Abstract
Purpose
The heavily forested Cache la Poudre (CLP) watershed in northern Colorado, USA, was impacted by the High Park wildfire in 2012. The wildfire burned land and vegetation immediately adjacent to the CLP River where blackened, ashy sediment samples were collected from five sites upstream of the City of Fort Collins drinking water intake to evaluate the spatial distribution and characteristics of burned sediments, along with quantifying and characterizing soluble compounds following a leaching experiment.
Materials and methods
At each site, samples were collected from three locations: (1) the edge of the bank adjacent to the water edge (downbank), (2) 1 m upslope of location 1 (midbank), and (3) 2 m upslope of location 1 (upperbank). All solid sediment samples were analyzed for elemental composition, and a subset of solid sediment samples were analyzed with 13C solid-state nuclear magnetic resonance spectroscopy. Sediments were mixed with the background CLP River water collected from upstream of the wildfire and allowed to leach for 6 and 24 h to determine the quantity and quality of water-soluble constituents. Filtered samples were analyzed for dissolved organic carbon (DOC), iron, manganese, and inorganic nutrient concentrations, by optical properties, and for disinfection byproduct (DBP) formation.
Results and discussion
Percent carbon and nitrogen content of the solid sediments were good predictors of leachate DOC concentration. The mean fluorescence index was higher for wildfire-impacted sediment leachates (1.50) compared to the background CLP River water (1.37), which may be due to changes in DOM molecular weight and oxidation of organic matter. All sediment leachates showed consistently higher haloacetonitrile and chloropicrin yields (DBP concentration/DOC concentration) compared to background CLP River water, whereas carbonaceous DBPs did not.
Conclusions
The collected sediments showed that burned material accumulated downstream near the river and was composed of inputs from burned soil and biomass along with the mobilization of unburned terrestrial material. The leachates of these sediments have different characteristics compared to the background CLP River water, indicating that DOM leached from sediments following a wildfire may increase aquatic DOC concentrations and N-DBP formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wildfire frequency has been increasing over the last several decades due to changes in seasonal precipitation patterns, climate, droughts, and forest disease outbreaks (Brown et al. 2004; Westerling et al. 2006). Many communities rely upon forested watersheds to provide high-quality source water supplies, which may be imperiled following a wildfire. In addition to impacting water quality, wildfires have been reported to change soil characteristics as a result of heating and combustion processes (Bodi et al. 2014). These changes to soil properties include water repellency (DeBano 2000; Specht and Frimmel 2000), nutrient content (Prieto-Fernandez et al. 1998; Prieto-Fernandez et al. 2004; Homann et al. 2011), and organic carbon content (Knicker 2007; Homann et al. 2011). Depending on heating temperatures, the effects of thermal alternation to soil samples can vary with changes to both physical properties and organic matter characteristics (Araya et al. 2016a, b). In a field study of prescribed burns and wildfires in pine forests in Oregon, wildfire severity was positively related to C and N losses in soil as a result of consumption of the organic soil horizon immediately following high severity fire conditions (Homann et al. 2011). However, Homann et al. (2011) also reported C and N deposition onto the soil surface during the year following the wildfire primarily from heat-killing needles from the overstory. This freshly deposited material was reported to have much lower C/N ratios than typical litterfall, potentially having implications for the recovery of a soil microbial community and vegetation (Homann et al. 2011). In addition to altering the concentration of C and N in soils, wildfires have been reported to alter the speciation of C, N, and P (reviewed in Knicker 2007).
Soil properties are known to impact dissolved organic carbon (DOC) concentration and character in nearby water bodies (Sanderman et al. 2009; Dick et al. 2015). Therefore, wildfire-induced changes to soils are likely involved in the water quality impacts observed in streams following wildfires. Soils mobilized by surface runoff and entrained in flowing water are referred to as sediments, which can settle out along the banks of streams, in slow-moving pools, or in lakes. For a watershed impacted by a prescribed burn, the eroded quantity (tons per hectare) of the sediment increased by one to three orders of magnitude for 2 years following the burn and up to 3 years for some areas of the watershed (Shakesby et al. 2015). Rainstorm events may be especially important for the transport of terrestrial material and soil organic matter to aquatic systems (Clark et al. 2007; Inamdar et al. 2011, 2012). Rainstorms following a wildfire in the Rocky Mountains mobilized more DOCs in a burned watershed than in an unburned reference watershed, but concomitant increases in DOC were not reported for high stream flows during spring snowmelt (Murphy et al. 2012; Writer et al. 2012).
The increased quantity of suspended sediments in streams following wildfires, especially during precipitation events, likely facilitates the dissolution of sediment-derived organic matter following wildfires and prescribed burns (Murphy et al. 2012; Writer et al. 2012; Vieira et al. 2015). In addition to hydrologic factors, soil and sediment characteristics may influence the amount and type of DOC mobilized. The C/N ratio of soils has been reported to explain most of the variability in riverine DOC flux for several biomes with increasing soil C/N ratio correlating with increasing riverine DOC flux (Aitkenhead and McDowell 2000). To date, this relationship has not been confirmed for wildfire-impacted watersheds with altered C/N ratios. However, for sediments collected after a prescribed burn, increases in N content and organic content were reported, suggesting that there may be increased mobilization of N-containing organic compounds following burning (Shakesby et al. 2015).
Wildfires are known to directly alter watershed-scale geochemical cycling and sediment transport (Williams and Melack 1997), often causing increases in nitrate and phosphate export, changes in stream water DOC concentrations, and mobilization of metals (Gresswell 1999; Writer et al. 2012), which are presumably derived from burned sediments (Doerr and Santin 2016; Santin and Doerr 2016). Wildfire effects on DOC concentrations in simulated laboratory studies and from field studies are mixed, likely due to differences in the precursor material burned in natural settings versus in laboratory settings. Revchuk and Suffet (2014) reported evidence for increased DOC export following a wildfire based on laboratory leaching experiments of recently charred material and 2-year-old aged char collected from field locations. However, following a fire in Glacier National Park, DOC concentrations in a stream draining a burned area showed “minimal” increase in DOC relative to an unburned area, indicating that changes in DOC concentration may vary depending on the wildfire and watershed (Mast and Clow 2008). In addition to altering ecologically relevant water quality parameters, e.g., nutrients and turbidity, wildfires have been reported to influence the formation of disinfection byproducts (DBPs) (Wang et al. 2015; Hohner et al. 2016), which form when natural waters are disinfected prior to distribution as finished drinking water (Liang and Singer 2003; Krasner et al. 2006; Hua and Reckhow 2007). DOC quantity and quality can have a significant impact on the concentrations of DBPs, which are regulated by the US Environmental Protection Agency, thus making control of their formation a priority for drinking water utilities (Singer et al. 1995).
