Abstract
Purpose
Despite the great number of studies about methane uptake and its response to grazing in the Inner Mongolia grasslands, only a few focused on the methanotrophic composition. This study aimed to investigate the methanotrophic community structure and abundance, then to analyze the abiotic driving factors of methanotrophic community structure in different enclosed times in this area.
Materials and methods
In this study we chose typical grasslands in the Xilin River Basin of Inner Mongolia, China to investigate methanotrophic community structure and abundance under different enclosure treatments as follows: 79E (grassland enclosed since 1979), 99E (grassland enclosed since 1999), and G (freely grazed grassland). A clone library was used to reveal the methanotroph community structure, and their relationships with abiotic factors were analyzed by redundancy analysis. Methanotroph abundance was determined by real-time PCR.
Results and discussion
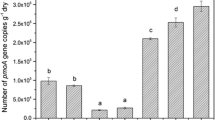
The OTUs of the three treatments mainly belonged to Type I methanotrophs, probably caused by the high pH value. Among all OTUs, only OTU1 belonged to upland soil cluster γ (USC-γ), whose abundance was the largest in all OTUs, indicating the USC-γ cluster was the main one to oxidize CH4 in the Inner Mongolia grasslands. Methanotrophic abundance (represented by the pmoA gene copies per gram of dry weight soil) decreased with the enclosure time as G (4.5 × 107) > 99E (2.8 × 107) > 79E (2.0 × 107), mainly caused by the lower soil moisture content in G. Lower soil moisture content facilitates more CH4 and O2 diffusive into soil thus leading to the proliferation of methanotrophs.
Conclusions
This study found a high abundance of methanotrophs in the soils of the Inner Mongolia grasslands, with the USC-γ cluster having the largest abundance, which may play a key role in oxidizing CH4 in the Inner Mongolia grasslands. Combined with those of previous studies, the results showed an obvious change of methanotrophic community composition with the increase of enclosure time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Methane (CH4) is the second largest greenhouse gas on the earth, responsible for approximately 20 % of the global warming induced by long-lived greenhouse gases since the pre-industrial era (Kirschke et al. 2013). The major sources of methane include wetlands (Belger et al. 2011), rivers (Sawakuchi et al. 2014), swamps (Chen et al. 2014), rice paddies (Bhatia et al. 2011; Liu et al. 2012b), and ruminant livestock (Moraes et al. 2014). The major sinks of methane are photochemical oxidation by the hydroxyl radical (OH) in the atmosphere (Cicerone and Oremland 1988) and biological oxidation by the methanotrophs in the aerobic soil (Curry 2007).
Though most of methanotrophs are neutrophilic and mesophilic (Trotsenko and Khmelenina 2002), they are also found to exist in the extreme environments, including the upper layer of deep pelagic seawater (Nercessian et al. 2005), the tundra soils (Vecherskaya et al. 1993), and the extremely hot environment (Sieburth et al. 1987; Bodrossy et al. 1995, 1997; Vecherskaya et al. 1993). They utilize methane as their single source of C and energy (Hanson and Hanson 1996). Based on cell morphology, phylogeny, and metabolic pathways, methanotrophs are divided into two distinct phylogenetic groups, i.e., Type I (utilizing ribulose monophosphate for formaldehyde assimilation) and Type II (using serine pathway for formaldehyde assimilation) (Hanson and Hanson 1996). Type I methanotrophs are often detected in environments with high pH and low methane concentrations (Hanson and Hanson 1996; Lin et al. 2004; Yun et al. 2012), while Type II favors environments of low pH, low oxygen and high methane concentrations (Putkinen et al. 2014). In addition, Type I methanotrophs can better adapt to the disturbance environment by responding sensitively to increasing substrate and oxygen levels (Steenbergh et al. 2010; Putkinen et al. 2014). Some biological factors influence the distribution of the two types of methanotrophs, e.g., earthworms were reported to stimulate activity of Type I methanotrophs compared to Type II methanotrophs (Kumaresan et al. 2011). Although methanotrophs were detected in anaerobic environment where methane was oxidized coupling to denitrification of nitrate and the reduction of sulfate (Boetius et al. 2000; Raghoebarsing et al. 2006), methanotrophs were also found to oxidize a significant amount of CH4 in aerobic soils (Hütsch 2001).
