Abstract
Purpose
Irrigation and fertilization accelerate the accumulation of environmental hormones such as 17α-Ethinyestradiol (EE2) and heavy metals such as Cd in soil, which in turn harms crops and human health. The objective of this study was to investigate the interaction of the plant ryegrass and EE2 degrading bacteria Hyphomicrobium sp. GHH on the remediation of EE2-Cd co-contaminated soil.
Materials and methods
The concentration of EE2 in soil was set as 25 mg kg−1, and the concentration of Cd was set as 0, 5, 20, and 80 mg kg−1, respectively. Pot experiments were carried out to investigate the control treatment without GHH inoculation and/or ryegrass cultivation (C), GHH inoculation alone (B), ryegrass cultivation alone (P), and combined use of ryegrass with GHH (B + P) on the EE2 and Cd removal from soil. The plant biomass, EE2 and Cd concentration in root and shoot of ryegrass, the bioconcentration factor (BCF), translocation factor (TF), phytoextraction efficiency of EE2 and Cd, and removal rate of EE2 and Cd from soil, as well as the soil urease activity and microbial biomass carbon (MBC) content, were determined to evaluate the interaction of plant and microbe on the soil remediation.
Results and discussion
After 28 days of treatment, in soil with spiked Cd at 5, 20, and 80 mg kg−1, the removal rate of EE2 from soil was: B + P > P > B > C; the effect on the Cd removal was B + P > P, while Cd cannot be removed by microbes directly. In B + P treatment, the removal rate was 89%, 80%, and 71%, respectively for EE2, and 0.81%, 0.43%, and 0.38% respectively for Cd. EE2 and Cd were mainly stored in root of ryegrass because that all the TFs were < 1. The inoculation of GHH significantly promoted plant growth and substantially increased the extraction efficiencies of EE2 and Cd by ryegrass (P < 0.05). Both cultivation of ryegrass and inoculation of GHH improved the soil environment, and the improving effect on the urease activity and MBC content was B + P > P > B > C. 5 mg kg−1 Cd increased soil urease activity and MBC content, and promoted the root growth. However, soil Cd at the concentration > 20 mg kg−1 caused irreparable harm to ryegrass and microorganisms; as the consequence, the EE2 extraction by plant significantly decreased (P < 0.05).
Conclusions
The combined use of ryegrass with GHH was effective to remediate the EE2-Cd co-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As a typical environmental endocrine disruptor, 17α-Ethinyestradiol (EE2) is receiving more and more attention. EE2 is the active ingredient of many acyeterions and is used in the treatment of diseases such as prostate cancer, breast cancer, menopause, postmenopausal syndrome, and osteoporosis (Kuster et al. 2005; Lima et al. 2011). EE2 is highly compatible with estrogen receptors (Tomšíková et al. 2012), and its estrogen potency is 2~30 times greater than the natural hormones (Aris et al. 2014). EE2 can inhibit the development of animal gonad and destroy animal endocrine systems (Xu et al. 2008; Hogan et al. 2008; Huang et al. 2015). Reports have shown that EE2 enters soils continuously with the use of sewage sludge, animal manure, and irrigation; as the consequence, EE2 deposits in soils due to its characteristics of being difficult to be degraded and being readily adsorbed onto soil surfaces (Hildebrand et al. 2006; Zhang et al. 2011). EE2 in soils can not only directly affect the microbial activities and plants’ growth, but also accumulate in plants (Karnjanapiboonwong et al. 2011; Schroeder et al. 2015) and consequently, harm to animal and human health through the food chain. However, researches on the removal of EE2 from soils have rarely been reported so far.
With the development of industry and agriculture, the input of organic pollutants and heavy metals into soils has been accelerated. Soil pollution in nature is usually characterized by co-presence of organic xenobiotics and heavy metals. Chinese Agricultural Pollution Prevention and Control Key Laboratory of the Ministry of Agriculture conducted a survey on 24 provinces and cities across the country, and the results showed that there were 320 seriously polluted areas, about 548 × 104 hm2, and more than 80% of the total area exceeded the pollution limit standard for heavy metals (Li et al. 2005). Among them, cadmium (Cd) has attracted much attention due to the frequently reported Cd rice events and the severe impair caused by “itai-itai disease”. The study of Baran et al. (2004) showed that the toxicity registered with co-presence of organic contaminants and heavy metals was stronger than that observed in the case of a single kind of pollution, which was probably due to that the excess heavy metals inhibited the microbial activities and thus, suppressed the decomposition of organic matter. On the other hand, in the co-contaminated soils, heavy metals may be complexed or bonded with organic compounds, which makes heavy metals easier to access the plants and endanger the plant health. In recent years, soil compound pollution has become a worldwide problem that seriously threatens the ecological environment and food security. So, it has been very urgent to remediate the soils polluted by organic pollutants and heavy metals.
