Abstract
The aim of this work was to examine the consequences of the wildfire on the differences in qualitative and quantitative composition of the soil, and Cu, Pb, Cd, and Zn levels in the soils and plant species of Geraniaceae family at Vidlic Mountain, Serbia. Main soil characteristics organic matter content, conductivity, redox potential, pH (H2O), pH (KCl), and chloride content in soil samples from post-fire areas and from fire non-disturbed areas were studied. The optimized three-step sequential extraction procedure was applied to the analysis of Cu, Pb, Cd, and Zn levels in the soils. Distribution of heavy metals in aerial parts and roots of examined species was investigated too. For some characteristic parameters of soil, like conductivity, redox potential, chloride content, the impact of fire on the soil habitats followed the same trend for all plants species. Content of copper, lead, and zinc in plant material derived from post-fire areas was generally greater than their content in the plants that grew on the area not exposed to fire. Although the content of cadmium was generally higher in all fractions of the samples from the locality not exposed to the fire, content of that metal in the plant parts was reversed. Most characteristics of soil are significantly altered as a consequence of fire. Majority of soil samples from the post-fire area had increased content of analyzed metals, except cadmium. Fire caused slightly increased bioavailability of the examined metals. The biggest difference in the content of the studied metals in the soil from the post-fire areas and the area not exposed to fire was in the fraction which includes metals associated with organic matter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wildfires, degrade ecosystems by increasing nutrient and soil losses through leaching and erosion, are one of the most widespread factors of ecosystem degradation around the world. Many forested landscapes experience a natural disturbance regime dominated by fire. Forest composition and fire severity are the key determinants of post-fire forest redevelopment. Fire consumes organic matter and creates dead wood; thus it can influence nutrient dynamics, productivity, and the habitat value of the forest for decades or centuries. Fire also creates a unique set of environmental conditions in the short term to which a diversity of biota are adapted. Indeed, there are plant species that are considered fire-dependant (Macdonald 2007).
Fires induce changes in soil nutrient balances and biogeochemical cycles as a result of biomass combustion and the increase of runoff and soil erosion because fires lead to significant removal of soil organic matter, deterioration of soil structure and porosity, considerable losses of nutrients through volatilization (Couto-Vazquez and Gonzalez-Prieto 2008).
During wildfire, some carbon compounds could be lost from the forest floor by volatilization. Also, a rearrangement of carbon moieties occurs within the organic matter, and refractory carbon forms are synthesized de novo (Gonzalez-Perez et al. 2004).
Soil is a variable mixture of minerals, organic matter, and water capable of supporting plant life on the earth’s surface. It is the final product of the weathering action of physical, chemical, and biological processes on rocks, which largely produces clay minerals. The weathering of parent rocks and minerals to form the inorganic soil components results ultimately in the formation of inorganic colloids which may be made available to plants as needed. Inorganic soil colloids often absorb toxic substances in soil (Manahan 2000).
The organic portion of soil consists of plant biomass in various stages of decay. High populations of bacteria, fungi, and animals such as earthworms may be found in soil. Soil contains air spaces and generally has a loose texture. The solid fraction of typical productive soil is approximately 5 % organic matter and 95 % inorganic matter. Although organic material comprises a relatively small portion of soil, it plays a key role in many soil processes. It serves as a source of food for micro-organisms and influences the physical properties of soil. Some organic compounds contribute to the weathering of mineral matte.
Heavy metals are natural components of the Earth’s crust. They cannot be degraded or destroyed and their presence in the mineral fraction has a great influence on soil geochemistry. Metals are also present in the organic fraction, frequently as bound forms, with some metal recycling occurring as a result of organic matter decomposition. Furthermore, heavy metals can be involved in a series of complex chemical and biological interactions. Trace metals can exist in a variety of forms in composts. These forms include water soluble, exchangeable, linked to organic substances, occluded or co-precipitated with oxides, carbonates and phosphates, or other secondary minerals and ions in the crystalline lattices of the primary minerals (Lake et al. 1984; Petruzzelli 1989; He et al. 1992; Iwegbue et al. 2006).
The distribution of metals between the specific forms varies widely according to the individual metal and the characteristics of the compost, which depend on the physical, chemical, and composting process. The most important factors which affect their mobility are pH, sorbent nature, presence and concentration of organic and inorganic complexing ligands, including humic and fulvic acids, root exudates, and nutrients. Biotic and abiotic redox reactions are very important in controlling the oxidation state and thus, the mobility and the toxicity of many elements (Violante et al. 2010).
Content of heavy metals in a soil is the result of inputs of the metal from three different types of sources: the minerals in the soil parent material (the weathered rock on which the soil has developed), anthropogenic inputs onto land, such as pesticides and manures, and deposition from the atmosphere (largely due to pollution). Heavy metals get into plants via adsorption which refers to binding of materials onto the surface or absorption which implies penetration of metals of metals into the inner matrix.
Plants have evolved highly specific and very efficient mechanisms to obtain essential micronutrients from the environment, even when present at low ppm levels. Plant roots, aided by plant-produced chelating agents and plant-induced pH changes and redox reactions, are able to solubilize and take up micronutrients from very low levels in the soil, even from nearly insoluble precipitates. Plants have also evolved highly specific mechanisms to translocate and store micronutrients. These same mechanisms are also involved in the uptake, translocation, and storage of toxic elements, whose chemical properties simulate those of essential elements (U.S. Department of Energy 1994).
All metals are toxic at higher concentrations, although many of them are essential, because they cause oxidative stress by formation of free radicals. Another reason why metals may be toxic is that they can replace essential metals in pigments or enzymes disrupting their function (Ghosh and Singh 2005).
Lead and cadmium are among the most dangerous metals that can cause acute and chronic pollution of the environment. Copper and zinc in increased concentrations may also cause harmful effects (Blagojevic et al. 2009).
Copper is an essential redox component for the normal healthy growth and reproduction of all higher plants and animals because it is required for a wide variety of processes, including the electron transfer reactions of respiration (cytochrome oxidase, alternate oxidase) and photosynthesis (plastocyanin), the detoxification of superoxide radicals (Cu–Zn superoxide dismutase) and lignification of plant cell walls (laccase) (Fox and Guerinot 1998). It is well known that copper and zinc when present in low concentrations, are important micronutrients, required for the activity of various types of enzymes, while in high concentrations, these two metals become toxic to plants. Copper, at concentrations higher than 1 µM, is a potent inhibitor of photosynthetic electron transport (Mohanty et al. 1989). Zinc is also one of the micronutrients essential for normal plant growth, but only a small Zn is non‐redox active but has a key structural and/or catalytic role in many proteins and enzymes. So, the amount of Zn is required (25–150 μg g−1 in dry tissue) (Adriano 1986). Plants take up zinc in its divalent form. The role of Zn in cells is based on its behavior as a divalent cation that has a strong tendency to form tetrahedral complexes. Zn is an essential catalytic component of over 300 enzymes, including alkaline phosphatase, alcohol dehydrogenase, Cu–Zn superoxide dismutase, and carbonic anhydrase. Zn also plays a critical structural role in many proteins (Fox and Guerinot 1998).
