Abstract
Purpose
The objectives of this study were to explore the influences of pH on the release of Cu, Zn, Cd, Pb, Ni, and Cr in sediments derived from the upstream, middle, and downstream reaches of Dongdagou stream in Gansu Province, Northwest China, and to examine the fractionation changes of heavy metals in the sediments after reaching their release equilibrium under different pH conditions.
Materials and methods
Sediment samples were obtained using a stainless steel grab sampler to collect the uppermost 10 cm of sediment from the channel bed. The pH-dependent release experiment was conducted in the solid-to-liquid ratio of 1:20 at different pH values (2, 4, 6, 8, 10, and 12) at room temperature. The total Cu, Zn, Cd, Pb, Ni, and Cr concentrations in the sediments were digested using an acid digestion mixture (HNO3 + HF + HClO4) in an open system. Metal fractionation of selected sediments was obtained using the Tessier sequential extraction procedure. Heavy metal concentrations in the samples were determined using atomic absorption spectrophotometry.
Results and discussion
The mean concentrations of heavy metals in sediments decreased in the following order: Zn (1676.67 mg kg−1) > Pb (528.65 mg kg−1) > Cu (391.34 mg kg−1) > Cr (53.48 mg kg−1) > Ni (34.27 mg kg−1) > Cd (11.53 mg kg−1). Overall, the solubility of Cu, Zn, Cd, Pb, and Ni decreased with increasing pH, and they were strongly released at pH 2. Moreover, the solubility of Cr increased with increasing pH, and its release was highest at pH 12. After reaching the release equilibrium of heavy metals under different pH conditions, the percentages of organic Cu, Zn, Cd, and Fe-Mn oxyhydroxide Pb decreased, compared to their initial fractions. The residual fractions of Ni and Cr were dominant, regardless of pH.
Conclusions
The average concentrations of Cu, Zn, Cd, and Pb in sediments were highly elevated compared with the soil background values in Gansu Province, China. The results of this pH-dependent release experiment showed that the release behaviors of Cu, Zn, Pb, and Cr followed an asymmetric V-shaped pattern, whereas Cd and Ni followed an irregular L-shaped pattern. The changes in the release of heavy metals in sediments were related to their redistribution between chemical fractionations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal pollution in sediments originated from mining and industrial activities is an increasing global problem (Fernandes et al. 2008; Kucuksezgin et al. 2008; Equeenuddin et al. 2013; Zhang et al. 2014). Heavy metals pose a serious threat to the aquatic environment as a result of their toxicity, non-biodegradable and persistent natures, and their ability to bioaccumulate in the food chain (Gopinath et al. 2010; Nobi et al. 2010; Zhang et al. 2014). Sediments are the main source and sink of heavy metal pollutants in aquatic ecosystems, serving an important role in heavy metal transport (Durán et al. 2012; Castillo et al. 2013; Superville et al. 2014). Sediment readily attenuates heavy metals as a result of its high sorption capacity (Prusty et al. 1994) and can release heavy metals into the water under different physical and chemical conditions (Wen and Allen 1999).
In general, the adsorption and desorption characteristics of sediment control the mobility and availability of heavy metals (Gäbler 1997; Krishnamurti et al. 1999; Burton et al. 2006). Heavy metal adsorption and desorption are influenced by several factors, including the following physicochemical properties of the sediment: inclusion of clay or silt minerals, organic matter content, cation exchange capacity, calcium carbonate content, and oxidation-reduction status (Antoniadis et al. 2008; Zhang et al. 2014). Adsorption and desorption are also influenced by the type of heavy metal (Jalali and Moharrami 2007; Ho et al. 2012) and the environmental conditions, such as temperature and the resuspension of sediment (Kristensen et al. 1992; Reitzel et al. 2013). In addition, biological perturbation (Battaglia et al. 1994; Chen and Lin 2001), dredging activity (Wang et al. 2014a, b; Fathollahzadeh et al. 2015), and aquatic environmental characteristics, such as cation and anion content in water (Zhong et al. 2006) and pH (Cappuyns and Swennen 2008; Equeenuddin et al. 2013), are also major influencing factors for adsorption and desorption of heavy metals in sediment. Furthermore, the release of heavy metals in sediment is influenced by the pH of the overlying water column, highlighting the importance of the aquatic environment in the release of heavy metals (Saeedi et al. 2013).
Many scientific studies have reported that pH is an important factor responsible for controlling the behavior of heavy metals in sediments through precipitation-dissolution and adsorption-desorption processes (Tipping et al. 2003; Cappuyns and Swennen 2008; Ho et al. 2012). Therefore, the pH-dependent release experiment was an important tool for evaluating the release of heavy metals at different pH values and may be used to assess the long-term release of contaminants (Ho et al. 2012). Different types of tests were used to assess pH-dependent leaching. A pHstat test was used to assess the changes in solubility with pH (Dijkstra et al. 2006). Previous studies (Ho et al. 2012) indicated that the leachabilities of Cu, Zn, Cd, and Pb were highest at pH 2, minimal amounts of Cu and Pb were released at pH 6, and minimal amounts of Cu and Zn were released at pH 8–9. Chen and Lin (2001) reported that the solubilization of metal in the bioleaching process was highly dependent on the pH and that its relationship with pH was non-linear. Peng et al. (2009) observed a decrease in pH resulting from the release of H+ into pore water, which caused the secondary release of heavy metals.
Heavy metals reside in different geochemical fractions of sediments, i.e., exchangeable, carbonate, organic, Fe-Mn oxyhydroxides and residual fractions (Tessier et al. 1979). Heavy metals associated with different fractions have different impacts on the environment (Tam and Wong 1996). The chemical fractions of heavy metals and the proportions of the different fractions of heavy metals in sediments are important factors that determine the migration and ecological effects of heavy metals in sediments. The effect of pH on the fraction of heavy metal species is important for controlling the transformations and bioavailability of heavy metals. Therefore, a fractionation study of heavy metals in sediments would provide information about their mobility and their potential as threats in aquatic environments (Equeenuddin et al. 2013). So far, many studies have focused on the content, fractionation, and sources of heavy metals in sediments (Andrade et al. 2001; Wang et al. 2014a, b; Maanan et al. 2015). However, little is known about the effects of pH on the release of heavy metals in sediments contaminated by mine-drainage streams (Lee et al. 2008; Equeenuddin et al. 2013).