Combing the advantages of phyto- and bioremediation, plant-microbe interaction recovery technique can shorten the remediation period, and is low-cost, and the most of all, can improve the bioremediation efficiency. Hence, it is very prospective for the treatment of polluted soils. Zhu et al. (2012) reported that with the combined use of the Cd-hyperaccumulator Sedum alfredii and DDT-degrading microbes, pollutants were effectively removed from the Cd-DDT co-contaminated soil, with the removal efficiency of 32.1~40.3% for Cd and 33.9~37.6% for DDT in a pot experiment over 18 months. Inoculation of DDT-degrading bacteria promoted the degradation of soil DDT, improved the root growth of S. alfredii, and promoted the extraction of Cd by plant. Li and Wong (2012) reported that plant cultivation benefited the growth of soil microbe. The addition of phenanthrene degrading bacteria Burkholderia cepacia increased the soil contents of available N and P, as well as the tolerance of plant to heavy metals (Li and Wong 2012). Combined application of S. alfredii and B. cepacia was more effective to remove phenanthrene from the soil, with the removal rate of 92.2~96.3%. Jeong et al. (2012) reported that P-solubilizing bacteria Bacillus megaterium has a potential of directly solubilizing P from soils (more than tenfold greater than the control without inoculation). Inoculation of B. megaterium significantly promoted the Cd accumulation in Brassicajuncea and Chinese jute (Abutilon theophrasti) by twofold relative to the uninoculated control. Cd-resistant bacteria (Pseudomonas sp. RJ10 and Bacillus sp. RJ16) produced indole-3-acetic acid and siderophores, enhanced plant growth, and increased the bioavailability of Cd in rhizosphere soils, thus promoting Cd uptake by rape (Brassica napus) (Sheng and Xia 2006) and tomato (Lycopersicon esculentum) (He et al. 2009). Further, Maria et al. (2011) demonstrated that inoculation with microbes significantly influenced the root biomass of S. caprea and the accumulation of Cd. The metal-resistant bacteria (Burkholderia sp. J62 and Pseudomonas thiver-valensis Y-1-3-9) could colonize the rhizosphere soils and root interiors of rape plants (Chen et al. 2013). Inoculation with these two bacteria in B. napus not only significantly increased the dry weights of B. napus root (by 52%), stem (by 38%), and leaf (by 32%), but significantly enhanced water-extractable Cd in the rhizosphere soils, and subsequently improved Cd uptake by root of B. napus (by 50%) and translocation from roots to above-ground tissues (by 16% in stem and 22% in leaf) (Chen et al. 2013).
Ryegrass is widely cultivated in south China because its advantages of rapid growth and large biomass. Studies have shown that ryegrass can grow well in the contaminated soils (Binet et al. 2000; He et al. 2014). Ryegrass accumulates effectively not only heavy metals such as Cd (He et al. 2014; He et al. 2019b), but organic pollutants such as PAHs and antibiotics in its root and shoot (Binet et al. 2000). However, minimal information is available regarding whether ryegrass can take up EE2 from soil. In our previous study, an EE2 degrading bacteria strain Hyphomicrobium sp. GHH (hereafter referred to as GHH) has been isolated (He et al. 2019) and preserved in the China Center for Type Culture Collection (CCTCC) with the number of CCTCC M 2017539. This study was conducted to investigate the effect of the combined use of ryegrass and GHH on the remediation of the EE2-Cd co-contaminated soil. Meanwhile, this study was also attempted to study the effects of ryegrass on the extraction of Cd and EE2 from the co-contaminated soil.
2 Materials and methods
2.1 Materials
The soil was collected from a farmland of Hangzhou suburb area, China. Soil was air-dried, ground, and sieved with 200 mesh sieve. The main physical and chemical characteristics of the soil were determined according to the conventional method of soil agrochemical analysis (MiPAF 2000) and presented as Table 1. EE2 was spiked to soil at 25 mg kg−1, and exogenous Cd (NO3)2 was added to soil at 0 mg kg−1, 5 mg kg−1, 20 mg kg−1, and 80 mg kg−1, respectively.
Ryegrass seeds were soaked in 2.5% H2O2 for 30 min and rinsed in deionized water. Then, 250 seeds were sown in each pot, to ensure there were 200 plants after germination. Ryegrass was cultivated in the greenhouse with the day/night time of 16/8 h and temperatures of day/night 25/15 °C. Plants were watered every day to maintain the soil moisture at 60% of the field’s water holding capacity.