Cadmium could cause a transient depletion of GSH and an inhibition of antioxidative enzymes, especially of glutathione reductase. Available data suggest that cadmium, when not detoxified rapidly enough, may trigger, via the disturbance of the redox control of the cell, a sequence of reactions leading to growth inhibition, stimulation of secondary metabolism, lignification, and finally cell death. This view is in contrast to the idea that cadmium results in unspecific necrosis. Plants in certain mycorrhizal associations are less sensitive to cadmium stress than non-mycorrhizal plant (Schutzendubel and Polle 2002).
Although Cd and Pb have no known role as nutrients, plants readily accumulate them in their system. Some trace elements, such as Cd and Zn, are rather mobile in soils and thus readily available for plants, although the uptake mechanisms are not well known. Trace elements can also penetrate into the leaves and their amount would depend upon the metal and plant species. For example, Cd, Zn, and Cu can penetrate into the leaf, while Pb is mostly adsorbed to the epicuticular lipids at the surface (Jeliazkova and Craker 1998).
Lead in soil is classified as a weak Lewis acid, which implies a strong covalent character to many of the ionic bonds it forms in soils and plants. Lead present in the soil is nearly always tightly bound to organic or colloidal material or in a precipitated form. Although Pb is not an essential nutrient for plants, majority of lead is easily taken up by plants from the soil and accumulated in root while only a small fraction was translocated upward to the shoots (Patra et al. 2004). At the root surface Pb binds to carboxyl groups of mucilage uronic acids. Mucilage binding restricts metal uptake into the root and establishes an important barrier protecting the root system. Pb inhibits chlorophyll synthesis by causing impaired uptake of essential elements such as Mg and Fe by plants (Burzynski 1987). It damages the photosynthetic apparatus due to its affinity for protein N- and S- ligands (Ahmed and Tajmir-Riahi 1993). Pb also causes strong dissociation of the oxygen evolving extrinsic polypeptide of PS II and displacement of Ca, Cl−, Mn from the oxygen-evolving complex (Rashid and Mukherji 1991). Lead is available to plants from soil and aerosol sources. The uptake, transport, and accumulation of Pb by plants are strongly depended on soil type and plant species, and they differ significantly with plant species.
Vidlic Mountain is located in the central area of the Balkan Peninsula, on the northeastern edge between Pirot and Sofia Valley predominantly in Serbia. The fire on Vidlic Mountain, caused by human factor, started on 20 July 2007, lasted for 10 days and burned over 2,500 ha of low vegetation, scrub, and forests (Ministry of Environment and Spatial Planning 2008). That year, the vegetation was totally destroyed. After the fire burned out, only a great amount of dust remained. In the area of beech forests, a biologically empty space was created. However, next year, at this place, with new environmental conditions, new phytocoenoses were formed.
Since the direct effects of fire on soil characteristics appear to be observable years after disturbance, the aim of this work was to examine the consequences of the 2007 fire on the differences in qualitative and quantitative composition of the soil and plants at Vidlic Mountain. The study was focused on Cd, Cu, Pb, and Zn, because these metals are abundant in agricultural soils (Forstner 1995), but at the same time they can be harmful to the living organisms at very high concentration. As the representative species in which content of heavy metals will be analyzed, we selected plants from Geraniaceae family (Geranium macrorrhizum L., Geranium robertianum L., Geranium bohemicum L.) because mentioned plants were grown at both sites: on the post-fire area and in natural environment not affect by fire.
Vidlic is the westernmost part of the mountain system Stara planina. Stara Planina (translated as “Old Mountain”), in other parts also called Balkan Mountain or simply Balkan, is a tertiary mountain 600 km in length that is situated in Serbia and Bulgaria, with the larger extent in Bulgaria. The Serbian part of Stara Planina is positioned on the Eastern border with the Republic of Bulgaria. On the Serbian side, it forms a belt that is approximately 100 km long and between 4 and 30 km wide. The karst plateau in Vidlic is elongated in the southeastern–northwestern direction. In contrast to other regions of mountain system, Stara planina and Mountain Vidlic are represented by unique geological structure and climate conditions. The geological structure of the Stara planina mountain is relatively simple, made of two main rock types: the Permian–Triassic red sandstone, the Triassic limestones and dolomites, yet this mountain is a treasury of geological heritage due to the variety of sediments of different ages. Geological composition of the Vidlic mountain is quite simple and its basic mass consists of limestones and dolomites (Petrovic 1971; Ciric 1989). Geological composition, tectonic events during the past periods, as well as flora and fauna are natural factors that have influenced the formation of soil media and its pedological types. The brown soil on limestone (which is relatively deep soil and if the limestone is softer and more porous it is deeper) and skeletal brown soil (which is a shallow and present incompletely developed land creation) prevail at the sampling area.
The purpose of present study was to investigate the consequences of the wildfire on soil characteristics (organic matter content, conductivity, redox potential, pH (H2O), pH (KCl), and chloride content), and Cu, Pb, Cd, and Zn content in soil and plant species of Geraniaceae family at Vidlic Mountain, Serbia. The above results are part of comprehensive studies in which the other plant families were tested in the same manner (Stankov Jovanovic et al. 2011).
All measurements, which include the parameters of soil and plants from family Geraniaceae from post-fire area, were carried out in parallel for the same plants collected from similar habitats in environment not disturbed by fire, in order to assess consequences of fire in real time.
Materials and methods
Soil sampling
Soil samples from six sampling points in post-fire areas on Vidlic Mountain, and areas nearby that were not affected by fire (natural environment), were collected in the end of April 2008 using a GPS instrument to establish the exact coordination points.
The sampling sites lay within an elevation range from 1,080 to 1,100 m.a.s.l. Environmental and geological characteristics of soil/plant sampling sites are shown in Table 1. From each sampling points, five soil samples were gathered and mixed properly to obtain a composite sample mixture. The soil samples were taken via a soil core sampler in such a way that the core of the soil was removed of radius 5 cm and depth 30 cm and placed in labeled cellophane bags. The sampling tools were washed with soap and rinsed with distilled water after each sampling. In the laboratory, bulk soil samples were spread on trays and were air dried at ambient conditions for 2 weeks. Then, the samples were grounded by mortar and pestle, sieved through a 2 mm mesh, and fine homogenized soil powder stored in tightly closed bottles. Map of the studied area is shown in Fig. 1.
