Abstract
A pot experiment was conducted to investigate the effect of three additives – citric acid (CA), polyaspartic acid (PASP), and FeCl3 – on the phytoextraction efficiency of cadmium (Cd) and lead (Pb) by ryegrass (Lolium perenneL.) from artificially contaminated soils with different heavy metal concentrations. The results showed that as the concentration of pollutants increased, the TI (tolerance index) and BCF (bio-concentration factor) of ryegrass gradually increased only when FeCl3 was applied. FeCl3 also exhibited the most significant biomass enhancement and heavy metal accumulation of ryegrass, as well as the highest phytoextraction efficiency in heavily-polluted soils. The overall orders of the optimal phytoextraction efficiency for the three additives in terms of their MER (metal extraction ratio) were: FeCl3 > PASP > CA. Therefore, FeCl3 can be used to improve the Cd and Pb phytoextraction efficiency of ryegrass in heavily-polluted soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The majority of polluted soils contain multiple pollutants, and the presence of a single contaminant is extremely rare. Cadmium (Cd), lead (Pb), copper (Cu), zinc (Zn), and arsenic (As) are the main pollution elements of croplands in China due to smelter waste discharge. Cd and Pb soil pollutants were ranked as the 1st and 2nd highest, with respective pollution percentages of 7.0% and 1.5% (Liu et al. 2013). Therefore, it is important to find an efficient and adaptable method to remediate soils co-contaminated with Cd and Pb.
Phytoextraction is the main and most useful phytoremediation technique for the removal of metals and is useful for in situ biotechnology applications (Lan et al. 2013). Hyperaccumulators have drawn much attention because of their ability to accumulate and tolerate a high degree of heavy metals (HMs) (Maxted et al. 2007). Their potential use in bioremediation is limited by their slow growth rate and low biomass (Mulligan et al. 2001). Recent research on phytoextraction has focused on identifying tolerant plants that can obtain high biomass and stable production, although they accumulate lower degrees of heavy metals (Komárek et al. 2008).
The most economical and effective approach is to identify plants that are easy to harvest, have certain heavy metal tolerances, fast growth, large biomass, strong adaptability, and those that can absorb and migrate heavy metals from the soil. Loliumperenne has a strong ability to recover and extract lead and cadmium in soil, and its adaptability, drought tolerance, cold resistance, tiller regeneration, fast growth rate, and high yield makes it popular for foraging metal-enriched soils (Jia et al. 2011).
Compared with conventional phytoextraction methods, enhanced phytoextraction has a higher metal extraction efficiency from polluted soils, since chelators can solubilize target metals from soils (Lasat 2002) which facilitates their uptake by plant roots for transport to shoots (Mcgrath et al. 2001). Non-biodegradable chelators such as ethylenediaminetetraacetic acid (EDTA) can pollute groundwater due to diffusion-controlled leaching in soil, and so more attention should be focused on environmentally-friendly additives. Citric acid (CA) can form soluble complexes with metal ions through its carbonyl groups and is also useful for enhanced phytoextraction since it rapidly biodegrades to CO2 and H2O and a low molecular weight organic acid. Polyaspartic acid (PASP) is an emerging macromolecular chelating agent which possesses carboxylic acid groups that can efficiently coordinate and form complexes with various HMs (Roqué et al. 2004). Furthermore, PASP degrades into environmentally-friendly ammonia, CO2, and H2O.
FeCl3 forms a complex with Cd2+ and Cl− which makes Cd become exchangeable and easily internalized by plants (Guo et al. 2016). FeCl3 was selected for use as a leaching agent in Cd-contaminated paddy soils based on its Cd-extraction efficiency, cost-effectiveness, and relatively low environmental impact (Makino et al. 2006). Three additives can also reduce soil pH and increase the activity and mobility of heavy metals. Therefore, we propose the use of CA, PASP, and FeCl3 as potential additives to enhance and remedy multiple HM-polluted soils. The aim of this study was to evaluate the potential of three additives to improve the phytoextraction of multiple HM-polluted soils using Loliumperenne under different pollution conditions.