Baiyin is an important copper resource-based city in China located in the central part of Gansu Province. This region contains extensive mineral resources, such as copper, lead, and zinc. Dongdagou stream is important for suburban drainage in Baiyin City. Nearly all of the non-ferrous metal mining and smelting plants, and several other factories are located along the upstream reaches of Dongdagou stream, which accept treated and untreated domestic wastewater and industrial sewage (Nan et al. 2002). In addition, Dongdagou stream is a tributary of the Yellow River. The water in Dongdagou stream is potentially subjected to acid-alkaline variations. Wastewater from metal mining and smelting plants can be highly acidic. Li et al. (2008) reported that the water pH of Dongdagou stream in Baiyin City was less than 2, and Equeenuddin et al. (2013) observed that the sediments in stream close to the collieries had a low pH of circa 2.5. The pH of acid mine drainage (AMD) water can reach as low as 2.1 (Alcolea et al. 2012). On the other hand, the wastewater from industrial sewage treatment plants has been found to be strongly alkaline, which is because most wastewater treatment plants use lime or limestone as a neutralizing agent to treat metal-polluted wastewater, which leads to strong alkalinity of wastewater. Chen et al. (2002) reported that the pH of the tailing wastewater in a lead-zinc mine was higher than 12. Bian et al. (2010) found that the pH of wastewater treatment plant of a non-ferrous metal smelting enterprise reached 12.5. If discharge of the strong acid and alkaline wastewater is substandard, it will destroy the ecosystem. Therefore, it is important to understand the influence of wide pH range of solution on the release behaviors of heavy metals in water-sediment systems of mine drainage streams for sediment remediation.
In this study, the influences of pH on the release of Cu, Zn, Cd, Pb, Ni, and Cr from sediment in Dongdagou stream were investigated. We studied the variations of the chemical fractionation, pH, and organic matter contents in sediments after reaching equilibrium regarding the release of heavy metals. The total concentrations of heavy metals in the sediments were also measured. This study is highly significant because it reveals the migration, transformation, and potential ecological risks of heavy metals and provides a better understanding of the significance of pH on the release of heavy metals when considering the treatment of contaminated sediments.
2 Materials and methods
2.1 Sampling and sample pretreatment
The study site was located in Dongdagou stream in Baiyin City in Gansu Province, Northwest China. Dongdagou stream is a suburban drainage stream that is contaminated by domestic wastewater and industrial sewage. Three sediment samples were collected from the upstream, middle, and downstream reaches of Dongdagou stream (represented as S1, S2, and S3, respectively; Fig. 1). Sediment samples of the uppermost 10 cm of the sediment surface were obtained using a stainless steel grab sampler. Samples were air-dried, powdered, passed through a 2-mm sieve, and homogenized before analyses.
2.2 Chemical analysis
The sediment pH was measured in a sediment/water suspension (1:2.5) at room temperature using a combined glass-calomel electrode. The electrical conductivity (EC) of the sediment in water (1:5) was measured using an EC meter. Organic matter was measured using potassium dichromate oxidation, and the detection limit was 0.5% (Nanjing Soil Institute 1977). The total Cu, Zn, Cd, Pb, Ni, Cr, Fe, and Mn concentrations in the sediments were digested using an acid digestion mixture (HNO3 + HF + HClO4) in an open system (see Table S1 in the Electronic Supplementary Material for details). Metal fractionation of selected sediments was obtained using the Tessier sequential extraction procedure (Tessier et al. 1979). Heavy metal concentrations in the samples were determined using atomic absorption spectrophotometry (Thermo Fishier, SOLAAR M6). The flame type was air-acetylene. The wavelengths of Cu, Zn, Cd, Pb, Ni, Cr, Fe, and Mn were 324.8, 213.9, 228.8, 283.3, 232.0, 357.9, 248.3, and 279.5 nm, respectively. The four-line deuterium lamp method was used to the background correction. Several physical and chemical properties of the sediments are presented in Table 1.
2.3 pH-dependent release and chemical fractionation experiment
Preliminary extraction kinetics of the sediment samples were investigated using acid-base solutions with different pH values, and the evolution of the extracted heavy metal concentrations over time showed that equilibrium was nearly reached at 48 h. Based on the preliminary extraction kinetics, this study chose 48 h as the equilibrium time in the pH-dependent release experiment. For this experiment, 1 g of sediment was shaken with 20 ml of the extractant solution at different pH values (2, 4, 6, 8, 10, and 12) at room temperature before immediately centrifuging at 4000 rpm for 10 min and passing the resulting supernatant solution through a 0.45-μm cellulose membrane filter. All extracting solutions used in this study were adjusted to a target pH value (2, 4, 6, 8, 10, or 12) by adding various amounts of HNO3 or NaOH.
After the pH-dependent release experiment, the chemical fractionation of the heavy metals in the sediment was measured again. Organic matter was also measured after the release experiments. pH was measured using the supernatant solution after reaching heavy metal equilibrium.
2.4 Quality control and assurance
The chemical analysis of each sample was conducted in triplicate relative to a control for analytical precision. For quality control and assurance, a standard reference sample GBW07408 (GSS-8) and blanks were included. We used blank duplicates, containing blank filter papers, which were run for each matrix and corrections to the analytical results, to determine the analytical precision and accuracy. All glassware and plastic containers were soaked in 10% (v/v) HNO3 for at least 12 h and thoroughly cleaned with Milli-Q water before utilization. All chemical reagents were guarantee reagent.
The detection limits of Cu, Zn, Cd, Pb, Ni, Cr, Fe, and Mn were 0.002, 0.002, 0.001, 0.02, 0.004, 0.003, 0.005, and 0.002 mg L−1, respectively. For certified reference sample GBW07408 (GSS-8) analyzed in this study, the recoveries of total metal content were 91–107%. For Cu, Zn, Cd, Pb, Ni, Cr, and Mn, relative standard deviation was <5% (see Table S2 in the Electronic Supplementary Material for details). The effectiveness of the Tessier sequential extraction procedure can be determined by comparing the sum of the metal contents in the individual fractions with the independent measurement of total metal content (Carter et al. 2006). The recoveries achieved by the Tessier sequential extraction procedure ranged from 90 to 106%. We include three experimental replicates of each sample in each assay, and the results showed that the relative standard deviation between experimental replicates was <5% for the metals we detected in this study.