To help solve this apparent disconnect between DOC concentrations from field and laboratory studies and to better understand how wildfire changes to water quality will affect the drinking water treatment processes, such as the formation of DBPs, it would be useful to conduct both field and laboratory studies on the same wildfire to link the two conditions. In this study, riverbank sediments were collected from a wildfire-affected watershed and leached in river water, to mimic water quality responses to wildfires. The aim was to evaluate the sediment characteristics along the lateral and longitudinal length of a reach of wildfire-impacted river and potential changes in water quality and DBP formation following a wildfire. Sediment leachates were filtered and characterized for general water quality parameters including DOC concentration, nutrients, and optical properties. In addition, a subset of the leachates was chlorinated at bench scale and analyzed for DBP formation. The characterization of the leachates represents an integrated signal from wildfire-induced chemical changes to burned soil, litter, and biomass, along with physical changes that likely resulted in the mobilization of an unburned terrestrial material to the river. Studying the solid material and the water-soluble material together provides a more comprehensive picture of the potential impacts of wildfires on drinking water sources and may be useful for understanding and predicting watershed recovery.
2 Materials and methods
2.1 Sediment collection following the High Park fire
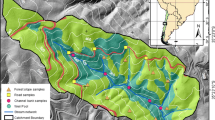
The High Park fire began on June 9, 2012, and was fully contained on July 2, 2012. The wildfire severity varied spatially from low to high (Fig. 1). Four rainstorms took place between June and October 2012: June 27, July 6, July 19, and July 25, 2012. Rainstorms were evidenced by changes in stream discharge from less than 10 cfs to greater than 700 cfs, and likely deposited wildfire-impacted soils and sediments along the banks of the Cache la Poudre (CLP) River.
Sediments were collected on October 31, 2012, from five sites along the CLP River (Fig. 1) within the burned area. The sediments were likely transported downstream after being entrained in the river before being deposited along the bank, possibly resulting in “pre-leaching” of highly soluble compounds prior to their collection. The most downstream sampling location, A, is immediately upstream of the City of Fort Collins drinking water utility intake. At each site (A–E; Fig. 1), sediments were collected from three riverbank locations (upperbank, midbank, and downbank) perpendicular to the stream bank spaced approximately 1 m apart. The “downbank” samples were collected at the edge of the river and the “upperbank” samples were collected furthest from the river.
The accumulation of black, charred sediment deposits along the riverbank was visibly distinct (i.e., loose black fines) from the native soil profile (i.e., fairly compact mineral soil). The sediment deposits consisted of a well-mixed layer of dark material with a discernible smoky odor, which was not present in upstream, unburned areas. The sediments were collected in bulk, ranging from 6 in.–2 ft deep. The samples contained a mixture of loose materials, including ash, charred organic material, and sediments, which were likely to be easily re-suspended during a rise in river elevation during higher flow events. An unburned soil sample was not collected for comparison, as the native riverbank soil from an upstream site would not represent the same precursor starting material of the organic-rich sediment deposits collected from the riverbank in the wildfire-impacted area. Rather, the sediment leachates are compared to baseline water quality and reactivity from a water sample collected upstream of the wildfire-impacted area of the watershed.
2.2 Laboratory sediment leaching
The sediment samples were dried first at room temperature (∼3–4 days) and then in a furnace at 105 °C for 2 h. The sediments were sieved through a 0.991-mm sieve, which represents fine silt and clays in the American Society for Testing Methods (ASTM) classification, and kept in the freezer until the leaching experiments were performed. For each riverbank location (upperbank, midbank, downbank), 200 g of sediment was placed in a 2-L Pyrex glass beaker and mixed with the CLP River water collected from a site upstream of the wildfire (Poudre Below Rustic (PBR); Fig. 1) for 2 min with a glass rod. The water used for leaching was a portion of a large (55 gal) sample collected in October 2013 from upstream of the wildfire that was stored in a large walk-in cooler. Thus, the water used to leach the sediments had the same characteristics and differences in the water chemistry of the leachates can be attributed to differences in sediment characteristics rather than differences in water quality prior to leaching. The samples were kept in the dark at room temperature for the allocated leaching duration (6 and 24 h). Leaching times represent high river flow (6-h leaching duration) and low river flow (24-h leaching duration). At the end of the leaching periods, the samples were decanted without disturbing the settled sediment, filtered (0.70 μm GF/F), and stored at 4 °C.
2.3 Analytical methodology
2.3.1 Solids analysis
Total carbon and nitrogen of the sediments were determined on a LECO Tru-Spec CN analyzer (Leco Crop., St. Joseph, MI, USA). Measurements were calibrated using a standard soil sample. Five sediment samples with high carbon concentrations were analyzed using 13C NMR spectroscopy. It was not possible to collect spectra for the samples with lower carbon concentrations. All samples were ground prior to NMR analysis. A 300-MHz Varian instrument with an operating 13C resonance of 75.4 MHz and 1H resonance of 299.9 MHz equipped with a 7.5 mm HX probe was used for all analyses housed at the Environmental Molecular Science Laboratory (EMSL) at the Pacific Northwest National Laboratory (Richland, WA). Samples were packed in a zirconia rotor and analyzed using a variable amplitude cross-polarization magic angle spinning coupled with total suppression of spinning sidebands (CPMAS/TOSS) pulse sequence with a spinning speed of 5 kHz. The pulse sequence begins with a hard 1H radiofrequency pulse followed by a variable amplitude CP pulse of 1 ms and the four pulse-toss sequence. The power for these toss pulses was optimized to minimize the sidebands. A 62.5-kHz 1H TPPM decoupling field was applied during the toss sequence with a 10-ms acquisition time. The number of scans was between 30,000 and100,000 and varied according to the amount of C present in the sample. Recycle delay between acquisitions varied between 1 and 2 s and was optimized for each sample by analyzing each sample’s proton signal. The data were processed with zero filling to 32 k and 200 Hz line broadening. The carbon chemical shift was calibrated using hexamethylbenzene as the external standard. Chemical shift regions were integrated according to broad functional group assignments: 0–25 ppm for terminal methyl, 25–45 ppm for methylene in rings/chains, 45–60 ppm for O-alkyl/N-alkyl, 60–90 ppm for carbohydrate/ether/alcohol, 90–160 ppm for aromatic/alkenes, and 160–190 ppm, for carboxyl/carbonyl/amide groups.