Plant sampling
Plant samples were collected simultaneously with the soil samples. Voucher specimens of analyzed plants are deposited at the Herbarium of the Faculty of Biology, Belgrade (BEOU) and voucher numbers are 16430 for G. robertianum L., 16431 for G. macrorrhizum L., and 16432 for G. bohemicum L. Before the analysis, the plant root and shoot parts were separated, air dried and cut into small pieces.
Acidity, redox potential, conductivity, organic matter, and chloride content determination in soil samples
Soil pH was determined in water (pH (H2O)) and 1 M KCl (pH (KCl)) at the ratio of soil: solution ratio 5 g soil: 25 mL liquid after a 2-h equilibration period.
The electrical conductivity (EC) and redox potential measurements in samples suspensions (25 mL liquid per 5 g soil) were done after 2 h mixing of soil sample with deionized water, applying standard conductometric method.
Organic carbon (OC) concentrations in soil samples were performed by Walkley–Black acid digestion method (Walkley and Black 1934) with some modification. A soil sample (0.5 g) is treated with 10 mL of K2Cr2O7 0.2 mol L−1 and 20 mL of concentrated H2SO4. The material was heated in a plate and homogenized carefully until reaching the temperature of 150 °C. After that, the material was removed from the plate, cooled to room temperature, and left to rest for 40 min. Later, 50 mL of distilled water was added and the sample shaken, then transferred the content into a 100-mL graduated flask and adjusted the volume, left to rest for 10–15 min, and then filtrated. The amount of 1 mL of concentrated phosphoric acid was added to the filtrate, and the extract was titrated with 0.25 mol L−1 of ammoniac ferrous sulfate solution, using diphenylamine 0.5 % as indicator. Soil organic carbon content is calculated from the difference in FeSO4 used between a blank and a soil solution (Pereira et al. 2006).
Total organic carbon is a component of soil organic matter, and about 58 % of the mass of organic matter existing as carbon. So, the proportion of organic matter in the soil sample was estimated as the amount of total organic carbon in a sample multiply by 100/58 (or 1.72).
Determination of cation-exchange capacity (CEC)
Exchangeable cations were extracted by 0.1 M CH3COONH4 (5 g soil per 25 mL solution), allowed it to stand overnight and filtered. Extraction was continued with three more portions of 25 mL of ethanol, all filtrates were merged and made up to 100 mL volume with deionized water. Concentrations of exchangeable cations were determined by flame atomic absorption spectrometry. Solid residue was mixed with 25 mL KCl (pH 2.5) solution, shaken for 30 min, and filtered into a 100 mL volumetric flask. Half of the filtrate was transferred into a 250-mL round-bottom flask, diluted with 50 mL of water, borate buffer was added, and distillation was performed. The distillate was analyzed using titrimetry (0.0095 M HCl standard solution) (Radojevic and Bashin 1999).
Soil samples treatment for pseudo total cation analysis
The total metal contents were determined after “aqua regia” digesting procedure. Exactly weighted soil samples (1 g) were first submerged with concentrated HNO3 (10 mL), allowed to stay overnight, and then heated to a small volume. After cooling, 5 mL of the mixture composed of H2O2 (30 %):H2O (v/v) = 3:2 was added and suspension was evaporated to a small volume. After cooling, 3 mL of H2O2 (30 %) was added and evaporation continued. After re-cooling, 10 mL of concentrated HCl was added and the mixture was left overnight. Obtained digestates were filtered, both the filter papers and residues rinsed, first with hot HCl and then hot deionized water. The digestates were then diluted to a final volume of 50 mL in accordance to the Method 3050B (US EPA 1996).
Sequential extraction of soils
The sequential extractions’ procedures are summarized in Table 2. The soil samples were consecutively shaken with these agents at given conditions and obtained suspensions were centrifuged (2,500 rpm) for 20 min (Quevauviller 1998).
Plant material digestion for heavy metal analysis
Plant samples were thoroughly washed to remove all adhered soil particles, and then air dried to a constant mass, root of each plant was separated from the shoot, and all parts were cut into small pieces, and finally sieved through 2 mm sieve. Content of heavy metals (Zn, Cu, Cd, and Pb) in the plant material was determined after acid digestion (Hoenig 2001; Tuzen 2003).
Instrumentation and chemicals
Measurements of soil pH were performed with pH-meter (Hach-sensION 3). For calibration, following buffer solutions were used : pH = 4 ± 0.02 (Titrisol, Merck, citrate buffer), pH = 7 ± 0.02 (Titrisol, Merck, phosphate buffer) and pH = 10 ± 0.02 (Titrival, Kemika, boric acid–potassium chloride-sodium hydroxide).
For conductivity measurement, conductivity-meter (Hach-sensION 5) was utilized. Hach sodium chloride, standard solution, 1,000 µS cm−1, was used as calibration solution.
Measurement of soil redox potential was carried out at pH-meter (Hach-sensION 3) with platinum electrode and silver–silver-chloride electrode as reference.
A Perkin Elmer AAS spectrophotometer 2380 (air/acetylene flame, hollow cathode lamp for absorption technique, deuterium lamp for back ground correction at the recommended current and conditions) was employed to measure the metal content of both plant and soil-digested samples. Calibration solutions were prepared as multi-element standards by dilution and mix of appropriate volumes of stock standard solutions (Merck, p.a. 1 g L−1 for each analyzed metal) in order to minimize the interference effects. The concentrations of elements in these multiple element calibration solutions were analogous to AAS linear range determination for each of the analyzed metals. All chemicals that used in the experiments were Merck, p.a. grade, unless it was stated otherwise. Deionized water with specific conductivity of 0.05 µS cm−1 was applied for washing of laboratory vessels and solution preparation.
Quality control
A determination of metals concentrations in digested samples was done applying calibration curve method. The analytical detection limits (triple standard deviation of the baseline noise/sensitivity) for each element were 0.009 ppm for Cd, 0.025 ppm for Cu, 0.06 ppm for Pb, and 0.008 ppm for Zn.
A standard reference material (LGC6187, leachable metals in river sediment, certified by LGC) was used in the sequential extraction, digestion, and analysis as part of the quality assurance/control protocol in the same way as soil samples to check the accuracy of the procedure. The recovery percentages were (101.4 ± 2.1 to 104.2 ± 2.5) % for Cd; (98.6 ± 1.2 to 101.0 ± 1.8) % for Cu; (95.6 ± 0.4 to 98.8 ± 0.7) % for Pb, and (98.2 ± 0.4 to 99.0 ± 0.5) %, for Zn. It is evident that the concentrations of heavy metals determined agreed well with the reported certified values, confirming the accuracy of the applied procedures.
