Abstract
Purpose
Rhizosphere and fertilization might affect soil microbial activities, biomass, and community. This study aimed to evaluate the impacts of Phyllostachys edulis (moso bamboo) rhizospheres on soil nutrient contents and microbial properties in a moso bamboo forest with different fertilizer applications and to link soil microbial activities with abiotic and biotic factors.
Materials and methods
The experiment included three treatments: (1) application of 45% slag fertilizer (45%-SF); (2) application of special compound fertilizer for bamboos (SCF); and (3) the control without any fertilizer application (CK). Simultaneously, bulk soils and 0.5, 2.5, 4.5, and 6.5-year-old (y) bamboo rhizosphere soils were selected. Soil nutrient contents were analyzed. Microbial activities were evaluated based on the activities of soil enzymes including β-glucosidase, urease, protease, phosphatase, and catalase. The total microbial biomass and community were assessed with the phospholipid fatty acids (PLFAs) method.
Results and discussion
In the CK and SCF treatments, organic matter contents of rhizosphere soils were significantly higher than those of bulk soils. Soil β-glucosidase, urease, protease, phosphatase, and catalase activities in rhizosphere soils were higher than those of bulk soils, with the sole exception of β-glucosidase of 0.5 y rhizosphere soil in the 45%-SF treatment. Compared with the CK treatment, fertilizer applications tended to increase soil total PLFAs contents and changed soil microbial community. Moso bamboo rhizospheres did not significantly increase the total microbial biomass. In the SCF treatment, the Shannon index of bulk soil was significantly lower than those of rhizosphere soils.
Conclusions
Our results suggested that both rhizospheres and fertilizer applications could change the soil microbial community structures and that moso bamboo rhizosphere could increase microbial activity rather than biomass in the forest soils with different fertilizer applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Accounting for approximately 1% of the global forest area, bamboo forest has experienced a stable and ongoing increase during the past decades and plays key functional roles in purifying air and reducing soil erosion and water runoff (Bystriakova et al. 2003; Zhou et al. 2011). Phyllostachys edulis (moso bamboo) is the most widespread bamboo species in Asian countries, and according to the latest survey, the moso bamboo covers approximately 4 million hectares in China (Ge et al. 2018). Moso bamboo plantations generate enormous benefits to local environmental protection and economic growth (Peng et al. 2013). Moso bamboo is the main source of non-wood forest product, and its timber has versatile applications in clothing ingredient, papermaking, laminated beams, and bamboo flooring (Sukmawan et al. 2016). Moreover, bamboo shoot is also an important and popular vegetable (Raghubanshi 1994; Sukmawan et al. 2016). Therefore, different management practices, such as understory vegetation removal, plowing, and shoot harvests, have been conducted in bamboo plantation to maintain high productivity (Raghubanshi 1994; Liu et al. 2011). Apart from the management practices above, fertilizer application is also one of the common intensive management regimes in forest plantation. As anthropogenic disturbances, these intensive management practices may generate inconsistent influences on soil nutrient contents and microbial properties (Cui et al. 2017; Bei et al. 2018). Cui et al. (2017) revealed that soil organic matter contents and bacterial communities in subtropical forest were resilient to changes derived from N additions. Other studies demonstrated that fertilization significantly changed soil microbial community and microbial carbon source utilization (Bei et al. 2018). Despite considerable efforts to elucidate the impacts of fertilizer applications on soil nutrient and environment, large knowledge gap still exists. The majorities of previous studies about fertilizers were mainly focused on agricultural soils, but little attention has been paid to the bamboo forest soils. On the other hand, the responses of soil nutrients and microorganisms could also be used to evaluate fertilizer performances and optimize fertilizer application rates (Wei et al. 2016; Geisseler et al. 2017). Therefore, more studies are still required to quantify the potential impacts of fertilizer application on microbial communities in the bamboo forest soils.
Moso bamboo plants could emerge from shoots and culms, due to its unique rhizome system, and this extensive rhizome system contributes moso bamboo to spread laterally and enables moso bamboo to inhabit even amidst harsh nutrient conditions (Chongtham et al. 2011; Chang and Chiu 2015). For moso bamboo species, there is a special rhizosphere and active rhizomatous clonal growth. New shoot of moso bamboo is produced in early spring, and the bamboo has special leaf life span, with the emergence and elongation of another new shoot in every 2 years (Song et al. 2016). Previous studies revealed that in the same soil, plant colonizing might erratically alter rhizosphere microbial community, depending on root exudates and rhizosphere sizes (Haichar et al. 2008; Bressan et al. 2009). Therefore, in moso bamboo forest, the rhizosphere effects might also be determined by ages of moso bamboo plants. However, to the best of our knowledge, little information is available about the rhizosphere effects of different age moso bamboo plants.