With an uptake rate of 4.3 Tg CH4 yr−1, grassland soils under well-aerated conditions are recognized as one of the major sinks of global soil CH4 (Dutaur and Verchot 2007). In China, compared with forest consuming 0.68 Tg CH4 yr−1, grassland is the second largest sink of CH4 that consumes 0.65 Tg CH4 yr−1 (Wang et al. 2014). The grasslands of Inner Mongolia are typical semi-arid, temperate grasslands with grazing as the dominant human disturbance. Subject to heavy grazing, the grasslands of Inner Mongolia are heavily degraded. Enclosure was taken as a measure to control such degradation by excluding grazing. Researchers have tried to understand the CH4 flux in enclosures, but very few focused on the methanotroph community composition of the enclosures (Zhou et al. 2008a, b). In this study, we investigated the same treatments in the same area with the previous one (Zhou et al. 2008a). Unlike the previous one, this study focused on analyzing the methanotroph community structure 7 years later, with a purpose of revealing its change during the enclosure and especially after the previous study. The hypothesis was that with the increasing enclosure time, the grassland gradually recovers to a good condition, the methanotroph community structure of surface soils may also change accordingly. For laying a basis for understanding CH4 uptake and grassland management in the Inner Mongolia, this study aimed to (1) investigate the methanotroph community structure and abundance of different enclosure time, and (2) find out the change of the methanotroph community structure and the abiotic driving factors for such change in different enclosure time.
2 Materials and methods
2.1 Site description and soil sampling
The experiment site in this study are located in the Xilin River catchment adjacent to the Inner Mongolia Grassland Ecosystem Research Station (43°26′–44°39′N, 115°33′–117°12′) of the Chinese Academy of Sciences (Fig. 1). The area belongs to the temperate terrestrial monsoon climate, with detailed climate information described by Zhou et al. (2008b). There were all together three experiment plots, including one that has been enclosed from grazing since 1979 (79E, 600 m × 400 m), one enclosed since 1999 (99E, 500 m × 300 m), and one being freely grazed with no enclosure (G) located besides 79E and 99E. The grazing intensity of the plot G is 2.0 sheep ha−1 yr−1. For each plot we collected samples in August 2012 as from three randomly chosen cores (2 cm in diameter, 10 cm in height). All nine soil samples were taken from 0–10 cm depths, sealed in plastic bags, and immediately transported in ice box to the laboratory. Each sample was divided into two parts; one part was stored at −4 °C for the analysis of physiochemical properties within less than one week after sampling; the other part was stored at −80 °C for molecular analysis.
2.2 Soil physicochemical properties measurements
In order to measure the dissolved organic carbon (DOC), NH4 +-N and NO3 −-N contents, fresh soil samples were sieved through a 2-mm mesh and then extracted with 2 M KCl. The other soil samples were air-dried and passed through a 0.25-mm sieve, then extracted with 0.2 M NaHCO3 to determine the PO4 3−-P content. All soil samples with extracted solution were run on a shaker under ambient temperature for 1 h; the supernatant was removed and then measured with a continuous flow analytical system (SKALAR San++, Skalar Co., Netherlands) for the contents of DOC, NH4 +-N, NO3 −-N, and PO4 3−-P. The soil moisture content was determined as the percentage of soil water weight in fresh soil weight, where soil water weight was calculated by subtracting the oven-dried soil weight from the fresh soil weight. Acidity meter (Sartorius PB-10, Gemany) was used to measure the soil pH, with soil and water ratio as 1:1.