Statistical evaluation of data
The evaluation of the obtained analytical data was performed by statistical means. The elimination of outliers was done by Grubb’s test, for each method the arithmetic mean, the standard deviation and the coefficient of variation were calculated by Statistica 7 program. The statistical significance was measured using the two-tailed Mann–Whitney U-test at a significance level of α = 0.05. The null hypothesis is that all samples were from the same population, while the alternate hypothesis is that they came from different populations corresponding to impact of wildfire.
Results
Main soil characteristics
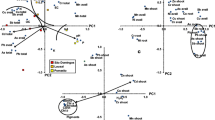
In order to examine impacts of fire on the areas populated with selected Geraniaceae species, main soil characteristics were determined. Parallel determinations were done for soil samples of corresponding Geraniaceae species from areas affected by the fire (post-fire environment) and nearby locations which had not suffered by fire (natural environment) and both sets of the results are shown in Fig. 2.
In Table 3, ΔpH of soils from post-fire area as well as from natural environment habituated by plant species G. robertianum, G. macrorrhizum, and G. bohemicum are presented.
Heavy metals
The sequential extraction protocol (Quevauviller 1998) was applied to the analysis of Cu, Pb, Cd, and Zn levels in the soils. Three independent fractions are produced by this protocol. A first fraction bound to carbonates (F1) corresponding to the acetic acid extractable metals or exchangeable fraction; the second one fraction bound to metal (oxy) hydroxides (F2) corresponding to the acidic hydroxylamine extractable metals or acid reducible fraction; and the third fraction bound to organic matter (F3) the acidic hydrogen peroxide extractable metals or organic fraction (Ramos et al. 1999; Yobouet et al. 2010). Non-residual fractions were composed of elements which could be bioavailable whereas the residual fraction presents elements which are never bioavailable. The concentrations in ppm of Cu, Zn, Cd, and Pb recovered with sequential extraction were calculated for each step (F1 to F3) as well as for the residual fraction (FR) are presented in Fig. 3.
The ways of plant intake of heavy metals can be estimated following their distribution in plants roots and shoots. Distribution of heavy metals in shoots/roots of selected Geraniaceae plant species is presented in Fig. 4.
Discussion
The effects of a fire may be very different on soils of different textures or chemical properties. Variation in fire effects may also occur within ecosystems because of differences in site characteristics, fuel conditions, and weather prior to, during, and after the fire. Erosion by wind (Aeolian), water, or gravity often, but not always, increases following fire. The severity and duration of the accelerated erosion depend on several factors, including soil texture, slope, recovery time of protective cover, the amount of residual litter and duff, and afterburn precipitation intensity. Raindrop splash, sheet and rill erosion, dry ravel, soil creep, and even mass wasting can occur. Several chemical changes in soils may occur as a direct result of fire, including a change in pH on some sites, the formation of water-repellant soil layers, hydrophobicity on some sites and changes in organic matter (National Wildfire Coordinating Group 2001).
Organic matter
The importance of soil organic matter in supplying nutrients contributes to cation-exchange capacity and improving soil structure. The effect of fire on earth organic matter may greatly vary depending on the type and intensity of a fire (high, low, etc.), the nature of burnt materials (wood, organic layover, dry grass), and type and humidity of the soil at the moment of fire. The effects of fire on processes in the soil and their intensity may therefore be quite variable and cannot be generalized (Jose et al. 2004). Depending on the type of fire, type of soil and its properties (porosity, aeration, and moisture at the moment of fire), as well as the quantity of charred organic remnants, the quantity of carbon in the soil surface layer may either increase or drop. The amount of soil organic matter consumed by fire depends on soil moisture content, amount and duration of heating, and amount of organic matter available for combustion or distillation. Changes in soil organic matter may also cause hydrophobicity. This phenomenon occurs during the combustion process when distilled aliphatic hydrocarbons migrate into the soil profile and condense on soil particles to form a water-repellent layer. Hydrophobicity, which typically results in reduced infiltration rates, appears to be most common in dry, coarse-textured soils that are heated to 176–204 °C. These effects, however, are usually short-lived, generally disappearing after the first year. (National Wildfire Coordinating Group 2001).
Soil samples of G. robertianum and G. macrorrhizum from post-fire area contain greater amounts of organic matter than soil samples from natural environment. The higher percentage of organic carbon from post-fire area demonstrates higher rate of biodegradation. The organic matter content in the soil samples from natural environment is higher than in post-fire environment samples in the case of G. bohemicum. The highest organic matter content was determined in the soil from the post-fire environment site of plant species G. robertianum (5.91 %). The smallest change in organic matter content was observed in the case of plant species G. bohemicum (5.18 % in soil samples from natural environment and 4.97 % in soil samples from post-fire area. The result from Mann–Whitney U-test (U = 98, p = 0.553) indicated that there is certainly sufficient information to accept the null hypothesis and to declare that there is no difference between the content of organic matter in the soil samples from natural and post-fire area.
Cation-exchange capacity
The cation-exchange capacity of a soil is greatly influenced by the organic matter level. A high organic matter soil will have a much higher cation-exchange capacity than a low organic matter soil. The highest cation-exchange capacity (CEC) was determined in the soil of G. robertianum L. from post-fire areas (65 ppm), while the lowest was determined in the soil of G. macrorrhizum L. from natural environment (6.7 ppm). The difference of soil CEC, after and before wildfire, is most significant for soil from habitat of plant G. robertianum L. (17.5 ppm natural environment and 65 ppm post-fire area). We ran a Mann–Whitney’s U-test to evaluate the difference in cation-exchange capacity of soil samples from natural and post-fire area, and we did not find a significant effect of fire (U = 1, p = 0.150).
Soil electrical conductivity
Electrical conductivity of the soil samples from natural environment is higher for all plant species. The most similar values of conductivity are in the soil samples of G. bohemicum 441 mS m−1 for species from natural environment and 409 mS m−1 for species from post-fire areas. The lowest values of conductivity were determined in soil from habitat of G. macrorrhizum (311 mS m−1) from post-fire areas, while the highest conductivity was measured in the soil from habitat of G. robertianum (497 mS m−1) from natural environment. A Mann–Whitney test indicated that electrical conductivity of the soil samples from natural and post-fire area is statistically different U = 0, p = 0.040.