Based on the above, the aims of this study were to (1) assess the effects of different fertilizer applications on nutrient contents and microorganisms in the moso bamboo forest soils; (2) quantify the impacts of different age moso bamboo rhizospheres on soil microbial activities, biomass, and communities; and (3) link microbial activities with nutrient contents and biomass of different microorganisms. Microbial activities were evaluated based on the activities of soil enzymes including β-glucosidase, urease, protease, phosphatase, and catalase. Simultaneously, geometric mean of enzymes activities (GMEA) was also calculated to integrate soil enzyme activities from variables with different units and variation range, for the GMEA was also a key parameter in assessing the whole soil microbial activities (Lu et al. 2015; Sanchez-Hernandez et al. 2018). The total microbial biomass and community were assessed with the phospholipid fatty acids (PLFAs) method, and total fungal, bacterial, and actinomycetal biomass were also quantified. The results of this study will be useful for expanding our horizons about the rhizosphere effects of moso bamboo and understanding the impacts of fertilizer applications on nutrient contents and microorganisms in bamboo forest soils.
2 Materials and methods
2.1 Experimental site and fertilizers
The experimental site was located in Guan Shan Foresty Farm (115° 29′ E, 27° 13′ N), Jiangxi Province, China, and this forestry farm was founded in 1963 and was set in the middle of the hillside. The local average temperature is 16.2 °C, and the annual average precipitation is 1950–2100 mm. The surface soil is classified into red soil developed from granite. All the climatic, environmental, and soil conditions are suitable for the growth of moso bamboo. Apart from the moso bamboo, some other species also exist in this forestry farm, including Cunninghamia lanceolata Lamb (Chinese Fir), Pinus massoniana Lamb (Masson Pine), and Robinia pseudoacacia Linn (Locust). In this study, 45% slag fertilizer and special compound fertilizer for bamboos were applied. These two fertilizers were manufactured by Wenshen Fertilizer Company (Hefei, China) and Liuyang Compound Fertilizer Company (Hunan, China), respectively, and nutrient contents of these two fertilizers are demonstrated in Table S1 (Electronic Supplementary Material—ESM).
2.2 Experimental design
The current experiment studied two factors, namely fertilizer applications and rhizosphere effects of different age moso bamboos. The different fertilization treatments were (1) application of 45% slag fertilizer (45%-SF); (2) application of special compound fertilizer for bamboos (SCF); and (3) the control without any fertilizer application (CK). Each treatment was tested in three replicate plots. In the bamboo forest, the plots with similar bamboo and soil characteristics were established before fertilization. The area of each plot was 400 m2, and the distance between different plots was 10 m. The fertilizers were applied into different plots at the rate of 15.0 kg, and after 1 year, bulk soils and 0.5, 2.5, 4.5, and 6.5-year-old (y) bamboo rhizosphere soils were randomly taken (0–10 cm) from each plot. The ages of bamboo were recognized with the method of leaf life span of moso bamboo. One part of the soil sample was air-dried and sieved to < 2 mm for determinations of soil nutrient contents, and the other part was kept at 4 °C prior to quantifying soil enzyme and microbial properties.
2.3 Determinations of soil nutrient contents
Soil nutrient contents were analyzed according to the methods described by Lu (2000). Soil organic matters (OM) were digested by oil-bath heating soil samples with the potassium dichromate and sulfuric acid (H2SO4), and total carbon (C) contents were quantified by titrating. Soil C contents were then converted into OM contents. Soil available nitrogen (AN) contents were measured by the alkaline-hydrolysis diffusion method. Soil samples were firstly digested with the sodium hydroxide (NaOH) melting method, and then the total phosphorus (TP) contents were analyzed by the molybdenum antimony colorimetric method with a spectrophotometer at 700 nm. Soil total potassium (TK) was obtained by heating the soil sample with hydrofluoric and perchloric acids in a crucible, and soil available K (AK) were extracted with 1 M ammonium acetate. The K contents were then quantified with the flame photometry.
2.4 Determinations of soil enzyme activities
Soil enzyme activities, including β-glucosidase, urease, protease, phosphatase, and catalase, were analyzed with the methods described by Guan (1986) with minor modifications. In each determination of soil enzyme, blanks without soils and samples without substrates of enzyme reaction were also included for the quality control.
Soil β-glucosidase determination the analytical enzyme activity was based on the reaction that soil β-glucosidase could specially hydrolyze p-nitrophenyl-β-d-glucoside to generate p-nitrophenol (pNP). Briefly, after pre-incubated with toluene, soil samples were incubated with the buffer (pH = 6.0 citrate-phosphate buffer) and p-nitrophenyl-β-d-glucoside at 37 °C for 1 h. After additions of calcium chloride (CaCl2) and NaOH solutions to terminate the enzyme action, the mixtures were centrifuged and filtered, and concentrations of generated pNP were quantified with a spectrophotometer at 410 nm. The result of soil β-glucosidase activity was converted into μg pNP g−1 dry soil d−1.
Soil urease determination
Fresh soil samples (approximately 5.0 g) were placed in a 50-mL Erlenmeyer flask and pre-incubated with toluene for 15 min. After additions of 20.0 mL of buffer (pH = 6.7 citric acid buffer) and 10.0 mL of substrate (urea), the mixtures were kept at 37 °C for 24 h. After filtering, 3.0 mL of filtrates was placed into 50.0 mL volumetric flask and then successively treated with 10.0 mL of double distilled H2O (ddH2O), 4.0 mL of sodium phenate, and 3.0 mL of sodium hypochlorite. Concentrations of indophenol blue were quantified within 1 h. The result of soil urease activity was expressed with the unit of mg NH4+-N g−1 dry soil d−1.
