Abstract
Fungi are key actors in controlling primary productivity. Depending on the fungal functional group, they may promote or decrease plant productivity. It is consensual that arbuscular mycorrhizal and some endophytic fungi contribute to promote plant productivity and defense against phytopathogenic organisms, including fungi. However, there is not much information about the relation between the distinct functional groups of fungi. In this chapter, we aim at understanding the importance of arbuscular mycorrhizal fungi (Glomus intraradices) and Piriformospora indica (Serendipita indica) inoculation in the tolerance of Tomato (Solanum lycopersicum) plants to fusarium wilt.
Tomato plants grown at two nutritional levels were inoculated with Glomus intraradices and/or Piriformospora indica (Serendipita indica) and then infected with Fusarium oxysporum. Plant biomass accumulation showed that plants inoculated with Glomus intraradices and Piriformospora indica (Serendipita indica) accumulated more biomass and were more tolerant to Fusarium wilt. The analysis of the root exudates showed that fungal infection changed the composition of the root exudates and pointed out the importance of antioxidant compounds.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction
Fungi cannot fix energy and nutrients directly, so they use the energy stored in plant and animal biomass to grow. They are a key group of organisms that interact with other organisms and the abiotic environment to regulate ecosystem processes. Fungi are able to share resources and span a range of spatial and temporal scales to mediate flows of energy and materials.
The role of fungi in primary production goes behind making nutrients available to plants. They form symbiotic associations with bacteria, algae, and plants and work as a supportive network for photobionts (Dighton 2016). These symbioses include mycorrhiza and endophytes, which can occur in multiple plant parts. Such interactions improve nutrient availability for primary production, confer plant tolerance to drought, salt, and heavy metals, and provide a degree of protection from pathogenic fungi or bacteria and herbivory (Smith and Read 1997).
However, fungi may also have negative effects on the primary production. Some of them are phytopathogenic and may attack plants below and above ground. The effects of these phytopathogens are particularly evident in agrosystems, especially in intensive monocrop agriculture (Termoshuizen 2014). Both plant pathogens and mycorrhiza may play an important role in regulating plant productivity and plant community composition. The degree of impact of the phytopathogen on plant host depends on the fungal species and on the environmental conditions. In many cases, the effect of the phytopathogen increases when plants are grown under suboptimal conditions and are already under stress. The role of mycorrhiza is probably to improve nutrient uptake by the host plant or to alter its physiology so the plant is better able to defend itself against the pathogen (Volpin et al. 1994; Sharma and Mukerji 2007) rather than direct competition between the symbiotic fungi.
In a meta-analysis of interactions between arbuscular mycorrhizal fungi (AMF) and phytopathogen fungi, Borowicz (2001) concluded that about 50% of the studies showed that AMF gave a degree of protection to their host plant against the phytopathogens. The effect of the pathogenic fungus was usually to reduce the growth of the AMF. The interactions between the functional groups of fungi resulted in the reduction of the growth of each one in about 16%.
The aim of this work was to understand if the presence of the endophyte Piriformospora indica (Serendipita indica) affected the relation between AMF and plant phytopathogenic fungi. Tomato (Solanum lycopersicum) plants were inoculated with AMF (Glomus intraradices, Gi) and or P. indica (Serendipita indica) (Pi) and then infected or not with Fusarium oxysporum under low and high nutrient availability. Results point out to a synergistic effect between P. indica (Serendipita indica) and Glomus intraradices toward plant defense against fusariosis.
3.2 Protocol Applied
F. oxysporum was isolated from the host plant. The stem of a diseased plant was cut lengthwise to reveal the xylem and trimmed of all the leaves and secondary roots leaving only the main stem and root. The stem was surface sterilized by soaking in 10% bleach solution for 5 min and then cut into 2–4 mm pieces. Sterilized stem pieces were incubated on Potato Dextrose Agar (PDA) plates under light conditions. Once the fungus was grown sufficiently from the pieces, mycelium isolates were transferred to new PDA plates and incubated for 10–14 days. Colonies of F. oxysporum were recognized by the characteristic reddish-purple centre surrounded by a pinkish white aerial mycelium. The morphological identification was confirmed using molecular methods (Amaral et al. 2013).