Redox potential
Redox potential (rH) is a measure of nutrient availability and mobility of heavy metals and also has important role in development of pedogenic properties such as soil color, iron depletions and concentrations (Sigg 2000). Redox potential measurements present a semi quantitative measure of the intensity of oxidation or reduction of a soil, which reflects many redox couples operating simultaneously in a dynamic system. The assessment of soil redox potential is particularly useful for characterizing the onset of reducing conditions in a soil caused by a lack of O2 and for partly interpreting their associated biogeochemical processes such as denitrification or bacterial degradation processes (Crawford et al. 2000). The redox potential is related to the concentration of several redox pairs in the soil, oxygen is the first acceptor that plays an important role (Aldridge and Ganf 2003). Soil samples from natural environment and post-fire area for all studied species have very similar values of the redox potential (Fig. 2). In all cases, values of redox potential of soil samples from natural environment were a very little higher for all plant species. Results of Mann–Whitney U-test indicate no significant difference between values of redox potential of soil samples from natural and post-fire area, U = 4, p = 0.850.
pH (H2O) and pH (KCl)
When nutrient metal ions are taken up by plant roots, hydrogen ion is exchanged for the metal ions. This process, plus the leaching of calcium, magnesium, and other metal ions from the soil by water containing carbonic acid, tends to make the soil acidic. Soil acts as a buffer and resists changes in pH. The buffering capacity depends upon the type of soil (Manahan 2000). Values of pH are very important. In a low pH environment, many metals are more soluble and thus can increase the movement of waste constituents. The ideal pH for many plants is slightly acidic, between 6.0 and 7.0. If the soil pH becomes too alkaline (pH > 8.5), iron, manganese, zinc, and other essential micronutrients are less available to plants. On the other hand, if the soil pH is too low (pH < 4.20), aluminum (Al), iron (Fe), and manganese (Mn) toxicity to plants may occur (US EPA 2006).
Wildfire can cause denaturation of organic acids. Significant increase in pH (even 3 pH units) can be observed immediately after fire at temperatures higher than 450–500 °C, when complete combustion occurs giving ash that is strongly basic (oxides, hydroxides, and carbonates of K, Ca and Mg). After the first precipitation, soil pH decreases due to the dissolution of basic compounds and their transport to water flows (Robichaud et al. 2000).
Elements critical for plant growth, such as nitrogen and phosphorus, become more available to plants after a fire that raises the pH of an acidic soil. Fire can significantly enhance site fertility when it raises the pH on cold, wet, acidic sites. Most cases of increased pH occurred on forest soils were the initial pH was acidic, and a large amount of organic material burned. Increases in nutrient availability may be highly significant. Rarely do arid or semi-arid soils, which are typically alkaline, exhibit increased pH after burning (National Wildfire Coordinating Group 2001).
pH (H2O), of soils from natural environment in distilled water are lower for G. robertianum and G. bohemicum while for G. macrorrhizum is vice versa. The values of pH (KCl) have the same behavior. The highest pH (H2O) is determined in soil of G. bohemicum from post-fire area (7.50). Also, in the soil of G. bohemicum is registered the most significant change in the pH (H2O), before (7.06) and after the fire (7.5). The lowest values of pH (H2O), were measured in soil samples of G. robertianum from both area: before (6.29) and after fire (6.59). The highest pH (KCl) was also determined in soil of G. bohemicum from post-fire area (7.12), while the lowest value of pH (KCl) was measured in soil of G. robertianum from natural environment.
The difference of pH (H2O) − pH (KCl) reveals the potential acidity or the exchange acidity of the soil. It highlights the displacement of the ions H+ adsorbed on the exchange sites of the adsorbing complex from soil toward the soil solution (argilo-humic colloids, complexes…) (Yobouet et al. 2010). The difference in soil pH in distilled water and in 1 M KCl solution was calculated in order to determine whether the particles of soil have neutral, positive, or negative charge. Sign of ΔpH is the opposite of soil particles charge. When the value of ΔpH is zero, the surface of particles of the soil is neutral. If other anions are present, they can be specifically adsorbed and thus may affect the pH decrease (Tan 1998). This difference is even lower than the soil is organic. Really, KCl allows a more significant displacement (than water) of the protons fixed by exchange with K+ in excess.
All soils have positive values of ΔpH which implies that the surface of soil colloidal particles is negatively charged. The presence of the organic matter decreases this exchange because of possible interaction of the mineral phases and the organic matter.
The results of the two-tailed Mann–Whitney U-test indicate no significant difference between pH (H2O) values of soils from natural and post-fire area U = 3, p = 0.550. There is satisfactorily information (U = 4, p = 0.850) to accept the null hypothesis and to declare that there is no difference between pH (KCl) values of soils from natural and post-fire area.
Chloride
The concentration of chloride in all soil samples was higher for post-fire area. The highest chloride concentration is determined in soil of G. macrorrhizum from post-fire area (125.67 ppm). Also, in the soil of G. macrorrhizum is registered the most significant change in the content of chloride before (54.3 ppm) and after the fire (125.67 ppm). The minimum chloride concentration was determined in soil from habitat of G. bohemicum (45.6 ppm) from natural environment. With an alpha level of 0.5 and U = 0, p = 0.045 the effect of the wildfire on the concentration of chloride in soil samples from natural and post-fire area was found to be statistically significant.
Heavy metals
Heavy metals occur naturally in soil, normally in low amounts, as result of weathering or other pedogenic processes acting on geological bedrock, where soils develop. Heavy metals are dangerous not only to plants and animals, but also to the entire environment. Some of the metals such as copper and zinc considered in the study are generally referred to as nutritional metals, at high concentration especially in the bioavailable form, they can become toxic. Lead and cadmium are two environmentally important very toxic heavy metals without any known biological function in plants, animals, and humans. The determination of total soil metal content alone is a poor indicator of metal toxicity since metals exist in different solid-phase forms that can vary greatly in terms of their bioavailability (Krishnamurti and Naidu 2002). Only a small portion of heavy metals and metalloids in soil is bioavailable. The mobility and availability of these pollutants are controlled by many chemical and biochemical processes such as precipitation–dissolution, sorption–desorption, complexation–dissociation, and oxidation–reduction. Not all the processes are equally important for each element, but all of them are affected by soil pH and biological processes.
The fraction bound to carbonates (F1) comprises metals adsorbed on the surface of soil. It is the most accessible and represents the exchangeable fraction. Metals on this fraction are easily mobile, can be easily transferred through rainfall to the other parts of the environment, and are assumed to be available. Concentrations of Cu, Zn, and Pb in Fraction 1 of soils from post-fire area were lower than metals concentrations in Fraction 1 of soils that were not exposed to the fire. In the case of cadmium concentration, the situation is reversed. In the Fraction 1 for all three examined plants, the concentration of cadmium in the soil samples from natural environment is higher than the one from the post-fire area.
Concentrations of Cu were in the range of 1.7 ppm for G. bohemicum to 3.1 ppm for G. robertianum in soil samples from natural environment, while in the samples from post-fire area concentrations of Cu were in the range of 2.2 ppm for G. bohemicum to 3.6 ppm for G. robertianum. Zn concentrations in soil samples from natural environment were in the interval ranging from 3.5 ppm for G. macrorrhizum to 9.1 for G. bohemicum. Soil samples of G. macrorrhizum from post-fire area have the smallest concentration of Zn 8.5 ppm, then, in the samples of G. bohemicum from post-fire area were 8.5 ppm of Zn. Cadmium levels were extremely low in exchangeable fraction for all samples tested and ranged from 0.1 to 1 ppm. Soil fractions 1 of all tested plants’ habitats are enriched in metal concentration for samples from natural environment except in the case of G. robertianum. Concentrations of Pb were in the range of 1.0 ppm for G. macrorrhizum to 1.5 ppm for G. robertianum in soil samples from natural environment, while in the samples from post-fire area concentrations of Cu were very slightly increased and ranged in the interval in the range of 1.2 ppm for G. macrorrhizum to 3.2 ppm for G. robertianum.