Soil phosphatase determination
Phosphatase activity was also determined with the pNP release method. Briefly, fresh soil samples (approximately 1.0 g) were treated with the toluene and then incubated with p-nitrophenyl-phosphate disodium at 37 °C for 1 h. The enzyme reaction was terminated by adding CaCl2 and NaOH solutions, and the concentrations of the pNP were quantified. The result of soil phosphatase activity was converted into mg pNP g−1 dry soil d−1.
Soil catalase determination
Fresh soil samples (approximately 5.0 g) were mixed with 40.0 mL of ddH2O and 5.0 mL of 0.3% hydrogen peroxide solution. The mixtures were shaken at 25 °C for 20 min. After terminating the enzyme action with the H2SO4 (3 mol L−1), the mixtures were filtered, and the filtrates were then titrated with 0.1 mol L−1 potassium permanganate (KMnO4) solution. Soil catalase activity was converted into mL KMnO4 g−1 dry soil d−1.
2.5 Determinations of soil phospholipid fatty acids
Soil phospholipid fatty acids (PLFAs) were analyzed with the method of Bardgett et al. (1996). Briefly, freeze-dried soil samples (8 g) were treated with a mixture of chloroform, methanol, and phosphate (1: 2: 0.8), and then the mixtures were shaken for 2 h. After centrifuging at the speed of 2000g for 10 min, lipids were separated with solid phase extraction chromatography, and the phospholipids were methylated with a mild-alkaline solution, purified, dried with N2, and dissolved in hexane. The PLFAs analyses were conducted on a gas chromatograph coupled with a mass spectrometer (Hewlett-Packard 6890). The PLFAs were identified by comparing their retention times and mass spectras with those from standard compounds and from spectral database. In the determination of soil PLFAs, blanks without soil samples were also included for quality control. Concentrations of PLFAs were quantified with an internal standard calibration method and converted into ng g−1 dry soil. The total fungal, bacterial (gram-positive and gram-negative bacteria), and actinomycetal PLFAs contents were calculated with all PLFA biomarkers of a specific group, and the biomarkers of different biological groups are demonstrated in Table S2 (ESM). The fungal to bacterial (F/B), actinomycetal to bacterial (A/B), and gram-positive to gram-negative bacterial (G+/G−) ratios were also calculated.
2.6 Calculation and statistical analysis
Geometric means of enzyme activities were calculated to integrate soil enzymes with different units
where Glu, Ure, Pro, Pho, and Cat were soil β-glucosidase, urease, protease, phosphatase, and catalase activities, respectively, in the same soil sample.
Soil microbial community diversities were calculated, including Shannon index for the species richness and Simpson index for the most common species, with the following equations:
where pi is the percentage value of a special PLFA content to the total PLFAs content in each sample.
Two-way analysis of variance was employed to determine significant differences among rhizospheres, fertilizer applications, and their interactions with SPSS v. 25.0, followed by Duncan’s multiple range test to detect significant differences (P < 0.05) among the different rhizospheres and fertilizer treatments. Principal component analysis (PCA) was used to separate soil microbial activity or community structure among the fifteen different groups (three fertilizer treatments * five different soils in each treatment).
Path analysis was conducted with SPSS Amos 23.0 to quantify direct and indirect impacts of soil properties and microbial biomass on soil enzyme activates. First, we hypothesized that soil organic matters and other nutrients could affect each other. Second, soil nutrient contents could generate direct impacts on soil fungal, bacterial, and actinomycetal biomass. Third, soil nutrient contents and different microbial biomass could directly affect the soil enzyme activities. The theoretical structural model was tested with the chi-squared fit statistic and other goodness-of-fit tests and then was adjusted.
3 Results
3.1 Effects of rhizosphere and fertilizer application on soil nutrient contents
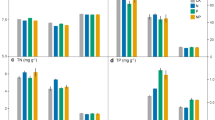
According to the results demonstrated in Table 1, the rhizosphere was a key factor affecting soil nutrient contents, with the sole exception of TP content, and the interactions of rhizosphere and fertilizer application could significantly (P < 0.05) affect the soil nutrient contents analyzed in this study. In the CK treatment, soil OM contents ranged from 22.0 ± 0.6 g kg−1 to 34.7 ± 1.2 g kg−1, and the OM contents of rhizosphere soils were significantly (P < 0.05) higher than that of the bulk soil. The same trend was also observed in the SCF treatment (Fig. 1(A)), but in the 45%-SF treatment, there was no significant difference in the OM content between the bulk and 2.5 y rhizosphere soils. The highest AN contents were observed in the 4.5 y, 6.5 y, and 2.5 y rhizosphere soils for the CK, 45%-SF, and SCF treatments, respectively (Fig. 1(B)). The AN contents of different soils in the SCF treatment were all significantly (P < 0.05) higher than their counterparts in the CK treatment, except for the 4.5 y rhizosphere soil. However, the TP contents of different soils in the CK treatment were significantly (P < 0.05) higher than those in SCF treatment (Fig. 1(C)). In the 45%-SF treatment, the TK content of the bulk soil was the lowest among the different five soils, while in the SCF treatment, the bulk soil had the highest TK content (Fig. 1(D)). The highest AK contents were observed in the 4.5 y rhizosphere, bulk and 0.5 y rhizosphere soils for the CK, 45%-SF, and SCF treatments, respectively (Fig. 1(E)).