Materials and Methods
PASP, CA and FeCl3 were purchased commercially (Sinopharm Chemical Reagent Co., Ltd, Shanghai, China). Ryegrass seeds were purchased from Import Grass Species Shop in Shanghai. All other chemicals and reagents were analytical grade. Soil samples were collected from the surface (0–20 cm depth) of an arable field outside the west gate of the Southeast University (Nanjing, China). Soils were air-dried, crushed, and then passed through a 2-mm diameter sieve, and thoroughly mixed. Physicochemical parameters of soils were measured (Table 1). Cd as Cd(NO3)2·4H2O and Pb as Pb(NO3)2 were added to the soils. After metals were added, soils were equilibrated for 20 days and subjected to five saturation cycles with deionized water and air dried, and then the concentrations of Cd and Pb in the contaminated soil were measured. According to the Soil Environmental Quality Standards (SEQSs) of China (GB 15618-2018), the risk screening value of Cd and the Pb intervention value in agricultural land were 0.6 ppm and 500 ppm. Artificially-contaminated soils were rated in three levels: lightly-polluted soil (0.55 ± 0.059 ppm Cd/408.5 ± 15.8 ppm Pb), moderately-polluted soil (1.26 ± 0.061 ppm Cd/949.4 ± 26.6 ppm Pb), and heavily-polluted soil (1.72 ± 0.19 ppm Cd/1293.4 ± 9.3 ppm Pb). Four treatments (CK, PASP, CA, FeCl3) were independently applied to each polluted soil, in replicates of three. Phytoextraction with additive application in lightly-polluted, moderately-polluted, and heavily-polluted soils were denoted as treatment-L, treatment-M, treatment-H, respectively, and the negative control was denoted as treatment-N.
The treated soils were packed into pots (1500 g per pot) with inner diameters of 15 cm and depths of 12 cm. Sand and gravel were passed over a 0.15-mm screen to help drain and avoid soil loss. Ryegrass seeds were soaked in distilled water after washing with 5% bleach for 10 min, and 20 seeds were spread in each pot. Deionized water (240 mL) was quantitatively added each week to ensure 80% soil water holding capacity (WHC). Three weeks after seed emergence, seedlings were thinned to five plants per pot with growth heights of approximately 10 cm. Plants were propagated in a greenhouse in natural sunlight under controlled conditions (50-60% relative humidity; 20–25°C). A dish with an 8 inch diameter was placed on the bottom of each pot to avoid cross-contamination. Fifteen days after propagating, PASP, CA, and FeCl3 aqueous solution treatments (10 mM) were applied using a hand-operated sprayer. Each treatment (50 mL/pot) was applied weekly to one set of pots for 21 days during the experiment. Fourteen days after being treated for 3 weeks, the potted plants were harvested for further analyses.
Prior to measuring, plants were cleaned with deionized water, and their roots were placed in a 20 mM Na-EDTA solution for 15 min to remove surface-adsorbed Cd2+ and Pb2+, and then rinsed with deionized water. Plants were dried in an oven at 105°C for 20 min, then at 75°C to a constant weight. Biomass (dry weights) was measured using an AdamLab PW124 model analytical balance. Roots and shoots were manually pulverized in liquid nitrogen, then ground and passed through a 0.15 mm sieve. Rhizosphere soil samples were taken from the pots by dividing the soil into quarters, whereupon two opposite quarters were discarded. The quartering process was repeated until the desired soil sample size was obtained. Then, the soil was allowed to naturally dry, and the granulometric fraction was pulverized in a planetary ball mill (PBM-H, DECO, China) with agate mortars, passed through a 0.075 mm sieve, and oven-dried at 75°C to a constant weight. Each sample (0.5 g, dry weight) was added to a Teflon digestion container, and 6 mL of nitric acid (65 wt%) and 2 mL of hydrogen peroxide (30 wt%) were successively added. The mixture was digested in a METASH MWD-850 microwave oven.