2.3 DNA extraction and construction of clone libraries
Soil DNA were extracted following the instructions of the soil DNA kit (E.Z.N.ATM) and were collected in 100 μl elution buffer. The concentration of the extracted DNA was analyzed by spectrophotometer (Maestrogen, MN-913, Taiwan). A microliter of DNA sample was used for PCR amplification. The primer pair A189 (GGNGACTGGGACTTCTGG) (Costello and Lidstrom 1999) and A682 (GAASGCNGAGAAGAASGC) (Holmes et al. 1995) was used to amplify the pmoA gene fragments in the first PCR. Hereafter, aliquots of PCR amplification products (2 μl) taken from the first round were used as the template in the second round of PCR amplification system using primer pair A189 and mb661 (CCGGMGCAACGTCYTTACC) (Costello and Lidstrom 1999). The PCR programs of two rounds were as follows: pre-degeneration at 95 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 30 s, and eventually extending at 72 °C for 5 min. The quality of PCR products was tested in 1.2 % agarose gel to confirm the specificity of PCR products. After that, the PCR products were purified by the gel extraction kit (E.Z.N.ATM). Then they were ligated into pMD19-T at 16 °C overnight and transformed into competent cell, smeared into LB medium and cultured. For each library, 100 colonies were randomly selected and screened using colony PCR with primer pair M13-47/RV-M. The positive colony PCR products were digested by restriction enzymes RsaI. The restriction fragment pattern was performed upon agarose gel electrophoresis and then the differences compared. The same patterns were treated as one Operational Taxonomic Unit (OTU) in each library (Han et al. 2009). The corresponding clones were grown overnight in LB. Then plasmids were extracted using plasmid minipuri kit (Felixbio, USA) and sequenced. Methanotroph DNA sequences were compared against those on the NCBI with the BLAST program and multiple sequence alignment analysis was performed with ClustalW2. The OTUs were defined as sequence groups in which sequences differed by ≤13 %. MEGA and ClustalX2 software were used to build phylogenetic tree which adopted neighbor-joining method based on alignments of DNA sequences (using Jukes-Cantor corrected distances). Bootstrap analysis was used to estimate the reliability of phylogenetic reconstructions (1,000 replicates). At last, the pmoA nucleotide sequences were submitted to Genbank by Bankit, as accession numbers KM355399, KM355405, KM355413, KM390980, KM390987, and KM390989.
2.4 Real-time PCR
Methanotroph abundance could be obtained by analyzing the pmoA copies in the soil using real-time PCR performed on a CFX96 real-time PCR detection system (Bio-Rad). The 25 μl reaction mixture consisted of 12.5 μl SYBR® Premix Ex Taq™ (CWBIO), 0.5 μl primer (10 μmol L−1), 1 μl DNA (as template), and water. A189/mb661 was the specific primers to amplify the methanotrophic pmoA fragments. The PCR reaction procedure was as follows: pre-degeneration at 95 °C for 10 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 30 s. A melting curve analysis was performed to confirm PCR product specificity after amplification by measuring fluorescence continuously as the temperature increased from 65 °C to 95 °C. Standard curves for real-time PCR were developed using plasmids as standards for quantitative analyses. The concentration of plasmid DNA was determined on a spectrophotometer (Maestrogen, MN-913, Taiwan), and the copy numbers of target genes were calculated directly from the concentration of the extracted plasmid DNA. Tenfold serial dilutions of a known copy number of the plasmid DNA were subject to real-time PCR assay in triplicate to generate an external standard curve. Data analysis was carried out with CFX96 software.
2.5 Data analyses
The coverage of clone library (C) was calculated using the equation:
Where n is the number of OTU containing only one pmoA gene sequence; N is the total number of sequences analyzed (Singleton et al. 2001).
Redundancy analysis (RDA) was used to analyze the relationship among treatments, methanotroph community structure, and soil physicochemical properties (Canoco for Windows 4.5). LSD analysis of variance (ANOVA) was used to analyze soil physicochemical properties and methanotrophic abundance of the three treatments. The statistical analyses were carried out using SPSS software (version 20). Statistical significance was set as P < 0.05 for all results.
3 Results
3.1 Variation in soil physicochemical properties among grasslands of different enclosure regimes
Soil physicochemical properties are shown in Table 1. No significant difference of pH was found among the grasslands of different enclosure conditions. Soil moisture content was significantly higher in 79E than in G, but showed no significant difference between 99E and the other two. The NH4 +-N content of 79E and 99E was significantly lower than that of G. The DOC was significantly higher in 79E and G than in 99E. The NO3 −-N and PO4 3−-P contents were significantly different among the three.
3.2 Phylogenetic analysis and methanotroph community composition
All together 394 pmoA sequences were found and classified into 6 OTUs. Apart from these six OTUs, nine reference sequences were also introduced, one pmoA-related sequence of Nitrosococ as an exogenous gene downloaded from GenBank to constitute the phylogenetic tree (Fig. 2). Of all six OTUs, five were Type I methanotrophs, and they were the main groups of methanotroph communities in these three sampling plots; only OTU5 belonged to Type II methanotrophs, found only in the G. Among all, only OTU1 belonged to upland soil cluster γ (USC-γ), whose abundance was the largest in all OTUs (Fig. 3).
The mean coverage values of clone libraries were 98 % for 79E, 99 % for 99E, and 96 % for G, indicating that the methanotrophic diversity could be well covered in each clone library. Within the 6 OTUs, 2 OTUs were found in79E, 1 in 99E and 5 in G. OTU1 was found in all three sampling sites; OTU2 was found only in 79E; other OTUs were found in G.