The isolated EE2 degrading bacteria strain GHH (He et al. 2019) was cultured in the mineral salt medium (MSM) (NH4Cl 2 g L−1, KH2PO4 0.5 g L−1, K2HPO4 0.5 g L−1, MgSO4·7H2O 0.2 g L−1, FeSO4·7H2O 0.01 g L−1, CaCl2 0.03 g L−1, MnSO4·H2O 0.001 g L−1, ZnSO4 0.001 g L−1, pH 7) for 24 h in an incubation shaker at 120 rpm and 30 °C. And then centrifuged at 8000 rpm for 10 min. After the bacterial solution was centrifuged at 8000 rpm for 10 min, the precipitate was washed twice with 0.85% sterile NaCl, then re-suspended in deionized water to OD600 value of 1, which was used as the GHH inoculum. On the 3rd day after seed germination, 10 mL of inoculum per pot was added to the soil and supplemented once on the 14th day.
2.2 Treatments done during pot experiment
Four treatments (with three replicates for each treatment) were set: control with no GHH inoculation or ryegrass cultivation (C), GHH inoculation alone (B), ryegrass cultivation alone (P), and GHH inoculation + ryegrass cultivation (B + P). On day 28 after the germination of ryegrass, samples were collected and the indicators were analyzed.
2.3 Determination of plant biomass
Plants were harvested and washed with deionized water. Then, plants were blot dried with absorbent paper and the taproot length was measured. The shoots were dried at 105 °C for 2 h, and then dried at 80 °C until the constant weight was obtained. The shoot dry weight was recorded. Other plant samples were placed in the sealed bags and cryopreservation for subsequent determination.
2.4 Analysis of EE2 content in plant and soil
EE2 in plant or soil was extracted based on the method of Flores et al. (2011). Fresh plant samples were ground and homogenized in liquid nitrogen. Shoot of 3 g or root of 1 g was put into a 50 mL polypropylene centrifuge tube, 10 mL of deionized water and 10 mL of acidified acetonitrile (containing 1% acetic acid) were added, and shaked and mixed for 1 min with the vortex mixer, then ultrasonized for 15 min. Salt extractant (MgSO4 4 g, NaCl 1 g, Na3C6H5O7 1 g, and C6H5Na2O7 0.5 g) was added and shaken immediately with a vortex mixer for 1 min. The mixture was centrifuged at 5000 rpm for 10 min, placed at − 20 °C for 1~2 h, and then 6–8 mL of the supernatant solution was filtered through a 0.22 μm membrane and dried by nitrogen bellowing. The dried sample was dissolved in 1 mL methanol and used for measurement. The EE2 recovery number of internal standard addition from the extraction method for plant ranged between 88.0 and 98.5%.
The extraction method for soil EE2 was similar to that for plant, the difference was: soil of 2 g was used, the salt extractant for soil was 4 g MgSO4 and 1.5 g CH3COONa. After centrifugation, about 8 mL of the supernatant was transferred to a 15 mL polypropylene centrifuge tube containing 900 mg anhydrous MgSO4 and 60 mg C18. After vortex and centrifugation, 6 mL of the supernatant solution was filtered and dried by nitrogen blowing. Then, the methanol-dissolved sample of 1 mL was taken for analysis. The EE2 recovery number of internal standard addition from the extraction method for soil ranged from 96.0 to 105.0%.
The concentration of EE2 in plant or soil was analyzed using high-performance liquid chromatography (HPLC, Agilent 1260, USA) and column chromatography (AORBAX Eclipse XDB-C18, 5 μm, 4.6 mm × 150 mm). The HPLC operating conditions were modified according to (Karnjanapiboonwong et al. 2011): mobile phase acetonitrile:water 45:55, detection wavelength 280 nm, flow rate 0.8 mL min−1, column temperature 30 °C, and injection volume 20 μL.
2.5 Determination of Cd content in plant and soil
The determination of Cd was based on the assay method of Zhu et al. (2012). Plant root or shoot was dried in an oven, then pulverized with a pulverizer, and sieved with 100 mesh sieve. Plant root or shoot of about 0.1 g was added with 1.0 mL HClO4 and 4.0 mL concentrated HNO3, then digested in a PTFE digestion tank at 180 °C until the solution was completely clear. The digestion solution was filtered through a 0.22-μm filter, and the filtrate was fixed with a 50 mL volumetric flask.
Soil sample was air-dried and sieved with a 2-mm sieve. Soil sample of 0.3 g was added with 1.0 mL concentrated HNO3, 1.0 mL HClO4, and 1.0 mL HF, and the mixture was digested at 180 °C. The follow-up steps were the same as that for plant sample. The Cd content in plant or soil sample was determined by the atomic absorption spectrometry (ICE 3300, Thermo Scientific).