2.3.2 CLP River monitoring sample collection
As part of a previous study, surface water samples were collected biweekly through the first spring snowmelt (2013) following the High Park fire, including a site upstream of the burned area (PBR) (Fig. 1). CLP River water for the leaching experiments was also collected from the PBR sampling site. Starting in August, the samples were collected monthly through October. Samples were filtered through pre-combusted GF/F glass fiber filters and were stored at 4 °C until analysis, as described in Sect. 2.2.
2.3.3 CLP River monitoring and leachate sample analysis
DOC and total dissolved nitrogen (TDN) were measured with a TOC-VSCH (Shimadzu Corp., Japan) analyzer. UV254 was measured using a UV-Vis spectrophotometer (Cary 100, Agilent Technologies) with a 1-cm path length quartz cuvette. Specific ultraviolet absorption at 254 nm (SUVA254) was determined by dividing UV254 absorbance by the DOC concentration (Weishaar et al. 2003). Total dissolved phosphorous (TDP) analysis followed Lachat QuikChem 8500 spectrophotometric flow injection methods. Nitrate and nitrite analyses were done with an analytical flow solution IV spectrophotometric analyzer (OI Analytical, USA). Ammonium was measured using a BioTek Synergy 2 Microplate Reader. Dissolved organic nitrogen (DON) was determined by the difference between TDN and the sum of all inorganic nitrogen species (nitrate, nitrite, and ammonium). Fe and Mn concentrations were measured at the University of Colorado Laboratory for Environmental and Geological Studies (LEGS) using ICP-AES. The samples for the metal analysis were made by mixing 1.5 g of solid material with 100 mL of CLP River water and filtering after 24 h.
Size distribution of the molecules in the solution was determined using a high-pressure liquid chromatograph equipped with a protein-pak column (Waters), UV detector (280 nm), and polystyrene sulfonate standards (210, 1000, 4300, 6800, and 17,000 Da). A phosphate buffer solution (pH = 6.8) was used as the mobile phase at a flow rate of 0.7 mL/min. Larger molecular weight compounds travel through the column more quickly than smaller molecular weight compounds due to their exclusion from small pores within the column. Therefore, retention time is negatively related to the log of the molecular weight of the molecules in solution.
Fluorescence excitation emission matrix (EEM) plots were recorded using a John Yvon Horiba FluoroMax-4 spectrofluorometer over excitation wavelengths of 240 to 450 nm in 10-nm increments, and fluorescence intensity was measured at emission wavelengths from 300 to 600 nm in 2-nm increments. Slit widths of 5 nm and integration times of 0.25 s were used for both excitation and emission wavelengths. The signal was collected in Sc/Rc mode to incorporate instrument-specific correction factors. EEMs were corrected for primary and secondary inner filter effects using the corresponding UV-Vis absorbance spectrum. EEMs were blank subtracted and Raman-normalized based on the Raman peak area for 18.2 MΩ MilliQ water collected at an excitation wavelength of 350 nm. Corrected EEMs are presented in Raman units (RU). Lamp scans and cuvette contamination checks were performed daily. EEMs were corrected and analyzed using MATLAB software. The fluorescence index (FI) is reported as the ratio of the emission at 470 nm to that at 520 nm with an excitation at 370 nm. The expected range for the FI lies between 1.2 and 1.9. Lower values are associated with DOM from terrestrial origin, and higher values are associated with DOM from microbial sources (McKnight et al. 2001). More recently, the FI has been shown to relate to shifting of the peak location, which may indicate other factors than DOM precursor material influencing the FI value (Korak et al. 2014).
Eight leachates were selected for bench-scale chlorination tests and DBP analysis based on water quality and DOM properties (e.g., DOC, SUVA254; Table S1, Electronic Supplementary Material). Chlorination methods followed uniform formation conditions (UFCs) (Summers et al. 1996), which mimic typical chlorination conditions in US drinking water treatment plants. Samples were chlorinated at room temperature with a buffered sodium hypochlorite dosing solution (pH 8) at 20 °C. A borate buffer was added to the samples to maintain a pH of 8 (±0.3). Chlorine doses were selected from a preliminary demand test to target a chlorine residual of 1.0 mg/L (±0.4 mg/L). Chlorinated samples were incubated in the dark for 24 h (±1 h) at room temperature (20 ± 1.0 °C). Chlorine residuals were measured with the N,N-diethyl-phenylenediamine (DPD) colorimetric method (SM4500-Cl G) and quenched with ammonium chloride immediately following 24 h prior to DBP analysis.
Chlorinated samples were analyzed for DBPs following EPA Method 551.1 (1995) for total trihalomethanes (TTHM) and haloacetonitriles (HAN). EPA method 552.2 was followed for analysis of haloacetic acids (HAAs). An Agilent 6890 Gas Chromatography System with an electron capture detector was used. Statistical analyses, including pairwise comparisons of DBP yields and DOM quality, were performed in JMP Pro 11.0 software using a threshold of 0.05 for p values. TTHM species include chloroform, bromodichloromethane (DCBM), chlorodibromomethane (CDBM), and bromoform. HAN4 species include dichloroacetonitrile (DCAN), trichloroacetonitrile (TCAN), dibromoacetonitrile (DBAN), and bromochloroacetonitrile (BCAN). HAA species include chloroacetic acid (MCAA), bromoacetic acid (MBAA), dichloroacetic acid (DCAA), trichloroacetic acid (TCAA), and dibromoacetic acid (DBAA).