Tomato seeds (Wt and AVP1OX), in the proportion of 1:10 (seeds: solution), were surface sterilized first with sodium hypochlorite 10% (v/v) for 5 min and then with 70% alcohol for 3 min. Seeds were then rinsed five times with sterile water. After sterilization, seeds were plated in polypropylene trays filled with 20 cm3 autoclaved substrate composed of sand and vegetable soil in the proportion 1:2. Seedlings were allowed to grow for 15 days in a growth chamber (16/8 h light/dark; 350 μmol m−2 s−1 at the leaf surface level during the light period, 25 ± 1 °C, 70% humidity).
Rhizoglomus intraradices, formerly Glomus intraradices BEG 24 (Gi), was obtained from the Bank Exchange of Glomales (BEG, Dijon—France). Spores were multiplied, in association with Sorghum tricolor roots grown in culture pots (5 L) filled with sterilized sand:vermiculite (1:1) mixture, and allowed to grow for three months under greenhouse conditions. The mixture of soil, fungal spores, hyphae fragments, and roots of colonized S. tricolor was used as AMF inoculant.
Inoculant quality was assessed by fungal spore counting. Fungal spores were extracted from the inoculant mixture by wet sieving (Vierheilig et al. 1998), followed by centrifugation in 20 and 60% sucrose gradient. Fifty grams of each were suspended in 2 L of water, and the suspension passed through overlapping sieves of 710 and 45 μm. The material collected in the 45 μm sieve was transferred to 50 mL tubes containing the sucrose gradient and centrifuged at 2000 g for 1 min. Spores were collected and transferred to crosshatch Petri dishes for counting.
P. indica (Serendipita indica) was multiplied in PDA plates. 0.5 cm diameter discs collected from the periphery of 9-day-old fungal colonies were used as inoculants.
After 15 days of growth, tomato seedlings were transplanted into pots (500 mL) filled with autoclaved sand:clay (2:1) mixture. AMF inoculation was performed by applying, at a depth of 2–3 cm below surface level, 30 mL per pot of the AMF inoculants containing Gi spores and hyphae, and Pi inoculation was performed by introducing a disc of culture in contact with the root. Non-inoculated treatment (control) received the same volume of substrate (30 mL). Seedlings were divided into two groups: one received just water and the other received 100 mL of full-strength Hoagland solution. Seedlings were irrigated with sterile water in order to maintain substrate water content between 40 and 60% of the substrate water capacity. Fifteen days after the inoculation, seedlings were (or not) infected with 1 mL of 104 mL−1 spores of F. oxysporum per plant. Plants were allowed to grow for more 30 days. Each treatment had 15 replicates.
Disease incidence was calculated as the ratio between the number of infected plants and the total number of plants multiplied by 100. Disease severity was determined according to Wellman (1939), where numerical values were assigned to five groups (g1 = 0.5, 1; g2 = 2; g3 = 3, 4; g4 = 5, 6; g5 = ≥7). Disease severity was calculated by the following formula: Disease severity = (ng1 + 2 ng2 + 5 ng3 + 10 ng4 + 20 ng5)/(n diseased plants). For confirmation of F. Oxysporum f. sp. Lycopersici infection, segments of 2-cm length starting upwards the shoot basis were dipped in 70% ethanol, flamed, and put into Petri dishes containing PDA amended with antibiotics to prevent bacterial growth (Steinkellner et al. 2011). The identification of F. oxysporum f. sp. Lycopersici was done according to Nelson (1990) by visual and microscopic analyses.
At the end of the experiment, five plants per treatment receiving low nutrient availability were gently removed from the substrate and washed thoroughly with water. Roots were submerged in acetate buffer (25 mM, pH 5.5) for 6 h, in a proportion of 10 mL:g root fresh weight. At the end, root exudates were stored at −20 °C until further processing. After that plant root and shoot fresh weight were determined. One root exudate per plant and five per treatment were prepared.