2.6 Determination of urease activity and microbial biomass carbon (MBC) content in soil
Soil urease activity was determined as described by Guo et al. (2012). Air-dried soil sample of 5 g was put into a 50-mL erlenmeyer flask, and added with 1 mL toluene, mixed, and then left to stand for 15 min. After adding 10 mL of 10% CON2H4 and 20 mL citrate buffer (pH 6.7), the sample was placed in the shaker at 37 °C for 24 h and shaken thoroughly. Then, the mixture was centrifuged at 10,000 rpm for 10 min. The supernatant was taken out and diluted 10 times with distilled water. Diluted supernatant of 400 μL was transferred to a 2-mL centrifuge tube, and 80 μL of 1.35 M C6H5ONa and 60 μL NaClO (active chlorine 0.9%) were added. The sample was mixed thoroughly and let stand for 20 min, then diluted with 460 μL distilled water. The absorbance was measured at 630 nm using an ultraviolet-spectrophotometer (Thermo Genesys 10, USA), and the concentration of NH3–N in sample was determined from a standard curve. The urease activity was expressed as U = y × 10 × V × W × T, where y was the concentration of NH3–N, 10 was the dilution ratio; V was the total volume of the reaction system, 2 mL; W was the sample weight, 0.25 g; and T was the reaction time, 1 day.
The determination of soil microbial biomass carbon (MBC) content was according to the method of Lu (1999). Fresh soil (equivalent to a dry weight of 10 g) was put into a 250-mL erlenmeyer flask, and added arginine aqueous solution with 0.3 mg per gram of soil sample. The mixture was cultured at 25 °C for 2 h, then placed in a − 15 °C refrigerator for 4 h. Sample was taken out and thawed at room temperature. Then, 20 mL KCl at 2 mol L−1 was added, and the mixture was shaken at 25 °C for 15 min. The solution was centrifuged and the supernatant was filtered with a 0.22-μm filter. The ammonium content of the filtrate was measured immediately by the indophenol blue colorimetric method, and the MBC content in soil was calculated as ω(C) = 0.01 × NH4+–N(mg/kg), where ω(C) was the mass fraction of MBC (Bc), mg/kg; 0.01 was the coefficient of conversion to Bc by arginine-induced transformation.
2.7 Data analysis and statistical method
The bioconcentration factor (BCF), translocation factor (TF), the phytoextraction efficiencies of EE2 and Cd by ryegrass, and the EE2 and Cd removal rates from soil were calculated. BCF was expressed as the ratio of the EE2 or Cd concentration in root to the EE2 or Cd concentration in soil. TF was defined by the ratio of the EE2 or Cd concentration in shoot to the EE2 or Cd concentration in root. Plant extraction efficiency for EE2 or Cd was calculated by the total amount of EE2 or Cd in plant to the original amount in soil. Removal rate of EE2 or Cd was the percentage change of the EE2 or Cd concentration in the soil before and after the treatment.
All data were expressed as mean ± standard deviation of the three replicates. Data were statistically analyzed by ANOVA and mean values were compared using Duncan’s honestly significant difference test, at the significance level of P < 0.05. All data were analyzed using SPSS 19.0.
3 Results and discussion
3.1 Plant growth
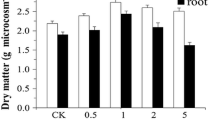
As shown in Fig. 1, in the EE2 contaminated soil, the addition of 5 mg kg−1 Cd promoted the growth of ryegrass, but as the Cd concentration continued to increase, plant growth was inhibited. The toxic stress of heavy metals on plants was due to the fact that heavy metals reduced or inhibited the synthesis of chlorophyll and enzymes involved in the photosynthesis; meanwhile, heavy metals influenced the absorption and transport of water and nutrients by plants (Fidalgo et al. 2011).
Root length (a) and shoot dry biomass (b) of ryegrass on day 28. Means with different letters indicate significantly different values according to Duncans multi-range test at P < 0.05. The majuscule shows the significant difference between soils with different concentrations of Cd at the same treatment (P < 0.05), and the minuscule indicates the significant difference between different treatments at the same concentration of Cd in soil (P < 0.05), the same for the following figures
Inoculation of EE2 degrading bacteria GHH promoted the growth of ryegrass. In the soil with spiked Cd at 0, 5, 20, and 80 mg kg−1, the root length increased by 7.24%, 12.90%, 11.82%, and 12.28%, respectively, and the shoot dry biomass increased by 2.99%, 0.73%, 11.29%, and 11.23%, respectively, as compared with the non-inoculated treatments. Soil microorganisms can dissolve the minerals such as phosphorus in the soil, and promote plant’s uptake of minerals and hence, improve the plant growth (Ganesan 2008; Ma et al. 2009). Microorganisms can also help to increase the plant’s resistance to the pollution stresses by synthesizing plant hormones such as auxins and cytokinins (Bianco and Defez 2009). In addition, microorganisms induce the synthesis of enzymes, thereby promoting the division and growth of plant cells (Glick 2010). On the other hand, microorganisms improve the rhizosphere environment and promote the release of root exudates. The organic acids secreted by roots may complex or chelate with heavy metal ions, thus reducing the biotoxicity of heavy metals (Liao et al. 2006).