Burned watershed areas for each classified wildfire severity (low, moderate, and high) draining to each sediment collection site were determined using the ArcMap 10.3 software. A 10-m resolution digital elevation map (DEM) of Larimer County, CO, a shapefile containing the Burned Area Emergency Response (BAER) wildfire severity for the High Park fire, and GPS coordinates from a Garmin handheld GPS unit were used for the GIS analysis. Briefly, the CLP watershed boundary and stream network were determined using a flow accumulation method and the watershed areas draining to each sediment sampling location were calculated using the watershed tool and geometry calculator.
3 Results and discussion
3.1 Sediment site burn severities and drainages
The percent drainage area burned (all severities) decreased from downstream, site A, to upstream, site E. At site A (Fig. 1), 1.7, 7.3, and 3.6% of the watershed area was burned at high, moderate, and low severity, respectively (87.4% unburned). Similarly, at site B, 1.7, 7.2, and 3.5% of the watershed area was burned at high, moderate, and low severity, respectively (87.6% unburned). Site C had a lower percent burned area with 0.95, 4.3, and 2.3% of the drainage burned at high, moderate, and low severity, respectively (92.5% unburned). At site D, 0.74, 2.9, and 1.4% of the watershed area was burned at high, moderate, and low severity, respectively (94.9% unburned), and at site E, 0.63, 2.4, and 1.1% of the watershed area was burned at high, moderate, and low severity, respectively (95.9% unburned). Overall, the total area draining the sediment collection sites was primarily unburned. However, the sediments along the riverbank had clear indications of being wildfire impacted, such as a dark black color and smoky smell. These evident impacts are likely due the proximity of the fire to river and the increased erosion potential of burned land (Moody and Martin 2001; Writer et al. 2012).
3.2 Solid-state characterization
Elemental analysis of the sediments showed that the downbank locations (nearest stream) had the lowest percent C and N, whereas the midbank locations generally had the highest percent carbon (C) (Table 1). Erosion at the downbank location may have washed high carbon sediments downstream, contact with flowing water may have “pre-leached” soluble carbon compounds, or initially, the sediments had lower carbon content. The midbank site would have had less contact with water to erode or leach sediments. There was likely limited deposition of wildfire-impacted sediments at the upperbank location because it was not reached by the river water level following deposition. There was also a noticeably higher percent C and N at site A, located furthest downstream and draining the largest burned area which is indicative of wildfire impacts and consistent with the finding that wildfires can increase soil C content, attributed to recalcitrant-burned biomass and re-colonization of burned land with N-fixing vegetation (Raison 1979; Johnson and Curtis 2001; Heath et al. 2015). Site A sediments also had a higher percent Fe compared to all of the other sites. A general longitudinal decreasing trend in percent Fe moving upstream was observed. Thus, the increased Fe concentrations measured at site A are likely due to heterogeneity in the watershed where geologic minerals may be the source of Fe and the physical changes following the High Park fire that destabilized watershed areas mobilizing Fe-containing minerals from unburned soil (Pereira et al. 2014).
13C NMR analysis was performed on the five samples with the highest percent C from the preliminary elemental analysis. The selected samples for the analysis included site A (upperbank, midbank, and downbank), site B (upperbank), and Site C (midbank) (Table 2). Many of the other solid samples had very low C content and obtaining meaningful 13C NMR spectra were not possible. For these five sites, the spectra were dominated by aromatic C compounds (Table 2, spectra shown in Fig. S1, Electronic Supplementary Material), which could be representative of condensed (160–90 ppm region) combustion products from wildfire, commonly referred to as black carbon (Knicker et al. 2008; Knicker et al. 2012; Merino et al. 2014) and charred material derived from pulp, leaves, and sawdust (Knicker et al. 2008). The presence of these heteroatoms is likely a result of incomplete combustion of precursor soil and biomass material (Knicker et al. 2008). These types of aromatic organic molecules are also found in DBP precursor material (Chang et al. 2001; Yang et al. 2008). The content of carbohydrates and other aliphatic compounds was low for all of the soil samples that were analyzed (Table 2). These compounds are often found in higher abundance in unburned soils and are likely destroyed during wildfires leading to lower abundances detected in this study (VanBergen et al. 1997; De la Rosa et al. 2013).
For site A, an NMR spectrum was obtained for all three riverbank locations due to the high percent C, facilitating a discussion regarding the possible mobilization distance/washing effect of fire-impacted soil organic matter (SOM) along the banks of the river. The total contribution of aromatic structures to the spectrum was highest (64.1%) for the downbank location compared to the midbank (52.0%) and the upperbank (53.2%) locations, indicating that a greater amount of fire-impacted SOM was deposited closest to the river, and was spread approximately evenly 1 (midbank) and 2 m (upperbank) from the river. In contrast, an opposite relationship, higher relative abundance for the upperbank and midbank locations, was determined for the signal derived from carbohydrates in hexose, some alcohols, and amino acids (90–60 ppm). Differences in the signals between bank locations reflect the impact of distance from the river upon SOM composition with the downbank location showing more recalcitrant, less soluble carbon structures consistent with wildfire impacts. Comparing site A midbank to site C midbank, we observe very similar contributions from each structural entity. However, site A and B upperbank SOM compositions were more different and reasons for this remain unclear. Heterogeneity in SOM source material or variability in accumulation and erosion may explain these differences. Compared to a 13C NMR study of whole soil and extracts collected from the Colorado montane environment, the aromatic carbon content of the High Park fire sediments (upperbank, midbank, and downbank) was higher than for soils that have not been affected by a wildfire recently (Keeler et al. 2006).
3.3 Concentration and character of water-soluble constituents
Leaching experiments were performed by mixing the sediments with CLP River water collected from the PBR site, upstream of the burned area. Sediments were leached for 6 or 24 h, and then filtered for relevant source water quality analyses. Dissolved metals, among other solutes, can be a concern for drinking water utilities, and elevated levels have been observed in stream water, soils, and vegetation following wildfires (Costa et al. 2014; Mitic et al. 2015). The concentrations of water-extractable Mn were in general highest for the downbank samples compared to the midbank and upperbank samples collected from the same site (Table S2, Electronic Supplementary Material). There was also an increase in the average Mn concentrations of the leachates with distance downstream. Fe concentrations were higher at site B compared to all of the other sites except for site A, which also had high Fe concentrations compared to the more upstream sites (Table S2, Electronic Supplementary Material). The mobilization of metals following wildfire was hypothesized to be from entrained sediment (Costa et al. 2014), and the leaching study here supports that conclusion. Thus, efforts to prevent excessive erosion of soils into surface waters following a wildfire may help to avoid elevated concentrations of metals.
