Abstract
Purpose
Microalgae biodiesel has attracted considerable attention as a potential substitute for fossil fuels and biodiesel from food crops. Nevertheless, its reported climate impacts in the scientific literature vary significantly. This article describes and synthesizes the range of results found in the life cycle assessment (LCA) literature regarding microalgae biodiesel studies to investigate whether particular parameters, e.g. technologies, were associated with higher or lower greenhouse gas (GHG) emissions so that a best practice can be inferred from currently available LCA data and thereby recommended.
Methods
A systematic literature review and meta-regression analysis (MRA) of 36 LCA studies that report on the GHG emissions of microalgae biodiesel was conducted. An assessment of key aspects, including modelling choices and technologies, was performed. Furthermore, MRA models were formulated considering several variables of interest describing both technical and modelling choices to identify the main causes for the variability in GHG emissions per MJ of biodiesel. Variables chosen include: microalgae species; culture medium; cultivation system; source of CO2; extraction technology; conversion technology; system boundary; geographical scope; inclusion or exclusion of capital goods; and how multifunctionality was handled.
Results and discussion
The reviewed studies altogether reported 308 results ranging from −0.7 to 3.8 kg CO2 eq. MJ−1biodiesel, portraying 19 different system configurations. Despite the comprehensive range of variables assessed, the models generated could not plausibly explain that the variability in GHG emissions depends either on the technologies considered or on the methodological choices adopted. However, the following relationships could be observed: location in Europe and high oil productivity were associated with lower emissions, whilst dry extraction should be avoided for leading to higher GHG emissions, on average.
Conclusions
There is a large degree of variability within the technologies considered, as well as the methodological choices adopted, so that no robust conclusions could be drawn from the MRA. Notwithstanding, average GHG emissions reported were more than twice as high as fossil diesel and, while there are some studies showing large benefits, none of the various algae technologies performed consistently better than fossil diesel, questioning the climate-mitigation potential of microalgae biodiesel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microalgae biofuels have attracted considerable attention as a replacement for fossil fuels due to algae’s fast and season independent growth, and relatively high lipid content (Fortier et al. 2014). In spite of these advantages, a life cycle assessment (LCA) should be performed to evaluate whether biofuel produced from algal feedstock leads to lower life-cycle impacts than fossil fuels. Numerous LCA studies of microalgae biofuels have been published covering very different processes and leading to contrasting results and conclusions. Previous reviews of microalgae biofuels LCAs focused on both qualitative analysis of methodological aspects, such as system boundaries and handling of co-products (e.g., Collet et al. 2015; Collotta et al. 2016), and comparison of impact assessment results with fossil fuels and other biofuels (e.g., Figueiredo et al. 2017; Carneiro et al. 2017). These reviews showed high heterogeneity between studies regarding both methodological choices and reported impacts, including GHG intensity results, and highlighted the difficulty in selecting preferable pathways due to high variability.
The variability in LCA results of microalgae biodiesel has been approached using harmonization techniques. Liu et al. (2012) developed a meta-model by harmonizing six studies and adopting common methodological choices in the modelling assumptions and system boundaries, so as to reduce differences in results. Tu et al. (2017) proposed a harmonized inventory data set to minimize the variation in results and applied it to assess impacts of algae biofuel production pathways in the U.S. (Tu et al. 2018). These studies required the adjustment of several system (Liu et al. 2012; Tu et al. 2017) and technical (Tu et al. 2017) features to consistently reduce variability (Tu et al. 2017) or to find a consistent scope of assessment to assess the inherent variability of different production pathways (Liu et al. 2012).
Another approach to summarize LCA results and elucidate variables of interest is the application of meta-regression analysis (MRA) techniques. MRA can be regarded as a statistically harmonization method complementary to other harmonization methods used in the LCA literature, such as the work of Tu et al. 2017 and Liu et al. (2012). MRA reduces subjectivity by using raw data with as little data manipulation as possible – it does not eliminate subjectivity in the modelling that originates the data but aims to discern statistically significant patterns from the raw data (Menten et al. 2013).
An MRA of life-cycle GHG emissions of advanced biofuels was performed by Menten et al. (2013) covering microalgae biodiesel studies published in 2009–2010 (8 studies; 69 results). The descriptive variables in the regression model included system boundaries (well-to-wheels or well-to-gate), type of co-products (e.g., glycerine, electricity, heat) and multifunctionality approach (e.g., allocation, substitution), but left out the different technologies used in the various stages of the algae biofuel pathway (except cultivation). Since then, the LCA literature on the topic has expanded, with studies covering different locations, algae species, and productivity ranges, testing different harvesting, drying, extraction and conversion technologies, and culture mediums, and assessing multiple uses for by-product valorisation. These aspects introduce new layers of variability not evaluated in the previous MRA.
This article describes and synthesizes the new range of LCA literature results, including studies published from 2009 to 2017, and builds on previous work by Figueiredo et al. (2015, 2017), by (i) performing a qualitative assessment of the literature, focusing on key aspects, including modelling choices and technologies to identify variables of interest; (ii) assessing the variability in the reported GHG intensity; and (iii) quantifying the effect of the most influential variables on the GHG intensity of microalgae biodiesel through MRA, expanding the range of variables analysed in Menten et al. (2013). The main goal is to (i) investigate whether it is possible to identify specific variables (e.g., technologies, modelling choices) that may be significantly associated with higher or lower GHG emissions per MJ of biodiesel and (ii) provide recommendations on which technologies should be avoided or promoted based on currently available LCA data.
2 Methods
2.1 Overview of microalgae biodiesel life-cycle chain
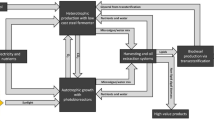
A microalgae biodiesel production chain typically includes the following life-cycle stages: microalgae cultivation, harvesting and drying, oil extraction, conversion, distribution and use (e.g. in a vehicle). Figure 1 shows the available technologies for each life-cycle stage. The diversity of technologies indicates that microalgae biodiesel production processes are at the early stage of development and the optimal technologies are not yet clearly established.