The fraction bound to iron and manganese oxides (F2) is sensitive to the redox potential changes and represents the fraction which can be solubilized in reducible conditions. Content of Cu, Zn and Pb in acid reducible fractions F2 of soil samples was higher for all post-fire soil samples. The highest concentration of copper was found in soil samples of G. robertianum from the post-fire area 2.4 ppm, while the lowest content of this metal, 0.9 ppm, was in the soil samples of G. macrorrhizum from natural environment. Copper content in soil samples from both examined localities for G. macrorrhizum is very similar 0.9 ppm from natural environment and 1.0 ppm from post-fire area. Cadmium levels are very low in acid reducible fractions for all samples regardless the area and ranged in the interval in the range of 0.1 ppm for G. robertianum and G. bohemicum from natural environment to 0.3 ppm for G. macrorrhizum from post-fire soil samples.
The lowest content of lead was in the soil samples of G. robertianum from natural environment while the soil samples of G. robertianum from post-fire have the biggest concentration of Pb 7.1 ppm.
The fractions bound to organic matter (F3) are temporarily inaccessible. They can be solubilized by chemical oxidation. In so-called organic or sulfides soil fraction (F3), the presence of Zn is noticeable for all soil samples from post-fire areas (from 55.5 ppm for G. robertianum to 67 ppm for G. macrorrhizum). Also, concentration of Pb is high in all soil samples from post-fire areas (from 41.5 ppm for G. bohemicum to 53 ppm for G. macrorrhizum). Concentrations of Cu were in the range of 3.0 ppm for G. macrorrhizum to 4.7 ppm for G. robertianum in soil samples from natural environment, while in the samples from post-fire area concentrations of Cu were in the range of 6.2 ppm for G. macrorrhizum to 9.1 ppm for G. robertianum. Copper was also distributed almost exclusively between the organic and residual phases (Bacon et al. 2005), because the affinity of Cu for organic matter/sulfides supported by the high stability constants of Cu complexes with organic ligands and low constants of solubility product of copper sulfide. Concentrations of Cd in organic or sulfides soil fraction (F3) were extremely low in all samples tested and ranged from 0.2 to 0.4 ppm. In all tested samples, presence of Cd was higher in soil samples of examined plants from post-fire areas (from 0.2 ppm for G. robertianum to 0.4 ppm for G. macrorrhizum). Concentration of Cd in soil samples from both examined localities for G. robertianum is the same 0.2 ppm.
The residual fraction (FR) includes mainly metals built in the crystal lattice of minerals. In natural conditions, they are practically inaccessible for living organisms and can be considered as an inert phase corresponding to the part of the metal which cannot be easily mobilized. Concentrations of Cu, Zn, and Pb in residual fraction (FR) of soils from post-fire area were lower than metals’ concentrations in residual fraction of soils that were not exposed to the fire. In the case of cadmium concentration, the situation is reversed. In the residual fraction for all three examined plants, the concentration of cadmium in the soil samples from natural environment is higher than the one from post-fire area.
Copper levels are samples from natural environment that were from 0.1 ppm for G. macrorrhizum to 0.8 ppm for G. bohemicum and from 3.1 ppm for G. robertianum to 3.7 for G. bohemicum in the samples of soils that were exposed to the fire. Lead is most abundant in residual fraction for all samples and its range of concentration varies from 2.1 ppm (for G. macrorrhizum from natural environment) to 47.1 ppm (for G. macrorrhizum from post-fire area). Results of the statistical analysis of copper content in the tested fractions showed that no differences between copper content in soils from natural and post-fire area in fractions F1 and F2. The difference between content of copper in soils from natural and post-fire area in fractions F3 and F4, is significant U = 0, p = 0.050 (in both cases).
Lead appeared to be very abundant in the residual fraction especially in the soil samples from post-fire region.
Cadmium was present in residual fraction of natural environment soils in slightly increased concentrations. The lowest content of Cd was in the soil samples of G. macrorrhizum from post-fire area 0.1 ppm, while the soil samples of G. bohemicum from natural environment have the biggest concentration of Cd 0.5 ppm.
Zinc levels in residual fraction were in the range from 0.1 ppm for G. robertianum to 4.5 ppm for G. macrorrhizum soils from natural area, while in the post-fire area it varies from 1.5 for G. robertianum to 44.5 ppm for G. macrorrhizum.
Results of the statistical analysis of metal content in the tested fractions of soil showed that there are differences between content of Zn, Cd, and Pb in soils from natural and post-fire area (U = 19.5, p = 0.001 for Zn, U = 35, p = 0.031 for Cd and U = 33, p = 0.022 for Pb). The two-tailed Mann–Whitney test indicated that content of copper in the soil samples from natural and post-fire area is not statistically different U = 51, p = 0.230. So, it suggests that the fire has different effects on the content of certain heavy metals in soil. The content of Zn, Cd, and Pb in the soil was affected by the fire, while the copper content in the soil did not change under the influence of fire.
The ways of plant intake of heavy metals can be estimated following their distribution in plants’ roots and shoots.
In all analyzed plants from both regions, copper is abundant very poorly (less than 4.4 ppm). The highest concentration of Cu was measured in shoots sample of G. macrorrhizum from the habitat outside the burnt areas and it was 4.42 ppm. In root samples of all investigated plants from post-fire region, copper was below the limit of detection.
Except the sample of the root of G. bohemicum from area that was not exposed to the fire, all of the samples have cadmium content in the range of 0.21–1.93 ppm. The significantly higher content of cadmium (8.9 ppm) was measured in a sample of the roots of G. bohemicum from post-fire area. Samples of aerial parts (shoots) of G. macrorrhizum from the territory which was not affected by the fire, containing more cadmium compared to the corresponding samples from the post-fire region. Samples of shoots and root of G. macrorrhizum from natural area contain approximately the same amount of cadmium (1.81 ppm for plants from natural environment and 1.79 ppm for plants from post-fire region). The elevated concentrations of Cd were registered in all parts of G. bohemicum and G. robertianum from post-fire area, while in the case of G. macrorrhizum plants samples from natural area have increasing concentrations of that metal.
Roots samples of G. robertianum and shoots samples of G. bohemicum from both studied regions do not contain lead. Also, lead is below the limit of detection in roots samples of G. bohemicum from post-fire area, and shoots samples of G. robertianum from post-fire area. The highest lead content, 5.71 ppm, was determined in shoots samples of G. robertianum from natural environment.
