Abstract
Postoperative delirium (POD) is a common neuropsychiatric complication in geriatric inpatients after hip fracture surgery and its occurrence is associated with poor outcomes. The purpose of this study was to investigate the relationship between preoperative biomarkers in serum and cerebrospinal fluid (CSF) and the development of POD in older hip fracture patients, exploring the possibility of integrating objective methods into future predictive models of delirium. Sixty hip fracture patients were recruited. Blood and CSF samples were collected at the time of spinal anesthesia when none of the subjects had delirium. Patients were assessed daily using the 4AT scale, and based on these results, they were divided into POD and non-POD groups. The Olink® platform was used to analyze 45 cytokines. Twenty-one patients (35%) developed POD. In the subsample of 30 patients on whom proteomic analyses were performed, a proteomic profile was associated with the incidence of POD. Chemokine (C-X-C motif) ligand 9 (CXCL9) had the strongest correlation between serum and CSF samples in patients with POD (rho = 0.663; p < 0.05). Although several cytokines in serum and CSF were associated with POD after hip fracture surgery in older adults, there was a significant association with lower preoperative levels of CXCL9 in CSF and serum. Despite the small sample size, this study provides preliminary evidence of the potential role of molecular biomarkers in POD, which may provide a basis for the development of new delirium predictive models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Delirium is a neuropsychiatric disorder characterized by an acute change in attention, awareness, and cognition [1]. The term postoperative delirium (POD) refers to a condition of acute cognitive dysfunction that occurs in the hospital up to 1 week after surgery and is a frequent perioperative complication, especially among older adults [2]. The incidence of POD after hip fracture has been reported to range from 11 to 51% and occurs in up to 50–70% of high-risk patient groups [3]. Furthermore, POD is associated with adverse outcomes such as longer length of stay, functional decline, persistent cognitive impairment, institutionalization, decrease in quality of life, premature mortality, and increased healthcare expenditure [4, 5].
Although studies have contributed some evidence in recent years, the pathophysiological mechanism of delirium remains largely unknown, and a multifactorial etiology has been suggested [6, 7]. Evidence supports that maladaptive neuroinflammatory response plays an important role in the development of POD [8, 9]. Several precipitating factors of delirium such as surgery, infection, or trauma cause endothelial damage, which increases blood–brain barrier (BBB) permeability allowing immune cells, cytokines, and other neuroinflammatory products to penetrate the brain parenchyma. These mediators activate the microglia, leading to neuronal dysfunction and delirium [10]. This mechanism has been observed particularly in older adults and patients with cognitive impairment undergoing hip fracture surgery [11, 12]. Some studies have found that tumor necrosis factor-α (TNF-α), proinflammatory cytokines such as interleukins (IL1, IL6, and IL8), and other novel neuroinflammatory biomarkers (neopterin) are elevated in the serum of these patients before surgery, but their study in cerebrospinal fluid (CSF) has yielded mixed results [13, 14]. However, studies carried out so far have important limitations because most do not account for geriatric syndromes, nor do they measure delirium in a daily pattern during hospitalization; furthermore, delirium characteristics such as subtype and severity are seldom assessed.
Importantly, there is no effective treatment for POD once it is established, so the best approach is its prevention. Several clinically based predictive models of delirium have been developed in recent years such as the DEAR tool [15,16,17], but they have some limitations because their application depends on the interpretation of the clinician who performs them, and consequently, they can be subjective and imprecise. For this reason, the possibility of integrating objective measures into predictive models of delirium needs to be explored. Serum biomarkers are quantifiable, reproducible, and minimally invasive and could be useful to better understand the pathophysiology of delirium and identify patients at higher risk of developing it.
The present study aimed to comprehensively characterize patients with delirium both from a clinical point of view and using a proteomic approach, in order to evaluate whether changes in the preoperative immunological profile could be associated with POD after hip fracture surgery. We hypothesized that older hip fracture patients with altered cytokine profiles before surgery could be at higher risk of developing delirium.