Life-cycle chain (well-to-wheels) of microalgae biofuel, including alternative technologies for each phase. * Optional step – depends on the bio oil recovery technology. PBR: Photo-bioreactor; OP: Open pond; CF: Centrifugation; CFP: Chamber filter press; DAF: Dissolved air flotation; VBD: Vacuum belt dryers; DE: Dry extraction; WE: Wet extraction; SC-CO2: Supercritical CO2 extraction; HTL: Hydrothermal liquefaction; P: Pyrolysis; Trans: Transesterification; HT: Hydrotreating; U&DT: Ultrasonication and direct transerterification; SC-CH3OH: Supercritical methanol; MBR: Microalgae biomass residues
The cultivation of microalgae can be conducted in three ways (Stephenson et al. 2010):
-
i)
In photo-bioreactors (PBR), which are closed systems with a productivity of cellular biomass generally higher than in open ponds, but more expensive to build and operate;
-
ii)
In open ponds (OP), also termed raceways, which are open systems, where a paddlewheel is used to mix the biomass. These systems can suffer from high losses by evaporation, requiring large volumes of fresh water. Furthermore, productivity can be affected by contamination with microorganisms (Stephenson et al. 2010); and
-
iii)
In hybrid systems, which are a combination of PBR and OP. A small volume is cultured in a closed system (Khoo et al. 2011) (PBR) where the inoculum is prepared, which is then transferred to an OP where the algae grow.
The growth media can be: (i) fresh water or marine water, depending on the algal species, with synthetic nutrients (synthetic medium); and (ii) wastewater (WW) containing the nutrients required for microalgae growth. The CO2 required during microalgae growth can be sourced from pure CO2 from e.g. an ammonia plant (e.g., Hou et al. 2011; Campbell et al. 2011; Monari et al. 2016), CO2 extracted from natural deposits (e.g., Connelly et al. 2015; Liu et al. 2013), and flue gas from an adjacent plant (e.g., Brentner et al. 2011; Orfield et al. 2015; Mu et al. 2017). The latter can be provided by direct injection or first separated into pure CO2 via monoethanolamine scrubbing and then injected into the cultivation system (Zaimes and Khanna 2014).
For harvesting, a number of technologies are available and different combinations are employed. For instance, microalgae may be first decanted, filtered, and/or flocculated and centrifuged or filtered. Depending on the bio-oil extraction technology, microalgae may need to be dried (e.g., solar drying; heat drying with different fuels). In the bio-oil extraction step, oil can be extracted from seven ways:
-
i)
Dry lipid extraction (DE), which is similar to the extraction of other vegetable oils (e.g., rapeseed, soybean). Microalgae biomass is previously dried and then exposed to extraction by a solvent (e.g. hexane, most common);
-
ii)
Wet lipid extraction (WE), in which wet microalgae biomass is exposed to extraction by a solvent (e.g. hexane);
-
iii)
Supercritical CO2 extraction (SC-CO2), which uses a supercritical fluid (CO2) to extract thermally sensitive lipid fractions without degradation; it has the advantages of avoiding the use of organic solvents and allowing for the selection of the relevant lipids for biodiesel production during the extraction process (Brentner et al. 2011);
-
iv)
Hydrothermal liquefaction (HTL), a thermochemical process in which algae is heated in a pressurized wet slurry; under these conditions, algal cells are broken down to mainly liquid components (Gollakota et al. 2018);
-
v)
Pyrolysis (P), a thermochemical process that decomposes biomass into liquid oil, gases and solid products, at temperatures ranging from 350 to 700 °C and in the absence of air (Goyal et al. 2008). For this process, algae need to be dry;
-
vi)
Ultrasonication with direct transesterification (U&DTrans), where bio-oil extraction and transesterification occur simultaneously. Methanol is added directly to dried biomass using sulphuric acid as a catalyst (Brentner et al. 2011); and
-
vii)
Supercritical methanol (SC-CH3OH), which combines lipid extraction and transesterification of oil from wet microalgae, requiring no catalyst (Brentner et al. 2011).
Besides oil, the extraction process also produces microalgae biomass residue (MBR), for which different management options can be applied, such as: direct land application as fertilizer; anaerobic digestion, generating biomethane that can be combusted for heat and electricity in a combined heat and power plant; and sacharification and fermentation to produce ethanol. When microalgae is cultivated in a synthetic medium, MBR can also be used in food supplements, pharmaceutical and cosmetic products, and for animal feed.
The conversion phase can be made, for example, through transesterification or hydrotreating. The process of transesterification involves the catalysed reaction of lipids with methanol, producing fatty acid methyl esters (i.e. biodiesel) and the co-product glycerine. Glycerine can be used for energy purposes, for animal feed or for pharmaceutical products, among other uses. The hydrotreating process involves an hydrogenation step, which produces synthetic hydrocarbons, followed by hydrocracking and distillation (at the end of hydrotreating, bio jet-fuel, naphtha and a small quantity of biodiesel are produced) (Cox et al. 2014). Finally, biodiesel is distributed and combusted in a vehicle.