The biggest difference in metal content between studied three plant species growing at two sites (natural environment and post-fire area) was noticed for zinc (141.34 ppm in root samples of G. macrorrhizum from natural environment and 23.8 ppm in shoots samples of G. macrorrhizum from post-fire area). The slightest difference in zinc content in the samples of roots and shoots of plants from natural environment and post-fire area was the case of plants G. bohemicum. Investigated plants from the post-fire area showed higher average concentrations of Zn, in comparison to the control group from the natural environment, which is closely associated with previous investigation of soil and plant samples of four plant species of Lamiaceae family growing on Vidlic Mountain (Stankov Jovanovic et al. 2011).
We ran a Mann–Whitney’s U-test to evaluate the difference in the content of Cu in tested plant species of Geraniaceae family from natural and post-fire area. It was found as a significant effect of wildfire ( U = 0.5, p = 0.001) on content of Cu in tested plant species from natural and post-fire area. Results of the statistical analysis indicated that content of Zn, Cd, and Pb in tested plant from natural and post-fire area was not statistically different (U = 14, p = 0.537 for Zn, U = 17, p = 0.877 for Cd, U = 12, p = 0.352 for Pb).
Obtained data for analyzed heavy metals content were within the limits of their average abundance in the Earth’s crust (Radojevic and Bashin 1999), which suggests that both natural environment and the area that has suffered from the fire are still unpolluted by heavy metals (Stankov Jovanovic et al. 2011).
There is limited number of studies dealing with influence of fire on metal content in soil and plants samples. However, the method of preparation of samples of soil or plant material is not the same as the one we applied. In some case (Chrysikou et al. 2008), soil and vegetation samples were collected 15 days after the ultimate fire extinction, so no rain events occurred between the fire and sample collection, while in some cases sampling was extended for several seasons (1.5 years) (Burke et al. 2010). The soil was collected from a depth of 0–5 cm (Chrysikou et al. 2008), t 0–10 cm (Guerraa et al. 2013). In some studies, soil samples collected 4 years before the fire were used as reference samples (Guerraa et al. 2013). Also, the concentrations of different elements were investigated: Zn, Co, Cd, and Pb (Sosorova et al. 2013), Zn, Cu, Sn, Sb and Pb Chrysikou et al. (2008), Zn, Cu, Pb, Ni and Cr (Bogacz et al. 2011), Hg (Burke et al. 2010).
The metal concentration in plants depends on a species and its environment. It is very probable that different growth conditions present in the same place and in different years affect the elemental composition in plants (Sosorova et al. 2013).
Keeping all this in mind, it is clear that it is difficult to compare our results to previously mentioned studies, except with published results of investigation of soil and plant samples of four plant species of Lamiaceae family (Ajuga genevensis L., Lamium galeobdolon (L.) L., Teucrium chamaedrys L., Acinos alpinus (L.) Moench.) growing on Vidlic Mountain (Stankov Jovanovic et al. 2011). Our results are in good agreement with previously published for soil and plant samples from Vidlic Mountain.
Conclusion
Soil samples of G. robertianum and G. macrorrhizum from post-fire area contain greater amounts of organic matter than soil samples from natural environment. Electrical conductivity of the soil samples from natural environment was higher for all plant species, while the concentration of chloride in all soil samples was higher for post-fire area. Soil samples from natural environment and post-fire area for all studied species have very similar values of the redox potential. pH (H2O), of soils from natural environment in distilled water are lower for G. robertianum and G. bohemicum while for G. macrorrhizum is vice versa. The values of pH (KCl) have the same behavior. All soils have positive values of ΔpH which implies that the surface of soil colloidal particles is negatively charged.
Results of the statistical analysis of metal content in the tested fractions of soil showed that there are differences between content of Zn, Cd, and Pb in soils from natural and post-fire area. The statistical analysis indicated that content of copper in the soil samples from natural and post-fire area is not statistically different. It suggests that the fire has different effects on the content of certain heavy metals in soil. The content of Zn, Cd, and Pb in the soil was affected by the fire, while the copper content in the soil did not change under the influence of fire.
The fire has different influences on the content of some heavy metals in plants then the influence in the soil. Copper is an element on which fire has got different effects than on cadmium, zinc, and lead. Results of the statistical analysis indicated that content of Zn, Cd, and Pb in tested plants from natural and post-fire area was not statistically different.
References
Adriano DC (1986) Trace elements in the terrestrial environment. Springer, New York
Ahmed A, Tajmir-Riahi HA (1993) Interaction of toxic metal ions Cd2+, Hg2+ and Pb with light-harvesting proteins of chloroplast thylakoid membranes. An FTIR spectroscopic study. J Inorg Biochem 50:235–243
Aldridge KT, Ganf GG (2003) Modification of sediments redox potential by three contrasting. Mar Freshw Res 54:87–94
Bacon JR, Hewitt IJ, Cooper P (2005) Reproducibility of the BCR sequential extraction procedure in a long-term study of the association of heavy metals with soil components in an upland catchment in Scotland. Sci Total Environ 337:191–205
Blagojevic N, Damjanovic-Vratnica B, Vukasinovic-Pesic V, Djurovic D (2009) Heavy metals content in leaves and extracts of wild-growing Salvia officinalis from Montenegro. Pol J Environ Stud 18:167–173
Bogacz A, Wozniczka P, Labaz B (2011) Concentration and pools of heavy metals in organic soils in post-fire areas used as forest and meadows. J Elem 16:515–524
Burke MP, Hogue TS, Ferreira M, Mendez CB, Navarro B, Lopez S, Jay JA (2010) Effect of wildfire on soil mercury concentrations in Southern California watersheds. Water Air Soil Pollut 212:369–385
Burzynski M (1987) The uptake and transpiration of water and the accumulation of lead by plants growing on lead chloride solutions. Acta Soc Bot Pol 56:271–280
Chrysikou L, Gemenetzis P, Kouras A, Manoli E, Terzi E, Samara C (2008) Distribution of persistent organic pollutants, polycyclic aromatic hydrocarbons and trace elements in soil and vegetation following a large scale landfill fire in northern Greece. Environ Int 34:210–225