Nutrient levels in surface waters may enhance algal growth and contribute to the pool of DBP precursors. CLP River water samples for the year following the wildfire showed elevated levels of nutrients at a wildfire-impacted site relative to a site upstream of the burned area (Hohner et al. 2016). It was hypothesized that the increases in surface water nutrient concentrations resulted from the leaching of eroded, burned material. Using the leaching studies to test this hypothesis, no discernable spatial trends were found for total dissolved phosphorous (TDP) related to the upstream/downstream sediment sites (Table S3, Electronic Supplementary Material), with values that varied from one another, but were quite patchy. However, laterally from the river TDP was the lowest for the downbank locations, while the upperbank and midbank locations did not show a trend, with values that varied between sites and were higher than the downbank values. At sites A and C, the midbank location had the highest TDP concentrations, whereas at sites B and E, the upperbank location had higher TDP concentrations compared to the other locations. This finding is somewhat unexpected, as water quality studies (Murphy et al. 2006; Miller et al. 2013) and soil studies (Norouzi and Ramezanpour 2013; Xue et al. 2014) have both reported increased concentrations of phosphorus in wildfire-impacted areas. However, a study of the effects of fire severity on ash chemical properties reported a decrease in TP with increasing fire severity (as estimated using ash color) indicating that fire severity may play an important role in soil chemical properties and water-extractable constituent concentrations (Pereira et al. 2012). Based on the BAER severity map for the High Park fire (Fig. 1), the fire impacts were very patchy in severity throughout the watershed, which may have led to the variability in the TDP concentrations leached from sediment samples collected from different areas. It is also possible that there were wildfire impacts on phosphorus (P) concentrations that were not captured in this study because in the short time between the High Park fire and collection of the sediments, P was abiotically removed (e.g., leached by stream water or sorbed to sediments) or biologically assimilated, and wildfire impacts on P were short lived in this system (Son et al. 2015).
Similar to TDP, the leachates showed no consistent TDN trend from the downstream to upstream sediment collection sites (Table S3, Electronic Supplementary Material). Site E had higher NO2 − + NO3 − than all other sites, except for site B. It is possible that forest ecology may play an important role in stream water nutrient concentrations for the area (Rhoades et al. 2013). NH4 + concentrations were higher for site A leachates, the most downstream site, than for any of the other sites (Table S3, Electronic Supplementary Material). Site B had the lowest NH4 + concentration. Similarly, the DON values at site A were higher than any of the other sites. Lee and Westerhoff (2005) reported that when DON is less than 40% of the TDN, calculating by difference can lead to inaccurate results when compared to samples pretreated with dialysis. For most of the leachates, DON was less than 40% of TDN making the calculation of DON potentially erroneous. The form of nitrogen found in the heated material has been shown in laboratory experiments to depend on heating duration (Knicker et al. 1996). Thus, water-soluble N content may be driven by wildfire intensity and/or duration, which is unknown for the environmental samples. However, there was a general increasing trend in DON and NH4 + concentrations with distance downstream, consistent with wildfire impacts mobilizing organic N and NH4 + that have been reported for soils following wildfires (Covington et al. 1991; Certini 2005).
3.4 Characterization of DOM leached from sediments
Another concern following a wildfire is the potential for increased mobilization of terrestrial DOM, resulting in higher DOC concentrations and DBP formation during drinking water treatment. Water-soluble organic carbon (WSOC) concentrations were calculated as the mass of carbon released into a solution divided by the mass of the sediments leached (Table 1). The yields of organic carbon in the solution per unit sediment after 6 h were the greatest for site A (mean 0.134 mg-C/g-sediment) with a decreasing trend upstream from site B (mean 0.065 mg-C/g-sediment) to site E (mean 0.053 mg-C/g-sediment). The spatial trend is likely influenced by a combination of hydrologic transport of wildfire-impacted sediments to the downstream sites, and the higher percentage area burned draining to sites A and B. Without a time-series dataset, the influence of hydrology and spatial distribution of wildfire severity cannot be decoupled. In addition to the higher percentages of C and N in the solid material, sites A and B leached more carbon into the solution when mixed with river water. The WSOC concentration showed a strong positive relationship with the percent nitrogen and carbon in the sediment samples as determined by elemental analysis (Fig. 2). The higher %N content of the solid sediments is characteristic of wildfire impacts and may enhance the solubility of carbon containing compounds, specifically aromatic ones that may result from burned biomass (Knicker et al. 2005; Knicker 2007). It is also possible that the wildfire resulted in the addition of carbon and nitrogen from partially combusted biomass to the soils, with a fraction soluble in the form of WSOC (Johnson and Curtis 2001).
The average SUVA254 values of the leachates showed an increasing trend with increasing lateral distance from the river, with the less wildfire-impacted upperbank locations having higher SUVA254 values than the midbank or downbank locations (Table S3, Electronic Supplementary Material). A longitudinal trend was also observed, with the downstream site A leachates having the lowest SUVA254 values (mean SUVA254 3.8) and site B and D leachates (mean SUVA254 4.4 for both) having the highest average SUVA254 values. These trends do not correlate with the elemental analysis of the solid samples, indicating that the C or N content did not simply drive the aromaticity of the leachates. Higher SUVA254 values following a wildfire were observed in a previous study (Writer et al. 2012); however, it is unclear whether the increase is attributed to enhanced erosion of unburned soil following the wildfire or due specifically to the mobilization of burned soil and biomass material. When the sediment leachates are compared to the upstream CLP River monitoring site, PBR, clearly higher SUVA254 values were observed for the leachates (Fig. 3). Based on the leaching experiments, it appears that the erosion and leaching of unburned terrestrial material may contribute to some of the changes to water quality in combination with burned material following a wildfire.