2.2 Selection process for the literature review
An online search of articles published between 2009 and 2017 consisting of LCA studies of microalgae biodiesel was performed in Web of Knowledge. The keywords used for the literature search (in TITLE) included a combination of synonyms of the terms “life cycle assessment” (OR “LCA” OR “life cycle analysis” OR “life cycle” OR “lifecycle” OR “life-cycle”) OR “greenhouse gas emissions” (OR “GHG”), AND “algae” (OR “algal” OR “microalgae” OR “microalgal”), AND “biofuel” (OR “biodiesel” OR “bioenergy”). The search retrieved 87 results. From these, only peer-reviewed journal articles written in English were considered (−5). Comments on published articles were excluded (−4) and articles with corrigendum were accounted for as a single reference (−2). Review articles were also not included (−8), but the references cited were scanned for relevant articles not retrieved in the previous literature search. The articles included in previous reviews by Figueiredo et al. (2015, 2017) were also skimmed. Articles were selected for further analysis if they detailed information on the methodology, assumptions, data used, and the GHG balances of the system (see Tables S1 and S2 in the Online Resource 1). To allow for comparisons, the results of the reviewed studies were normalized to 1 MJ of biodiesel (measured in terms of the lower heating value). Studies were excluded mainly due to: (i) addressing other bioenergy routes than biodiesel (e.g. bio jet fuel); (ii) lack of transparency and insufficient quantitative information (e.g. the study considered other functional unit than MJ of biodiesel and it was not possible to convert the results due to lack of data), (iii) the assessment being limited to one single life-cycle stage of biodiesel production, and (iv) the study focused the analysis in other metrics than GHG emissions (e.g., energy use, water footprint). In the end, 36 articles were included in the review and provided 308 estimates of life cycle GHG emissions of microalgae biofuel.
2.3 Meta-regression model and variables
The MRA was performed using STATA® (StataCorp 2012). A General-to-Specific methodology was employed to specify the regression model, i.e. estimating a general model containing a wide range of potentially important variables and then stepwise exclude the least significant variable, one by one, until only statistically significant (p < 0.05) variables remain in a specific or final model.
The proposed model is a multiple linear regression model with the following structure:
where Y is the dependent variable that represents the GHG emissions associated with one MJ of produced biodiesel; Xj = (X2,…,Xk) are the independent variables (or regressors); β1 is a common factor (intercept); and βj = (β2,…, βk) are the partial regression coefficients that measure characteristics of the biodiesel case study under analysis; i = (1,…,n) is the ith observation, and ε is the error term. The model was estimated using ordinary least square (OLS) method assuming a linear relationship between the dependent and independent variables. Due to the skewness of the original data (see Fig. S1 in Online Resource 1), we log transformed both the continuous independent and depend variable data to approximately conform to normality; therefore, a log-log model was used. Inevitably, all observations containing negative GHG emissions per MJ were excluded (<6%). However, as there were few negative values in the sample, the risk that these observations would change the interpretation of the relationship (the direction of the coefficient) is small. To account for potential non-linear relationships a quadratic term was included in the robustness checks.
2.4 Selection of variables of interest
The selection of variables of interest was based on a qualitative review of the LCA studies on microalgae biodiesel previously selected, focusing on: (i) relevant aspects related with microalgae biodiesel production systems which differed between studies and may influence GHG emission results (e.g., biomass productivity, lipid content, and microalgae species – additional details for each study can be found in Table S1 in Online Resource 1); and (ii) the main methodological choices adopted in the LCA studies (e.g., geographical scope, system boundary, and multifunctionality – additional details for each study can be found in Table S2 in Online Resource 1). Studies were performed using laboratory or pilot scale data, which may limit its use to represent full commercial applications.
The microalgae species used in LCA studies were set as variables. Different species of microalgae were reported, including both marine and freshwater species. One study reported results for more than one microalgae species. The most common genus of microalgae was Chlorella spp. (31%), particularly Chlorella vulgaris (19%), followed by Nannochloropsis ssp. (22%). Scenedesmus was mentioned in two studies (5%). In addition to those, three other species of microalgae were identified (Pleurochrysis carterae, Tetraselmis, Neochloris) and 42% of the studies did not indicate the kind of microalgae considered.
Another variable included was the type of culture medium: synthetic medium (included in 81% of the studies) and wastewater (25%). Two studies (5%) compared both culture mediums.
Both lipid content and microalgae productivity show a large variation in the reviewed studies and are seen as key factors for life cycle GHG emissions of microalgae biofuels (Menten et al. 2013). Lipid content varied from 2% to 61%. The productivities varied from 2 g DM m−2 day−1 (Sills et al. 2013) to 646 g DM m−2 day−1 (Brentner et al. 2011). From the point of view of biodiesel production, the important factor is the oil productivity, i.e. the product of lipid content and microalgae productivity; therefore, oil productivity was selected as a variable of interest.
The CO2 source also differed between studies and was set as variable. We distinguish studies that use CO2 from natural or industrial origins and studies that use waste CO2 (from flue gas from adjacent plant).
Regarding the different technology pathways, all technologies found in the literature for the different biodiesel production steps (see section 2.1), namely microalgae cultivation (OP [69% of the studies], PBR [25%], hybrid [19%]), bio-oil extraction (DE [61%], WE [31%], HTL [19%], P [2.5%]) and biodiesel conversion (transesterification, hydrotreating), were set as variables.
Key LCA methodological choices were also selected as variables of interest. Three alternative system boundaries were identified and set as variables. The most common was well-to-wheels (WtW) (53%), also referred to as cradle-to-combustion, cradle-to-grave, or pond-to-wheels, and covers the entire life cycle including the combustion of the biodiesel. One third considered a well-to-gate (WtG) boundary, also denominated as cradle-to-gate, which includes emissions only up to the gate of the biodiesel conversion process. Finally, 14% of the studies considered a well-to-pump (WtP) boundary (also known as well-to-tank), which also includes the distribution of the biodiesel, but not its combustion.
The inclusion or exclusion of capital goods in the system boundary was also assessed. Most of the studies excluded capital goods from the system boundary (53%), whilst one third accounted for the infrastructures and 14% did not provide any information on that aspect.