3 Results and discussion
3.1 Sediment characteristics
The physicochemical characteristics and concentrations of Cu, Zn, Cd, Pb, Ni, Cr, Fe, and Mn in the studied sediment are presented in Table 1. The pH of the sediments varied from 6.59 to 7.97 (i.e., slightly acidic to mildly alkaline). The EC of the sediments varied from 261 to 316 μS cm−1, which indicated that the conductivity of the sediments was weak. The organic matter contents in the three sediment samples ranged from 1.13 to 1.77%. The total metal trace element concentrations (including Cu, Zn, Cd, and Pb) in three sediments were very high. Among the trace elements, Zn had the highest concentration detected, ranging from 1290.51 to 2048.73 mg kg−1, followed by Pb (370.03–657.31 mg kg−1), Cu (330.34–447.60 mg kg−1), Mn (306.15–452.04 mg kg−1), Cr (51.26–56.40 mg kg−1), Ni (26.83–46.17 mg kg−1), and Cd (9.49–13.70 mg kg−1). The concentrations of Fe detected in the sediments ranged from 3.07 to 3.82%. The highest concentrations of Cu, Zn, and Cd were measured in the sample from S1, which is located in the upstream reach of Dongdagou stream. The highest concentrations of Pb and Fe were detected in the sample from S2 (middle reaches of Dongdagou stream), and the highest concentrations of Ni, Cr, and Mn were measured in the sample from S3 (downstream reaches of Dongdagou stream).
The mean concentrations of the elements in the sediments from Dongdagou stream and the other sites are provided in Table 2. These results suggested severe pollution in the Dongdagou stream sediment. The average concentrations of Cu, Zn, Cd, and Pb in the Dongdagou stream sediment were highly elevated compared to the soil background values in Gansu Province, China (China National Environmental Monitoring Center 1990). The concentrations of Cu, Zn, Cd, and Pb in the Dongdagou stream sediment were approximately 16 times, 24 times, 96 times, and 28 times higher than the soil background values in Gansu Province, respectively. The average concentrations of Ni, Cr, and Mn in the sediment samples from Dongdagou stream were lower than the soil background values in Gansu Province. The mean concentrations of Cu and Pb in the sediment samples from Dongdagou stream were higher than those of other contaminated locations, except for the Odiel River in Spain. The concentrations of Zn in the sediment samples from Dongdagou stream were lower than the concentrations measured in the Scheldt River in Belgium but exceeded the concentrations observed at other locations. The Cd concentrations in the sediment from Dongdagou stream were higher than at other polluted locations, except for the Malter Reservoir in Germany. The concentrations of Cr in the sediment from Dongdagou stream were quite low compared to other locations. The concentrations of Ni and Mn in the sediment samples from Dongdagou stream were lower than those measured in the Makum coalfield in India, the Cam River in Vietnam, and the Scheldt River in Belgium. However, the Ni and Mn concentrations in the Dongdagou stream sediments were higher than those in the Okpara River in Nigeria. The concentrations of Fe in sediment samples from Dongdagou stream were lower than the Fe concentrations measured in the Odiel River in Spain and the Cam River in Vietnam and were similar to the concentrations measured in the Scheldt River in Belgium.
3.2 Heavy metal release as a function of pH
pH was used to measure the acidity and alkalinity of the sediment and strongly influenced the solubility of heavy metals. Different patterns of heavy metal release with pH were observed (Fig. 2). The pH-dependent release behaviors of Cu, Zn, Pb, and Cr followed an asymmetric V-shaped pattern. The solubility of Cu, Zn, and Pb increased with decreasing pH, although small concentrations of these elements were also released in the alkaline pH range. The solubility of Cr increased with increasing pH. Interestingly, Cr was also released from the sediment at acidic pH values. The pH-dependent release behavior of Cd and Ni followed an irregular L-shaped pattern. Particularly, the solubility of Cd and Ni decreased as the pH increased, and small amounts of Cd and Ni were released in the alkaline pH range. Many scientific studies have reported that the pH-dependent leaching behaviors of Cu, Zn, Cd, Pb, and Ni follow an asymmetric V-shaped pattern (Tack et al. 1996; Cappuyns and Swennen 2008; Ho et al. 2012). The different patterns of heavy metal release, as a function of pH, may be related to the nature of the heavy metals and the physicochemical properties of the sediments.