Methods
Study design and participants
This was a prospective cohort study. Between August 2021 and December 2021, we approached sixty consecutive hip fracture patients aged 75 years or older undergoing subarachnoid anesthesia, who were admitted to the Orthopedic ward of Hospital Universitario de Navarra (Pamplona, Spain). Patients were excluded if (1) they had preoperative delirium, (2) advanced dementia (a score > 5 at Global Deterioration Scale [18]), (3) severe dependence (a score < 20 at Barthel Index [19]), (4) terminal disease (life expectancy < 3 months), (5) were unable to communicate in Spanish, and (6) were not willing or not capable to provide informed consent.
Clinical assessments
Medical records were reviewed, and patients and relatives were interviewed preoperatively and daily after surgery. A Comprehensive Geriatric Assessment (CGA) was performed at the time of enrolment that included functional status (Barthel Index and Lawton and Brody scale [20]), frailty (FRAIL scale [21]), nutrition (Mini-Nutritional Assessment-Short Form (MNA-SF) [22]), grip strength (measured with JAMAR 5030J1 Hand Dynamometer), quality of life (EuroQol Scale-5D [23]), falls, sensory impairment, depression (Yesavage Geriatric Depression Scale [24]), polypharmacy, and demographic factors such as provenance and education level. Possible confounding factors, including fracture characteristics, type of anesthesia, type of surgery, and peri- and post-operative complications such as infectious events or postoperative anemia were registered for all patients.
The presence or absence of delirium was scored daily until discharge by two geriatricians using the Spanish version of the 4AT scale [25, 26]. Information for the 4AT was based on a psychiatric examination of the patient, review of medical and nursing records, and information given by the patient’s closest relative. All 60 patients were non-delirious before surgery. Delirium symptom severity was assessed postoperatively with the validated Memorial Delirium Assessment Scale (MDAS) [27]. The peak MDAS was defined as the highest total delirium severity score recorded in the postoperative period. Delirium subtype was assessed using the Delirium Motor Subtype Scale-4 [28]. Preexisting cognitive impairment was based on medical history and the Informant Questionnaire on Cognitive Decline short form (IQCODE-sf). The informant was asked to recall the cognitive situation 2 weeks prior to the hip fracture and compare it with the situation 10 years earlier. Patients with a mean score of 3.9 or higher were considered to have global cognitive impairment [29, 30].
Sample collection and laboratory assessments
Blood and CSF were collected before surgery and processed in no more than 1 h from their sampling. The blood sample (8 mL obtained by venous puncture) was collected the morning prior to surgery and placed in a polypropylene plastic tube at room temperature (25 °C) for 30 min until blood coagulation occurred; then, the tube was centrifuged in a fixed-angle rotor at 1960 g for 10 min at room temperature. After centrifugation, the serum in the upper layer was carefully extracted and divided into 0.5 mL aliquots and immediately stored at − 80 °C. CSF samples were collected during canulation for the introduction of spinal anesthesia, prior to the administration of any anesthetic. Lumbar punctures were performed with a 25-gauge needle between the L3–L4 and L4–L5 intervertebral space. From each patient, 2 mL of CSF was collected in polypropylene tubes which were transported to the laboratory and centrifuged at 720 g for 10 min at 4 °C, divided into 0.5 μL aliquots, and stored at − 80 °C. Samples of 30 patients (both serum and CSF), in which 15 had delirium and 15 did not, were selected and sent on dry ice for analysis at Cobiomic Bioscience (Parque Científico Tecnológico de Córdoba, Córdoba, Spain). We used Olink® technology to assess cytokine and chemokine levels. The Olink® reagents are based on the proximity extension assay (PEA) technology, where 45 oligonucleotide-labeled antibody probe pairs are each allowed to bind to their respective target protein present in the sample. Following the hybridization of the matched oligo sequences, a PCR reporter sequence is formed by a proximity-dependent DNA polymerization event. This is then amplified and subsequently detected and quantified using real-time polymerase chain reaction (PCR). The assay is performed in a 48-plex format without any need for washing steps, and results are reported in standard concentration units (pg/mL). When cytokines are within the lower and upper limits of quantification (LLOQ and ULOQ) for each assay, the values are not included in the analysis. Details about PEA technology, assay performance, and validation data are available from the manufacturer (www.olink.com), and all the biomarkers analyzed with this technology are detailed in Supplementary Table 1.