Root exudates were fractioned on an Amberlite XAD-1180 (Fluka Chemie GmbH) to hydrophilic and lipophilic compounds using MQ water and absolute ethanol as eluants. For GC-MS analyses, the hydrophilic fraction was hydrolyzed with 10% HCl for 3 h at room temperature, followed by derivatization (Kanani et al. 2008). Lyophilized samples were dissolved in 50 μl of a solution of methoxyamine hydrochloride in pyridine (20 mg/mL). After incubation at room temperature for 18 h, 50 μl N-methyl-N-TMS-trifluoroacetamide was added for derivatization into trimethyl-silyl ethers and esters. 0.5 μl of the obtained solution was injected into an AutoSystem XL gas chromatograph (Perkin Elmer Inc., Waltham, MA, USA) in the split-less mode following the procedure described by Hage-Ahmed et al. (2013). The obtained chromatograms were integrated with Turbomass 4.1.1 (Perkin Elmer Inc.), and the peak areas of measured compounds were converted to relative amounts (% of the total peak area of the chromatogram). Mass spectra were tentatively identified by comparison with commercial (NIST 08, National Institute of Standards and Technology, Gaithersburg, MD) and noncommercial databases (Kopka et al. 2005) and grouped into classes of compounds. For HPLC-UV analyses of lipophilic analytes, dried exudates were diluted in methanol (10 mg/ml). The analyses were carried out using a Dionex Summit HPLC with a photodiode array detector following the procedure described by Hage-Ahmed et al. (2013).
Germination of F. oxysporum spores (100 μl 107 microconidia/mL) was assayed in 500 μl root exudates or acetate buffer (25 mM, pH 5.5) in 24-well culture plates and incubated at 24 °C in the dark for 20 h, under shaking 200 rpm. After 20 h, plates were observed under the microscope and 200 spores in each well checked for germination.
The degree of colonization of the tomato roots was assessed, based on 100 root fragments from five plants of each treatment (Giovanetti and Mosse 1980) and stained with trypan blue (Phillips and Hayman 1970). Briefly, thin roots were rinsed in running water, cut into pieces of 1 cm, and treated with KOH 10% for 1 h. Then, roots were rinsed five times with sterile water, treated with HCl 1% for 3 min, and stained with trypan blue 0.05% diluted in lactoglycerol 0.02%. Roots were observed with a stereoscope at 10–40× magnification.
3.3 Salient Features
The Fusarium wilt symptoms include loss of turgor and leaf chlorosis, starting from the lower leaves, sometimes followed by leaf abscission and plant death. It may be manifested on only one side of the host plant (Schwartz et al. 2005). Symptoms of F. oxysporum infection were visible on control and Gi-inoculated plants. Symptomatic plants were much smaller, chlorotic, and wilted.
The observation of root segments showed that all plants inoculated with Gi or Pi were colonized independently of the presence of F. oxysporum. Colonization rates ranged between 40 and 60% without presenting any relation with treatments.
Nutrient availability in the root medium determined plant biomass accumulation only in plants not inoculated with Gi or Pi. As it would be expected, higher nutrient availability allowed higher plant biomass accumulation and higher resistance to F. oxysporum infection. Plant inoculation with Gi increased biomass accumulation to the levels of the non-inoculated plants grown under high nutrient availability and substantially increased plant tolerance to F. oxysporum. Plant inoculation with Pi increased biomass accumulation by 10–20% in relation to control plants, but although small, the effect of the infection with F. oxysporum was evident. However, plants inoculated with both Gi and Pi produced high biomass accumulation without any effect of the F. oxysporum (Fig. 3.1). The simultaneous inoculation of the plants with both fungi (Gi and Pi) reduced disease incidence and severity to less than 25% of that observed for the control plants.
Biomass accumulation of plants grown with the distinct treatments. Control plants were not inoculated. L—low nutritional level. H—high nutritional level. F—Fusarium oxysporum. Gi—Glomus intraradices. Pi—Piriformospora indica (Serendipita indica). GiPi—Co-inoculation of Glomus intraradices and Piriformospora indica (Serendipita indica). Values represent the mean percentage of the control plants under low nutritional level and the bars the standard deviation. N = 10
Disease incidence and severity were lower for control plants grown with higher than with lower nutrient availability. The same tendency was observed for Gi- and Pi- inoculated plants (Table 3.1). There was no correlation between disease incidence or severity and the degree of Gi or Pi root colonization. Inoculation with Gi and/or Pi was effective in decreasing the incidence and the severity of Fusarium wilt. In particular, the co-inoculation of the plants with Gi and Pi reduced disease incidence in 50% and severity in more than 75%, even at low nutrient availability (Table 3.1).