The solubilities of Cu, Zn, and Pb (relative to total concentration) as a function of pH were very similar for the different samples. The release of Cu was highest at pH 2 (S1, 16.56%; S2, 21.32%; S3, 6.35%; percentage here and below refers to % of the total content) and lowest at pH 4 (S1, 0.09%; S2, 0.13%; S3, 0.12%). Considerable concentrations of Cu were also released at pH 12 (S1, 1.25%; S2, 2.35%; S3, 5.51%). For Zn, its release was highest at pH 2 (S1, 5.80%; S2, 2.64%; S3, 1.23%), and minimal concentrations were released at pH 4–10 in S1 (0.02%), at pH 10 in S2 (0.04%), or at pH 8–10 in S3 (0.03%). Considerable concentrations of Zn were also released at pH 12 (S1, 0.35%; S2, 0.12%; S3, 0.73%). The adsorption edge for Cu and Zn occurred at approximately pH 4; below this pH, no uptake was likely to occur (Millward and Moore 1982). Pb was strongly released at pH 2 (S1, 52.20%; S2, 21.64%; S3, 1.98%) and pH 12 (S1, 2.29%; S2, 0.26%; S3, 1.09%), and minimal Pb concentrations were measured at pH 4–10. The release of Cd and Ni decreased with increasing pH; i.e., it was highest at pH 2 (Cd: S1, 27.59%; S2, 12.58%; S3, 6.01%; Ni: S1, 18.38%; S2, 22.56%; S3, 4.70%) and lowest at pH 12 (Cd: S1, 0.09%; S2, 0.21%; S3, 0.25%; Ni: S1, 0.31%; S2, 0.25%; S3, 0.18%). Tack et al. (1996) and Van Herreweghe et al. (2002) observed that Cu, Zn, Cd, and Pb became more mobile with decreasing pH (considering a pH range of 2–8) because the dissolution of metal hydroxides and carbonates increased and the adsorption at cation exchange surfaces of sediments decreased. At pH 9–11, their solubilities increased to a certain extent because heavy metals can form stable and soluble complexes with hydroxyl groups and dissolved organic carbon or can be released with the dissolution of sulfide minerals. Belzile et al. (2004) and Guven and Akinci (2013) suggested that high pH values promote the adsorption and precipitation of heavy metals in sediment, while low pH may weaken the strength of metal associations and decrease metal retention by sediment. The release of Cr was contrary to the release of other heavy metals tested, with the highest concentrations of Cr released at pH 12 (S1, 3.06%; S2, 5.11%; S3, 2.41%) and very little Cr released at pH 4–10. A considerable Cr concentration was also released at pH 2 (S1, 1.04%; S2, 1.84%; S3, 0.42%). Additionally, a low pH could reduce the negative surface charges of organic matter, clay particles, and Fe-Mn-Al oxides, resulting in the solubilization of sulfides (Du Laing et al. 2009). Adsorption sites in sediments are pH-dependent, with the number of negative sites for cation sorption decreasing with decreasing pH. Also, under alkaline conditions, heavy metals can precipitate as oxides, hydroxides, carbonates, and phosphates (Lindsay 1979). Because the release of heavy metals changed significantly due to acidification, it should be noted that only a relatively small amount of heavy metals had increased solubility at alkaline pHs. From a practical point of view, research of heavy metal release at alkaline pHs could be used for the remediation of sediments contaminated with heavy metals. Consequently, an increase in sediment pH would result in decreased Cu, Zn, Cd, Pb, and Ni solubilities, but the mobility of Cr would significantly increase upon alkalization. Under the same pH conditions, the solubilities of different heavy metals in the same sediment sample were different. At pH 2, the release of heavy metals from sample S1 decreased in the following order: Pb (52.20%) > Cd (27.59%) > Ni (18.38%) > Cu (16.56%) > Zn (5.80%) > Cr (1.04%). The release of heavy metals from sample S2 decreased in the following order: Ni (22.56%) > Pb (21.64%) > Cu (21.32%) > Cd (12.58%) > Zn (2.64%) > Cr (1.84%). The release of heavy metals from the S3 sample decreased in the following order: Cu (6.35%) > Cd (6.01%) > Ni (4.70%) > Pb (1.98%) > Zn (1.23%) > Cr (0.42%). At pH 12, the release of heavy metals from the three sediment samples decreased in the following order: for S1, Cr (3.06%) > Pb (2.29%) > Cu (1.25%) > Zn (0.35%) > Ni (0.31%) > Cd (0.09%); for S2, Cr (5.11%) > Cu (2.35%) > Pb (0.26%) > Ni (0.25%) > Cd (0.21%) > Zn (0.12%); and for S3, Cu (5.51%) > Cr (2.41%) > Pb (1.09%) > Zn (0.73%) > Cd (0.25%) > Ni (0.18%). At pH 4–10, the release of heavy metals from the three sediment samples was similar. Under the same pH conditions, the differences of the heavy metal release rates among the three sediment samples may be due to the different physical and chemical properties of the sediment; therefore, the different release rates of heavy metals from the same sediment sample at various pHs may be due to differences in the chemical speciation of the heavy metals in the sediment.
The variations of the pH and organic matter contents in the release experiments are shown in Table 3. The results suggest that strongly acidic and alkaline solutions had a significant influence on the sediment pH after treatment. The solution pH after reaching the release equilibrium decreased at pH 2 and increased at pH 12 when compared to the initial sediment pH. The pH of the solution could be considered with the sediment pH after the release equilibrium of heavy metals. This result demonstrated that the solution pH plays a significant role in heavy metal release from the studied sediment, especially at pH 2 and 12. However, the pH of the solution after reaching release equilibrium changed little at pH 4–10 when compared to the initial pH of the sediment. This result indicated that the sediment had a higher buffering capacity, which likely caused a slight increase in the heavy metal contents at pH 4–10. The final organic matter contents of the three sediment samples were slightly lower than the initial organic matter contents and decreased with increasing pH. A similar observation was previously reported by Spain (1990) and Motavalli et al. (1995). These results suggest that organic matter was not the deciding factor in the increase in heavy metal solubility at pH 2–8 in the studied sediments. Daldoul et al. (2015) also reported that Zn, Cd, and Pb mobilization did not have a great affinity for organic matter.
To further understand the effects of heavy metal release on aquatic sediment in Dongdagou stream, this study referred to the environmental quality standards for surface water (State Environmental Protection Administration 2003) and integrated wastewater discharge standard (State Environmental Protection Administration 1997) to illustrate the environmental risk caused by heavy metal release. The concentrations of heavy metals released under different pH conditions from the three sediment samples are given in Table 4. At pH 2, the concentrations of Cu, Zn, Cd, and Pb released from sample S1 exceeded category 5 of the standard limits of the environmental quality standards for surface water and the maximum allowable discharge concentration of the integrated wastewater discharge standard. The concentrations of Cu, Zn, Cd, and Pb in sample S1 were 3.71, 2.97, 19, and 96.6 times higher than the category 5 standard limits of the environmental quality standards for surface water, respectively, and 1.86, 1.19, 1.9, and 9.66 times higher than the maximum allowable discharge concentrations of the integrated wastewater discharge standards, respectively. The concentration of Ni release from sample S1 was 13.5 times higher than the category 5 standard limits of the environmental quality standards for surface water. At pH 4–10, the concentrations of all heavy metals release in sample S1 did not exceed the standard values. At pH 12, the released heavy metal concentrations, except for Pb in sample S1, did not exceed the standard values. For sample S2, the released concentrations of Cu and Pb at pH 2 exceeded the category 5 standard limits of the environmental quality standards for surface water and the maximum allowable discharge concentration of the integrated wastewater discharge standards. The released concentrations of Cu and Pb were 4.22 and 71.1 times higher than the category 5 standard limits of the environmental quality standards for surface water, and 2.11 and 7.11 times higher than the maximum allowable discharge concentrations of the integrated wastewater discharge standards, respectively. The released concentrations of Zn and Cd at pH 2 exceeded the category 5 standard limits of the environmental quality standards for surface water. At pH 4–12, the released concentrations of Cu, Zn, Cd, and Pb did not exceed the standard values. The released concentrations of Ni at pH 2, 6, and 8 exceeded the category 5 standard limits of the environmental quality standards for surface water. The released concentrations of Cr at pH 12 exceeded the category 5 standard limits of the environmental quality standards for surface water. For sample S3, the released concentrations of Cu, Cd, Pb, and Ni at pH 2 were higher than the category 5 standard limits of the environmental quality standards for surface water. The released concentrations of Pb at pH 12 were also higher than the category 5 standard limits of the environmental quality standards for surface water. The released concentrations of Zn and Cr did not exceed the standard values.