Standard protocol approval, registration, and patient consents
This study was conducted in accordance with the Declaration of Helsinki (World Medical Association) and was approved by the Navarra Clinical Research Ethics Committee on June 25, 2021 (PI_2021/68). Data and samples were collected after informed consent from patients at the time of enrolment. There was no financial compensation for the participants.
Statistical analyses
Variables were tested for normality using the Shapiro–Wilk method. Consequently, non-parametric (Mann–Whitney U) or parametric (independent t-test) tests were used to compare the two groups (patients who developed POD versus patients who did not develop POD) regarding baseline characteristics measured in continuous variables. For dichotomous or nominal variables, Fisher’s exact or Pearson’s X2 was used. Data were presented as mean and standard deviation unless stated otherwise. For descriptives and testing of group differences, the statistical software used was SPSS version 26 (International Business Machines Corporation (IBM), Armonk, New York, USA). p-value of < 0.05 was considered significant.
We used Tukey’s fences method to detect observations out of the normal range by using interquartile ranges, which are often used for detecting outliers in various fields [31]. One hundred twenty-two outliers were excluded from the analysis out of the 2700 values analyzed using the Olink platform. Before performing Tukey’s fences, the normality of the data was checked before fitting the curve. Features with > 70% missing values in the real samples or > 10% outlier values in the serum and CSF samples were deleted first. Thirty-eight biomarkers passed quality control in serum, and 27 passed quality control in CSF (Supplementary Table 2). Serum biomarkers in pg/mL values were analyzed using two unpaired t‐tests, the Benjamini–Hochberg method for p-value correction with 5% false discovery rate, and a distribution boxplot. p-values < 0.05 were considered statistically significant after correction with the Benjamini–Hochberg method. A principal component analysis (PCA) and volcano plot assessed the distribution of the groups, using singular value decomposition with imputation (pre-normalized data, no transformation), and visualized using ClustVis [32]. Spearman’s correlation matrices were calculated including clinical variables, sex and age, and significant biomarkers for patients who developed POD versus patients who did not develop POD, using the R package Corrplot (version 0.84 (https://cran.r-project.org/package=corrplot)).
Results
Clinical results
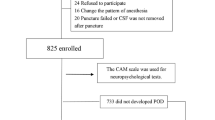
From August 2021 to December 2021, 60 of 138 hip fracture patients fulfilled the criteria for participation and provided informed consent (Fig. 1). Twenty-one of these 60 patients developed POD (35%), and thirty-nine did not (65%).
The characteristics of patients with and without POD are shown in Table 1. Patients who developed POD had more cognitive impairment (p < 0.001), higher dependency (p = 0.005), and worse nutritional status (p = 0.013).
Characteristics of the surgery and clinical complications after surgery are shown in Table 2. No significant differences were found between the type of hip fracture, time from surgery to sitting, femoral nerve block, blood transfusion or bladder catheterization, and the incidence of POD. However, patients who spent a longer period of time without walking after surgery had higher incidence of POD (p = 0.003). In addition, patients who developed delirium had more infections (p < 0.001) and used more psychotropic drugs (p < 0.001) and more opioids (p = 0.013) during hospitalization than patients without delirium.
Laboratory results
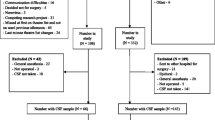
Serum and CSF samples of 30 patients were analyzed (15 patients with POD and 15 patients without POD), and the results are shown in Supplementary Table 2. A score plot was generated to show the separation between the POD and non-POD groups. The PCA analysis did not reveal any abnormal deviations between the two groups (Fig. 2).