3.2 Interaction of ryegrass and GHH on Cd removal from co-contaminated soil
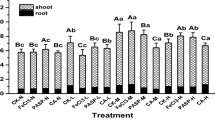
Ryegrass has been previously reported to be effectively on remediation of the single Cd polluted soil (He et al. 2014; He et al. 2019b). In this study, with the increase of soil Cd concentration, the concentrations of Cd in roots and shoots of ryegrass increased significantly (P < 0.05). In the P treatment with soil spiked Cd at 5, 20, and 80 mg kg−1, Cd concentration was 16.01, 50.98, and 274.76 mg kg−1 respectively in root of ryegrass, and 4.32, 17.19, and 73.87 mg respectively in shoot. Moreover, in the P treatment, the extraction efficiency of Cd at day 28 was 0.3~0.41% (Table 2), indicating that ryegrass could also be applied to extract Cd from the compound polluted soil, whereas Cd was mainly stored in root of ryegrass since all the TFs were < 1 (Table 2).
With the increase of soil Cd concentration, the toxicity of Cd to plant increased, and the plant biomass declined (Fig. 1); consequently, the extraction efficiency of Cd decreased significantly (P < 0.05) (Table 2). At the spiked Cd concentration of 20 and 80 mg/kg, the extraction efficiency of Cd in B + P treatment decreased by 47.38% and 53.02%, respectively, with comparison to that in soil with spiked Cd at 5 mg kg−1.
Table 2 and Fig. 2 indicated that soil Cd was only extracted by plant, and the microorganisms did not show to directly remove Cd. However, inoculation of EE2 degrading bacteria GHH improved the growth of ryegrass (Fig. 1), and promoted the concentration of Cd in plant tissues (P < 0.05) (Fig. 3), and consequently, significantly enhanced the extraction of Cd by ryegrass (P < 0.05) (Table 2 and Fig. 2). In the soil with spiked Cd at 5, 20, and 80 mg kg−1, the extraction efficiency of Cd in the B + P treatment was 0.81%, 0.43%, and 0.38%, respectively, which was 97.64%, 45.04%, and 20.33% higher respectively than that in the P treatment (Fig. 2). Similarly, Liu et al. (2015) reported that in the soils added with 25 and 50 mg Cd kg−1, inoculated with mycorrhizal fungus (Glomus versiforme) increased 33% and 47% in the shoot biomass of S. nigrum, and 62% and 46% in the root, respectively; meanwhile, Cd uptakes by S. nigrum increased 69% and 157% in the shoots, and 243% and 136% in the roots, respectively. Li et al. (2007) reported that the inoculation of microorganisms in soil promoted the growth of S. alfredii and increased its extraction of Cd and Zn by 296% and 135%, respectively. It has been found that some organic acids secreted by roots could change the bioavailability of heavy metals and promote the roots’ uptake of heavy metals (Chen et al. 2003).
3.3 Combined effect of ryegrass and GHH on the EE2 removal from co-contaminated soil
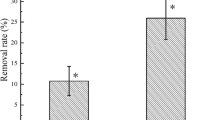
As shown in Fig. 4, in the soil with the same Cd concentration, the promoting effect of treatment on the EE2 removal rate was in the order: B + P > P > B > C. This result suggested that the interaction of ryegrass with GHH was more favorable than the treatment of ryegrass or GHH alone for the removal of soil EE2. In particular, in the soil with spiked Cd at 0, 5, 20, and 80 mg kg−1, the removal rate of EE2 in the B + P treatment was 90%, 89%, 80%, and 71%, respectively, indicating that the combination of ryegrass and GHH was effective to remove EE2 from soil. Root secretions can provide nutrients for soil microorganisms and increase the microbial activities, thereby promoting the degradation of organic contaminants (Gerhardt et al. 2009; Jha et al. 2018). Moreover, soil microorganisms reduce the concentration of soil organic pollutants, thus alleviating the stress of organic pollutants to plant, which is in turn conducive to plant growth and the phytoextraction of the pollutants.
As shown in Fig. 5, in the Cd-EE2 co-contaminated soil, inoculation of GHH significantly improved the EE2 uptake and accumulation by ryegrass. In the soil with spiked Cd at 0, 5, 20, and 80 mg kg−1, EE2 concentration in root increased by 3.73%, 12.40%, 35.94%, and 69.56%, respectively, and increased by 55.76%, 71.80%, 16.71%, and 35.18% respectively in shoot. Although stored mainly in root, EE2 was effectively extracted by ryegrass with the extraction efficiency of 12.19% in the P treatment and 14.02% in the B + P treatment of C (Table 3). Organic matters can be actively or passively absorbed by plant root (Chiou et al. 2001; Su and Zhu 2007), and further translocated to the stem and leaf. Studies have shown that the ability of plants to transport organic matters depends on the octanol-water distribution coefficient Koc. If log Koc < 0.5, organic matters cannot pass cell walls and enter into the plant root; if log Koc > 3, organic matters can be absorbed by root, but the translocation capacity is limited so that were mainly stored in root (Schnoor et al. 1995). The Koc value of EE2 is 4.76 (Schroeder et al. 2015); therefore, EE2 was mainly stored in root of ryegrass, and the transport from root to shoot is slow (Table 3).