Dealing with multifuncionality in LCA studies is a challenging and controversial issue (Malça and Freire 2006) and different choices may yield very different results. The reviewed studies used different approaches to deal with multifunctionality. One quarter considered exclusively the substitution method or system expansion, including alternative functions for the co-products (Sander and Murthy 2010; Sills et al. 2013; Zaimes and Khanna 2013; Mu et al. 2014; Soh et al. 2014; Soratana et al. 2014; Chowdhury and Freire 2015; Monari et al. 2016; Mu et al. 2017). Different substitution options were compared in 8% of the studies (Zaimes and Khanna 2013; Soratana et al. 2014; Zaimes and Khanna 2014). One quarter of the revised studies considered exclusively an allocation method based on mass, energy or economic value (Brentner et al. 2011; Hou et al. 2011; Yanfen et al. 2012; Frank et al. 2013; Collet et al. 2014; Maranduba et al. 2015; Orfield et al. 2015; Souza et al. 2015; Bello et al. 2017). A combination of system expansion and allocation was used by 14% of the studies, namely considering the anaerobic digestion of MBR to energy and/or fertilizers combined with economic or energy allocation between biodiesel and glycerine (Stephenson et al. 2010; Frank et al. 2012; Adesanya et al. 2014; Woertz et al. 2014) or biodiesel and methane or ethanol (Soratana et al. 2013). Finally, 14% of the studies compared the substitution and allocation methods (Batan et al. 2011; Passell et al. 2013; Adesanya et al. 2014; Zaimes and Khanna 2014; Yuan et al. 2015). Detailed information concerning the allocation method considered by each study is shown in Table S2 (Online Resource 1). The different ways in which multifunctionality was handled were also set as variable of interest.
Studies also differed in the alternative functions for the co-products. Microalgae biomass residue was used for: i) animal feed; ii) anaerobic digestion with combustion of methane for electricity and digested biomass for fertilizer; iii) co-generation; and iv) ethanol production (substituting ethanol from corn). Glycerine was either used by pharmaceutical industries, displacing fossil glycerine, or combusted for energy production. For the particular cases in which WW was used as culture medium, different ways of dealing with the co-production of biodiesel and biological nutrients removal (BNR) were considered: not accounting for the nutrient gains or the environmental burdens associated with the use of WW effluent prior to microalgae cultivation and the avoided burdens from using microalgae as a BNR process (Soratana et al. 2014; Woertz et al. 2014; Monari et al. 2016); substitution, assuming that the conventional BNR process in a wastewater treatment plant is replaced by the microalgae cultivation system (and hence applied credits from avoided BNR) (Mu et al. 2014); substitution, assuming avoided production of synthetic fertilizers (Chowdhury and Freire 2015); substitution of both conventional BNR process and synthetic fertilizers production (Soratana et al. 2013); and allocation of the environmental burdens from WW treatment prior to microalgae cultivation to biodiesel production (Sander and Murthy 2010).
The geographical location of the case studies was selected as a variable of interest. Location can influence insolation and the GHG intensity of background processes, such as energy used in microalgae production. Half of the reviewed studies were located in North America (USA) and one-sixth in Europe (two in the UK, two in France, one in Denmark, and one undefined countries). Remaining locations were Australia, China, and Brazil, with two studies each, and India, Israel and Singapore (all with one study). The geographical scope of three studies was undefined.
Table 1 presents all independent variables (Xj in Eq. 1) included in the meta-regression analysis, which were selected based on the identified potential factors for the higher variability associated with the GHG emissions of 1 MJ of biodiesel. In addition, the year of publication was considered as variable of interest. It is important to note that, with the exception of microalgae oil productivity and publication year, all variables are discrete, and therefore are inevitably represented in a binary manner, i.e. quantified as either 0 or 1.
3 Results and discussion
3.1 Reported GHG intensity of microalgae biodiesel
Figure 2 presents the GHG emissions reported in the 36 studies, which range between −700 and 3800 g CO2 eq. MJ−1 (corresponding to 308 data points, mean: 245 g CO2 eq. MJ−1). In Fig. 3, the reported results were grouped by: (a) culture medium (synthetic or wastewater); (b) CO2 source (pure CO2 or waste CO2); (c) cultivation system (OP, PBR and hybrid); (d) extraction technology (dry, wet, HTL, pyrolysis or other); (e) conversion technology (hydrotreating or transesterification); (f) location (North America, Europe, Asia, Australia and Latin America); (g) system boundary (WtW, WtG and WtP); and (h) multifunctionality approach (mass, energy, and price allocation, substitution or hybrid).
GHG intensity of microalgae biofuel reported in the reviewed studies grouped by: (a) culture medium (synthetic or wastewater); (b) CO2 source (pure CO2 or waste CO2); (c) cultivation system (open pound [OP], photobioreactor [PBR] and hybrid); (d) extraction technology (dry, wet, hydrothermal liquefaction [HTL], pyrolysis and other); (e) conversion technology (hydrotreating and transesterification); (f) location (North America [NA], Europe [EU], Asia [AS], Australia [AU] and Latin America [LA]); (g) system boundary (well-to-wheels [WtW], well-to-gate [WtG] and well-to-pump [WtP]); and (h) multifunctionality approach (mass, energy, and price allocation, substitution [Subs] and hybrid); n indicates the number of observations
About half of the reported biodiesel results are higher than fossil diesel GHG intensity, i.e. 94 g CO2 eq. MJ−1 (European Parliament Union and Council of the European Union 2018). The results with the highest GHG emissions (above 1000 g CO2 eq. MJ−1) were essentially associated with five studies (Stephenson et al. 2010; Mu et al. 2014; Soh et al. 2014; Soratana et al. 2014; Monari et al. 2016). These were caused by low lipid content (2%) (Soh et al. 2014) and a combination of the worst possibilities (low microalgae productivity, low lipid content, low technological efficiency, high energy consumption) (Mu et al. 2014). Results from Monari et al. (2016), and Stephenson et al. (2010) were associated with a high energy consumption in cultivation (pumps, paddlewheels), harvesting (blowers and centrifugation) and oil extraction. Soratana et al. (2014) did not discuss the high environmental impacts associated with biodiesel production, because the study was focused on assessing different pathways for co-products. Studies reporting results over 2500 g CO2 eq. MJ−1 (Soratana et al. 2014; Monari et al. 2016) all used wastewater as culture medium (Fig. 3a), PBR as cultivation system (Fig. 3c), transesterification as extraction technology (Fig. 3e) and substitution as the approach to deal with co-products (Fig. 3h).