Effects of fertilizer applications on (A) organic matter, (B) available N, (C) total P, (D) total K, and (E) available K contents in the rhizosphere of different age bamboos and bulk soils. Different low case letters indicate significant differences (P < 0.05) among different soils for a particular treatment, and different capital letters indicate significant differences (P < 0.05) among different treatments for a particular soil
3.2 Effects of rhizosphere and fertilizer application on soil enzyme activities
The rhizospheres of moso bamboos, fertilizers, and their interactions could all significantly affect soil enzyme activities in this study (Table 1). As demonstrated in Fig. 2(A), the β-glucosidase activity of different soil samples in the CK treatment tended to be lower than their counterparts in the 45%-SF treatment. In the CK and SCF treatments, the lowest β-glucosidase activities were both presented in the bulk soils, with enzyme activities being 6.32 ± 1.32 to 15.76 ± 0.44 μg pNP g−1 dry soil d−1, respectively. However, in the 45%-SF treatment, the 0.5 y rhizosphere soil had the lowest β-glucosidase activity (17.49 ± 1.44 μg pNP g−1 dry soil d−1). For soil urease, the highest enzyme activities were all observed in the 6.5 y rhizosphere soils, and the bulk soils all had the lowest enzyme activities (Fig. 2(B)). In the three different treatments, the highest protease activities were demonstrated in different rhizosphere soils, but the lowest protease activities were always in the bulk soils (Fig. 2(C)). Compared with the CK treatment, fertilizer applications significantly (P < 0.05) increased phosphatase activities in the bulk soils, and the phosphatase activity of bulk soil in the CK treatment was 80.2% and 61.1% of those in the 45%-SF and SCF treatments (Fig. 2(D)). The catalase activities were enhanced after applying the 45%-SF, relative to the CK treatment (Fig. 2(E)). In the three different treatments, the phosphatase and catalase activities of bulk soils were significantly (P < 0.05) lower than those in the rhizosphere soils, and the same trend was also observed in the geometric means of enzyme activities (Fig. 2(F)). Combining all the results presented in Fig. 2, we concluded that soil microbial activities were increased by the moso bamboo rhizospheres, regardless of bamboo ages.
Effects of fertilizer applications on (A) β-glucosidase, (B) urease, (C) protease, (D) phosphatase, and (E) catalase activities and (F) geometric means of enzyme activities in the rhizosphere of different age bamboos and bulk soils. Different low case letters indicate significant differences (P < 0.05) among different soils for a particular treatment, and different capital letters indicate significant differences (P < 0.05) among different treatments for a particular soil
3.3 Effects of rhizosphere and fertilizer application on soil microbial biomass
Compared with the CK treatment, fertilizer applications tended to increase soil total PLFAs contents in the different soils, and in the SCF treatment, the PLFAs contents of different rhizosphere soils were all significantly (P < 0.05) higher than their counterparts in the CK treatment (Fig. 3(A)). Fungi and bacteria had the similar trends in the PLFAs contents among the five soils in different treatments, and the highest fungal and bacterial PLFAs were both observed in the bulk soils of the 45%-SF treatment, with the value being 1.80 × 103 and 1.00 × 104 ng g−1 dry soil, respectively (Fig. 3(B and C)). For soil actinomycete, the bulk soils of fertilizer application treatments had the highest contents, but in the CK treatment, the 2.5 y rhizosphere soil had the highest actinomycetal PLFAs (Fig. 3(D)). In the bulk soils, fertilizer applications tended to increase the fungal/bacterial and actinomycetal/bacterial PLFAs content ratios (Fig. S1A and S1B—ESM). However, for the 6.5 y rhizosphere soils, compared with the CK treatment, the PLFAs content ratios of gram-positive/gram-negative bacteria were significantly decreased by the fertilizer applications (Fig. S1C—ESM). In the three different treatments, there were no significant differences in total microbial PLFAs contents between the bulk and rhizosphere soils, and the same results were also observed in Table 1 that the rhizosphere had negligible impacts on the total, fungal, bacterial, or actinomycetal PLFAs.
Effects of fertilizer applications on (A) total, (B) fungal, (C) bacterial, and (D) actinomycetal PLFAs contents in the rhizosphere of different age bamboos and bulk soils. Different low case letters indicate significant differences (P < 0.05) among different soils for a particular treatment, and different capital letters indicate significant differences (P < 0.05) among different treatments for a particular soil
3.4 Effects of rhizosphere and fertilizer application on soil microbial community diversity
Both in the CK and 45%-SF treatments, there were no significant differences in the Shannon index between the bulk and rhizosphere soils (Fig. 4(A)). In contrast, in the SCF treatment, the Shannon index of bulk soil was significantly lower than those of rhizosphere soils. Relative to the CK treatment, the 45%-SF application generated negligible impacts on the Simpson index in the different soils. The Simpson indices of bulk soils in the CK and 45%-SF treatments were significantly lower than that in the SCF treatment whose bulk soil had the highest Simpson index among the five different soils (Fig. 4(B)). Although the microbial community diversities kept relatively stable under the conditions of different fertilizer applications, the interactions of rhizospheres and fertilizer applications indeed affected the Shannon and Simpson indices (Table 1).