Principal component (PCA) of POD and non-POD groups. Principal component analysis (PCA) was created to investigate possible outliers in the data set. Score plot showing the separation between the POD and without POD group. The red points correspond to patients with POD, and the blue points correspond to patients without POD
The volcano plot in Fig. 3 shows the differences between POD and non-POD groups. Those who developed POD had significantly higher levels of CXCL12 and EGF in serum as well as higher levels of CSF3 and TGFA in CSF compared to patients without POD. However, patients who developed POD had significantly lower levels of CSF3, CXCL9, IL10, CCL2, and CXCL8 in serum and lower levels of CCL3, CXCL9, and CCL4 in CSF compared to patients without POD.
Biomarkers identification according to serum and cerebrospinal fluid levels into POD and non-POD groups. The volcano plot for detected biomarkers; the X-axis represents the log2 fold-change value, while the Y-axis represents the − log10 p-value; the gray point represents the biomarkers without significant difference. The red point represents the biomarkers increase with significant difference, while blue point represents the biomarkers decrease with significant difference, in patients with POD compared to patients without POD
We examined the correlation between cytokine concentrations in serum and CSF in both POD and non-POD groups, with adjustments made for sex and age using the Spearman rank (Rho) correlation. The analysis unveiled significant negative associations between serum EGF and serum CXCL9 levels (rho = − 0.754; p < 0.05), CSF TGFA and CSF CXCL9 (rho = − 0.714; p < 0.01), and CSF CSF3 and CSF CCL3 (rho = − 0.843; p < 0.05) in the non-POD group. Furthermore, other noteworthy positive relationships were observed between serum CSF3 and serum IL10 (rho = 0.603; p < 0.05), CSF CSF3 and serum CXCL12 (rho = 0.737, p < 0.05), CSF CSF3 and serum CXCL8 (rho = 0.816, p < 0.05), and CSF CCL4 and CSF CCL3 (rho = 0.64, p < 0.05) in the non-POD group.
On the other hand, significant negative relationships were found between serum CSF3 and serum CXCL9 (rho = − 0.688; p < 0.05), serum EGF and serum CXCL9 (rho = − 0.635; p < 0.05), and serum EFG and serum CXCL8 (rho = − 0.605; p < 0.05) in POD group. Similarly, other significant positive relationships were found between serum CXCL8 and serum IL10 (rho = 0.538; p < 0.05), serum CSF3 and serum CXCL8 (rho = 0.597; p < 0.05), serum CCL2 and serum CSF3 (rho = 0.599; p < 0.05), CSF CCL3 and serum CXCL12 (rho = 0.621; p < 0.05), CSF CCL4 and serum CCL2 (rho = 0.596; p < 0.05), and CSF CCL4 and CSF CCL3 (rho = 0.812; p < 0.001) in POD group.
The only cytokine that had a significantly positive correlation in both serum and CSF of patients with POD was CXCL9 (rho = 0.663; p < 0.05) (Fig. 4).
Matrix of pairwise Spearman correlations among biomarkers according to serum (s) and cerebrospinal fluid (c) levels for POD and non-POD groups adjusted by sex and age. Color represents the level of Spearman’s correlations (blue means positive correlation, and red means negative correlation). Significant protein pair correlations were indicated as ***p < 0.001, **p < 0.01, and *p < 0.05
Discussion
In this study, we investigated the association between POD and 45 cytokines and chemokines in preoperative serum and CSF in older hip fracture patients, and we identified 12 potential biomarkers of delirium risk. Four of them were found to be up-regulated (serum CXCL12, serum EFG, CSF CSF3, and CSF TGFA), and eight were down-regulated (serum CSF3, serum CXCL9, serum CXCL8, serum IL10, serum CCL2, CSF CCL3, CSF CXCL9, and CSF CCL4). An interesting finding was the significant correlation between lower levels of CXCL9 in serum and CSF in patients with POD compared to patients without POD.