In order to understand the mechanisms involved in the increased tolerance of tomato plants to Fusarium wilt after inoculation with Gi and Pi, F. oxysporum spores were germinated in root exudates obtained from the distinct treatments. Only low nutrient availability treatments were considered. In comparison with buffer, root exudates promoted F. Oxysporum germination. However, the germination of F. Oxysporum spores was much lower (10–30%) when roots were inoculated with Gi and/or Pi. It is interesting that only in the presence of Pi root infection with F. oxysporum affected the rate of spore germination in the root exudates (Fig. 3.2).
Germination rate of the Fusarium oxysporum spores in buffer or in the root exudates of the low nutritional level treatments. F—Fusarium oxysporum. Gi—Glomus intraradices. Pi—Piriformospora indica (Serendipita indica). GiPi—Co-inoculation of Glomus intraradices and Piriformospora indica (Serendipita indica). Values represent the mean percentage of the control plants under low nutritional level and the bars the standard deviation. N = 5
3.3.1 Chemical Composition of the Root Exudates
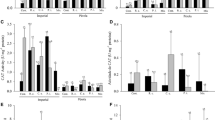
In the polar fraction of the root exudates, sugars and organic acids had the highest peak areas ranging between 30–50% and 28–48%, respectively (Fig. 3.3); amino acids represented a much lower percentage of the pic area of the polar fraction of the root exudates. Taking into consideration the general composition of the root exudates, those of the control plants, especially when not infected with F. oxysporum, differed from the other root exudates. Control plants had root exudates less enriched in sugars and enriched in amino acids and organic acids. According to their prevalence in the analyzed root exudates, glucose, fructose, malate, citrate, and succinate were the polar metabolites selected for further analysis (Fig. 3.4). Glucose and fructose were more prevalent whenever root was colonized by fungi. With the exception of the control plants, no significant differences were detected due to the presence of F. oxysporum. As far as organic acids are concerned, malate and citrate were the more abundant across treatments. And, contrary to sugars, the presence of fungi in the rhizosphere significantly decreases the presence of malate in the rhizosphere. From the roots colonized with fungi, those colonized with both Gi and Pi (in the presence or not of F. oxysporum) presented the lowest relative amounts of malate in the root exudates. No significant differences were observed for citrate concentrations across treatments.
Relative peak area (TIC) of selected compounds present in the root exudates from GC–MS analyses (mean ± Sd, n = 5). The low level nutritional treatments comprised: F—Fusarium oxysporum. Gi—Glomus intraradices. Pi—Piriformospora indica (Serendipita indica). GiPi—Co-inoculation of Glomus intraradices and Piriformospora indica (Serendipita indica). Values represent the mean percentage of the control plants under low nutritional level and the bars the standard deviation. N = 5
Relative peak area (TIC) of selected compounds present in the root exudates from GC–MS analyses grouped into substance classes and treatments (mean ± Sd, n = 5). The low level nutritional treatments comprised: F—Fusarium oxysporum. Gi—Glomus intraradices. Pi—Piriformospora indica (Serendipita indica). GiPi—Co-inoculation of Glomus intraradices and Piriformospora indica (Serendipita indica). Values represent the mean percentage of the control plants under low nutritional level and the bars the standard deviation. N = 5
In the nonpolar fraction of the root exudates, five main substances were identified (Fig. 3.5), namely, tryptophan, protocatechuic acid, chlorogenic acid, salicylic acid, and caffeic acid. The relative peak area ranged from 0 to 8%. Chlorogenic and caffeic acids were the only compounds where differences among treatments could be detected. Roots colonized with Pi presented higher levels of both acids in relation to the other ones.