3.3 Chemical fractionation and transformation of heavy metals in sediments after reaching the release equilibrium state
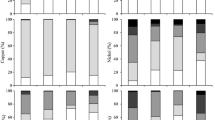
To understand the possible behavior of heavy metals under different physiochemical conditions at the sediment-water interface, a chemical fractionation study was carried out to obtain information about their associations with various geochemical phases in samples S1, S2, and S3 (Fig. 3 and Table S3 in the Electronic Supplementary Material). The sediments in our study were air-dried, which led to the oxidation of the anoxic sediments, and it may redistribute the metals initially contained in the sulfidic phase (Larner et al. 2008). Therefore, the metal fractions mentioned in the study referred to the fractions of the oxidized sediments. Cu was mainly associated with the organic (S1, 53.59%; S2, 46.26%; S3, 49.94%) and residual (S1, 38.35%; S2, 44.81%; S3, 41.89%) fractions. Zn was preferably associated with the residual (S1, 53.73%; S2, 64.94%; S3, 46.27%) and organic (S1, 37.57%; S2, 30.62%; S3, 34.12%) fractions. Cd was predominantly associated with the organic (S1, 45.47%; S2, 48.53%; S3, 31.38%) and residual (S1, 22.05%; S2, 35.42%; S3, 24.98%) fractions, followed by the exchangeable, carbonate, and Fe-Mn oxyhydroxide fractions. Pb was dominantly found in the Fe-Mn oxyhydroxide fraction (S1, 40.72%; S2, 46.44%; S3, 48.24%). Ni was mainly associated with the residual fractions (S1, 33.06%; S2, 28.57%; S3, 34.43%), followed by the carbonate, exchangeable, Fe-Mn oxyhydroxide and organic fractions in the S1 and S2 samples or followed by the Fe-Mn oxyhydroxide, carbonate, exchangeable, and organic fractions in the S3 sample. Cr was uniformly fractionated in the residual fractions (S1, 92.75%; S2, 92.46%; S3, 92.32%), while the non-residual fractions were negligible.
The changes in the fractionation behaviors of the investigated heavy metals after reaching release equilibrium with respect to pH are shown in Fig. 4 and Table S4 (Electronic Supplementary Material). Both Cu and Zn mainly occurred in the residual and organic fractions at all pH values. The percentage of organic matter in the sediment decreased compared to the initial fractions (here, initial fraction refers to the fraction of air-dried sediments). However, the exchangeable Cu (S1, 2.18%; S2, 1.91%; S3, 0.58%) and Zn (S1, 0.91%; S2, 0.66%; S3, 1.05%) concentrations were much higher in the sediments at pH 2. At pH 12, the heavy metals were associated more with the carbonate fraction (Cu: S1, 11.28%; S2, 14.45%; S3, 18.29%; Zn: S1, 3.82%; S2, 2.07%; S3, 9.69%). Equeenuddin et al. (2013) reported that exchangeable Zn at low pH and carbonate-associated Zn at high pH were prevalent in sediments, and in a strongly acidic environment, higher concentrations of all metals were available in their exchangeable fraction. Cd mainly occurred in the organic and residual fractions, and compared to the initial fractions, the proportion of Cd in the organic fractions decreased and the proportion of Cd in the residual fractions increased. Cu, Zn, and Cd have great affinities for organic matter because of their abilities to form stable complexes with humic ligands (Chander et al. 1994; Dijkstra et al. 2006). Pb was uniformly fractionated into Fe-Mn oxyhydroxides and residual fractions. The percentage of Fe-Mn oxyhydroxide fraction decreased compared to initial fractions. However, the fraction of Pb into the exchangeable fraction was significant at pH 2 (S1, 14.27%; S2, 7.54%; S3, 0.28%), as well as carbonate-associated Pb at pH 12 (S1, 25.61%; S2, 36.57%; S3, 30.98%). Kelderman and Osman (2007) reported that the oxidation of the heavy metal-sulfide bindings led to an increase of redox potential, which increased the exchangeable and carbonate Cu, Zn, and Pb concentrations. Forstner and Wittmann (1981) reported that the carbonate showed a high affinity for Pb, which may co-precipitate with carbonate minerals at high pH. There was also a preferential association of Ni and Cr toward the residual fraction. The fraction of Ni into an exchangeable fraction was not observed. A minor amount of Cr was also fractionated into an exchangeable fraction. The residual Ni and Cr fractions were dominant, regardless of the pH (Equeenuddin et al. 2013). The sequential extraction procedures simulated to a certain extent different environmental conditions of the sediment. But, the various fractions of heavy metals do not necessarily reflect the relative scavenging action of sediment phases (Tessier et al. 1979). The fractions of heavy metals in sequential extraction procedures are not static and changed with different extraction steps. For example, Tack and Verloo (1996) reported that the carbonate could not dissolve completely under the analytical conditions of the Tessier method.