Box plots of optical properties including FI and SUVA254 values for the CLP River water (PBR site) and sediment leachates. The centerline of the box represents the mean with the top and bottom representing the upper and lower quartiles. For the CLP River water, 95% confidence intervals are represented by the whiskers and the dots are the highest and lowest values
The FI is another optical parameter used to understand the DOM precursor material and chemical characteristics (McKnight et al. 2001; Korak et al. 2014). The FI values were not substantially different between the 6- and 24-h leachates (Table S3, EEMs of site A are presented in Fig. S3, Electronic Supplementary Material). The data obtained for different sediment sites along the river and at different lateral riverbank locations did not show clear trends (Table S3, Electronic Supplementary Material). However, the leachates had higher FI values compared to the background CLP River water (Fig. 3). Although the FI values can be skewed by Fe interactions, the metal concentrations measured for the leachates were not high enough to significantly influence the FI values (Poulin et al. 2014). The FI seems to be a particularly sensitive metric for wildfire-impacted DOM, and it is unlikely that the FI strictly represents the microbial to terrestrial continuum described by McKnight et al. (2001), as wildfires are likely not selectively destructing terrestrial material or adding microbial material causing the increase in FI.
Thus, molecular weight changes that shift fluorescence spectral characteristics may partially explain the higher FI associated with the wildfire-impacted sediment leachates. Using preparative scale size exclusion chromatography (SEC), a general trend of increasing FI with decreasing molecular weight was reported for the surface water samples (Romera-Castillo et al. 2014). A blue shift of the fluorescence, caused by increased oxidized functional groups following fire, could also explain the higher FI values (Coble 1996; Korak et al. 2014), which relates well with the primarily aromatic composition of the sediments from the NMR analysis. There were no significant differences in the MW estimates of the five sediment sites despite considerable variability (Table S2, Electronic Supplementary Material). It is possible that the sediments contained larger molecular weight, fire-derived compounds that were not soluble in river water and were thus not captured by the SEC. Wildfire may produce low-solubility, larger molecular weight aromatic organic compounds and/or degrade higher molecular weight compounds to lower molecular weight compounds, water-soluble compounds. To date, there are no other studies of wildfire-impacted sediments leachates that have evaluated DOM molecular weight using SEC.
3.5 DBP reactivity of wildfire impacted sediment leachates
Eight of the leachates were chlorinated and analyzed for DBP formation based on water quality and DOM properties (e.g., DOC, SUVA254; Table S1, Electronic Supplementary Material). DBP yields were calculated (DBP concentration/DOC concentration) in order to gain an understanding of the reactivity of the precursor material leached from the sediments on a per unit carbon basis. DBP yields for carbonaceous DBPs (C-DBPs; TTHMs and HAA5s) and nitrogenous DBPs (N-DBPs; HAN4 and chloropicrin) are presented in Table 3. Site D showed the highest TTHM and HAA5 yields, appearing to have the greatest C-DBP reactivity compared to the other sites. The site A downbank leachate (24 h) showed the highest HAN4 and chloropicrin yields overall. Assuming that the sediments from site A were the most wildfire-affected, these findings suggest that the burned material may be more reactive for forming N-DBPs and is enriched in N-DBP precursors compared to carbonaceous precursors, consistent with other work (Wang et al. 2015). The higher nitrogen content of the more downstream, wildfire-impacted sediments, specifically at site A, also supports the higher N-DBP reactivity. The leachates had higher HAA5 yields and slightly lower TTHM yields than the background CLP River water, although they were not substantially different (Fig. 4). In addition, both HAN4 and chloropicrin yields were higher for the leachates compared to the background water (Fig. 4). The mobilization of the N-DBP precursors may be one of the greatest impacts of soluble compounds from the burned sediments compared to the background CLP River water.
Box plots of the DBP yields for CLP River water (PBR site) and the sediment leachates. The centerline of the box represents the mean with the top and bottom representing the upper and lower quartiles. For the CLP River water, 95% confidence intervals are represented by the whiskers and the dots are the highest and lowest values
4 Conclusions
Overall, this study confirms that water-soluble compounds leached from sediments following a wildfire contributed measured changes to water quality, reported and suggested elsewhere, but not directly compared to surface water quality changes in the same area (Revchuk and Suffet 2014; Wang et al. 2015). In addition, we found evidence that spatial distribution of the sediments may be hydrologically influenced with the more downstream site, which drains a larger burned area, showing greater wildfire impacts in the form of higher C and N content in the sediments. This study also highlights that changes in stream water quality characteristics are likely altered due to a combination of inputs from burned soil and biomass along with the mobilization of unburned terrestrial material that was not heated, but rather had increased mobility following the wildfire. Hydrology and erosion impart different characteristics on sediments and may account for spatial variability in solid sediments and leachate characteristics. However, it is clear that the leachates of these wildfire-impacted sediments have different characteristics compared to the background CLP River water, indicating that utilities may need to alter their treatment processes to handle increased sediment loads, raised nutrient and TOC concentrations, and to control N-DBPs.
References
Aitkenhead JA, McDowell WH (2000) Soil C: N ratio as a predictor of annual riverine DOC flux at local and global scales. Global Biogeochem Cy 14:127–138
Araya SN, Fogel ML, Berhe AA (2016a) Thermal alteration of soil organic matter properties: a systematic study to infer response of Sierra Nevada climosequence soils to forest fires. Soil. doi:10.5194/soil-2016-57
Araya SN, Meding M, Berhe AA (2016b) Thermal alteration of soil physico-chemical properties: a systematic study to infer response of Sierra Nevada climosequence soils to forest fires. Soil 2:351–366
Bodi MB, Martin DA, Balfour VN, Santin C, Doerr SH, Pereira P, Cerda A, Mataix-Solera J (2014) Wild land fire ash: production, composition and eco-hydro-geomorphic effects. Earth-Sci Rev 130:103–127
Brown, T.J.; Hall, B.L.; Westerling, A.L. (2004) The impact og twenty-first century climate change on wildland fire danger in the western United States: An applications perspective. Climate Change, 62:365-388.