In this study, the degradation of EE2 attributed to the interaction of GHH with the soil indigenous microorganisms. However, in soil with spiked Cd at 0, 5, 20, and 80 mg kg−1, the degradation rate of EE2 in the B treatment was 47%, 48%, 30%, and 32% higher respectively than that of C (Fig. 4), indicating that the degradation of soil EE2 was mainly due to the inoculation of GHH.
As shown in Fig. 4, there was no significant difference of the removal rate of EE2 between soils at 0 and 5 mg Cd kg−1 with the same treatment (P > 0.05). While, with the increase of soil Cd concentration, both the EE2 degradation by microorganisms and the EE2 extraction by ryegrass decreased gradually (Table 3 and Fig. 4). As compared to the soil with no spiked Cd, the removal rate of soil EE2 in the C, B, P, B + P treatment decreased by 2%, 21.43%, 6.67%, and 11.11%, respectively, in the soil at spiked Cd of 20 mg kg−1, and decreased by 35.71%, 32.86%, 18.67%, and 21.11% respectively at spiked Cd of 80 mg kg−1. This was probably because that the toxicity of Cd inhibited the activities of microorganisms and the plant biomass as well.
3.4 Urease activity and MBC content in soil
Soil enzymes play an important role in promoting the decomposition of organic matters and the recycling of nutrients in soil. So, they are of great significance in the evaluation of soil fertility, land use, and environmental assessment (Wang et al. 2014). Soil urease can hydrolyze urea and help the absorption of N by plant. MBC provides readily available nutrients, and is the power of the decomposition and mineralization of soil organic contaminants. MBC is closely related to nutrient cycles of soil C, N, P, and S. As a part of soil activated carbon, MBC can reflect the small change of soil full carbon, participate directly in the soil biochemical conversion processes, and promote the soil nutrient availability; therefore, MBC is vital for soil fertility and plant nutrition.
As shown in Fig. 6, in the soil with the same Cd concentration, there was no significant difference of soil urease activity between the GHH inoculation treatment and C. However, ryegrass cultivation significantly increased the urease content of soil (P < 0.05). It could also be found from Fig. 6 that both the planting of ryegrass and the inoculation of GHH were effective on increasing the soil MBC content. In the same Cd concentration soil, the MBC content was in the order: B + P > P > B > C. Plant root can directly secrete enzymes into soil. Plant residues (including stems and leaves) entering soil may cause the changes in the amount of soil organic matter and the microflora, so as to provides enzymes indirectly for soil. In addition, organic matters such as sugars and acids secreted by plant root can improve the rhizosphere environment and stimulate the growth of rhizosphere microorganisms (Jha et al. 2018). The increase of urease and MBC not only improved soil ecological environment, but enhanced the plant’s absorption of N, P, and other nutrients, thus promoting plant growth (Fig. 1) and further enhancing the phytoextraction of contaminants from soil (Fig. 5).
With the increase of soil Cd concentration, both of the urease activity and the MBC content increased first and then decreased (Fig. 6). In the soil with spiked Cd at 5 mg kg−1, the urease activity and MBC content increased as compared to those of the soil without Cd addition. With the gradually increase of soil Cd concentration, the enzyme activity was inhibited and the MBC content decreased (Fig. 6). This could be due to the toxic excitatory effect. That is, when an organism is stimulated by external pollutants, it compensates. If the compensatory is greater than the inhibition of the contaminant, the effect is to promote; but, as the concentration of pollutants increases, the inhibition exceeds the compensation, and the inhibition performs (Calabrese and Baldwin 2003).
4 Conclusions
The combined use of ryegrass with GHH was effective to remediate the EE2-Cd co-contaminated soil. In the soil with spiked Cd at 5, 20, and 80 mg kg−1, the effect on the EE2 and Cd removal from soil for 28 days was P + B > P > B. Among which, the removal rate by B + P treatment was 89%, 80%, and 71% respectively for EE2, and 0.81%, 0.43%, and 0.38% respectively for Cd. Soil EE2 and Cd could be effectively extracted by ryegrass and stored mainly in root. Both ryegrass cultivation and GHH inoculation significantly increased soil urease activity and MBC content, and improved the soil environment, with the promoting effect in the order: B + P > P > B > C. Inoculation with GHH significantly promoted the growth of ryegrass and enhanced the phytoextraction efficiency of EE2 and Cd from soil. Low concentration of Cd increased soil urease activity and MBC content, as well as the growth of plant root; nevertheless, higher concentrations of Cd inhibited the microbial activity and plant growth, and the removal of soil contaminants was correspondingly decreased. In conclusion, this plant-microbe interaction recovery technique was promising to play an effective role in the remediation of EE2-Cd polluted soil.