3.3 Methanotrophic abundance
Methanotrophs showed great abundance (2.0–4.5 × 107 pmoA gene copies per gram of soil) in the Inner Mongolia grasslands (Fig. 3). The methanotrophic abundance of G (4.5 × 107 pmoA gene copies per gram of soil) was significantly higher than that in 79E (2.0 × 107 pmoA gene copies per gram of soil), but 99E (2.8 × 107 pmoA gene copies per gram of soil) showed no significant difference with the other two. The pmoA gene copies of each OTU were also obtained based on the proportion in clone library. Among all, OTU1 (1.9 × 107 pmoA gene copies per gram of soil in 79E, 2.8 × 107 pmoA gene copies per gram of soil in 99E, and 3.5 × 107 pmoA gene copies per gram of soil in G) constituted the majority of the three types of grassland, accounting for 94 % of 79E, 100 % of 99E, and 76 % of G, respectively.
3.4 Relationship between abiotic factors and methanotrophic community structure
The correlation analysis results between methanotrophic community structure and soil physicochemical properties were shown in Fig. 5. OTU1 had a positive correlation with NO3 −-N, but a negative correlation with pH and DOC. OTU1 was found not discernibly related to NH4 +-N, PO4 3−-P, or soil moisture content. OTU2 had a positive correlation with pH, soil moisture content and DOC, but a negative correlation with NO3 −-N, NH4 +-N and PO4 3−-P. Of all the abiotic factors, pH was most closely related to OTU2. It appeared that there was a positive correlation between OTU3, OTU4, OTU5, OTU6, and NH4 +-N, PO4 3−-P, DOC, while a negative correlation with NO3 −-N, WC, and pH. NH4 +-N, PO4 3−-P, and NO3 −-N were the most closely related abiotic factors for OTUs which only existed in G.
4 Discussion
4.1 Effect of enclosure time on the methanotroph community composition
Previous studies demonstrated that the Inner Mongolia grassland is a CH4 sink (Ma et al. 2006; Liu et al. 2007; Holst et al. 2008; Chen et al. 2010; Geng et al. 2010) with USC-γ as the main methanotrophic cluster oxidizing atmospheric CH4 (Zhou et al. 2008a). Different from those results, this study found in all three treatments only one genotype (OTU1) belonging to USC-γ cluster. But similar to the former results, we also found through real-time PCR that USC-γ (8.2 × 107 pmoA gene copies per gram of soil) was the dominant methanotrophic cluster, accounting for 87 % in abundance of all three treatments (Fig. 3).
Among the six OTUs found in the three types of grassland, five were Type I methanotrophs, and only one (OTU 5) was a Type II methanotroph that existed exclusively in G. This indicated that enclosure time did not affect Type I methanotrophs, which kept playing a major role in oxidizing methane in all three types of grassland, no matter whether it was enclosed from grazing, or how long it was enclosed (Fig. 2). This was probably related to the fact that the soil pH did not decrease significantly with enclosure (Table 1). The comparatively high pH value could lead to the wide existence of Type I methanotrophs (Hanson and Hanson 1996). The fact that Type II methanotrophs barely existed in G should be resulted from its sensitivity to higher CH4 concentrations at soil pore spaces caused by the lower soil moisture in the sampling plot G.
