (version 17.0; SPSS Inc., Chicago, USA) software was used to perform standard statistical tests, including one-way analysis of variance (ANOVA), Student-Newman-Keuls (SNK) test, and Pearson correlation analysis. Pearson correlation analysis was used to determine the correlation between the genus abundance and As concentration. Moreover, the porewater Fe(II) concentration, the porewater As concentration, Chao 1 estimator, or Shannon index among different SMFCs were compared using one-way analysis of variance. Mean values are shown in text followed by 1 standard error (±SE). Statistical significance was denoted at p < 0.05.
2.8 Accession number of nucleotide sequences
The 16S rRNA gene-based Illumina sequencing reads have been deposited at GeneBank with accession number SRP056410.
3 Results
3.1 The changes of porewater As and Fe(II) in SMFCs
The concentrations of As and Fe(II) in porewater of the SMFCs (As0, As1, As2, As3) were tested at the end of the first and second wet phases (Table 1). In all the treatments, the porewater As levels were significantly lower than the total soil As. Arsenic concentrations increased with As addition; in the meantime, they decreased with incubation time. The top average concentrations of As dropped from 8.64 μM in wet 1 phase to 3.77 μM in wet 2 phase. The porewater Fe(II) was undetectable in wet 1 phase and ranged from 6.88 μM (As0) to 28.5 μM (As3) in wet 2 phase. The porewater Fe(II) increased with increasing As concentrations in paddy soils.
3.2 Time-dependent current density in the SMFCs with different As addition
An overview of the current density in treatments As0 and As3 is given in Fig. 1. The variations in current density in treatments As1 and As2 were similar to that of As0 (Fig. S1, Electronic Supplementary Material). The currents in all the SMFCs increased rapidly in the initial 5 days and reached the maximum in day 16, when the SMFCs were switched to dry stage.
The average current density recorded on day 16 were 48.1, 46.2, 45.4, and 38.1 mA m−2 for treatments As0, As1, As2, and As3, respectively. High As addition into paddy soils decreased current density suggesting inhibition on the respiration processes of microbial organisms on electrode surfaces. When the voltages reduced to zero, the SMFCs were flooded again. During the wet 2 phase, the voltage variances in all treatments showed no difference.
3.3 Polarization properties of the SMFCs
Although the voltage variances of the SMFCs with different As addition were similar for all the treatments, there is a clear difference of the potentials between cathodes and anodes measuring with reference electrodes and electrochemical stations. The polarization curves were tested at the end of the wet 1 phase (day 16) (Fig. 2) and wet 2 phase (day 40) (Fig. S4, Electronic Supplementary Material). Based on the polarization curves, the maximum power outputs were calculated. In the wet 1 phase, the order of the maximum power for the 4 treatments is As2 > As1 ~ As0 > As3.
According to the wet 1 polarization curves, it is shown that low As enhanced the maximum cathode potentials while high As greatly suppressed. The average maximum potentials of cathodes were +254, +409, +520, and +84 mV for As0, As1, As2, and As3, respectively. Compared to cathode, As had slight effect anodes. The maximum average potentials of anodes decreased from −170 mV (As0) to −241 mV (As3). The slope of the curves indicated that the cathodes’ potentials were the main factors limiting the current output of the SMFCs, except for the As3 treatment. However, As showed no significant effect on the SMFC performance in the wet 2 phase, in terms of the power, the electrodes potentials among all treatments.
3.4 SEM analysis
Biofilms of anodes were observed by SEM to characterize their biofilm structure and morphology in Fig. 3. The SEM micrograph of As0 showed bacteria attaching to carbon fiber directly. However, the SEM micrograph of As1, As2, and As3 showed that more bacteria presented in the void spaces of the carbon fiber. Compared with As0, As2 and As3 had more putative nanowires, which might be used for electron transfer between bacteria.
3.5 The anode microbial communities under various As contamination
The Miseq results showed that the As addition affected the microbial communities on anodes in the wet 2 phase. Moreover, there was no significant effect on the diversity of microbial community in cathodes (Table 1). For community richness, the highest Chao1 estimator for anode was found in the As0 treatment and followed by the As1, As2, and As3 treatment in sequence. For community diversity, the Shannon indexes for anodes were in the following sequence: As0-Shannon > As1-Shannon ~ As2-Shannon > As3-Shannon.
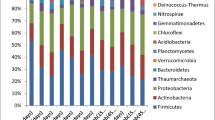
Figure 4a showed the relative abundance of bacterial community in enriched biofilms of anodes at class level. It was clear that the microbial composition of the treatment As0 was the most diverse, and that of the treatment As3 was the least, as expected from the corresponding Chao1 and Shannon indexes patterns (Fig. 4a and Table 1). Furthermore, bacterial community of treatment As0 was enriched for Nitrospira, Deltaproteobacteria, Clostridia, Betaproteobacteria, Anaerolineae, and Acidobacteria. High concentrations of As decreased the relative abundance of Nitrospira, Anaerolineae, and Acidobacteria, while increasing those of Deltaproteobacteria and Betaproteobacteria. Notably, the relative abundance of Deltaproteobacteri significantly increased when As levels were elevated (p < 0.05), which were 15, 31, 36, and 43 % for treatments As0, As1, As2, and As3, respectively.