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143:1–10
Chang EE, Chiang PC, Ko YW, Lan WH (2001) Characteristics of organic precursors and their relationship with disinfection by-products. Chemosphere 44:1231–1236
Clark JM, Lane SN, Chapman PJ, Adamson JK (2007) Export of dissolved organic carbon from an upland peatland during storm events: implications for flux estimates. J Hydrol 347:438–447
Coble PG (1996) Characterization of marine and terrestrial DOM in seawater using excitation emission matrix spectroscopy. Mar Chem 51:325–346
Costa MR, Calvao AR, Aranha J (2014) Linking wildfire effects on soil and water chemistry of the Marao River watershed, Portugal, and biomass changes detected from Landsat imagery. Appl Geochem 44:93–102
Covington WW, Debano LF, Huntsberger TG (1991) Soil-nitrogen changes associated with splash pile burning in pinyon-juniper woodlands. For Sci 37:347–355
De la Rosa JM, Gonzalez-Perez JA, Gonzalez-Vila FJ, Knicker H (2013) Medium term effects of fire induced soil organic matter alterations on Andosols under Canarian pine (Pinus canariensis). J Anal Appl Pyrol 104:269–279
DeBano LF (2000) The role of fire and soil heating on water repellency in wildland environments: a review. J Hydrol 231:195–206
Dick JJ, Tetzlaff D, Birkel C, Soulsby C (2015) Modelling landscape controls on dissolved organic carbon sources and fluxes to streams. Biogeochemistry 122:361–374
Doerr SH, Santin C (2016) Global trends in wildfire and its impacts: perceptions versus realities in a changing world. Philos T R Soc B 371:50345–50345
Gresswell, R.E. (1999) Fire and aquatic ecosystems in forested biomes of North America. Transactions of 10.1007/s11368-016-1604-1 the America Fisheries Society, 128:193-221
Heath JT, Chafer CJ, Bishop TFA, Van Ogtrop FF (2015) Wildfire effects on soil carbon and water repellency under eucalyptus forest in Eastern Australia. Soil Res 53:13–23
Hohner AK, Cawley KM, Oropeza J, Summers RS, Rosario-Ortiz FL (2016) Drinking water treatment response following a Colorado wildfire. Water Res 43:1118–1129
Homann PS, Bormann BT, Darbyshire RL, Morrissette BA (2011) Forest soil carbon and nitrogen losses associated with wildfire and prescribed fire. Soil Sci Soc Am J 75:1926–1934
Hua G, Reckhow DA (2007) Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res 41:1667–1678
Inamdar S, Finger N, Singh S, Mitchell M, Levia D, Bais H, Scott D, McHale P (2012) Dissolved organic matter (DOM) concentration and quality in a forested mid-Atlantic watershed, USA. Biogeochemistry 108:55–76
Inamdar S, Singh S, Dutta S, Levia D, Mitchell M, Scott D, Bais H, McHale P (2011) Fluorescence characteristics and sources of dissolved organic matter for stream water during storm events in a forested mid-Atlantic watershed. J Geophys Res-Biogeo 116:23
Johnson DW, Curtis PS (2001) Effects of forest management on soil C and N storage: meta analysis. For Ecol Manag 140:227–238
Keeler C, Kelly EF, Maciel GE (2006) Chemical-structural information from solid-state C-13 NMR studies of a suite of humic materials from a lower montane forest soil, Colorado, USA. Geoderma 130:124–140
Knicker H (2007) How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 85:91–118
Knicker H, Almendros G, GonzalezVila FJ, Martin F, Ludemann HD (1996) C-13- and N-15-NMR spectroscopic examination of the transformation of organic nitrogen in plant biomass during thermal treatment. Soil Biol Biochem 28:1053–1060
Knicker H, Gonzalez-Vila FJ, Polvillo O, Gonzalez JA, Almendros G (2005) Fire-induced transformation of C- and N-forms in different organic soil fractions from a Dystric Cambisol under a Mediterranean pine forest (Pinus pinaster). Soil Biol Biochem 37:701–718
Knicker H, Hilscher A, Gonzalez-Vila FJ, Almendros G (2008) A new conceptual model for the structural properties of char produced during vegetation fires. Org Geochem 39:935–939
Knicker H, Nikolova R, Dick DP, Dalmolin RSD (2012) Alteration of quality and stability of organic matter in grassland soils of Southern Brazil highlands after ceasing biannual burning. Geoderma 181:11–21
Korak JA, Dotson AD, Summers RS, Rosario-Ortiz FL (2014) Critical analysis of commonly used fluorescence metrics to characterize dissolved organic matter. Water Res 49:327–338
Krasner SW, Weinberg HS, Richardson SD, Pastor SJ, Chinn R, Sclimenti MJ, Onstad GD, Thruston AD (2006) Occurrence of a new generation of disinfection byproducts. Environ Sci Technol 40:7175–7185
Lee, W.T.; Westerhoff, P. (2005) Dissolved organic nitrogen measurement using dialysis pretreatment. Environmental Science and Technology, 39:879-884
Liang L, Singer PC (2003) Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environ Sci Technol 37:2920–2928
Mast MA, Clow DW (2008) Effects of 2003 wildfires on stream chemistry in Glacier National Park, Montana. Hydrol Process 22:5013–5023
McKnight DM, Boyer EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT (2001) Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol Oceanogr 46:38–48
Merino A, Ferreiro A, Salgado J, Fonturbel MT, Barros N, Fernandez C, Vega JA (2014) Use of thermal analysis and solid-state C-13 CP-MAS NMR spectroscopy to diagnose organic matter quality in relation to burn severity in Atlantic soils. Geoderma 226:376–386
Miller WW, Johnson DW, Gergans N, Carroll-Moore EM, Walker RF, Cody TL, Wone B (2013) Update on the effects of a Sierran wildfire on surface runoff water quality. J Environ Qual 42:1185–1195
Mitic VD, Jovanovic VPS, Ilic MD, Mandic SDN (2015) Impact of wildfire on soil characteristics and some metal content in selected plants species of Geraniaceae family. Environ Earth Sci 73:4581–4594
Moody JA, Martin DA (2001) Initial hydrologic and geomorphic response following a wildfire in the Colorado Front Range. Earth Surf Proc Landf 26:1049–1070
Murphy JD, Johnson DW, Miller WW, Walker RF, Carroll EF, Blank RR (2006) Wildfire effects on soil nutrients and leaching in a Tahoe Basin watershed. J Environ Qual 35:479–489
Murphy SF, McCleskey RB, Writer JH (2012) Effects of flow regime on stream turbidity and suspended solids after wildfire, Colorado Front Range. Wildfire and Water Quality: Processes, Impacts and Challenges 354:51–58