For the genotype composition of the methanotroph community, the study found they varied with the enclosed time. In order to compare the genotypes composition of the methanotroph community with 7 years ago, we collected the genotypes analyzed by Zhou et al. (2008a) and performed sequence alignment analysis using ClustalW2 to these genotypes together with those in this study. The result showed no same genotypes existing between the two samplings 7 years apart. This was considered to be caused by climatic change, which is accompanied by changes in soil physicochemical properties and the atmospheric CH4 concentration (Dalla Valle et al. 2007; Kirschke et al. 2013). The latter two would in turn induce occurrence of different genotypes of methanotrophs (Dubey and Singh 2001; Veraart et al. 2015). After 7 years, however, the genotypes of 99E showed an obvious change. In Zhou et al.’s research (2008a), the 99E (7-year enclosure at their research time) and G (7 years ago) shared the same five genotypes. But in our study, 7 years after theirs, the 99E (13-year enclosure) had only one genotype consistent with G. This indicated that the methanotroph community structure of 99E (13-year enclosure) had undergone a qualitative change. Clustering analysis showed that the methanotroph community composition of 79E (33-year enclosure) and 99E (13-year enclosure) was significantly different from that of G (Fig. 4b). The result was also inconsistent with that by Zhou et al. (2008a), that 99E (7-year enclosure at the time of their research) first clustered with G, and then with 79E (Fig. 4a). The inconsistency between the results was probably caused by the state shift of the methanotroph community composition in grassland recovery succession analogical to a clear shift of the methanotroph community composition in Sphagnum-associated peatland succession (Putkinen et al. 2014). With increasing enclosure time, the surface soil becomes loose, the density reduces and moisture content increases (Liu et al. 2012a). The vegetation restoration level and soil physicochemical properties in 99E gradually shifted from grazing environment to a relatively stable state of enclosure site (79E). At the same time, the methanotroph community composition also changed accordingly. Figure 5 shows that NH4 +-N and PO4 3−-P can drive the change of methanotrophic community structure in 99E. Previous studies have demonstrated that NH4 +-N and PO4 3−-P have an effect on the methanotroph community structure (Dubey and Singh 2001; Zhang et al. 2014; Veraart et al. 2015). For instance, NH4 +-N may lead to the dominance of Methylobacter in landfill biocover soils (Zhang et al. 2014). PO4 3−-P can increase methanotroph abundance, but little is known about its effect on methanotroph community structure (Veraart et al. 2015). The lower NH4 +-N and PO4 3−-P concentration induced by the longer enclosed time (Table 1) may cause a certain genotype reduction or disappearance. In addition, the inconsistence of results might also be caused by the different analysis methods. Zhou et al. (2008a) used the DGGE to analyze the methanotroph community structure, while this study used the clone library. However, the comparison qualitatively indicated the trend of change in the composition of methane oxidizing microbes. For more reliable conclusions, more available results based on the same methods should be integrated in comparison.
4.2 The effect of enclosure on methanotrophic abundance
The present study collected data about methanotroph abundance to further compare them between grassland and other ecosystems (Table 2). The comparison showed no great variation of methanotroph abundance among all ecosystems, where the order of magnitude for pmoA gene copies per gram of soil reached 107-108 (Abell et al. 2009; Yun et al. 2012, 2014; Zheng et al. 2012; Deng et al. 2013). For forest and grassland, the soil is under well-aerated conditions (Wang et al. 2014). Soils can supply sufficient O2 for methanotroph amplification. Although wetlands and rice paddies are water logged all the year around or just seasonally, plant roots can also transport O2 to the rhizosphere, resulting in rapid proliferation of methanotrophs (Hanson and Hanson 1996). Therefore, these ecosystems have similarly abundant methanotrophs in the surface soils, although there is large divergence among their habitats due to difference of water regime.
One result of this study was that 79E had significantly lower methanotrophic abundance (Fig. 3) than G. This was considered to be related to soil moisture content, which is considered to play an important role for methanotroph dynamics (Amaral et al. 1998; Del Grosso et al. 2000; He et al. 2011). Soil moisture content was found to have a significantly negative correlation with methanotrophic abundance (Adamsen and King 1993; Castro et al. 1995; Horz et al. 2005; Zheng et al. 2012). Lower soil moisture content facilitates more CH4 and O2 diffusive into soil, thus leading to the proliferation of methanotrophs (Horz et al. 2005; Zheng et al. 2012). In our results, the soil moisture content in the 79E sampling plot was 23.50 %, significantly higher than that of G (17.92 %, P = 0.03). Therefore, the significantly lower methanotrophic abundance in 79E was probably attributable to the higher soil moisture content (Table 1), which is adverse to methanotroph proliferation. In nutrient-rich soils, moderate grazing enhanced carbon input quality by inversely increasing the abundance of some palatable plants which stimulate the growth of microbial biomass over long time scales (Sankaran and Augustine 2004). In our study sites, grazing might increase the labile C inputs that could be easily utilized to produce CH4 when it moved to the anaerobic layer of soil (Van den Pol-van Dasselaar and Oenema 1999; Chacon et al. 2006). The elevated concentration of methane then stimulated the increase of methanotrophic abundance. In addition, the increasing CH4 concentration of soil pore spaces caused by the lower soil moisture may also directly influence the methanotrophic abundance in G.