The total concentrations of Cd and Pb in soil extracts and dried plant samples were determined using an atomic absorption spectrophotometer (SP-3803AA; Shanghai, China) following a previously-published method (Zhang et al. 2010). The Cd and Pb contents in standard soil materials (SRM 2710a, Montana I Soils, NIST) were 0.086 ± 0.023 ppm and 16.3 ± 2.4 ppm, respectively. The Cd and Pb contents in standard plant materials (GBW07602, Shrub Branches, CNAC) were 0.32 ± 0.07 ppm and 47.5 ± 3.3 ppm, respectively. Standard reference materials were also analyzed as part of the QA/QC protocol, and the relative standard deviation of each heavy metal was less than 10%. Recovery rates of 95 ± 5% were obtained using a series of GSS standard samples and a series of GSV standard samples, as well as the blank in each batch of soil samples.
Calculations of the translocation factor [TF = Metal concentration in shoots/metal concentration in roots (ppm)] was adopted from Trotta et al. (2006). The bio-concentration factor (BCF), and the ratio of the metal concentration in the different plant organs to the metal concentration in the soil, were adopted from Tu et al. (2002). The tolerance index [TI = biomass of an enriched soil (g)/biomass of a control soil (g)] was adopted from Baker et al. (1994). The metal extraction ratio (MER) – the ratio of metal accumulation in the shoots to that in the soil – was adopted from Mertens et al. (2005), and is calculated using the following equation:
where Cshoot is the metal concentration in the harvested shoots, Mshoot is the shoot biomass, Csoil is the metal concentration in the soil, and Msoil is the mass of the potted soil.
Statistical analysis was performed using SPSS version 16.0 for Windows software package (SPSS, Chicago, IL, USA). Summarized results are presented as the treatment means ( ± standard deviation, SD) from three replicate measurements of three independent experiments. Data were tested at significance levels of p < 0.05 by two-way ANOVA.
Results and Discussion
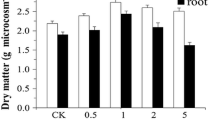
A high plant biomass yield is the primary indication of successful phytoextraction. According to Fig. 1, the change in the ryegrass biomass was similar under different treatments, which first increased and then decreased as the pollution concentration increased, reaching a maximum in moderately-polluted soil. Bidar et al. (2007) found that low concentrations of Cd2+ can promote an increase in ryegrass biomass by inducing the synthesis of phytochelatins (PCs) and stimulating the expression of stress protein genes such as DanJ-like proteins and heat shock proteins (HSP). Low concentrations of Pb2+ can also promote chloroplast enzyme activity and accelerate the synthesis of chlorophyll. In contrast, high concentrations of Cd2+ inhibit protein synthesis in plants, causing stunted plant growth, while Pb2+ affects the respiration of plants and the normal structure of organelles, and inhibits photosynthesis and mitosis of root cell. (Tewari et al. 2002; Alaboudi et al. 2018). It indicated that the concentration of heavy metals had various effects on the ryegrass biomass.
Effects of additive application on the biomass of shoots and roots in Lolium perenne. The values shown are the mean ± SD (n = 3). Different capital letters above bars indicate statistically significant differences using different treatments, and different lowercase letters indicate the same treatment with statistically significant differences at different contamination levels (p < 0.05)
Compared with the control group, all ryegrass biomass values decreased after being treated with the three additives with low pollution concentrations. FeCl3 had a significantly negative effect, and reduced the biomass by 24.54%. The averaged biomass under the low pollution condition is in descending order as follows: CK > PASP > CA > FeCl3. However, as the concentration increased to the heavily-polluted level, FeCl3 and PASP promoted the biomass slightly, which increased by 13.34% and 11.73%, respectively, while the plant biomass was still lower than the control group when treated with CA. The averaged biomass in heavily-polluted soil is in descending order as FeCl3 > PASP > CK > CA. This indicates that the additives had different effects on plant growth at varying degrees of pollution. Similar results have been reported which showed that the biomass response of S.orientalis treated with ethylenediamine disuccinic acid (EDDS) was different in soils contaminated with 10 ppm and 100 ppm Cd-contamination (Lan et al. 2013). This indicated that, due to the positive effect of HMs and FeCl3 in moderately-polluted soil, the biomass of the FeCl3-M showed a maximum increase of 63.6%, compared with FeCl3-L. The biomass and the promoting efficiency of shoots were significantly higher than that of the roots. This difference may be explained by the formation of a complex between additives and HMs which tended to remain primarily in the roots (Sun et al. 2009). The stability of high-concentration complexes decreased, making it easy for them to re-decompose into a large amount of free metallic ions which affected the normal growth and physiological metabolism of the roots.