Effects of fertilizer applications on (A) Shannon index and (B) Simpson index of the microbial community in the rhizosphere of different age bamboos and bulk soils. Different low case letters indicate significant differences (P < 0.05) among different soils for a particular treatment, and different capital letters indicate significant differences (P < 0.05) among different treatments for a particular soil
3.5 Combined effects of rhizosphere and fertilizer application on soil microorganisms
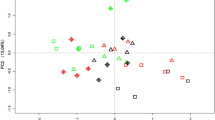
The analysis of soil sample locations showed that both the fertilizer applications and rhizosphere were the factors influencing soil microbial activities (Fig. 5(A)). For soil enzyme activities, the sum of PC1 and PC2 could explain 66.57% of the total variance. As depicted in the two-dimensional PCA plot, the bulk soils of different treatments were located towards the left side of PC1, with the PC1 values of bulk soils being significantly lower than those of the rhizosphere soils. Relative to the CK treatment, the soil samples of the 45%-SF treatment, both the bulk and rhizosphere soils, tended to shift to the positive side of PC2 (Fig. 5(A)). For the microbial community structure, the PC1 and PC2 explained 65.27% and 7.30% of the total variance, respectively. In the CK and 45%-SF treatments, relative to the bulk soil, microbial community structure was only significantly affected by the 4.5 y rhizosphere, while in the SCF treatment, both the 0.5 y and 4.5 y rhizospheres could significantly change soil microbial community structure (Fig. 5(B)). Moreover, for all the 4.5 y rhizosphere soils, fertilizer application was a key factor affecting microbial community structure.
The path analyses of the factors comprehensively affecting soil microbial biomass and enzyme activities were well validated through the statistical test (Fig. 6). Soil nutrient contents could explain 32.7% of the variance of fungal biomass. Soil OM, AN, AK, and fungal and actinomycetal biomass could explain 88.2% of the total variance of soil bacterial biomass. Moreover, fungi could generate positive effects on soil bacterial and actinomycetal biomass. The OM content was positively correlated with the β-glucosidase, urease, protease, and phosphatase activities, but negatively correlated with the catalase activity. The increases of AN content probably enhanced urease and protease activities, but the increases of TP and TK might inhibit catalase activity. For the soil β-glucosidase, soil OM, TP TK, and bacterial, actinomycetal, and fungal biomass could explain 39.7% of the total variance (Fig. 6(A)). The determined soil properties and microbial biomass could explain 31.4%, 47.1%, and 55.1% of the total variances for the protease, phosphatase, and catalase activities, respectively.
Path analysis of the factors affecting (A) β-glucosidase, (B) urease, (C) protease, (D) phosphatase, and (E) catalase activities. Solid arrows denoted positive effects and dash arrows denoted negative effects. Data on arrows were standardized direct effects, and the statistical significances of standardized direct effects were accepted at *P < 0.05 and ** P < 0.01
4 Discussion
4.1 Effects of rhizosphere and fertilizer application on soil nutrient contents
In general, soil OM contents were increased by fertilizer applications (Srinivasarao et al. 2014; Chen et al. 2016; Tian et al. 2017), and this trend was clearly demonstrated in the SCF treatment (Fig. 1(A)). Plant rhizosphere could generate positive or negative impacts on soil OM content, depending on plant species and soil environmental conditions (Lützow et al. 2006). The plant litters and root exudates could increase soil OM contents through different stabilization mechanisms: (1) complexation with soil metal ion or absorption by soil minerals; (2) enclosure in soil aggregates and inaccessibility to soil microorganisms; and (3) selective preservation due to discrepant recalcitrance to soil decomposer (Lützow et al. 2006; Murphy et al. 2015). On the other hand, plant root could facilitate soil aeration by forming air channel and improving the physical property of soil aggregate, and a varying proportion of soil OM might be lost via soil respiration and mineralization of N-rich compounds (Murphy et al. 2015). The bulk soil had the lowest OM contents in the CK treatment, which is consistent with the previous study (Geisseler et al. 2017), but the 45%-SF and SCF treatments had different trends of soil OM contents among the bulk and rhizospheres soils. The reason might be due to the differences in nutrient ratios of the applied fertilizers. In the SCF treatment, the applied fertilizer was specifically designed for the moso bamboo, and nutrient contents might match with soil OM and moso bamboo growth better.
Previous studies demonstrate that available N and K contents tended to increase after fertilizer applications (Mbuthia et al. 2015; Li et al. 2017). However, we observed that fertilizer applications generated discrepant impacts on these available nutrient contents (Fig. 1(B and E)). We attributed it as a comprehensive result of direct nutrient additions, plant uptakes, and microbial transformations (Laird et al. 2010). It is well accepted that within the rhizosphere, nutrient cycling could be accelerated by root exudates stimulating microbial activity and turnover (Hamilton and Frank 2001). Meanwhile, moso bamboo and soil microorganism also took up the nutrients from forest soils, decreasing the available nutrient contents. Consequently, similar to the soil OM content, the available nutrient contents were also adjusted by moso bamboo and soil microbial conditions.