4.3 The abiotic driving factors of methanotrophic community structure
The soil abiotic parameters have an important influence on methanotrophic community structure. Kumaresan et al. (2011) showed that it is complex interactions of several abiotic factors rather than any single factor that is responsible for methanotrophic population in landfill cover soil. Our study also found, the methanotrophic community structure being driven by several abiotic factors. In 79E, OTU1, and OTU2 were the main genotypes. NO3 −-N, DOC, and pH were the main driving factors for these two genotypes (Fig. 5). Therefore, NO3 −-N, DOC, and pH may be the main contributors to the methanotrophic community structure of 79E. In 99E, the NO3 −-N and DOC were the driving factors for OTU1, the only genotype in 99E. In G, the significantly higher concentrations of NH4 +-N, PO4 3−-P than those in 79E and 99E (Table 1) should be the factors promoting the existence of OTU3, OTU4, OTU5, and OTU6 unique to G. The occurrence of the genotypes in G (OTU1, OTU3, OTU4, OTU5, and OTU6) was mainly driven by the combined effect of NH4 +-N, PO4 3−-P, NO3 −-N, and DOC. Though our study mainly focused on the effect of abiotic factors, we understand that there still exist many biotic factors to influence the methanotrophic community structure (Kumaresan et al. 2011). The effects of biotic factors (other microbes) on the methanotrophic community structure need to be further studied.
5 Conclusions
This study found that USC-γ cluster is the predominant methanotrophs to uptake CH4 in the Inner Mongolia grasslands. Different from results of earlier studies, the USC-γ cluster constituted the major abundance but not the genotype. 99E, the transition phase from grazing to enclosure, had a methanotroph community composition changing with time. NH4 +-N and PO4 3−-P are considered to be the main driving factors for the methanotroph community structure. These results indicated that enclosure can change the soil methanotroph community structure, then may influence CH4 uptake. But the relationship between methanotroph community structure and CH4 flux need to be further explored in the Inner Mongolia grasslands.
References
Abell GC, Stralis-Pavese N, Sessitsch A, Bodrossy L (2009) Grazing affects methanotroph activity and diversity in an alpine meadow soil. Environ Microbiol Rep 1:457–465
Adamsen APS, King GM (1993) Methane consumption in temperate and sub-arctic forest soils-rates, vertical zonation, and responses to water and nitrogen. Appl Environ Microbiol 59:485–490
Amaral JA, Ren T, Knowles R (1998) Atmospheric methane consumption by forest soils and extracted bacteria at different pH values. Appl Environ Microbiol 64:2397–2402
Belger L, Forsberg BR, Melack JM (2011) Carbon dioxide and methane emissions from interfluvial wetlands in the upper Negro River basin, Brazil. Biogeochemistry 105:171–183
Bhatia A, Ghosh A, Kumar V, Tomer R, Singh SD, Pathak H (2011) Effect of elevated tropospheric ozone on methane and nitrous oxide emission from rice soil in north India. Agric Ecosyst Environ 144:21–28
Bodrossy L, Murrell JC, Dalton H, Kalman M, Puskas LG, Kovacs KL (1995) Heat-tolerant methanotrophic bacteria from the hot water effluent of a natural gas field. Appl Environ Microbiol 61:3549–3555
Bodrossy L, Holmes EM, Holmes AJ, Kovács KL, Murrell JC (1997) Analysis of 16S rRNA and methane monooxygenase gene sequences reveals a novel group of thermotolerant and thermophilic methanotrophs, Methylocaldum gen. nov. Arch Microbiol 168:493–503
Boetius A et al (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407:623–626
Castro MS, Steudler PA, Melillo JM, Aber JD, Bowden RD (1995) Factors controlling atmospheric methane consumption temperate forest soils. Glob Biogeochem Cycles 9:1–10
Chacon N, Silver WL, Dubinsky EA, Cusack DF (2006) Iron reduction and soil phosphorus solubilization in humid tropical forests soils: the roles of labile carbon pools and an electron shuttle compound. Biogeochemistry 78:67–84
Chen W et al (2010) Annual methane uptake by typical semiarid steppe in Inner Mongolia. J Geophys Res 115:1–10
Chen GC et al (2014) Rich soil carbon and nitrogen but low atmospheric greenhouse gas fluxes from North Sulawesi mangrove swamps in Indonesia. Sci Total Environ 487:91–96