Community analyses of enriched anode biofilms using Miseq of the 16S rRNA gene, reported at the class level. a Each type of soil had three MFC replications. “Others” represents low abundance (less than 1 %) bacteria. Community analyses of 16S rRNA gene sequences identified by genus within the Deltaproteobacteria. b Others include low abundance (less than 1 %) bacteria within the Deltaproteobacteria
Further analyses of the anodes communities of genus classification showed the evolution of the class Deltaproteobacteria in details in Fig. 4b. The relative abundance of Geobacter spp. increased from 1 % for the treatment As0 to 18 % for the treatment As3. The composition of microbial communities of biofilms in cathodes was shown in Fig. S3 (Electronic Supplementary Material), and no significant difference was found among As treatments.
3.6 Correlation analysis of the genus abundance and As concentration
Table S1 (Electronic Supplementary Material) showed that the correlation between Geobacter and As concentration is significant at the 0.05 level. Linear regression analysis was used to study the relationship between the relative abundance of Geobacter and As concentration (Fig. 5). A significant positive correlation with As concentrations was found for the genus Geobacter (R 2 = 0.9306).
4 Discussion
The response of soil functional microbial community to elevated As levels was revealed by using soil MFCs and Miseq techniques. The addition of As to soils significantly changed the cathode and anode potentials in the first wet stage. In the meantime, the microorganisms on cathode and anode exhibited different variation pattern in the treatments with different As levels.
In wet 1 phase, the cathode potentials showed higher sensitivity to As than the anode. The low level of As addition slightly enhanced the cathode potentials; however, it dramatically decreased the potentials when the As levels were elevated to 266 mg kg−1 (8.64 μmol L−1 in pore water). The disparity among cathodes disappeared in wet 2 phase, in terms of chemical potentials and microbial community (Fig. S2 and Fig. S3, Electronic Supplementary Material). Two reasons may explain the homogenization of the cathodes in wet 2 phase. One is the ageing effect during dry-wet alternation, which reduced the mobile As in pore water and overlay water. When As solution were added to soils, some of them are immobilized or form compounds by adsorption or precipitation (Jalali and Khanlari 2008; Huang et al. 2015). Especially, the iron oxides formed in dry phase would have strong adsorption affinity to As (Xu et al. 2008). As shown in Table 1, the concentrations of porewater As in wet 1 phase were much lower than that in wet 2 phase. Our previous study found that As bioaccessibility in soils could reached approximately stable levels with 1 or 2 weeks of ageing (Tang et al. 2007). Li et al. (2009) studied the effect of water management regimes on As mobilization and reported that aerobic treatments rapidly decreased the concentration of As in soil solution after 120 days of flooding. Another is that the disturbance of daily water refilling caused rapid exchange of microbes between surface water and cathode, which lead to similar microbial community structure on the cathode surface in wet 2 phase, but not on the anode (Fig. 2).
The ARB on anodes showed high tolerance to As toxicity, which is supported by the polarization curves and microbial community data in phase 1 (Figs. 2 and 4). The relative abundance of Deltaproteobacteria was the highest in the highest As contamination, although the diversity of microbial community significantly decreased with the As addition. Deltaproteobacteria was also found to be dominant in arsenic removal water treatment system (Upadhyaya et al. 2012). Several mechanisms have been proposed for As detoxification in microbes: As(V) reduction, methylation, and excretion, which are widely found in anaerobic bacteria (Zhu et al. 2014; Oremland and Stolz 2003; Paez-Espino et al. 2009). The anaerobic bacteria are proposed to adopt the ancient high As environment and evolve the anaerobic arsenate respiration (Zhu et al. 2014). Most of the known ARB are obligate anaerobes, which might possess high As tolerance ability. The rapid shift of oxygen, arsenite, and methylated As in anaerobic condition might also confer the ARB with high arsenic resistance higher priority to inoculate the anode, especially for Geobacter. Strong competitive advantage of Geobacter was observed in high As treatment (Fig. 4b). The study on microbial communities in Maine groundwater also found that the highest percentage of Geobacter existed in the wells with the highest As concentration by fluorescence in situ hybridization (Weldon and MacRae 2006). The existence of genes for As(V) respiration in several Geobacter genomes may enhance the As resistance of certain Geobacter species (Lear et al. 2007; Giloteaux et al. 2012; Hery et al. 2008). Ohtsuka et al. (2013) reported Geobacter sp. OR-1 isolated from Japanese paddy soil was a dissimilatory arsenate-reducing bacterium. Arsenate respiratory reductase genes (arrA) were closely related to those also found in Geobacter uraniumreducens and Geobacter lovleyi (Giloteaux et al. 2012; Hery et al. 2010). Although some other Geobacter species like Geobacter sulfurreducens were unable to conserve energy for growth via the dissimilatory reduction of As(V), they could tolerate high (500 μM) concentration of As(V) (Islam et al. 2005). For methylation, Jia et al. (2013) used primers of arsenite S-adenosylmethionine methyltransferase (ArsM) and identified the prokaryotic arsM genes in Geobacter metallireducens GS-15 from rhizosphere with wide ranging As concentrations.