A risk assessment code (RAC) was applied to evaluate the environmental risk of heavy metal pollution in the sediment (Ghrefat and Yusuf 2006; Li et al. 2015). The risk assessment code assessed the availability of heavy metal in the sediment by applying a scale to the percentage of the total heavy metal in exchangeable and carbonate fractions. A five-level risk classification has been categorized in terms of RAC: no risk (<1%), low risk (1–10%), medium risk (11–30%), high risk (31–50%), and very high risk (>50%) (Perin et al. 1985). The environmental risk assessment of heavy metals in three sediment samples before and after the release experiments according to the RAC is shown in Table 5. This assessment indicated that the Cu, Zn, and Cr concentrations in sample S1 had low risk, the Cd and Pb concentrations in sample S1 presented a medium risk, and the Ni concentrations in sample S1 presented a high risk. The values of risk assessment for the heavy metals in sample S1 decreased as follows: Ni > Pb > Cd > Cu > Cr > Zn. After reaching heavy metal release equilibrium under different pH conditions, the RAC values of Cu, Zn, and Cr in sample S1 gradually increased compared to their initial values; however, the risk level was generally low. Moreover, the Cu concentration was labeled medium risk at pH 12. The RAC values of Cd, Pb, and Ni in sample S1 decreased compared to the initial values. The Cd concentrations were considered medium risk at pH 4–12, but at pH 2, the Cd concentrations were considered low risk. The Pb concentrations presented medium risk at all pH values. It should be noted that the risk level of Ni changed from high risk to low risk after reaching release equilibrium. For sample S2, Cu, Pb, and Cr concentrations were considered a low risk, Zn presented no risk, Cd showed a medium risk, and Ni posed a high risk. The RAC values of heavy metals in sample S2 decreased as follows: Ni > Cd > Pb > Cr > Cu > Zn. After the release of heavy metals under different pH conditions, the RAC values of the Cu, Zn, Pb, and Cr concentrations increased compared to the initial values. The Cu concentrations showed low risk at pH 2–10 and medium risk at pH 12. Zn presented no risk at pH 2–10 and low risk at pH 12. The risk level of the Pb concentrations changed from low risk to medium risk. At pH 12, the Pb concentrations posed a high risk. The Cr concentrations showed a low risk from pH 2 to 12. The RAC values of Cd and Ni decreased compared to the initial values. The risk level of Cd concentrations changed from medium risk to low risk compared to the initial values. At pH 12, the Cd concentration posed a medium risk. The risk level of the Ni concentration changed from high risk to low risk. For sample S3, the Cu, Zn, Pb, and Cr concentrations showed a low risk, Cd concentrations presented a medium risk, and Ni concentrations posed a high risk. The RAC values of heavy metals in sample S3 decreased as follows: Ni > Cd > Pb > Cu > Zn > Cr. After the release of heavy metals under different pH conditions, the RAC values of Cu, Zn, Cd, Pb, and Cr increased compared to the initial values. Copper concentrations showed a low risk at pH 2 and pH 6 and presented a medium risk at pH 4, 8, 10, and 12. Similar to S2, Zn concentrations showed a low risk in S3. Cadmium concentrations showed a medium risk at pH 2–10 and a high risk at pH 12. The risk level of Pb changed from low risk to medium risk after equilibrium was reached. Chromium concentrations in S3 presented a low risk. The RAC values of Ni decreased compared to initial values, changing from high risk to low risk.
4 Conclusions
The average concentrations of Cu, Zn, Cd, and Pb in sediments from the study area were highly elevated compared with the soil background values in Gansu Province, China. The pH-dependent release behaviors of Cu, Zn, Pb, and Cr followed an asymmetric V-shaped pattern, whereas Cd and Ni followed an irregular L-shaped pattern. pH played a significant role in controlling the releasing of heavy metals in the studied sediment. The release equilibrium concentrations of Cu, Zn, Cd, Pb, and Ni in solution at pH 2 exceeded the category 5 standard limits of environmental quality standards for surface water or the maximum allowable discharge concentrations of integrated wastewater discharge standard.
The changes in the release of heavy metals in the studied sediments were related to their redistribution between chemical fractionations. After reaching the release equilibrium of heavy metals under different pH conditions, the percentages of organic Cu, Zn, Cd, and Fe-Mn oxyhydroxide Pb decreased, compared to their initial fractions. However, higher concentrations of Cu, Zn, and Pb were available in their exchangeable fraction at pH 2, while more fractionating into the carbonate fraction occurred at pH 12 (after release equilibrium) compared to other pH values. Fraction of Cd into the exchangeable part was significantly influenced by pH. The residual fractions of Ni and Cr were dominant, regardless of pH. The risk assessment coding results indicated that pH played a significant role in increasing the RAC values of Cu, Zn, Pb, and Cr and decreasing the RAC values of Cd and Ni after release equilibrium was reached.