4.2 Effects of rhizosphere and fertilizer application on enzyme activities
As a descriptive parameter of soil microbial population, soil enzyme patterns and their activities can directly reflect the bio-availabilities of different substrates and the nutrient demands of plants and microorganisms (Sinsabaugh et al. 2008; Nannipieri et al. 2012; Fraser et al. 2013; Mbuthia et al. 2015). The supplies of exogenous C, including fertilizer applications and root exudates, have the potential to increase soil β-glucosaminidase activities, and the trend demonstrated in the SCF treatment mostly agreed with the existing results that fertilizer applications stimulated the mineralization of recalcitrant C (Mbuthia et al. 2015). For the rhizosphere, root exudates and plant litters could enhance soil OM contents, and in this study, enhanced activities of extracellular enzymes involved in the decomposition of OM (β-glucosidase and protease) were reflected with the trends of soil OM contents demonstrated in the path analysis. It has been reported that N bio-availability could accelerate decompositions of dead plant and insect (Craine et al. 2007; Baumann et al. 2009). The increases of both protease and urease activities further confirmed that decompositions of N-rich compounds were enhanced by moso bamboo rhizospheres (Fig. 2(B and C)). Within the rhizosphere, plants could cooperate with indigenous microorganisms to develop several mechanisms to meet the P requirements of plants and soil microorganisms. Apart from altering soil physical structure, plant roots could generate (1) protons adjusting soil pH and solubilizing P; (2) extracellular enzymes mineralizing organic compound enriched with P; and (3) citrates complexing aluminum and iron to release inorganic P (Shen et al. 2002; Rengel and Marschner 2005). Consequently, rhizosphere is one of the hotspots of mineralizations of organic P compounds in soils (Rengel and Marschner 2005). Moreover, we also noticed that in the bulk soils, fertilizer applications also increased the phosphatase activity, similar to what has already reported in previous studies (Liang et al. 2017). The reason accounting for these phenomena might be that fertilizers containing adequate nutrients stimulated activities of soil microorganisms related to P biogeochemical cycling. Generally, catalase activity is positively correlated with the soil fertility, and catalase mediates H2O2 transformation, and thus protects cells from damages due to reactive oxygen (Zhang et al. 2011). In this study, the catalase activities of the rhizosphere soils were consistently higher than those of bulk soils, regardless of fertilizer applications (Fig. 1(E)), which might also be a benefit generated from the cooperation of bamboo root and soil microorganism.
4.3 Effects of rhizosphere and fertilizer application on soil microbial biomass and community
Currently, there is no consensus result or conclusion about soil microbial biomass in the rhizosphere and bulk soils. Sanaullah et al. (2011) demonstrated that microbial biomass C in rhizosphere soil was significantly higher than that in unplanted control, whether at drought or optimum growth conditions. However, in the chickpea and durum wheat field, there were no significant differences in soil microbial biomass between the rhizosphere and bulk soils (Tang et al. 2014). In our current study, we revealed that moso bamboo rhizospheres generated discrepant (positive, no significant and negative) impacts on soil microbial biomass (Fig. 3), which is consistent with the result of Massaccesi et al. (2015). Apart from the determined methods, plant species might also be a key reason accounting for these differences. Within the rhizosphere, soils in proximity to the roots, process similar rhizodeposition, and the differences of soil structure, nutrient transformation, and root exudates between bulk and rhizosphere soils are material conditions conducive to induce modifications of soil microbial biomass and community (Chang and Chiu 2015; Massaccesi et al. 2015; Mori et al. 2016).
A meta-analysis conducted by Geisseler et al. (2017) showed that fertilizer applications had the potential to increase soil microbial biomass (Fig. 2). In this study, the increases in microbial biomass could be attributed to comprehensive improvements in soil nutrient contents and microbial population. Applied fertilizer could provide required N, P, and other nutrients for microbial growth and reproduction. Apart from the direct nutrient effect, the indirect stimulations among different microbial groups might also account for these results (Fig. 6). Fungi could provide bacteria with resources that bacteria were not able to acquire on their own, and different microbial groups might generate synergistic impacts (Romaní et al. 2006). This possibility was supported by the results demonstrated in the path analysis. For example, applied fertilizers could increase soil fungal biomass, which, in turn, generated positive stimulations to bacterial and actinomycetal biomass (Fig. 6).
4.4 Linkages among soil enzyme activities, microbial biomass, and nutrient contents
Rhizospheric processes resulting from plant-soil-microorganism interactions could improve plant tolerance and enhance plant productivity (Mori et al. 2016). Relative to the bulk soils, there were higher enzyme activities in the rhizosphere soils, which may be strategies of moso bamboo growth and soil microorganism flourishing. The plants could make the best use of environmental conditions and nutrient contents to stimulate soil microorganisms to benefit its own growth. The C bio-availability had a determining impact in the microorganism-mediating process, especially for extracellular enzymes which exert essential roles in the biogeochemistry (Fontaine et al. 2004; Yarwood et al. 2006). Rhizosphere priming effect on soil OM decomposition was the major factor controlling soil C dynamics (Cheng 2009). The higher β-glucosaminidase and protease confirmed a high metabolism and turnover rate of rhizosphere microorganisms (Fig. 1(A and B)). The turnovers of rhizosphere microorganisms are generally at the scales of hours or days, which are significantly shorter than those of plant roots. The linkages of short microbial turnover and rhizosphere priming effect have important implications for the growth of moso bamboo (Cheng 2009). Due to increased microbial turnover and mineralization rates of soil OM, moso bamboo has the advantages over soil microorganisms to obtain the N, P, and K nutrients. On the other hand, the OM decomposition could also supply energy to soil microorganisms to flourish and further mineralize organic compounds containing N and P. Consequently, the activities of soil enzymes related to soil C, N, and P cycling in the rhizosphere soils tended to be higher than their counterparts in the bulk soils.