The porewater Fe(II) was undetectable at the end of wet 1 phase, when the cell current significantly increased (Table 1 and Fig. 1). This suggested the ARB inoculated the anode before the abundant Fe(II) ion released to porewater. The chemical-inert anode may compete with iron oxides for microbial extracellular respiration and slow down the Fe(III) reduction. Our previous study also found the concentration of porewater Fe(II) was lower in closed circuit MFCs than that in open circuit MFCs (Wang et al. 2015). At the end of wet 2 phase, the porewater Fe(II) ranged from 6.88 to 28.5 μmol L−1 at wet 2 phase. The highest Fe(II) concentration was found in the treatment of highest As, which is consistent with our previous finding that the ARB possess higher growth advantages in high As condition.
5 Conclusions
By using MFCs to enrich ARB, we revealed that As contamination could modulate the ARB community in paddy soils. The ARB, which shared the mechanisms of extracellular respiration of Fe(III) reducing bacteria, showed great As tolerance, which might be explained by their versatile detoxification mechanisms. This study implies in a dynamic environment, e.g., intermittently flooded paddy field or intertidal zone, the As contamination may increase the competition the microbial community respiring extracellular minerals and further change the Fe and As behavior.
References
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows integration and analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Dunaj SJ, Vallino JJ, Hines ME, Gay M, Kobyljanec C, Rooney-Varga JN (2012) Relationships between soil organic matter, nutrients, bacterial community structure, and the performance of microbial fuel cells. Environ Sci Technol 46(3):1914–1922
Edvantoro BB, Naidu R, Megharaj M, Singleton I (2003) Changes in microbial properties associated with long-term arsenic and DDT contaminated soils at disused cattle dip sites. Ecotoxicol Environ Saf 55(3):344–351
Giloteaux L, Holmes DE, Williams KH, Wrighton KC, Wilkins MJ, Montgomery AP, Smith JA, Orellana R, Thompson CA, Roper TJ (2012) Characterization and transcription of arsenic respiration and resistance genes during in situ uranium bioremediation. ISME J 7(2):370–383
Gomez E, Durillon C, Rofes G, Picot B (1999) Phosphate adsorption and release from sediments of brackish lagoons: pH, O2 and loading influence. Water Res 33(10):2437–2447
Hery M, Gault AG, Rowland HAL, Lear G, Polya DA, Lloyd JR (2008) Molecular and cultivation-dependent analysis of metal-reducing bacteria implicated in arsenic mobilisation in south-east asian aquifers. Appl Geochem 23(11):3215–3223
Hery M, Van Dongen BE, Gill F, Mondal D, Vaughan DJ, Pancost RD, Polya DA, Lloyd JR (2010) Arsenic release and attenuation in low organic carbon aquifer sediments from West Bengal. Geobiology 8(2):155–168
Huang B, Li Z, Huang J, Chen G, Nie X, Ma W, Yao H, Zhen J, Zeng G (2015) Aging effect on the leaching behavior of heavy metals (Cu, Zn, and Cd) in red paddy soil. Environ Sci Pollut Res 22(15):11467–11477
Husson O (2013) Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362(1–2):389–417
Islam FS, Pederick RL, Gault AG, Adams LK, Polya DA, Charnock JM, Lloyd JR (2005) Interactions between the Fe(III)-reducing bacterium Geobacter sulfurreducens and arsenate, and capture of the metalloid by biogenic Fe(II). Appl Environ Microbiol 71(12):8642–8648
Jalali M, Khanlari ZV (2008) Effect of aging process on the fractionation of heavy metals in some calcareous soil of Iran. Geoderma 143(1–2):26–40
Jia Y, Huang H, Zhong M, Wang FH, Zhang LM, Zhu YG (2013) Microbial arsenic methylation in soil and rice rhizosphere. Environ Sci Technol 47(7):3141–3148
Lear G, Song B, Gault AG, Polya DA, Lloyd JR (2007) Molecular analysis of arsenate-reducing bacteria within Cambodian sediments following amendment with acetate. Appl Environ Microbiol 73(4):1041–1048
Li R, Stroud J, Ma J, McGrath S, Zhao F (2009) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43(10):3778–3783
Li G, Sun GX, Williams PN, Nunes L, Zhu YG (2011) Inorganic arsenic in Chinese food and its cancer risk. Environ Int 37(7):1219–1225
Logan BE, Regan JM (2006) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14(12):512–518