Relative peak area (TIC) of selected compounds present in the root exudates from relative peak area (TIC) of HPLC–UV analyses (mean ± Sd, n = 5). The low level nutritional treatments comprised: F—Fusarium oxysporum. Gi—Glomus intraradices. Pi—Piriformospora indica (Serendipita indica). GiPi—Co-inoculation of Glomus intraradices and Piriformospora indica (Serendipita indica). Values represent the mean percentage of the control plants under low nutritional level and the bars the standard deviation. N = 5
3.4 Interpretation
In this study, we attempt to highlight the potential importance of fungi as regulators of ecosystem functions, namely of primary productivity. Depending on the functional group considered, fungi may contribute to increase or decrease plant biomass productivity. It is consensual that AMF and some endophytic fungi promote while phytopathogenic fungi decrease plant productivity. However, the rhizosphere is a very heterogeneous place and plants are simultaneously colonized by many fungi belonging to distinct functional groups. The output of this interaction is not well known and the results obtained so far are contradictory. However, soil microbes belonging to Trichoderma (Melo 1998) and Bacillus genera demonstrated to be efficient in controlling Fusarium wilt. Our dataset showed that Gi and Pi may be very efficient in controlling F. oxysporum disease (Fig. 3.1 and Table 3.1). Although these effects are very dependent on all biotic and abiotic variables, they show the potential of AMF and endophyte fungi to control Fusarium wilt.
The mechanisms behind these interactions are complex and result from multifactorial responses. However, root exudates are certainly involved. The effect may be a direct one resulting from the production of compounds by the AMF or endophytic fungi, competition between the AMF and endophytic fungi with the phytopathogen, or induction of plant defense pathways due to the priming effect of AMF and endophytic fungi. Independently of the mechanism involved, a change in the composition of the root exudates must be observed (Fig. 3.2–5). Although our results must be taken with caution due to technical limitations in the analysis of the composition of the root exudates, some changes were consistent with the treatments. The fungal (beneficial or pathogenic) colonization increased the sugar component of the root exudates, which makes sense since the fungus increases the root sink for carbon (Fig. 3.1). The opposite was observed for malate.
Priming of the immune system of the plant leads to a systemic protection against pathogens in combination with changes in the secondary metabolism (Jung et al. 2012), which may explain the relative increase in the amount of chlorogenic and caffeic acids when roots were colonized with Pi (Fig. 3.5). Chlorogenic acids are a group of phenolic secondary metabolites produced by certain plant species and an important component of coffee (Coffea spp.). Chlorogenic acid has been implicated in biotic and abiotic stress responses, while the related shikimate esters are key intermediates for lignin biosynthesis (Lallemand et al. 2012). Elevated levels of chlorogenic acid in transgenic tomato plants increased protection from UV light (Clé et al. 2008) and enhanced microbial resistance (Niggeweg et al. 2004). More recently, it has been shown that chlorogenic acid can act as a pest resistance factor in ornamental plants (Leiss et al. 2009). Meanwhile, the closely related shikimate esters are known to be key intermediates in the synthesis of lignin (Hoffmann et al. 2004; Chen and Dixon 2007).
3.5 Conclusion
Our results point out the relevance of mycorrhizal and endophytic fungi in the regulation of phytopathogenic fungi and show that the effect may be mediated by changes in the composition of the root exudates calling attention to chlorogenic acid, which is an important group of antioxidants, soluble esters formed between phenolic hydroxycinnamates and quinic acid. However, root exudates consist of many different compounds with different effects on microorganisms in the rhizosphere. Therefore, other compounds may be responsible or involved in the biological control of Fusarium wilt mediated by AMF Gi and Pi. Taking into consideration that fruits and vegetables are major sources of antioxidants, and high levels of these compounds in the diet are believed to contribute to improved health condition (Bazzano et al. 2002; Astley 2003), it would also be important to investigate the levels of these compounds in comestible parts of the plants.
These results apart from highlighting the importance of AMF and AMF-like fungi such as P. indica (Serendipita indica) in biocontrol also raise the question of crop management, since intensive soil management destroys the mycelium networks and may substantially minimize the work of fungi in controlling primary productivity.