5 Conclusions
In the CK treatment, the OM contents of the rhizosphere soils were significantly higher than those of the bulk soils. The interactions of rhizosphere and fertilizer could significantly affect AN, TP TK, and AK contents in the bamboo forest soils. Soil enzyme activities in the rhizosphere soils tended to be higher than those of the bulk soils. The moso bamboo rhizospheres did not significantly increase the total microbial biomass, but compared with the CK treatment, fertilizer application had the potential to increase soil microbial biomass whether in the bulk or rhizosphere soils. Both fertilizer applications and moso bamboo rhizospheres could significantly affect soil microbial community structures, and moso bamboo rhizosphere could increase soil microbial activity rather than biomass.
References
Baumann K, Marschner P, Smernik RJ, Baldock JA (2009) Residue chemistry and microbial community structure during decomposition of eucalypt, wheat and vetch residues. Soil Biol Biochem 41:1966–1975
Bardgett RD, Hobbs PJ, Frostegård Å (1996) Changes in soil fungal: bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol Fertil Soils 22:261–264
Bei S, Zhang Y, Li T, Christie P, Li X, Zhang J (2018) Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil. Agric Ecosyst Environ 260:58–69
Bressan M, Roncato M-A, Bellvert F, Comte G, el Zahar Haichar F, Achouak W, Berge O (2009) Exogenous glucosinolate produced by Arabidopsis thaliana has an impact on microbes in the rhizosphere and plant roots. ISME J 3:1243–1257
Bystriakova N, Kapos V, Lysenko I, Stapleton C (2003) Distribution and conservation status of forest bamboo biodiversity in the Asia-Pacific Region. Biodivers Conserv 12:1833–1841
Chang EH, Chiu CY (2015) Changes in soil microbial community structure and activity in a cedar plantation invaded by moso bamboo. Appl Soil Ecol 91:1–7
Chen C, Zhang J, Lu M, Qin C, Chen Y, Yang L, Huang Q, Wang J, Shen Z, Shen Q (2016) Microbial communities of an arable soil treated for 8 years with organic and inorganic fertilizers. Biol Fertil Soils 52:455–467
Cheng W (2009) Rhizosphere priming effect: its functional relationships with microbial turnover, evapotranspiration, and C–N budgets. Soil Biol Biochem 41:1795–1801
Chongtham N, Bisht MS, Haorongbam S (2011) Nutritional properties of bamboo shoots: potential and prospects for utilization as a health food. Compr Rev Food Sci Food Saf 10:153–168
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
Cui J, Wang J, Xu J, Xu C, Xu X (2017) Changes in soil bacterial communities in an evergreen broad-leaved forest in east China following 4 years of nitrogen addition. J Soils Sediments 17:2156–2164
Haichar FZ, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T, Achouak W (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Lett 7:314–320
Fraser FC, Hallett PD, Wookey PA, Hartley IP, Hopkins DW (2013) How do enzymes catalysing soil nitrogen transformations respond to changing temperatures? Biol Fertil Soils 49:99–103
Ge X, Zhou B, Wang X, Li Q, Cao Y, Gu L (2018) Imposed drought effects on carbon storage of moso bamboo ecosystem in southeast China: results from a field experiment. Ecol Res 33:393–402
Geisseler D, Linquist BA, Lazicki PA (2017) Effect of fertilization on soil microorganisms in paddy rice systems—a meta-analysis. Soil Biol Biochem 115:452–460
Guan SY (1986) Soil enzymology and research method. Agricultural Press (Beijing, In Chinese)
Hamilton EW, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–2402
Laird DA, Fleming P, Davis DD, Horton R, Wang B, Karlen DL (2010) Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158:443–449
Li R, Tao R, Ling N, Chu G (2017) Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: implications for soil biological quality. Soil Tillage Res 167:30–38
Liang X, Jin Y, He M, Liu Y, Hua G, Wang S, Tian G (2017) Composition of phosphorus species and phosphatase activities in a paddy soil treated with manure at varying rates. Agric Ecosyst Environ 237:173–180
Liu J, Jiang P, Wang H, Zhou G, Wu J, Yang F, Qian X (2011) Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For Ecol Manag 262:1131–1137
Lu H, Li Z, Fu S, Méndez A, Gascó G, Paz-Ferreiro J (2015) Combining phytoextraction and biochar addition improves soil biochemical properties in a soil contaminated with Cd. Chemosphere 119:209–216
Lu RK (2000) Analytical methods for soil and agro-chemistry. China Agricultural Science and Technology Press, Beijng (in Chinese)
Lützow MV, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57:426–445
Massaccesi L, Benucci G, Gigliotti G, Cocco S, Corti G, Agnelli A (2015) Rhizosphere effect of three plant species of environment under periglacial conditions (Majella Massif, central Italy). Soil Biol Biochem 89:184–195