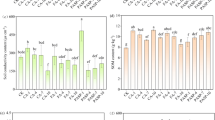
The tolerance index is another feature that affects the phytoremediation process, and values greater than the threshold value are important for phytoremediation studies (Bluskov et al. 2005). In Fig. 2, ryegrass was observed to be the most tolerant in heavily-polluted soils treated with FeCl3 and PASP, having shoot TI values of 1.19 ± 0.148 and 1.15 ± 0.132, respectively, which reflected a net increase in biomass and indicated an effective Cd and Pb extraction ability. The averaged TI values in heavily-polluted soils are listed in descending order as follows: FeCl3 > PASP > CA. High TI values were also accompanied by high HM accumulation, indicating efficient Cd and Pb transport from the roots to the shoots (Diwan et al. 2010) when promoted by FeCl3 and PASP. This possibly occurs because the stimulation of tolerance mechanisms in ryegrass, such as powerful antioxidant defense systems (Khan et al. 2009), and a decrease in the root-to-shoot transport (TF) play major roles in Cd and Pb tolerance (Table 2). A significant decline in the TI was observed when treatments were applied to lightly-polluted soils compared to CK-L, with shoot TI values near 0.77–0.94. This indicated that additives reduced the tolerance of ryegrass at low contamination levels, and the potential reason is that the complex formed by Cd2+ and Pb2+ and additives reduced the promotion of plant biomass by low concentrations heavy metals. (Solis-Dominguez et al. 2007).
Effects of the application of chelants on the tolerance index (TI) of roots and shoots in Lolium perenne. The threshold for TI is 1. Values shown are the mean ± SD (n = 3). Different letters above the bars indicate statistically significant differences from the different treatments at p < 0.05. An asterisk (*) indicates that the TI of the root significantly differed from the corresponding TI of shoots (p < 0.05)
HM uptake is an important parameter used to understand the cellular response of plants and is one of the necessary conditions to achieve successful phytoextraction (Diwan et al. 2010). According to Fig. 3, the HM content of roots was much higher than that of the shoots. Combined with the low TI of the roots ( < 1) in Fig. 2, this indicated that the growth of the ryegrass root system was significantly inhibited due to heavy metal stress. In Fig. 3a, b, the application of three additives under moderately- and heavily-polluted levels promoted the absorption of Cd and Pb in roots, and the Cd and Pb content of roots increased as the pollution level increased. This suggests that the additives may help ryegrass prevent metal precipitation by forming metal complexes that easily enriched in the roots (Yeh et al. 2015).
The Cd and Pb content in different tissues of Lolium perenne. Values shown are the mean ± SD (n = 3). Different capital letters above bars indicate statistically significant differences from different treatments, and different lowercase letters indicate the same treatment with statistically significant differences at different contamination levels (p < 0.05)
In Fig. 3c, d, the three additives promoted the absorption of Cd of ryegrass shoots. The promoting effects of FeCl3 increased as the pollution level increased, and the promoting effect of FeCl3 was the most evident in the heavily-polluted soil, where it increased the Cd and Pb content in the shoots by 157.7% and 366.05%, respectively. The averaged Pb content of shoots in heavily-polluted soil is listed in descending order as follows: FeCl3 > PASP > CA > CK. Earlier reports have also indicated that FeCl3 can provide cations capable of exchanging soil-adsorbed HMs and ligands to form soluble metal complexes which enhance the accumulation in shoots (Xiong and Feng 2001). The promoting effects of PASP first increased and then decreased, and the maximum Cd and Pb content increased by 74.54% and 66.65%, respectively at the medium level. This indicated that an increase in HMs can decrease the solubilizing capacity of PASP, which reduces the mobility of HMs and inhibits transportation from the roots to shoots (Lingua et al. 2014). The averaged Cd content of shoots in moderately-polluted soil is listed in descending order as follows: PASP > CA > FeCl3 > CK. The promoting effect of CA in three polluted soils was relatively stable compared with FeCl3 and PASP. However, the Cd content increased by 184.6% with CA in the lightly-polluted soil, demonstrating that the promoting effect of CA was the most significant in the lightly-polluted soil among the three additives. The averaged Cd content in shoots in the lightly-polluted soil is listed in descending order as follows: CA > FeCl3 > PASP > CK.