Norouzi M, Ramezanpour H (2013) Effect of fire on soil nutrient availability in forests of Guilan, north of Iran. Carpathian Journal of Earth and Environmental Sciences 8:157–170
Pereira P, Ubeda X, Martin D, Mataix-Solera J, Cerda A, Burguet M (2014) Wildfire effects on extractable elements in ash from a Pinus pinaster forest in Portugal. Hydrol Process 28:3681–3690
Pereira P, Ubeda X, Martin DA (2012) Fire severity effects on ash chemical composition and water-extractable elements. Geoderma 191:105–114
Poulin BA, Ryan JN, Aiken GR (2014) Effects of iron on optical properties of dissolved organic matter. Environ Sci Technol 48:10098–10106
Prieto-Fernandez A, Acea MJ, Carballas T (1998) Soil microbial and extractable C and N after wildfire. Biol Fert Soil 27:132–142
Prieto-Fernandez A, Carballas M, Carballas T (2004) Inorganic and organic N pools in soils burned or heated: immediate alterations and evolution after forest wildfires. Geoderma 121:291–306
Raison RJ (1979) Modification of the soil environment by vegetation fires, with particular reference to nitrogen transformations—review. Plant Soil 51:73–108
Revchuk AD, Suffet IH (2014) Effect of wildfires on physicochemical changes of watershed dissolved organic matter. Water Environ Res 86:372–381
Rhoades CC, McCutchan JH, Cooper LA, Clow D, Detmer TM, Briggs JS, Stednick JD, Veblen TT, Ertz RM, Likens GE, Lewis WM (2013) Biogeochemistry of beetle-killed forests: explaining a weak nitrate response. Proc Natl Acad Sci U S A 110:1756–1760
Romera-Castillo C, Chen M, Yamashita Y, Jaffe R (2014) Fluorescence characteristics of size-fractionated dissolved organic matter: implications for a molecular assembly based structure? Water Res 55:40–51
Sanderman J, Lohse KA, Baldock JA, Amundson R (2009) Linking soils and streams: sources and chemistry of dissolved organic matter in a small coastal watershed. Water Resour Res 45:13
Santin C, Doerr SH (2016) Fire effects on soils: the human dimension. Philos T R Soc B 371:50171–50171
Shakesby RA, Bento CPM, Ferreira CSS, Ferreira AJD, Stoof CR, Urbanek E, Walsh RPD (2015) Impacts of prescribed fire on soil loss and soil quality: an assessment based on an experimentally-burned catchment in central Portugal. Catena 128:278–293
Singer, P.C.; Obolensky, A.; Greiner, A. (1995) DBPs in chlorinated North Carolina drinking waters. Journal of the American Water Works Association, 87:83-92
Son, J.H.; Kim, S.; Carlson, K.H. (2015) Effects of wildfire on river water quality and riverbed sediment phosphorus. Water Air and Soil Pollution, 226:26
Specht CH, Frimmel FH (2000) Specific interactions of organic substances in size-exclusion chromatography. Environ Sci Technol 34:2361–2366
Summers RS, Hooper SM, Shukairy HM, Solarik G, Owen D (1996) Assessing the DBP yield: uniform formation conditions. J Am Water Works Ass 88:80–93
VanBergen PF, Bull ID, Poulton PR, Evershed RP (1997) Organic geochemical studies of soils from the Rothamsted classical experiments—1. Total lipid extracts, solvent insoluble residues and humic acids from Broadbalk wilderness. Org Geochem 26:117–135
Vieira DCS, Fernandez C, Vega JA, Keizer JJ (2015) Does soil burn severity affect the post-fire runoff and interrill erosion response? A review based on meta-analysis of field rainfall simulation data. J Hydrol 523:452–464
Wang JJ, Dahlgren RA, Ersan MS, Karanfil T, Chow AT (2015) Wildfire altering terrestrial precursors of disinfection byproducts in Forest Detritus. Environ Sci Technol 49:5921–5929
Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. (2006) Warming and earlier spring increase western US forest wildfire activity. Science, 313:940-943
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic matter. Environ Sci Technol 37:4702–4708
Williams, M.R.; Melack, J.M. (1997) Effects of prescribed burning and drought on the solute chemistry of mixed-conifer forest streams of the Sierra Nevada, California. Biogeochemistry, 39:225-253
Writer JH, McCleskey RB, Murphy SF (2012) Effects of wildfire on source-water quality and aquatic ecosystems, Colorado Front Range. Wildfire and Water Quality: Processes, Impacts and Challenges 354:117–122
Xue L, Li QJ, Chen HY (2014) Effects of a wildfire on selected physical, chemical and biochemical soil properties in a Pinus massoniana forest in South China. Forests 5:2947–2966
Yang X, Shang C, Lee W, Westerhoff P, Fan CH (2008) Correlations between organic matter properties and DBP formation during chloramination. Water Res 42:2329–2339
Acknowledgments
The authors thank three anonymous reviewers. The authors acknowledge support from the Water Research Foundation (project no. 4524). We would like to thank Ms. Clare Steninger and Mr. Brent Morgensen for their help with collecting sediment samples; Leigh Gilmore and John Meyer for processing samples (filtering, turbidity, UV, etc); Dorothy Noble for running the DBP analysis; and Jesse Nestler for helping with the SEC analysis. Thanks to Deborah Martin, USGS, Boulder, CO, for helpful comments. Funding for the NMR work was provided by the USDA NIFA AFRI postdoctoral fellowship no. 2012-01330. A portion of the research was performed using EMSL, a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory. We would like to thank Dr. Sarah Burton and Dr. Andrew Lipton for their assistance with NMR setup and acquisition.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Claudio Zaccone
Electronic supplementary material
Electronic Supplementary Material The supporting information section contains the 13 C NMR spectra, selected fluorescence excitation emission matrices, a table of size exclusion chromatography number averaged and MW estimations, size exclusion chromatograms, and a table describing the reasons for choosing samples for bench scale chlorination experiments.
ESM 1
(DOCX 8287 kb)
Rights and permissions
About this article
Cite this article
Cawley, K.M., Hohner, A.K., McKee, G.A. et al. Characterization and spatial distribution of particulate and soluble carbon and nitrogen from wildfire-impacted sediments. J Soils Sediments 18, 1314–1326 (2018). https://doi.org/10.1007/s11368-016-1604-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1604-1