In ten studies, negative GHG emissions were reported due to: i) using substitution or system expansion to deal with co-products (see Table S2 in Online Resource 1) (Sander and Murthy 2010; Batan et al. 2011; Adesanya et al. 2014; Mu et al. 2014; Cox et al. 2014; Chowdhury and Freire 2015; Monari et al. 2016); and ii) accounting for biogenic CO2 absorbed during photosynthesis but not when biodiesel is combusted (Pardo-Cárdenas et al. 2013; Mu et al. 2014; Bennion et al. 2015; Malik et al. 2015; Monari et al. 2016). In addition to the large variation between the different studies, many studies show very wide ranges, because different scenarios comparing technologies, co-product uses and future system changes were assessed.
Although we grouped the results of different studies according to different aspects (e.g., technologies, culture medium, multifunctionality approach), it was not possible to draw specific conclusions regarding the best pathways, because of the number of available technologies for each life-cycle phase and the number of possible combinations (19 different system combinations were evaluated, as shown in Table S3 in the Online Resource 1). A meta-regression analysis is thus presented in an attempt to extrapolate clearer conclusions.
3.2 Meta-regression analysis results
Estimated results for the microalgae biodiesel MRA are presented in Table 2. We included in the model all variables at p < 0.05 significance levels. The standard errors of the coefficient estimates are reported in parenthesis. N indicates the number of observations used to perform the regression and the strength of the regression is reported as adjusted R-square (Adj. R2). The final model after employing the General-to-Specific methodology is as follows:
Our model suggests the following preferences for minimizing GHG emissions of microalgae biodiesel production:
-
Location: preference for EU;
-
Cultivation system: avoidance of PBRs;
-
Oil extraction technology: avoidance of dry extraction;
-
Conversion technology: avoidance of transesterification.
Despite the statistical significance of the variables (at 95%), the Adj. R2 of the model generated is not indicative of goodness of fit (48% of the variability around the mean is explained by the model), which is also shown in Fig. 4. In some instances, the model generated goes with the expectations, for example, the productivity of oil is negatively correlated with GHG emissions, indicating that a higher productivity is associated with a lower emission level, which confirms the findings of other scholars (e.g. Menten et al. 2013). PBRs appear to be associated with higher GHG emissions, as reported for instance in Menten et al. (2013) and Stephenson et al. (2010), but contrary to Tu et al. (2018) conclusions. The study presenting the highest GHG emissions per MJ of biodiesel (above 3.5 kg CO2 eq. MJ−1) uses PBR as cultivation system (Soratana et al. 2014), which might be driving the MRA results, and is not included in Tu et al. (2018) analysis – this hypothesis is tested in the robustness check (section 3.2.1).
Actual versus predicted values. The actual values refers to the dependent variable, ln(Y g CO2 eq. MJ−1) and the predicted values are based on the model specification of the dependent variable presented in Table 2
In terms of oil-extracting technologies, our model indicates that none of the technologies considered is statistically significant, with the exception of dry extraction, which is correlated positively with GHG emissions. In terms of the processing of algae oil into biodiesel, our model shows that transesterification is associated with higher emissions than hydrotreatment, in opposition to Menten et al. (2013) conclusions. Again, this result might be prompted by the high GHG emissions reported in one study (Soratana et al. 2014).
In terms of methodological choices, a mid-level system boundary (WtP) is unexplainably associated with higher emissions, as is the handling of co-production via substitution. The later suggests that dealing with multifunctionality in any way other than substitution (e.g. allocation) may underestimate emissions relative to using a system-expansion-and-substitution approach, which is the prioritized algorithm by the ISO 14044:2006 standards. Nevertheless, handling co-products via substitution often leads to net negative emissions as a result of the avoided burdens from displaced products; the use of a log-log model, which excludes negative values of GHG emissions, may overestimate the effect of substitution in microalgae biodiesel GHG emissions. Furthermore, location in the EU is associated with lower emission levels, which contradicts Menten et al. (2013) findings. The location may influence several parameters, such as insolation and the GHG intensity of energy used in microalgae production processes, but these aspects could not be captured by our model as the studies do not provide sufficient information to allow for a detailed assessment. Variables that presented no statistically significant effect include the algae species, the CO2 source, the culture medium, and the publication year.
3.2.1 Robustness checks
The data consists of several binary variables that are mutually exclusive within each category but not across categories, hence, imperfect multicollinearity is likely to exist between the independent variables. Multicollinearity produces unexpectedly large standard errors of the OLS estimate, and reduces the precision of the estimate coefficients and weakens the statistical power of the regression model. Hence, the stepwise approach of sequentially removing the least significant variables based on their associated p values might exclude the wrong variables. The presence of imperfect multicollinearity suggests it will be problematic to estimate accurately one or more of the partial effects using the data at hand (Stock and Watson 2015).
To test the robustness of the regression model an alternative approach, using the Variance Inflation Factor (VIF) as the exclusion criteria, was used in the general-to-specific procedure, as suggested by Zuur et al. (2010). The VIF estimates how much the variance of a regression coefficient is inflated due to multicollinearity in the model, hence, independent variables in the model with the highest VIF-value were excluded sequentially until no multicollinearity was detected (VIF below 2). Then, subsequent exclusion of variables by significance was performed. This procedure resulted in a different model specification (as shown in Table S4 in Online Resource 1), which underpins the concerns about the robustness of the baseline model as different significant variables were identified (ExtTec_Wet; ExtTec_HTL; Loc_NA, all with a negative contribution to GHG emissions).