BCF and TF are the defining parameters in phytoremediation and provide the basis for metal uptake, storage in the roots, and mobilization into aerial plant parts (Diwan et al. 2010). In Table 2, the application of additives effectively promoted the BCF of Cd in roots and shoots, and the BCF of Cd in shoots under CA-L treatment reached a maximum of 0.62 ± 0.054, an increase of 181.8%. The BCF of Cd and Pb in the roots was far higher than that in the shoots, and roots had a high Cd enrichment ability (BCF > 1), reaching a maximum of 12.7 ± 1.54 when treated by FeCl3-H. These findings agree with earlier reports which indicated that ryegrass is capable of accumulating Cd and Pb mainly in its roots, and metals were found in the cell walls and vacuoles, indicating that this plant species has a metal sequestration mechanism (He et al. 2013, 2014). The BCF of Cd was stable across the polluted concentration range, which signified that ryegrass may be efficiently used for phytoremediation across a wide and fluctuating concentration range of Cd contamination (Zurayk et al. 2001). As the HM content increased, the BCF of Pb in the roots also increased, while the TF gradually decreased, which indicated that ryegrass could potentially be used in phytostabilization to reduce the mobility of Pb accumulated and precipitated within the root zone (Zhao et al. 2016). As a non-hyperaccumulator with BCF and TF shoot values ( < 1), ryegrass can produce much higher shoot biomasses and may achieve a considerable HM phytoextraction efficiency (Hu et al. 2013). Since the BCF of Cd in shoots and roots was higher than that of Pb, ryegrass was the most efficient in Cd uptake and enrichment, followed by Pb. The low BCF of Pb also illustrated that it had a tolerance mechanism of and exclusion to Pb (Sun et al. 2008) which indicates that ryegrass has great phytoextraction potential for soils heavily polluted with Cd and Pb.
The MER expresses the extraction capacity as a ratio which takes into account the produced biomass and the soil volume to be cleaned more informative (Mertens et al. 2005). According to Fig. 4, there was a very similar MER change between Cd and Pb, which suggested that the three additives potentially enhanced the combined phytoextraction of Cd and Pb by ryegrass. FeCl3, PASP, and CA have the most significant phytoextraction enhancement effects in heavily, moderately and lightly polluted soils, respectively (p < 0.05). The highest average MERs of CK, FeCl3, PASP, and CA were 0.094%, 0.21%, 0.18%, and 0.15% for Cd, and 0.002%, 0.011%, 0.007%, and 0.005% for Pb, respectively. Sun et al. (2008) reported that the MERs of the Cd-hyperaccumulator Solanum nigrum in co-contaminated soil (10 ppm Cd/250 ppm As) were 0.22% for Cd and 0.006% for As, respectively. This suggests that the FeCl3-enhanced phytoextraction of Cd by ryegrass can achieve a favorable efficiency similar to other hyperaccumulators. The optimal MER of each additive is listed in descending order as follows: FeCl3 > PASP > CA > CK. The MER of FeCl3-H showed a 3.4-fold increase for Cd and 13.3-fold for Pb compared to CK-H. For phytoremediation projects in the field, the cost of additives must be considered, and the price of additives are in the following order: FeCl3 < CA < PASP. These results indicate that FeCl3-H has potential commercial viability, and also demonstrated the highest enhancement in the phytoextraction of Cd and Pb by ryegrass.