References
Aris AZ, Shamsuddin AS, Praveena SM (2014) Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. Environ Int 69:104–119
Baran S, Bielińska JE, Oleszczuk P (2004) Enzymatic activity in an airfield soil polluted with polycyclic aromatic hydrocarbons. Geoderma 118(3–4):221–232
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60(11):3097–3107
Binet P, Portal JM, Leyval C (2000) Dissipation of 3-6-ring polycyclic aromatic hydrocarbons in the rhizosphere of ryegrass. Soil Biol Biochem 32:2011–2017
Calabrese EJ, Baldwin LA (2003) Toxicology rethinks its central belief. Nature 421(6924):691–692
Chen YX, Lin Q, Luo YM, He YF, Zhen SJ, Yu YL, Tian GM, Wong MH (2003) The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 50(6):807–811
Chen Z, Sheng X, He L, Huang Z, Zhang W (2013) Effects of root inoculation with bacteria on the growth, Cd uptake and bacterial communities associated with rape grown in Cd-contaminated soil. J Hazard Mater 244–245:709–717
Chiou CT, Sheng GY, Manes M (2001) A partition-limited model for the plant uptake of organic contaminants from soil and water. Soil Biol Biochem 35(7):1437–1444
Fidalgo F, Freitas R, Ferreira R, Pessoa AM, Teixeira J (2011) Solanum nigrum L. antioxidant defence system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress. Environ Exp Bot 72:312–319
Flores MIA, Romero-Gonza’lez R, Frenich AG, Vidal JLM (2011) QuEChERS-based extraction procedure for multifamily analysis of phytohormones in vegetables by UHPLC-MS/MS. J Sep Sci 34(13):1517–1524
Ganesan V (2008) Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizopseudomonad. Curr Microbiol 56(4):403–407
Gerhardt KE, Huang XD, Glick BR, Greenberg BM (2009) Phytoremediation and rhizoremediation of organic soil contaminants: potential and challenges. Plant Sci 176(1):20–30
Glick BR (2010) Using soil bacteria to facilitate phytoremediation. Biotechnol Adv 28(3):367–374
Guo H, Yao J, Cai MM, Qian YG, Guo Y, Richnow HH, Blake RE, Doni S, Ceccanti B (2012) Effects of petroleum contamination on soil microbial numbers, metabolic activity and urease activity. Chemosphere 87:1273–1280
He L, Chen Z, Ren G, Zhang Y, Qian M, Sheng X (2009) Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria. Ecoto Environ Safe 72:1343–1348
He SY, Wu QL, He ZL (2014) Synergetic effects of DA-6/GA(3) with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere 117:132–138
He SY, Guo HH, He ZL, Yang CP, Yu T, Chai QW, LU L (2019) Interaction of Lolium perenne and Hyphomicrobium sp. GHH enhances the removal of 17α-ethinyestradiol (EE2) from soil. J Soils Sediments 19:1297–1305
He SY, Guo HH, He ZL, Wang L (2019b) Effects of new-type cleaning agent MGDA with plant growth regulator DA-6 on phytoextraction of Cd-contaminated soils by ryegrass. Pedosphere 29:161–169
Hildebrand C, Londry KL, Farenhorst A (2006) Sorption and desorption of three endocrine disrupters in soils. J Environ Sci Heal B 41(6):907–921
Hogan NS, Duarte P, Wade MG, Lean DRS, Trudeau VL (2008) Estrogenic exposure affects metamorphosis and alters sex ratios in the northern leopard frog (Rana pipiens): identifying critically vulnerable periods of development. Gen Comp Endocrinol 156(3):515–523
Huang B, Sun WW, Li XM, Liu JL, Li Q, Wang RM, Pan XJ (2015) Effects and bioaccumulation of 17β-estradiol and 17α-ethynylestradiol following long-term exposure in crucian carp. Ecotox Environ Safe 112:169–176
Jeong S, Moon HS, Nam K, Kim JY, Kim TS (2012) Application of phosphate-solubilizing bacteria for enhancing bioavailability and phytoextraction of cadmium (Cd) from polluted soil. Chemosphere 88:204–210
Jha P, Panwar J, Jha PN (2018) Mechanistic insights on plant root colonization by bacterial endophytes: a symbiotic relationship for sustainable agriculture. Environ Sustain 1(1):25–38
Karnjanapiboonwong A, Chase DA, Canas JE, Jackson WA, Maul JD, Morse AN, Anderson TA (2011) Uptake of 17α-ethynylestradiol and triclosan in pinto bean, Phaseolus vulgaris. Ecotoxicol Environ Saf 74(5):1336–1342