Chemokine (C-X-C motif) ligand 9 (CXCL9) is a small cytokine belonging to the CXC chemokine family that is also known as monokine induced by gamma interferon (MIG). CXCL9 induces chemotaxis, promotes differentiation and multiplication of leukocytes, and causes tissue extravasation. It has been found that CXCL9 increases with age [33] and is an important factor in age-related chronic inflammation, being involved in cardiac aging, adverse cardiac remodeling, and poor vascular function. Age-related elevation in CXCL9 leads to endothelial cell senescence and predicts subclinical levels of cardiovascular aging in healthy individuals [34]. CXCL9 has also been shown to be associated with falls and hip fracture in older population [35] and frailty [36, 37]. Furthermore, data in the literature indicates a significant role of CXCL9 and its receptor (CXCR3) in the central nervous system (CNS), in both physiological and pathological processes [38, 39]. CXCL9 is expressed in human brain-derived microvascular endothelial cells and astrocytes and is especially involved in Th1 response. In addition, it has been shown that CXCL9 is able to induce the activation of extracellular signal-regulated kinases (ERK1/2) in cortical neurons and might be involved in a neuronal–glial interaction. The up-regulation of this chemokine and its receptor was also identified in Alzheimer’s disease (AD) brains, finding higher levels of CXCL9 in AD patients compared to patients with mild cognitive impairment or without cognitive impairment [40, 41]. On the other hand, CXCL9 exhibited the lowest levels in participants with 1 or more ε4 alleles in a study carried out to identify a panel of plasma biomarkers of AD [42]. These dissenting results support the fact that lower levels of CXCL9 were associated with POD in our study. These discrepancies could be justified by the multiple mechanisms implicated in delirium development (tissue damage, infection, pain, polypharmacy, hypoxia…), the heterogeneity in the methodology of the studies carried out (different biochemical analyses and clinical assessments), and the small sample size of our study. Other factors to consider are the systemic inflammatory response syndrome of our patients (all of them had hip fractures, with the consequent tissue damage compared to other studies where the patients did not have it) and the complexity for chemokine receptor CXCR3 activation that is involved in wound healing with different signaling pathways [43].
Serum stromal cell-derived factor 1 (CXCL12) is involved in Alzheimer’s disease (AD) pathophysiology [44], and pro-epidermal growth factor (EGF), whose up-regulation has been associated with cognitive impairment in Parkinson’s disease [45, 46], showed higher levels in patients with POD compared to patients without POD in our study. These findings support the possible relationship between CXCL12, EGF, and POD.
Granulocyte colony-stimulating factor (CSF3) showed significantly higher levels in CSF but lower levels in the serum of patients with POD in our study. Previous studies have found that its up-regulation improves neuroplasticity [47], and its administration in rats protects against cognitive impairment [48].
Transforming growth factor alpha (TGFA) showed higher levels in CSF of patients who developed POD in our study and has been associated with AD and vascular dementia [49, 50], which could explain the pathophysiological substrate for delirium.
Interleukin-10 (IL10), whose down-regulation has been found in patients with AD and delirium [51, 52], showed lower levels in the serum of patients who developed POD in our study, which is consistent with previous literature.
C–C motif chemokine 2 (CCL2), Interleukin-8 (CXCL8), C–C motif chemokine 3 (CCL3), and C–C motif chemokine 4 (CCL4) showed significantly lower levels in patients with POD in our study. However, higher levels have been associated with dementia [53, 54], delirium among critically ill patients [55], and neuropsychiatric disorders in Parkinson’s disease [56, 57] in previous literature.
Although the etiology of POD is both complex and elusive, in our study age, comorbidity, sensory impairment, depression, worse functional status, cognitive impairment, malnutrition, frailty, and polypharmacy were identified as clinical predisposing factors of POD, which is consistent with previous literature [58, 59]. In addition, infections and immobility after hip fracture surgery were the only significant precipitating factors of POD during the postoperative period, according to similar results obtained in other studies [1, 17].