An additional issue questioning the validity of our models is the low explanatory power, the R2. In an attempt to address this issue, several alternative model specifications were tested. First, a quadratic term was included to allow for curvature in the linear relationship between the dependent variable and oil productivity. The quadratic term was highly insignificant, and thus removed. Second, we included an interaction term to deal with the outliers in the data. There are a number of ways to deal with outliers, although they are often seen as data problems that must be fixed, outliers can also be of substantive interest and studied as unique phenomena that may lead to novel theoretical insights (Aguinis et al. 2013). In our case, no discernible insights could be extrapolated, except for the inexistence of clear patterns, which is an insight in itself, suggesting that LCA results are very variable between themselves and should not be compared. Since the exclusion of outliers post hoc might affect the objectivity of the results, they were not excluded. Instead, we investigated the traits among the outliers and added an interaction term explaining these common traits (see Table S4 in Online Resource 1).
The common traits are handling co-production via substitution and the use of PBRs and transesterification. There are 21 such observations. However, there is no intuitive explanation to why these three traits would yield the extreme estimate of emissions, as this group consists of observations in the upper and the lower tails of the emission distribution, ranging between −210 and 3800 g CO2 eq. MJ−1, although negative values were excluded from the MRA after the log transformation. Emissions on the upper tail of the emission distribution correspond to two studies: Monari et al. (2016) and Soratana et al. (2014). In both studies, GHG emissions are dominated by the biodiesel production process train and credits from substitution only account for less than 3% of those emissions. The high energy consumption in PBRs for CO2 pumping and nutrient mixing is reported by Monari et al. (2016) as the main reason for the high GHG emissions associated with biodiesel from microalgae. On the other hand, emissions on the lower tail of the emission distribution are associated with three studies: Batan et al. (2010) and Monari et al. (2016) when using waste CO2, whose negative results are mainly due to considering CO2 sequestration by the algae, but not biodiesel combustion; and Mu et al. (2014), which considers credits from avoided wastewater treatment, besides CO2 sequestration. Regardless of the interpretation of these common traits, the predictive power (R2) is improved when accounting for these by using an interaction term and different conclusions are supported regarding the other explanatory factors. For instance, whilst oil productivity and studies located in Europe are associated with lower GHG emissions and dry extraction with higher emissions as in our first regression model (see section 3.2), the new model (with an interaction term, see Table S4) also associates the use of CO2 from industrial or natural origin to higher GHG emissions and the use of synthetic medium to lower GHG emissions.
Notwithstanding, the application of the different methods to generate regression models did not circumvent the fact that no explainable patterns could be observed from the data and chosen variables.
4 Conclusions
We conducted a systematic literature review and meta-regression analysis of studies that report on the climate change effects of microalgae biodiesel, and reported their differences. The reviewed 36 studies altogether reported 308 data points in terms of g CO2 eq. MJ−1. The published values are widely variable, ranging from −0.7 to 3.8 kg CO2 eq. MJ−1biodiesel. Despite the high variability demonstrated, results suggest that reported GHG emissions from microalgae biodiesel are, on average, 2.6 times as high as those from its fossil counterpart, i.e. 94 g CO2 eq. MJ−1 (European Parliament Union and Council of the European Union 2018). To investigate whether significant variables and preferred technologies can be inferred from currently available LCA data, regression models were formulated considering several variables of interest describing a number of technical and modelling choices. Despite the comprehensive range of variables included in the models tested, none of the models generated could plausibly explain that the variability depends on the specific technologies considered nor on the methodological choices adopted. However, some relationships could be observed across most models. Specifically, our models indicate that location in the EU and high oil productivity are associated with lower emissions, as expected, whilst dry extraction should be avoided as it is associated with higher GHG emissions, on average.
The data collected does not follow any robust patterns, except confirm previous findings (e.g., importance of oil productivity and avoidance of energy-intensive processes such as dry extraction). While there were some studies showing large benefits, there was wide variation between the various algae technologies and it transpired that none performed consistently better than fossil diesel. The reported high levels of, and large variability in, GHG emissions question the climate-mitigation potential of microalgae biodiesel.
References
Adesanya VO, Cadena E, Scott SA, Smith AG (2014) Life cycle assessment on microalgal biodiesel production using a hybrid cultivation system. Bioresour Technol 163:343–355. https://doi.org/10.1016/j.biortech.2014.04.051
Aguinis H, Gottfredson RK, Joo H (2013) Best-practice recommendations for defining, identifying, and handling outliers. Organ Res Methods 16:270–301. https://doi.org/10.1177/1094428112470848
Batan L, Quinn J, Bradley T, Willson B (2011) Net energy and greenhouse gas emissions evaluation of biodiesel derived from microalgae. Environ Sci Technol 45:1160–1160. https://doi.org/10.1021/es1038479