Lorenz N, Hintemann T, Kramarewa T, Katayama A, Yasuta T, Marschner P, Kandeler E (2006) Response of microbial activity and microbial community composition in soils to long-term arsenic and cadmium exposure. Soil Biol Biochem 38(6):1430–1437
Lovley DR (2008) Extracellular electron transfer: wires, capacitors, iron lungs, and more. Geobiology 6(3):225–231
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286
Marin A, Masscheleyn P, Patrick W Jr (1993) Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant Soil 152(2):245–253
Masscheleyn PH, Delaune RD, Patrick WH (1991) Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ Sci Technol 25:1414–1419
Meliker JR, Franzblau A, Slotnick MJ, Nriagu JO (2006) Major contributors to inorganic arsenic intake in southeastern Michigan. Int J Hyg Envir Heal 209(5):399–411
Miceli JF III, Parameswaran P, Kang DW, Krajmalnik Brown R, Torres CI (2012) Enrichment and analysis of anode-respiring bacteria from diverse anaerobic inocula. Environ Sci Technol 46(18):10349–10355
Ohtsuka T, Yamaguchi N, Makino T, Sakurai K, Kimura K, Kudo K, Homma E, Dong DT, Amachi S (2013) Arsenic dissolution from Japanese paddy soil by a dissimilatory arsenate-reducing bacterium Geobacter sp. OR-1. Environ Sci Technol 47(12):6263–6271
Oremland RS, Stolz JF (2003) The ecology of arsenic. Science 300(5621):939–944
Paez-Espino D, Tamames J, de Lorenzo V, Cánovas D (2009) Microbial responses to environmental arsenic. Biometals 22(1):117–130
Sun GX, Williams PN, Zhu YG, Deacon C, Carey AM, Raab A, Feldmann J, Meharg AA (2009) Survey of arsenic and its speciation in rice products such as breakfast cereals, rice crackers and Japanese rice condiments. Environ Int 35(3):473–475
Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuita K (2004) Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38(4):1038–1044
Tang XY, Zhu YG, Shan XQ, McLaren R, Duan J (2007) The ageing effect on the bioaccessibility and fractionation of arsenic in soils from China. Chemosphere 66(7):1183–1190
Upadhyaya G, Clancy TM, Brown J, Hayes KF, Raskin L (2012) Optimization of arsenic removal water treatment system through characterization of terminal electron accepting processes. Environ Sci Technol 46(21):11702–11709
Wang XJ, Chen XP, Yang J, Wang ZS, Sun GX (2009) Effect of microbial mediated iron plaque reduction on arsenic mobility in paddy soil. J Environ Sci 21(11):1562–1568
Wang N, Chen Z, Li HB, Su JQ, Zhao F, Zhu YG (2015) Bacterial community composition at anodes of microbial fuel cells for paddy soils: the effects of soil properties. J Soils Sediments 15(4):926–936
Weldon JM, MacRae JD (2006) Correlations between arsenic in Maine groundwater and microbial populations as determined by fluorescence in situ hybridization. Chemosphere 63(3):440–448
Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, Feldmann J, Meharg AA (2006) Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ Sci Technol 40(16):4903–4908
Xu XY, McGrath SP, Meharg AA, Zhao FJ (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42(15):5574–5579
Zhu YG, Sun GX, Lei M, Teng M, Liu YX, Chen NC, Wang LH, Carey AM, Deacon C, Raab A, Meharg AA, Williams PN (2008a) High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ Sci Technol 42(13):5008–5013
Zhu YG, Williams PN, Meharg AA (2008b) Exposure to inorganic arsenic from rice: a global health issue? Environ Pollut 154(2):169–171
Zhu YG, Yoshinaga M, Zhao FJ, Rosen BP (2014) Earth abides arsenic biotransformations. Annu Rev Earth Planet Sci 42(1):443–467
Acknowledgments
The work is financially supported by the National Natural Science Foundation of China (No. 41430858 and 41571305) and the Natural Science Fund for Colleges and Universities of Jiangsu Province (15KJB610014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Human and animal rights and informed consent
This article does not contain any studies with animals or human participants performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Additional information
Responsible editor: Galina Machulla
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 5175 kb)
Rights and permissions
About this article
Cite this article
Wang, YJ., Chen, Z., Liu, PP. et al. Arsenic modulates the composition of anode-respiring bacterial community during dry-wet cycles in paddy soils. J Soils Sediments 16, 1745–1753 (2016). https://doi.org/10.1007/s11368-016-1369-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1369-6