Cicerone RJ, Oremland RS (1988) Biogeochemical aspects of atmospheric methane. Glob Biogeochem Cycles 2:299–327
Costello AM, Lidstrom ME (1999) Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol 65:5066–5074
Curry CL (2007) Modeling the soil consumption of atmospheric methane at the global scale. Glob Biogeochem Cyles 21. doi:10.1029/2006GB002818
Dalla Valle M, Codato E, Marcomini A (2007) Climate change influence on POPs distribution and fate: a case study. Chemosphere 67:1287–1295
Degelmann DM, Borken W, Drake HL, Kolb S (2010) Different atmospheric methane-oxidizing communities in European beech and Norway spruce soils. Appl Environ Microbiol 76:3228–3235
Del Grosso SJ et al (2000) General CH4 oxidation model and comparisons of CH4 oxidation in natural and managed systems. Glob Biogeochem Cyles 14:999–1019
Deng Y, Cui X, Luke C, Dumont MG (2013) Aerobic methanotroph diversity in Riganqiao peatlands on the Qinghai-Tibetan Plateau. Environ Microbiol Rep 5:566–574
Dubey SK, Singh JS (2001) Plant-induced spatial variations in the size of methanotrophic population in dryland and flooded rice agroecosystems. Nutr Cycl Agroecosyst 59:161–167
Dutaur L, Verchot LV (2007) A global inventory of the soil CH4 sink. Global Biogeochem Cy 21. doi:10.1029/2006GB002734
Geng YB, Luo GQ, Yuan GF (2010) CH4 uptake flux of Leymus chinensis steppe during rapid growth season in Inner Mongolia, China. Sci China-Earth Sci 53:977–983
Han B et al (2009) Diversity and activity of methanotrophs in alkaline soil from a Chinese coal mine. FEMS Microbiol Ecol 70:196–207
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60:439–471
He PJ, Yang N, Fang WJ, Lu F, Shao LM (2011) Interaction and independence on methane oxidation of landfill cover soil among three impact factors: water, oxygen and ammonium. Front Environ Sci Eng China 5:175–185
Holmes AJ, Costello A, Lidstrom ME, Murrell JC (1995) Evidence that particulate methane monooxygenase may be evolutionarily related. FEMS Microbiol Lett 132:203–208
Holst J, Liu C, Yao Z, Brüggemann N, Zheng X, Giese M, Butterbach-Bahl K (2008) Fluxes of nitrous oxide, methane and carbon dioxide during freezing–thawing cycles in an Inner Mongolian steppe. Plant Soil 308:105–117
Horz HP, Rich V, Avrahami S, Bohannan BJM (2005) Methane-oxidizing bacteria in a California upland grassland soil: diversity and response to simulated global change. Appl Environ Microbiol 71:2642–2652
Hütsch BW (2001) Methane oxidation in non-flooded soils as affected by crop production—invited paper. Eur J Agron 14:237–260
Kirschke S et al (2013) Three decades of global methane sources and sinks. Nat Geosci 6:813–823
Kumaresan D, Hery M, Bodrossy L, Singer AC, Stralis-Pavese N, Thompson IP, Murrell JC (2011) Earthworm activity in a simulated landfill cover soil shifts the community composition of active methanotrophs. Res Microbiol 162:1027–1032
Lin JL, Radajewski S, Eshinimaev BT, Trotsenko YA, McDonald IR, Murrell JC (2004) Molecular diversity of methanotrophs in Transbaikal soda lake sediments and identification of potentially active populations by stable isotope probing. Environ Microbiol 6:1049–1060
Liu C et al (2007) Winter-grazing reduces methane uptake by soils of a typical semi-arid steppe in Inner Mongolia, China. Atmos Environ 41:5948–5958
Liu FC, Li HI, Dong Z, Zhang H (2012a) Advances in research on enclosure effects on vegetation restoration and soil physicochemical property of degraded grassland. Sci Soil Water Conserv 10:116–122
Liu GC et al (2012b) Microbial community composition controls the effects of climate change on methane emission from rice paddies. Environ Microbiol Rep 4:648–654
Ma X, Wang S, Wang Y, Jiang G, Nyren P (2006) Short‐term effects of sheep excrement on carbon dioxide, nitrous oxide and methane fluxes in typical grassland of Inner Mongolia. New Zeal J Agric Res 49:285–297
Moraes LE, Strathe AB, Fadel JG, Casper DP, Kebreab E (2014) Prediction of enteric methane emissions from cattle. Glob Chang Biol 20:2140–2148
Nercessian O, Noyes E, Kalyuzhnaya MG, Lidstrom ME, Chistoserdova L (2005) Bacterial populations active in metabolism of C1 compounds in the sediment of Lake Washington, a freshwater lake. Appl Environ Microbiol 71:6885–6899