References
Amaral DOJ, Almeida CMA, Malafaia CB, Silva MLRB, Correia MTS, Lima VLM, Silva MV (2013) Identification of races 1, 2 and 3 of Fusarium oxysporum f. sp. lycopersici by molecular markers. Afr J Microbiol Res 7:2324–2331. doi:10.5897/AJMR12.2234
Astley S (2003) Dietary antioxidants: past, present and future? Trends Food Sci Technol 14:93–98
Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK (2002) Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-Up Study. Am J Clin Nutr 76:93–99
Borowicz VA (2001) Do arbuscular mycorrhizal fungi alter plant–pathogen relations? Ecology 82:3057–3068
Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25:759–761
Clé C, Hill LM, Niggeweg R, Martin CR, Guisez Y, Prinsen E, Jansen MA (2008) Modulation of chlorogenic acid biosynthesis in Solanum lycopersicum: consequences for phenolic accumulation and UV-tolerance. Phytochemistry 69:2149–2156
Dighton J (2016) Fungi in ecosystem processes, 2nd edn. CRC Press, New York, pp 155–189
Giovanetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Hage-Ahmed K, Moyses A, Voglgruber A, Hadacek F, Steinkellner S (2013) Alterations in root exudation of intercropped tomato mediated by the arbuscular mycorrhizal fungus Glomus mosseae and the soilborne pathogen Fusarium oxysporum f.sp. lycopersici. J Phytopathol 161:763–773
Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, Pollet B, Legrand M (2004) Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyl transferase affects phenylpropanoid biosynthesis. Plant Cell 16:1446–1465
Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ (2012) Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol 38:651–664
Kanani H, Chrysanthopoulos PK, Klapa MI (2008) Standardizing GC–MS metabolomics. J Chromatogr B 871:191–201
Kopka J, Schauer N, Krueger S (2005) GMD.CSB.DB: the Golm Metabolome Database. Bioinformatics 21:1635–1638
Lallemand LA, Zubieta C, Lee SG, Wang Y, Acajjaoui S, Timmins J, McSweeney S, Jez JM, McCarthy JG, McCarthy AA (2012) A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiol 160:249–260
Leiss KA, Maltese F, Choi YH, Verpoorte R, Klinkhamer PG (2009) Identification of chlorogenic acid as a resistance factor for thrips in Chrysanthemum. Plant Physiol 150:1567–1575
Melo IS (1998) Agentes microbianos de controle de fungos fitopatogênicos. In: Melo IS, Azevedo JL (eds) Controle Biológico, vol 1. Embrapa Meio Ambiente, Jaguariúna, pp 17–67
Nelson EB (1990) Exudate molecules initiating fungal responses to seeds and roots. Plant Soil 129:61–73
Niggeweg R, Michael AJ, Martin C (2004) Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat Biotechnol 22:746–754
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–160
Schwartz HF, Steadman JR, Hall R, Forster RL (eds) (2005) Compendium of bean diseases, 2nd edn. American Phytopathological Society, St. Paul, p 120
Sharma MPAG, Mukerji KG (2007) Arbuscular mycorrhiza mediated plant pathogen interactions and the mechanisms involved. In: Chincholkar SB, Mukerji KJ (eds) Biochemical control of plant diseases. Haworth Press, New York, pp 47–74
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic Press, San Diego, pp 110–130
Steinkellner S, Hage-Ahmed K, Garcıa-Garrido JM, Illana A, Ocampo JA, Vierheilig H (2011) A comparison of wild-type, old and modern tomato cultivars in the interaction with the arbuscular mycorrhizal fungus Glomus mosseae and the tomato pathogen Fusarium oxysporum f.sp. lycopersici. Mycorrhiza 22:189–194
Termoshuizen AJ (2014) Root pathogens. In: Dighton J, Krumins JA (eds) Interactions in soil: promoting plant growth. Springer Science and Baseness Media, Dordrecht, pp 119–137
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Volpin HE, Okon Y, Kapulnik Y (1994) A vesicular-arbuscular mycorrhiza (Glomus intraradices) induces a defense response in alfalfa roots. Plant Physiol 104:683–689
Wellman FL (1939) A technique for studying host resistance and pathogenicity in tomato Fusarium wilt. Phytopathology 29:945–956
Acknowledgment
Ajit Varma is thankful to Department of Science and Technology and Department of Biotechnology for partial financial funding and to DST-FIST for providing confocal microscope facility.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Cruz, C., Ramos, A., Babalola, O.O., Kamel, H., Dias, T., Varma, A. (2017). Soil: Do Not Disturb, Mycorrhiza in Action. In: Varma, A., Prasad, R., Tuteja, N. (eds) Mycorrhiza - Function, Diversity, State of the Art. Springer, Cham. https://doi.org/10.1007/978-3-319-53064-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-53064-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53063-5
Online ISBN: 978-3-319-53064-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)