References
Alcolea A, Vázquez M, Caparrós A, Ibarra I, García C, Linares R, Rodríguez R (2012) Heavy metal removal of intermittent acid mine drainage with an open limestone channel. Miner Eng 26:86–98
Andrade S, Poblet A, Scagliola M, Vodopivez C, Curtosi A, Pucci A, Marcovecchio J (2001) Distribution of heavy metals in surface sediments from an antarctic marine ecosystem. Environ Monit Assess 66(2):147–158
Antoniadis V, Robinson JS, Alloway BJ (2008) Effects of short-term pH fluctuations on cadmium, nickel, lead, and zinc availability to ryegrass in a sewage sludge-amended field. Chemosphere 71(4):759–764
Battaglia F, Morin D, Ollivier P (1994) Dissolution of cobaltiferous pyrite by thiobacillus ferrooxidans and thiobacillus thiooxidans: factors influencing bacterial leaching efficiency. J Biotechnol 32(1):11–16
Belzile N, Chen YW, Gunn JM, Dixit SS (2004) Sediment trace metal profiles in lakes of Killarney Park, Canada: from regional to continental influence. Environ Pollut 130(2):239–248
Bian D, Ren Q, Tian X, Li G (2010) The process of wastewater treatment plant of a non-ferrous metal smelting enterprise. Environ Eng 28(4):9–12 (in Chinese)
Burton ED, Phillips IR, Hawker DW (2006) Factors controlling the geochemical partitioning of trace metals in estuarine sediments. Soil Sediment Contam 15(3):253–276
Cappuyns V, Swennen R (2008) The application of pHstat leaching tests to assess the pH-dependent release of trace metals from soils, sediments and waste materials. J Hazard Mater 158(1):185–195
Cappuyns V, Swennen R, Devivier A (2004) Influence of ripening on pHstat leaching behaviour of heavy metals from dredged sediments. J Environ Monit 6(9):774–781
Carter J, Walling DE, Owens PN, Leeks GJL (2006) Spatial and temporal variability in the concentration and speciation of metals in suspended sediment transported by the River Aire, Yorkshire, UK. Hydrol Process 20(14):3007–3027
Castillo MLA, Trujillo IS, Alonso EV, Torres AGD, Pavón JMC (2013) Bioavailability of heavy metals in water and sediments from a typical Mediterranean Bay (Málaga Bay, Region of Andalucía, Southern Spain). Mar Pollut Bull 76(1–2):427–434
Chander DVR, Venkobachar C, Raymahashay BC (1994) Retention of fly ash-derived copper in sediments of Pandu River near Kanpur, India. Environ Geol 24(2):133–139
Chen SY, Lin JG (2001) Bioleaching of heavy metals from sediment: significance of pH. Chemosphere 44(5):1093–1102
Chen J, Pan X, Hu H (2002) Treatment of lead containing and high pH tailing wastewater in a lead-zinc mine. Nonferrous Met 54(2):108–110 (in Chinese)
China National Environmental Monitoring Center (1990) The soil background values of China. China Environmental Science, Beijing, pp 334–379 (in Chinese)
Daldoul G, Souissi R, Souissi F, Jemmali N, Chakroun HK (2015) Assessment and mobility of heavy metals in carbonated soils contaminated by old mine tailings in North Tunisia. J Afr Earth Sci 110:150–159
Dijkstra JJ, van der Sloot HA, Comans RN (2006) The leaching of major and trace elements from MSWI bottom ash as a function of pH and time. Appl Geochem 21(2):335–351
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407(13):3972–3985
Durán I, Sánchez-Marín P, Beiras R (2012) Dependence of Cu, Pb and Zn remobilization on physicochemical properties of marine sediments. Mar Environ Res 77(6):43–49
Equeenuddin SM, Tripathy S, Sahoo PK, Panigrahi MK (2013) Metal behavior in sediment associated with acid mine drainage stream: role of pH. J Geochem Explor 124(1):230–237
Fathollahzadeh H, Kaczala F, Bhatnagar A, Hogland W (2015) Significance of environmental dredging on metal mobility from contaminated sediments in the Oskarshamn Harbor, Sweden. Chemosphere 119:445–451
Fernandes C, Fontaínhas-Fernandes A, Cabral D, Salgado MA (2008) Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz-Paramos lagoon, Portugal. Environ Monit Assess 136(1–3):267–275
Forstner U, Wittmann GTW (1981) Metal pollution in the aquatic environment. Springer, Berlin, p 486
Gäbler HE (1997) Mobility of heavy metals as a function of pH of samples from an overbank sediment profile contaminated by mining activities. J Geochem Explor 58(58):185–194
Ghrefat H, Yusuf N (2006) Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam, Jordan. Chemosphere 65(11):2114–2121
Gopinath A, Nair SM, Kumar NC, Jayalakshmi KV, Pamalal D (2010) A baseline study of trace metals in a coral reef sedimentary environment, Lakshadweep Archipelago. Environ Earth Sci 59(6):1245–1266
Guven DE, Akinci G (2013) Effect of sediment size on bioleaching of heavy metals from contaminated sediments of lzmir Inner Bay. J Environ Sci 25(9):1784–1794
Ho HH, Swennen R, Cappuyns V, Vassilieva E, Van Gerven T, Van Tran T (2012) Potential release of selected trace elements (As, Cd, Cu, Mn, Pb and Zn) from sediments in Cam River-mouth (Vietnam) under influence of pH and oxidation. Sci Total Environ 435-436(7):487–498
Jalali M, Moharrami S (2007) Competitive adsorption of trace elements in calcareous soils of western Iran. Geoderma 140(1):156–163
Kelderman P, Osman AA (2007) Effect of redox potential on heavy metal binding forms in polluted canal sediments in delft (the netherlands). Water Res 41(18):4251–4261
Krishnamurti GSR, Huang PM, Kozak LM (1999) Sorption and desorption kinetics of cadmium from soils: influence of phosphate. Soil Sci 164(12):888–898
Kristensen P, Søndergaard M, Jeppesen E (1992) Resuspension in a shallow eutrophic lake. Hydrobiologia 228(1):101–109
Kucuksezgin F, Uluturhan E, Batki H (2008) Distribution of heavy metals in water, particulate matter and sediments of Gediz River (Eastern Aegean). Environ Monit Assess 141(1–3):213–225
Larner BL, Palmer AS, Seen AJ, Townsend AT (2008) A comparison of an optimised sequential extraction procedure and dilute acid leaching of elements in anoxic sediments, including the effects of oxidation on sediment metal partitioning. Anal Chim Acta 608(2):147–157
Lee G, Faure G, Bigham JM, Williams DJ (2008) Metal release from bottom sediments of Ocoee Lake No. 3, a primary catchment area for the Ducktown Mining District. J Environ Qual 37(2):344–352
Li X, Tang Z, Chu F (2008) Analysis on speciation and transportation of heavy metals in water and sediment in Baiyin Mine. Earth Environ 36(3):218–224 (in Chinese)