Metal extraction ratio (MER) of Lolium perenneL. under different treatments. Values shown are the mean ± SD (n = 3). Different capital letters above bars indicate statistically significant differences from different treatments, and different lowercase letters indicate the same treatment with statistically significant differences between different contamination levels (p < 0.05)
In conclusion, the results of this study have demonstrated that FeCl3 can more strongly enhance the phytoextraction efficiency of ryegrass than PASP and CA in heavily-polluted soil. Three additives were shown to increase the accumulation and BCF of Cd and Pb, but their enhancement effects in different polluted soils were affected by the HM content. FeCl3 had both the highest enhancement to BCF and also the highest biomass and tolerance in heavily-polluted soil. Based on the MER, the enhanced phytoextraction of ryegrass was similar to the phytoextraction effect of a previously-reported hyperaccumulator due to its high biomass of shoots, stable Cd enrichment, and tolerance of Pb. Therefore, ryegrass appears to be a promising plant for phytoextraction. Moreover, the results reflect the growth-promoting effect of additives on harvestable biomass which is essential for phytoextraction. While the results were based on pot experiments, further work would be required to verify the phytoextraction rates in field conditions.
References
Alaboudi KA, Ahmed B, Brodie G (2018) Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann Agric Sci 63:123–127
Baker AJM, Mcgrath SP, Sidoli C, Reeves RD (1994) The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants. Resour Conserv Recycl 11:41–49
Bidar G, Garçon G, Pruvot C, Dewaele D, Cazier F, Douay F, Shirali P (2007) Behavior of Trifolium repens and Lolium perenne growing in a heavy metal contaminated field: Plant metal concentration and phytotoxicity. Environ Pollut 147:546–553
Bluskov S, Arocena JM, Omotoso OO, Young JP (2005) Uptake, distribution, and speciation of chromium in Brassica juncea. Int J Phytoremediat 7:153–165
Diwan H, Ahmad A, Iqbal M (2010) Uptake-related parameters as indices of phytoremediation potential. Biologia 65:1004–1011
Guo X, Wei Z, Wu Q, Li C, Qian T, Zheng W (2016) Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: field experiments. Chemosphere 147:412–419
He S, Wu Q, He Z (2013) Effect of DA-6 and EDTA alone or in combination on uptake, subcellular distribution and chemical form of Pb in Lolium perenne. Chemosphere 93:2782–2788
He S, Wu Q, He Z (2014) Synergetic effects of DA-6/GA(3) with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere 117:132–138
Hu J, Wu S, Wu F, Leung HM, Lin X, Wong MH (2013) Arbuscular mycorrhizal fungi enhance both absorption and stabilization of Cd by Alfred stonecrop (Sedum alfredii Hance) and perennial ryegrass (Lolium perenne L.) in a Cd-contaminated acidic soil. Chemosphere 93:1359–1365
Jia Y, Tang SR, Ju XH, Shu LN, Tu SX, Feng RW, Giusti L (2011) Effects of elevated CO(2) levels on root morphological traits and Cd uptakes of two Lolium species under Cd stress. J Zhejiang Univ Sci B 12:313–325
Khan I, Ahmad A, Iqbal M (2009) Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicol Environ Safe 72:626–634
Komárek M, Tlustos P, Száková J, Chrastný V (2008) The use of poplar during a two-year induced phytoextraction of metals from contaminated agricultural soils. Environ Pollut 151:27–38
Lan J, Zhang S, Lin H, Li T, Xu X, Li Y, Jia Y, Gong G (2013) Efficiency of biodegradable EDDS, NTA and APAM on enhancing the phytoextraction of cadmium by Siegesbeckia orientalis L. grown in Cd-contaminated soils. Chemosphere 91:1362–1367
Lasat MM (2002) Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual 31:109–120