Putkinen A, Larmola T, Tuomivirta T, Siljanen HM, Bodrossy L, Tuittila ES, Fritze H (2014) Peatland succession induces a shift in the community composition of Sphagnum-associated active methanotrophs. FEMS Microbiol Ecol 88:596–611
Raghoebarsing AA et al (2006) A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440:918–921
Sankaran M, Augustine DJ, (2004) Large herbivores suppress decomposer abundance in a semiarid grazing ecosystem. Eco 85:1052–1061
Sawakuchi HO, Bastviken D, Sawakuchi AO, Krusche AV, Ballester MVR, Richey JE (2014) Methane emissions from Amazonian Rivers and their contribution to the global methane budget. Glob Chang Biol 20:2829–2840
Sieburth JN, Johnson PW, Eberhardt MA, Sieracki ME, Lidstrom M, Laux D (1987) The first methane-oxidizing bacterium from the upper mixing layer of the deep ocean: Methylomonas pelagica sp. nov. Curr Microbiol 14:285–293
Singleton DR, Furlong MA, Rathbun SL, Whitman WB (2001) Quantitative comparisons of 16S rRNA gene sequence libraries from environmental samples. Appl Environ Microbiol 67:4374–4376
Steenbergh AK, Meima MM, Kamst M, Bodelier PLE (2010) Biphasic kinetics of a methanotrophic community is a combination of growth and increased activity per cell. FEMS Microbiol Ecol 71:12–22
Trotsenko YA, Khmelenina VN (2002) Biology of extremophilic and extremotolerant methanotrophs. Arch Microbiol 177:123–131
Van den Pol-van Dasselaar A, Oenema O (1999) Methane production and carbon mineralisation of size and density fractions of peat soils. Soil Biol Biochem 31:877–886
Vecherskaya M, Galchenko V, Sokolova E, Samarkin V (1993) Activity and species composition of aerobic methanotrophic communities in tundra soils. Curr Microbiol 27:181–184
Veraart AJ, Steenbergh AK, Ho A, Kim SY, Bodelier PLE (2015) Beyond nitrogen: the importance of phosphorus for CH4 oxidation in soils and sediments. Geoderma 259:337–346
Wang J, Song C, Zhang J, Wang L, Zhu X, Shi F (2014) Temperature sensitivity of soil carbon mineralization and nitrous oxide emission in different ecosystems along a mountain wetland-forest ecotone in the continuous permafrost of Northeast China. Catena 121:110–118
Yun J, Zhuang G, Ma A, Guo H, Wang Y, Zhang H (2012) Community structure, abundance, and activity of methanotrophs in the Zoige wetland of the Tibetan Plateau. Microb Ecol 63:835–843
Yun J, Ju Y, Deng Y, Zhang H (2014) Bacterial community structure in two permafrost wetlands on the Tibetan Plateau and Sanjiang Plain, China. Microb Ecol 68:360–369
Zhang X, Kong JY, Xia FF, Su Y, He R (2014) Effects of ammonium on the activity and community of methanotrophs in landfill biocover soils. Syst Appl Microbiol 37:296–304
Zheng Y, Yang W, Sun X, Wang SP, Rui YC, Luo CY, Guo LD (2012) Methanotrophic community structure and activity under warming and grazing of alpine meadow on the Tibetan Plateau. Appl Microbiol Biotechnol 93:2193–2203
Zheng Y, Zhang LM, Zheng YM, Di H, He JZ (2008) Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices. J Soil Sediment 8:406–414
Zhou XQ, Wang YF, Huang XZ, Hao YB, Tian JQ, Wang JZ (2008a) Effects of grazing by sheep on the structure of methane-oxidizing bacterial community of steppe soil. Soil Biol Biochem 40:258–261
Zhou XQ, Wang YF, Huang XZ, Tian JQ, Hao YB (2008b) Effect of grazing intensities on the activity and community structure of methane-oxidizing bacteria of grassland soil in Inner Mongolia. Nutr Cycl Agroecosyst 80:145–152
Acknowledgments
This study was supported by the 100 Talents Program of The Chinese Academy of Sciences, 1000 Talents Program of Sichuan Province, the National Natural Science Foundation of China (No. 31570480) and Youth Science and Technology Innovation Team Support Foundation of Sichuan Province (Y5D2071100). The authors give special thanks to Ms. Wan Xiong for her edits and valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Ma, T., Chen, H., Wang, Y. et al. Effects of enclosure time on the community composition of methanotrophs in the soils of the Inner Mongolia grasslands. J Soils Sediments 16, 1022–1031 (2016). https://doi.org/10.1007/s11368-015-1305-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1305-1