Li R, Li R, Chai M, Shen X, Xu H, Qiu G (2015) Heavy metal contamination and ecological risk in Futian mangrove forest sediment in Shenzhen Bay, South China. Mar Pollut Bull 101(1):448–456
Lindsay P (1979) Chemical equilibria in soils. John Wiley and Sons, New York, p 450
Maanan M, Saddik M, Maanan M, Chaibi M, Assobhei O, Zourarah B (2015) Environmental and ecological risk assessment of heavy metals in sediments of Nador lagoon, Morocco. Ecol Indic 48:616–626
Millward GE, Moore RM (1982) The adsorption of Cu, Mn, and Zn by iron oxyhydroxide in model estuarine solutions. Water Res 16(6):981–985
Morillo J, Usero J, Gracia I (2002) Partitioning of metals in sediments from the Odiel River (Spain). Environ Int 28(4):263–271
Motavalli PP, Palm CA, Parton WJ, Elliott ET, Frey SD (1995) Soil pH and organic C dynamics in tropical forest soils: evidence from laboratory and simulation studies. Soil Biol Biochem 27(12):1589–1599
Müller J, Ruppert H, Muramatsu Y, Schneider J (2000) Reservoir sediments—a witness of mining and industrial development (Malter Reservoir, eastern Erzgebirge, Germany). Environ Geol 39(12):1341–1351
Nan Z, Li J, Zhang J, Cheng G (2002) Cadmium and zinc interactions and their transfer in soil-crop system under actual field conditions. Sci Total Environ 285(1–3):187–195
Nanjing Soil Institute (1977) Soil analysis. Shanghai Scientific (in Chinese)
Nganje TN, Adamu CI, Ugbaja AN, Ebieme E, Sikakwe GU (2011) Environmental contamination of trace elements in the vicinity of Okpara coal mine, Enugu, Southeastern Nigeria. Arab J Geosci 4(1):199–205
Nobi EP, Dilipan E, Thangaradjou T, Sivakumar K, Kannan L (2010) Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuar Coast Shelf Sci 87(2):253–264
Peng J, Song Y, Yuan P, Cui X, Qiu G (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161(4):633–640
Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Zanette ML, Orio AA (1985) Heavy metal speciation in the sediments of Northern Adriatic Sea. A new approach for environmental toxicity determination. In: Lekkas TD (Ed) Heavy metals in the environment (Vol. 2, pp. 454–456). Edinburgh: CEP Consultants
Prusty BG, Sahu KC, Godgul G (1994) Metal contamination due to mining and milling activities at the Zawar zinc mine, Rajasthan, India: 1. Contamination of stream sediments. Chem Geol 112(3):275–291
Reitzel K, Jensen HS, Egemose S (2013) pH dependent dissolution of sediment aluminum in six Danish lakes treated with aluminum. Water Res 47(3):1409–1420
Saeedi M, Li LY, Karbassi AR, Zanjani AJ (2013) Sorbed metals fractionation and risk assessment of release in river sediment and particulate matter. Environ Monit Assess 185(2):1737–1754
Spain AV (1990) Influence of environmental conditions and some soil chemical properties on the carbon and nitrogen contents of some tropical Australian rainforest soils. Aust J Soil Res 28(6):825–839
State Environmental Protection Administration (1997) Integrated wastewater discharge standard. China Environmental Science, Beijing, pp 272–290 (in Chinese)
State Environmental Protection Administration (2003) Environmental quality standards for surface water. China Environmental Science, Beijing, pp 207–218 (in Chinese)
Superville PJ, Prygiel E, Magnier A, Lesven L, Gao Y, Baeyens W, Ouddane B, Dumoulin D, Billon G (2014) Daily variations of Zn and Pb concentrations in the Deûle River in relation to the resuspension of heavily polluted sediments. Sci Total Environ 470-471:600–607
Tack FMG, Verloo MG (1996) Estimated solid phase distribution of metals released in the acid extractable and reducible steps of a sequential extraction. Int J Environ Anal Chem 64(3):171–177
Tack FM, Callewaert OWJJ, Verloo MG (1996) Metal solubility as a function of pH in a contaminated, dredged sediment affected by oxidation. Environ Pollut 91(2):199–208
Tam NFY, Wong YS (1996) Retention and distribution of heavy metals in mangrove soils receiving wastewater. Environ Pollut 94(3):283–291
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851
Tipping E, Smith EJ, Lawlor AJ, Hughes S, Stevens PA (2003) Predicting the release of metals from ombrotrophic peat due to drought-induced acidification. Environ Pollut 123(2):239–253
Van Herreweghe S, Swennen R, Cappuyns V, Vandecasteele C (2002) Chemical associations of heavy metals and metalloids in contaminated soils near former ore treatment plants: a differentiated approach with emphasis on pHstat-leaching. J Geochem Explor 76(2):113–138
Wang L, Yuan X, Zhong H, Wang H, Wu Z, Chen X, Zeng G (2014a) Release behavior of heavy metals during treatment of dredged sediment by microwave-assisted hydrogen peroxide oxidation. Chem Eng J 258:334–340
Wang J, Liu R, Zhang P, Yu W, Shen Z, Feng C (2014b) Spatial variation, environmental assessment and source identification of heavy metals in sediments of the Yangtze River Estuary. Mar Pollut Bull 87(1–2):364–373
Wen X, Allen HE (1999) Mobilization of heavy metals from Le An River sediment. Sci Total Environ 227(2–3):101–108
Zhang C, Yu Z, Zeng G, Jiang M, Yang Z, Cui F, Zhu M, Shen L, Hu L (2014) Effects of sediment geochemical properties on heavy metal bioavailability. Environ Int 73:270–281
Zhong A, Guo S, Li F, Li G, Jiang K (2006) Impact of anions on the heavy metals release from marine sediments. J Environ Sci 18(6):1216–1220
Acknowledgments
This work was supported by the Supervision of Ph. D. Degree Fund of Chinese Ministry of Education (20120211110018), the Fundamental Research Funds for the Central Universities in Lanzhou University (lzujbky-2015-150 and lzujbky-2016-261), and the National Natural Science Foundation of China (Nos. 91025015, 51178209, and 41501337).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Sophie Ayrault
Electronic supplementary material
ESM 1
(DOCX 40 kb)
Rights and permissions
About this article
Cite this article
Zang, F., Wang, S., Nan, Z. et al. Influence of pH on the release and chemical fractionation of heavy metals in sediment from a suburban drainage stream in an arid mine-based oasis. J Soils Sediments 17, 2524–2536 (2017). https://doi.org/10.1007/s11368-017-1730-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1730-4