Lingua G, Todeschini V, Grimaldi M, Baldantoni D, Proto A, Cicatelli A, Biondi S, Torrigiani P, Castiglione S (2014) Polyaspartate, a biodegradable chelant that improves the phytoremediation potential of poplar in a highly metal-contaminated agricultural soil. J Environ Manag 132:9–15
Liu L, Wang Y, Luo G, Chen X, Li X (2013) Heavy metal contamination of urban soil in an old industrial city; (Shenyang) in Northeast China. Geoderma 192:50–58
Makino T, Sugahara K, Sakurai Y (2006) Remediation of cadmium contamination in paddy soils by washing with chemicals: selection of washing chemicals. Environ Pollut 144:2–10
Maxted AP, Black CR, West HM, Crout NM, Mcgrath SP, Young SD (2007) Phytoextraction of cadmium and zinc from arable soils amended with sewage sludge using Thlaspi caerulescens: development of a predictive model. Environ Pollut 150:363–372
Mcgrath SP, Zhao FJ, Lombi E (2001) Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant Soil 232:207–214
Mertens J, Luyssaert S, Verheyen K (2005) Use and abuse of trace metal concentrations in plant tissue for biomonitoring and phytoextraction. Environ Pollut 138:1–4
Mulligan CN, Yong RN, Gibbs BF (2001) Remediation technologies for metal-contaminated soils and groundwater: an evaluation. Eng Geol 60:193–207
Roqué J, Molera J, Vendrell-Saz M, Salvadó N (2004) Crystal size distributions of induced calcium carbonate crystals in polyaspartic acid and Mytilus edulis acidic organic proteins aqueous solutions. J Cryst Growth 262:543–553
Solis-Dominguez FA, Gonzalez-Chavez MC, Carrillo-Gonzalez R, Rodriguez-Vazquez R (2007) Accumulation and localization of cadmium in Echinochloa polystachya grown within a hydroponic system. J Hazard Mater 141:630–636
Sun Y, Zhou Q, Diao C (2008) Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresource Technol 99:1103–1110
Sun YB, Zhou QX, Jing A, Liu WT, Rui L (2009) Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma 150:106–112
Tewari RK, Kumar P, Sharma PN, Bisht SS (2002) Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Sci 162:381–388
Trotta A, Falaschi P, Cornara L, Minganti V, Fusconi A, Drava G, Berta G (2006) Arbuscular mycorrhizae increase the arsenic translocation factor in the As hyperaccumulating fern Pteris vittata L. Chemosphere 65:74–81
Tu C, Ma LQ, Bondada B (2002) Arsenic accumulation in the hyperaccumulator Chinese brake and its utilization potential for phytoremediation. J Environ Qual 31:1671–1675
Xiong ZT, Feng T (2001) Enhanced accumulation of lead in Brassica pekinensis by soil-applied chloride salts. B Environ Contam Toxicol 67:67–74
Yeh TY, Lin CL, Lin CF, Chen CC (2015) Chelator-enhanced phytoextraction of copper and zinc by sunflower, Chinese cabbage, cattails and reeds. Int J Environ Sci Technol 12:327–340
Zhang X, Zhang S, Xu X, Li T, Gong G, Jia Y, Li Y, Deng L (2010) Tolerance and accumulation characteristics of cadmium in Amaranthus hybridus L. J Hazard Mater 180:303–308
Zhao L, Li T, Zhang X, Chen G, Zheng Z, Yu H (2016) Pb uptake and phytostabilization potential of the mining ecotype of Athyrium wardii (Hook.) grown in Pb-contaminated soil. Clean: Soil, Air, Water 44:1184–1190
Zurayk R, Sukkariyah B, Baalbaki R, Ghanem DA (2001) Chromium phytoaccumulation from solution by selected hydrophytes. Int J Phytoremediat 3:335–350
Acknowledgements
This work was financially supported by National Key R&D Program of China (2018YFD0800304) and Natural Science Foundation of Jiangsu Province (No. BK20171075).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, F., Xu, W. et al. Enhanced Phytoextraction for Co-contaminated Soil with Cd and Pb by Ryegrass (Lolium perenne L.). Bull Environ Contam Toxicol 103, 147–154 (2019). https://doi.org/10.1007/s00128-019-02661-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02661-7