Ciric J (1989) Geography of upper Ponišavlje and Luznica. In: Nikolic P (ed) Pirot proceedings, vol 16, pp 9–23, newspaper publishing institution, “Freedom”, Pirot
Couto-Vazquez A, Gonzalez-Prieto SJ (2008) Short- and medium-term effects of fire and fire-fighting chemicals on soil micronutrient availability. Sci Total Environ 407:297–303
Crawford JJ, Train SJ, Tuovinen OH (2000) Bacterial degradation of atrazine in redox potential gradients in fixed-film sand columns. Soil Sci Soc Am J 64:624–634
Forstner U (1995) Land contamination by metals—global scope and magnitude of problem. In: Herbert AE, Huang CP, Bailey GW, Bowers AR (eds) Metal speciation and contamination of soil. CRC Press, Boca Raton, FL, pp 1–34
Fox TC, Guerinot ML (1998) Molecular biology of cation transport in plants. Annu Rev Plant Physiol Plant Mol Biol 49:669–696
Ghosh M, Singh SP (2005) A review on phytoremediation of heavy metals and utilization of its byproducts. Appl Ecol Environ Res 3:1–18
Gonzalez-Perez JA, Gonzalez-Vila FJ, Almendros G, Knicker H (2004) The effect of fire on soil organic matter—a review. Environ Int 30:855–870
Guerraa MBB, Netoa EL, Priantia MTA, Pereira-Filhob ER, Schaefera CEGR (2013) Post-fire study of the Brazilian Scientific Antarctic Station: toxic element contamination and potential mobility on the surrounding environment. Microchem J 110:21–27
He XT, Traina SJ, Logan TJ (1992) Chemical properties of municipal solid waste compost. J Environ Qual 21:318–329
Hoenig M (2001) Preparation steps in environmental trace element analysis-facts and traps. Talanta 54:1021–1038
Iwegbue CMA, Egobueze FE, Opuene K (2006) Preliminary assessment of heavy metals levels of soils of an oil field in the Niger Delta, Nigeria. Int J Environ Sci Technol 3:167–172
Jeliazkova EA, Craker LE (1998) Heavy metals and seed germination in some medicinal and aromatic plants. HortScience 33:203–206
Jose S, Gillespie AR, Pallardy SG (2004) Interspecific interactions in temperate agroforestry. Agroforest Syst 61–62:237–255
Krishnamurti GSR, Naidu R (2002) Solid-solution speciation and phytoavailability of copper and zinc in soils. Environ Sci Technol 36:2645–2651
Lake DL, Kirk PWW, Lester JN (1984) Fractionation, characterization, and speciation of heavy metals in sewage and sludge amended soil: a review. J Environ Qual 13:175–183
Macdonald SE (2007) Effects of partial post-fire salvage harvesting on vegetation communities in the boreal mixedwood forest region of northeastern Alberta, Canada. For Ecol Manage 239:21–31
Manahan ES (2000) Environmental chemistry, 7th edn. CRC Press LLC, Boca Raton, FL
Ministry of Environment and Spatial Planning Republic of Serbia (2008) Report on fires in protected natural resources for 2007 year (in Serbian)
Mohanty N, Vass I, Demeter S (1989) Copper toxicity affects photosystem 11 electron transport at the secondary quinone acceptor, Q(B). Plant Physiol 90:175–179
National Wildfire Coordinating Group (2001) Fire effects guide. Chapter v—soils, water, and watersheds By Dr. Bob Clark. http://www.nwcg.gov/pms/RxFire/FEG.pdf
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52:199–223
Pereira MG, Valladares GS, Anjos LHC, Benites VM, Espíndula JRA, Ebeling AG (2006) Organic carbon determination in Histosols and soil horizons with high organic matter content from Brazil. Sci Agric 63:187–193
Petrovic J (1971) Odorovac cave, proceedings of the Faculty of Sciences 1, separat, University of Novi Sad, pp 228–258
Petruzzelli G (1989) Recycling wastes in agriculture: heavy metal bioavailubiity. Agric Ecosyst Environ 27:493–503
Quevauviller Ph (1998) Operationally defined extraction procedures for soil and sediment analysis. I. Standardization. Trac Trends Analyt Chem 17:289–298
Radojevic M, Bashin V (1999) Practical environmental analysis. RSC, Cambridge
Ramos L, Gonzalez MJ, Hernandez LM (1999) Sequential extraction of copper, lead, cadmium, and zinc in sediments from Ebro River (Spain): relationship with levels detected in earthworms. Bull Environ Contam Toxicol 62:301–308
Rashid P, Mukherji S (1991) Changes in catalase and ascorbic oxidase activities in response to lead nitrate treatments in mungbean. Indian J Plant Physiol 34:143–146
Robichaud PR, Beyers JL, Neary DG (2000) Evaluating the effectiveness of postfire rehabilitation treatments. USDA Forest Service, Gen Tech Rep RMRSGTR-63
Schutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Sigg L (2000) Redox potential measurements in natural waters: significant: concepts and problems. In: Schulz HD, Fischer WR, Bottcher J, Duijnisveld WHM (eds) Redox: fundamentals, processes and applications. Springer, Berlin, pp 1–11
Sosorova SB, Merkusheva MG, Ubugunov LL (2013) Pyrogenic changes in microelement content in soils and plants from the pine forests of western Transbaikal. Contemp Probl Ecol 6:499–508
Stankov Jovanovic VP, Ilic MD, Markovic MS, Mitic VD, Nikolic Mandic SD, Stojanovic GS (2011) Wild fire impact on copper, zinc, lead and cadmium distribution in soil and relation with abundance in selected plants of Lamiaceae family from Vidlic Mountain (Serbia). Chemosphere 84:1584–1591
Summary Report of a Workshop on phytoremediation research needs (1994) Mechanisms of plant uptake, translocation, and storage of toxic elements. Plume Focus Area, U. S. Department of Energy, Santa Rosa, California. http://www.osti.gov/bridge/servlets/purl/10109412-BckU4U/webviewable/10109412.pdf
Tan K (1998) Principles of soil chemistry, 3rd edn. Marcel Dekker Inc, Revised and expanded
Tuzen M (2003) Determination of heavy metals in soils, mushrooms and plant samples by atomic absorption spectromerty. Microchem J 74:289–297
(US EPA) United States Environmental Protection Agency (1996) Method 3050B: acid digestion of sediments, sludges and soils. http://www.epa.gov/wastes/hazard/testmethods/sw846/pdfs/3050b.pdf. Accessed December 1996
(US EPA) United States Environmental Protection Agency (2006) Process design manual: land treatment of municipal wastewater effluents. EPA 625/R-06/016, ORD, Cincinnati, OH
Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10:268–292
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Yobouet YA, Adouby K, Trokourey A, Yao B (2010) Cadmium, copper, lead and zinc speciation in contaminated soils. Int J Eng Sci Technol 2:802–812
Acknowledgments
The research was supported by the Serbian Ministry of Education and Science (Grant No. 172051).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitic, V.D., Stankov Jovanovic, V.P., Ilic, M.D. et al. Impact of wildfire on soil characteristics and some metal content in selected plants species of Geraniaceae family. Environ Earth Sci 73, 4581–4594 (2015). https://doi.org/10.1007/s12665-014-3744-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3744-1