Bello M, Ranganathan P, Brennan F (2017) Life cycle optimization for sustainable algal biofuel production using integrated nutrient recycling technology. ACS Sustain Chem Eng 5:9869–9880. https://doi.org/10.1021/acssuschemeng.7b01833
Bennion EP, Ginosar DM, Moses J, Agblevor F, Quinn JC (2015) Lifecycle assessment of microalgae to biofuel: comparison of thermochemical processing pathways. Appl Energy 154:1062–1071. https://doi.org/10.1016/j.apenergy.2014.12.009
Brentner LB, Eckelman MJ, Zimmerman JB (2011) Combinatorial life cycle assessment to inform process design of industrial production of algal biodiesel. Environ Sci Technol 45:7060–7067. https://doi.org/10.1021/es2006995
Campbell PK, Beer T, Batten D (2011) Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour Technol 102:50–56. https://doi.org/10.1016/j.biortech.2010.06.048
Carneiro MLNM, Pradelle F, Braga SL, Gomes MSP, Martins ARFA, Turkovics F, Pradelle RNC (2017) Potential of biofuels from algae: comparison with fossil fuels, ethanol and biodiesel in Europe and Brazil through life cycle assessment (LCA). Renew Sust Energ Rev 73:632–653. https://doi.org/10.1016/j.rser.2017.01.152
Chowdhury R, Freire F (2015) Bioenergy production from algae using dairy manure as a nutrient source: life cycle energy and greenhouse gas emission analysis. Appl Energy 154:1112–1121. https://doi.org/10.1016/j.apenergy.2015.05.045
Collet P, Hélias A, Lardon L, Steyer JP, Bernard O (2015) Recommendations for life cycle assessment of algal fuels. Appl Energy 154:1089–1102. https://doi.org/10.1016/j.apenergy.2015.03.056
Collet P, Lardon L, Hélias A, Bricout S, Lombaert-Valot I, Perrier B, Lépine O, Steyer JP, Bernard O (2014) Biodiesel from microalgae - life cycle assessment and recommendations for potential improvements. Renew Energy 71:525–533. https://doi.org/10.1016/j.renene.2014.06.009
Collotta M, Busi L, Champagne P, Mabee W, Tomasoni G, Alberti M (2016) Evaluating microalgae-to-energy -systems: different approaches to life cycle assessment (LCA) studies. Biofuels Bioprod Biorefin 10:883–895. https://doi.org/10.1002/bbb.1713
Connelly EB, Colosi LM, Clarens AF, Lambert JH (2015) Life cycle assessment of biofuels from algae hydrothermal liquefaction: the upstream and downstream factors affecting regulatory compliance. Energy and Fuels 29:1653–1661. https://doi.org/10.1021/ef502100f
Cox K, Renouf M, Dargan A, Turner C, Klein-Marcuschamer D (2014) Environmental life cycle assessment (LCA) of aviation biofuel from microalgae, Pongamia pinnata, and sugarcane molasses. Biofuels Bioprod Biorefin 8:579–593. https://doi.org/10.1002/bbb.1488
European Parliament Union, Council of the European Union (2018) Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the use of energy from renewable sources. Off J Eur Union 328:82–209
Figueiredo F, Garcia R, Castanheira E, Malça J, Freire F, Brandão M, Frank ED, Eng AG, Cowie A, Laurens LML (2017) Sustainability and life-cycle assessment of algal bioenergy. In: Laurens LML (ed) State of technology review – algae bioenergy. IEA bioenergy, pp 82–94
Figueiredo F, Garcia R, Margarida SG, Castanheira EG, Malça J, Oliveira AC, Matos C, Freire F (2015) Life-cycle assessment of microalgae biodiesel: a review. Energy for Sustainability 2015 - Sustainable cities: designing for people and the planet. Coimbra, Portugal
Fortier M-OP, Roberts GW, Stagg-Williams SM, Sturm BSM (2014) Life cycle assessment of bio-jet fuel from hydrothermal liquefaction of microalgae. Appl Energy 122:73–82. https://doi.org/10.1016/j.apenergy.2014.01.077
Frank ED, Elgowainy A, Han J, Wang Z (2013) Life cycle comparison of hydrothermal liquefaction and lipid extraction pathways to renewable diesel from algae. Mitig Adapt Strateg Glob Chang 18:137–158. https://doi.org/10.1007/s11027-012-9395-1
Frank ED, Han J, Palou-Rivera I, Elgowainy A, Wang MQ (2012) Methane and nitrous oxide emissions affect the life-cycle analysis of algal biofuels. Environ Res Lett 7. https://doi.org/10.1088/1748-9326/7/1/014030
Gollakota ARK, Kishore N, Gu S (2018) A review on hydrothermal liquefaction of biomass. Renew Sust Energ Rev 81:1378–1392. https://doi.org/10.1016/j.rser.2017.05.178
Goyal HB, Seal D, Saxena RC (2008) Bio-fuels from thermochemical conversion of renewable resources: a review. Renew Sust Energ Rev 12:504–517. https://doi.org/10.1016/j.rser.2006.07.014
Hou J, Zhang P, Yuan X, Zheng Y (2011) Life cycle assessment of biodiesel from soybean, jatropha and microalgae in China conditions. Renew Sust Energ Rev 15:5081–5091. https://doi.org/10.1016/j.rser.2011.07.048
Khoo HH, Sharratt PN, Das P, Balasubramanian RK, Naraharisetti PK, Shaik S (2011) Life cycle energy and CO2 analysis of microalgae-to-biodiesel: preliminary results and comparisons. Bioresour Technol 102:5800–5807. https://doi.org/10.1016/j.biortech.2011.02.055
Liu X, Clarens AF, Colosi LM (2012) Algae biodiesel has potential despite inconclusive results to date. Bioresour Technol 104:803–806. https://doi.org/10.1016/j.biortech.2011.10.077
Liu X, Saydah B, Eranki P, Colosi LM, Greg Mitchell B, Rhodes J, Clarens AF (2013) Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Bioresour Technol 148:163–171. https://doi.org/10.1016/j.biortech.2013.08.112
Malça J, Freire F (2006) Renewability and life-cycle energy efficiency of bioethanol and bio-ethyl tertiary butyl ether (bioETBE): assessing the implications of allocation. Energy 31:3362–3380. https://doi.org/10.1016/j.energy.2006.03.013
Malik A, Lenzen M, Ralph PJ, Tamburic B (2015) Hybrid life-cycle assessment of algal biofuel production. Bioresour Technol 184:436–443. https://doi.org/10.1016/j.biortech.2014.10.132
Maranduba HL, Robra S, Nascimento IA, da Cruz RS, Rodrigues LB, de Almeida Neto JA (2015) Reducing the life cycle GHG emissions of microalgal biodiesel through integration with ethanol production system. Bioresour Technol 194:21–27. https://doi.org/10.1016/j.biortech.2015.06.113