Mbuthia LW, Acosta-Martínez V, DeBruyn J, Schaeffer S, Tyler D, Odoi E, Mphesheaa M, Walker F, Eash N (2015) Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: implications for soil quality. Soil Biol Biochem 89:24–34
Mori A, Kirk GJ, Lee JS, Morete MJ, Nanda AK, Johnson-Beebout SE, Wissuwa M (2016) Rice genotype differences in tolerance of zinc-deficient soils: evidence for the importance of root-induced changes in the rhizosphere. Front Plant Sci 6:1160
Murphy CJ, Baggs EM, Morley N, Wall DP, Paterson E (2015) Rhizosphere priming can promote mobilisation of N-rich compounds from soil organic matter. Soil Biol Biochem 81:236–243
Nannipieri P, Giagnoni L, Renella G, Puglisi E, Ceccanti B, Masciandaro G, Fornasier F, Moscatelli MC, Marinari SARA (2012) Soil enzymology: classical and molecular approaches. Biol Fertil Soils 48:743–762
Peng Z, Lu Y, Li L, Zhao Q, Feng Q, Gao Z, Lu H, Hu T, Yao N, Liu K (2013) The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat Genet 45:456–461
Raghubanshi AS (1994) Effect of bamboo harvest on dynamics of nutrient pools, N mineralization, and microbial biomass in soil. Biol Fertil Soils 18:137–142
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312
Romaní AM, Fischer H, Mille-Lindblom C, Tranvik LJ (2006) Interactions of bacteria and fungi on decomposing litter: differential extracellular enzyme activities. Ecology 87:2559–2569
Sanaullah M, Blagodatskaya E, Chabbi A, Rumpel C, Kuzyakov Y (2011) Drought effects on microbial biomass and enzyme activities in the rhizosphere of grasses depend on plant community composition. Appl Soil Ecol 48:38–44
Sanchez-Hernandez JC, Del Pino JN, Capowiez Y, Mazzia C, Rault M (2018) Soil enzyme dynamics in chlorpyrifos-treated soils under the influence of earthworms. Sci Total Environ 612:1407–1416
Shen H, Yan X, Zhao M, Zheng S, Wang X (2002) Exudation of organic acids in common bean as related to mobilization of aluminum-and iron-bound phosphates. Environ Exp Bot 48:1–9
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Srinivasarao C, Venkateswarlu B, Lal R, Singh A, Kundu S, Vittal K, Patel J, Patel M (2014) Long-term manuring and fertilizer effects on depletion of soil organic carbon stocks under pearl millet-cluster bean-castor rotation in western India. Land Degrad Dev 25:173–183
Song X, Gu H, Wang M, Zhou G, Li Q (2016) Management practices regulate the response of Moso bamboo foliar stoichiometry to nitrogen deposition. Sci Rep 6:24107
Sukmawan R, Takagi H, Nakagaito AN (2016) Strength evaluation of cross-ply green composite laminates reinforced by bamboo fiber. Compos Part B-Eng 84:9–16
Tang X, Bernard L, Brauman A, Daufresne T, Deleporte P, Desclaux D, Souche G, Placella SA, Hinsinger P (2014) Increase in microbial biomass and phosphorus availability in the rhizosphere of intercropped cereal and legumes under field conditions. Soil Biol Biochem 75:86–93
Tian J, Lou Y, Gao Y, Fang H, Liu S, Xu M, Blagodatskaya E, Kuzyakov Y (2017) Response of soil organic matter fractions and composition of microbial community to long-term organic and mineral fertilization. Biol Fertil Soils 53:523–532
Wei W, Yan Y, Cao J, Christie P, Zhang F, Fan M (2016) Effects of combined application of organic amendments and fertilizers on crop yield and soil organic matter: an integrated analysis of long-term experiments. Agric Ecosyst Environ 225:86–92
Yarwood SA, Myrold DD, Högberg MN (2006) Termination of belowground C allocation by trees alters soil fungal and bacterial communities in a boreal forest. FEMS Microbiol Ecol 70:151–162
Zhang C, Liu G, Xue S, Song Z (2011) Rhizosphere soil microbial activity under different vegetation types on the loess plateau, China. Geoderma 161:115–125
Zhou G, Meng C, Jiang P, Xu Q (2011) Review of carbon fixation in bamboo forests in China. Bot Rev 77:262
Funding
This work was supported by the National Natural Science Foundation of China (31560204) and by Key Laboratory of State Forestry Administration on Forest Ecosystem Protection and Restoration of Poyang Lake Watershed. Manyun Zhang sincerely thanks the support of the Youth Talent Program of Anhui University (S01002142).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jianming Xue
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 377 kb)
Rights and permissions
About this article
Cite this article
Zhang, W., Liu, S., Zhang, M. et al. Phyllostachys edulis (moso bamboo) rhizosphere increasing soil microbial activity rather than biomass. J Soils Sediments 19, 2913–2926 (2019). https://doi.org/10.1007/s11368-019-02334-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02334-2