Kuster M, López de Alda MJ, Barceló D (2005) Estrogens and progestogens in wastewater, sludge, sediments, and soil. Water Pollut 5:1–24
Li WC, Wong MH (2012) Interaction of Cd/Zn hyperaccumulating plant (Sedum alfredii) and rhizosphere bacteria on metal uptake and removal of phenanthrene. J Hazard Mater 209-210:421–433
Li FY, Qu XY, Wu RL (2005) Theoretical basis and technology of bioremediation of contaminated soil. Chemical Industry Press, Beijing (in Chinese)
Li WC, Ye ZH, Wong MH (2007) Effects of bacteria on enhanced metal uptake of the Cd/Zn-hyperaccumulating plant, Sedum alfredii. J Exp Bot 58(15-16):4173–4182
Liao CS, Yen JH, Wang YS (2006) Effects of endocrine disruptor di-n-butyl phthalate on the growth of Bok choy (Brassica rapa subsp. chinensis). Chemosphere 65(10):1715–1722
Lima DLD, Calisto V, Esteves VI (2011) Adsorption behavior of 17α-ethynylestradiol onto soils followed by fluorescence spectral deconvolution. Chemosphere 84(8):1072–1078
Liu H, Yuan M, Tan SY, Yang XP, Jing YX (2015) Enhancement of arbuscular mycorrhizal fungus (Glomus versiforme) on the growth and Cd uptake by Cd-hyperaccumulator Solanum nigrum. Appl Soil Ecol 89:44–49
Lu RK (1999) Soil argrochemistry analysis protocoes. China Agriculture Science Press, Beijing (in Chinese)
Ma Y, Rajkumar M, Freitas H (2009) Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. J Environ Manag 90(2):831–837
Maria SD, Rivelli AR, Kuffner M, Sessitsch A, Wenzel WW, Gorfer M, Strauss J, Puschenreiter M (2011) Interactions between accumulation of trace elements and macronutrients in Salix caprea after inoculation with rhizosphere microorganisms. Chemosphere 84:1256–1261
MiPAF (Ministero delle Politiche Agricole e Forestali) (2000) Osservatorio nazionale pedologico e per la qualita del suolo, international society of soil science,Societa Italiana della Scienza del Suolo. Metodi di Analisi Chimica del Suolo.Franco Angeli Editore, Milano, Italy. fasciculated.D
Schnoor JL, Light LA, McCutcheon SC, Wolfe NL, Carreira LH (1995) Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29(7):318–323
Schroeder LM, Blackwell B, Klein D, Morse AN (2015) Rate uptake of three common pharmaceuticals in celery, Apium graveolens. Water Air Soil Poll 226(123):1–20
Sheng XF, Xia JJ (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64:1036–1042
Su YH, Zhu YG (2007) Transport mechanisms for the uptake of organic compounds by rice (Oryza sativa) roots. Environ Pollut 148(1):94–100
Tomšíková H, Aufartova J, Solich P, Novakova L, Ferrera ZS, Rodriguez JJS (2012) High-sensitivity analysis of female-steroid hormones in environmental samples. Ttac-Trend Anal Chem 34:35–58
Wang YY, Fang L, Lin L, Luan TG, Tam NFY (2014) Effects of low molecular-weight organic acids and dehydrogenase activity in rhizosphere sediments of mangrove plants on phytoremediation of polycyclic aromatic hydrocarbons. Chemosphere 99:152–159
Xu H, Yang J, Wang YX, Jiang Q, Chen H, Song HY (2008) Exposure to 17α-ethynylestradiol impairs reproductive functions of both male and female zebrafish (Danio rerio). Aquat Toxicol 88(1):1–8
Zhang X, Gao XJ, Li GX, Li QZ, Guo QH, Yan CZ (2011) Estrogenic compounds and estrogenicity in surface water, sediments, and organisms from Yundang Lagoon in Xiamen, China. Arch Environ Contam Toxicol 61(1):93–100
Zhu ZQ, Yang XE, Wang K, Huang HG, Zhang XC, Fang H, Li TQ, Alva AK, He ZL (2012) Bioremediation of Cd-DDT co-contaminated soil using the Cd-hyperaccumulator Sedum alfredii and DDT-degrading microbes. J Hazard Mater 235-236:144–151
Funding
This study was supported by the grant from National Natural Science Foundation of China (41501521, 41301327).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Claudio Bini
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, S., Li, Y., Guo, H. et al. Combined effect of ryegrass and Hyphomicrobium sp. GHH on the remediation of EE2-Cd co-contaminated soil. J Soils Sediments 20, 425–434 (2020). https://doi.org/10.1007/s11368-019-02358-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02358-8