Menten F, Chèze B, Patouillard L, Bouvart F (2013) A review of LCA greenhouse gas emissions results for advanced biofuels: the use of meta-regression analysis. Renew Sust Energ Rev 26:108–134. https://doi.org/10.1016/j.rser.2013.04.021
Monari C, Righi S, Olsen SI (2016) Greenhouse gas emissions and energy balance of biodiesel production from microalgae cultivated in photobioreactors in Denmark: a life-cycle modeling. J Clean Prod 112:4084–4092. https://doi.org/10.1016/j.jclepro.2015.08.112
Mu D, Min M, Krohn B, Mullins KA, Ruan R, Hill J (2014) Life cycle environmental impacts of wastewater-based algal biofuels. Environ Sci Technol 48:11696–11704. https://doi.org/10.1021/es5027689
Mu D, Ruan R, Addy M, Mack S, Chen P, Zhou Y (2017) Life cycle assessment and nutrient analysis of various processing pathways in algal biofuel production. Bioresour Technol 230:33–42. https://doi.org/10.1016/j.biortech.2016.12.108
Orfield ND, Levine RB, Keoleian GA, Miller SA, Savage PE (2015) Growing algae for biodiesel on direct sunlight or sugars: a comparative life cycle assessment. ACS Sustain Chem Eng 3:386–395. https://doi.org/10.1021/sc5004117
Pardo-Cárdenas Y, Herrera-Orozco I, González-Delgado ÁD, Kafarov V (2013) Environmental assessment of microalgae biodiesel production in Colombia: comparison of three oil extraction systems. CTyF - Ciencia, Tecnol y Futur 5:85–100
Passell H, Dhaliwal H, Reno M, Wu B, Ben Amotz A, Ivry E, Gay M, Czartoski T, Laurin L, Ayer N (2013) Algae biodiesel life cycle assessment using current commercial data. J Environ Manag 129:103–111. https://doi.org/10.1016/j.jenvman.2013.06.055
Sander K, Murthy GS (2010) Life cycle analysis of algae biodiesel. Int J Life Cycle Assess 15:704–714. https://doi.org/10.1007/s11367-010-0194-1
Sills DL, Paramita V, Franke MJ, Johnson MC, Akabas TM, Greene CH, Tester JW (2013) Quantitative uncertainty analysis of life cycle assessment for algal biofuel production. Environ Sci Technol 47:687–694. https://doi.org/10.1021/es3029236
Soh L, Montazeri M, Haznedaroglu BZ, Kelly C, Peccia J, Eckelman MJ, Zimmerman JB (2014) Evaluating microalgal integrated biorefinery schemes: empirical controlled growth studies and life cycle assessment. Bioresour Technol 151:19–27. https://doi.org/10.1016/j.biortech.2013.10.012
Soratana K, Barr WJ, Landis AE (2014) Effects of co-products on the life-cycle impacts of microalgal biodiesel. Bioresour Technol 159:157–166. https://doi.org/10.1016/j.biortech.2014.02.070
Soratana K, Khanna V, Landis AE (2013) Re-envisioning the renewable fuel standard to minimize unintended consequences: a comparison of microalgal diesel with other biodiesels. Appl Energy 112:194–204. https://doi.org/10.1016/j.apenergy.2013.05.082
Souza SP, Gopal AR, Seabra JEA (2015) Life cycle assessment of biofuels from an integrated Brazilian algae-sugarcane biorefinery. Energy 81:373–381. https://doi.org/10.1016/j.energy.2014.12.050
StataCorp (2012) Stata statistical software: release 12. StataCorp LLC, College Station, TX
Stephenson AL, Kazamia E, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Life-cycle assessment of potential algal biodiesel production in the United Kingdom: a comparison of raceways and air-lift tubular bioreactors. Energy and Fuels 24:4062–4077. https://doi.org/10.1021/ef1003123
Stock JH, Watson MW (2015) Introduction to econometrics, 3rd edition. Pearson/Addison Wesley
Tu Q, Eckelman M, Zimmerman J (2017) Meta-analysis and harmonization of life cycle assessment studies for algae biofuels. Environ Sci Technol 51:9419–9432. https://doi.org/10.1021/acs.est.7b01049
Tu Q, Eckelman M, Zimmerman JB (2018) Harmonized algal biofuel life cycle assessment studies enable direct process train comparison. Appl Energy 224:494–509. https://doi.org/10.1016/j.apenergy.2018.04.066
Woertz IC, Benemann JR, Du N, Unnasch S, Mendola D, Mitchell BG, Lundquist TJ (2014) Life cycle GHG emissions from microalgal biodiesel - a CA-GREET model. Environ Sci Technol 48:6060–6068. https://doi.org/10.1021/es403768q
Yanfen L, Zehao H, Xiaoqian M (2012) Energy analysis and environmental impacts of microalgal biodiesel in China. Energy Policy 45:142–151. https://doi.org/10.1016/j.enpol.2012.02.007
Yuan J, Kendall A, Zhang Y (2015) Mass balance and life cycle assessment of biodiesel from microalgae incorporated with nutrient recycling options and technology uncertainties. GCB Bioenergy 7:1245–1259. https://doi.org/10.1111/gcbb.12229
Zaimes GG, Khanna V (2014) The role of allocation and coproducts in environmental evaluation of microalgal biofuels: how important? Sustain Energy Technol Assessments 7:247–256. https://doi.org/10.1016/j.seta.2014.01.011
Zaimes GG, Khanna V (2013) Environmental sustainability of emerging algal biofuels: a comparative life cycle evaluation of algal biodiesel and renewable diesel. Environ Prog Sustain Energy 32:926–936. https://doi.org/10.1002/ep.11810
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgments
The authors thank Annette Cowie for her comments on earlier drafts. This work was supported by Fundação para a Ciência e a Tecnologia, Portugal (FCT) and the European Regional Development Fund under Project 3599-PPCDT (SABIOS project, PTDC/AAG-MAA/6234/2014 - POCI-01-0145-FEDER-016765), and FCT under research contract CEECIND/00417/2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Peter Rudolf Saling
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The opinions expressed in this article are the sole responsibility of the author(s) and should not be interpreted as reflecting the views of Sveriges Riksbank.
Electronic supplementary material
ESM 1
(PDF 319 kb)
Rights and permissions
About this article
Cite this article
Garcia, R., Figueiredo, F., Brandão, M. et al. A meta-analysis of the life cycle greenhouse gas balances of microalgae biodiesel. Int J Life Cycle Assess 25, 1737–1748 (2020). https://doi.org/10.1007/s11367-020-01780-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-020-01780-2