However, these predisposing and precipitating clinical factors are often difficult to quantify because they are subjective and, consequently, less accurate. In contrast, biomarkers are quantifiable, objective, and potentially reproducible. Moreover, serum biomarkers are minimally invasive which makes them accessible in different clinical settings. Although further research is needed, these findings emphasize the role of biomarkers in POD after hip fracture and its potential use through the development of integrating objective methods into future predictive models of delirium.
Strengths and limitations
We highlight several study strengths such as the collection of blood and CSF and the well-defined sample. However, some study limitations warrant mention. First, the small sample size requires that the results be interpreted with caution. Additionally, the sampling at a single time point results in the loss of information regarding the course of delirium during hospitalization. Second, our set of inflammatory biomarkers might not be an appropriate indicator of inflammation in the CNS because most of the cytokines and chemokines analyzed are not characteristic of a specific condition or process; thus, additional testing and evaluation might be required to identify specific markers of delirium in order to implement the understanding of the CNS disorders pathophysiology. Further research with larger sample sizes and comprehensive assessments of confounding factors is needed to validate these findings and determine the clinical utility of molecular biomarkers in predicting and managing POD.
Conclusion
In summary, we found 12 biomarkers associated with POD, and lower levels of CXCL9 had the most significant correlation between serum and CSF. These findings suggest that altered proteomic profile before surgery could be associated with POD development. Although further research is needed to confirm these results, biomarkers may offer a valuable tool for delirium risk assessment in addition to current predictive clinical models.
References
Inouye SK, Westendorp RGJ, Saczynski JS, Naeije G, Pepersack T. Delirium in elderly people. Lancet. 2014;383(9920):911–22. https://doi.org/10.1016/S0140-6736(13)60688-1.
Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456–66. https://doi.org/10.1056/nejmcp1605501.
Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatrics. 2007;19(2):197–214. https://doi.org/10.1017/S104161020600425X.
Zhou J, Liu C, Sun Y, Huang W, Ye K. Cognitive disorders associated with hospitalization of COVID-19: results from an observational cohort study. Brain Behav Immun. 2021;91:383–92. https://doi.org/10.1016/j.bbi.2020.10.019.
Oberai T, Woodman R, Laver K, Crotty M, Kerkhoffs G, Jaarsma R. Is delirium associated with negative outcomes in older patients with hip fracture: analysis of the 4904 patients 2017–2018 from the Australian and New Zealand hip fracture registry. ANZ J Surg. 2022;92(1–2):200–5. https://doi.org/10.1111/ans.17421.
Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–222. https://doi.org/10.1016/j.jagp.2013.09.005.
Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, Slooter AJC, Ely EW. Delirium. Nat Rev Dis Prim. 2020;6(1). https://doi.org/10.1038/s41572-020-00223-4.
Wang Y, Shen X. Postoperative delirium in the elderly: the potential neuropathogenesis. Aging Clin Exp Res. 2018;30(11):1287–95. https://doi.org/10.1007/s40520-018-1008-8.
Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–56. https://doi.org/10.1016/j.ebiom.2018.10.021.
Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019;128(4):781–8. https://doi.org/10.1213/ANE.0000000000004053.
Liu X, Yu Y, Zhu S. Inflammatory markers in postoperativedelirium (POD) and cognitive dysfunction (POCD): A meta-analysis of observational studies. PLoS One. 2018;13(4). https://doi.org/10.1371/journal.pone.0195659.
Beloosesky Y, Hendel D, Weiss A, Hershkovitz A, Grinblat J, Pirotsky A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. Journals Gerontol - Ser A Biol Sci Med Sci. 2007;62(4):420–6. https://doi.org/10.1093/gerona/62.4.420.
Hirsch J, Vacas S, Terrando N, Yuan M, Sands LP, Kramer J, et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. 2016;13(1):211. https://doi.org/10.1186/s12974-016-0681-9.
Miao S, Shen P, Zhang Q, Wang H, Shen J, Wang G, et al. Neopterin and mini-mental state examination scores, two independent risk factors for postoperative delirium in elderly patients with open abdominal surgery. J Cancer Res Ther. 2018;14(6):1234–8. https://doi.org/10.4103/0973-1482.192764.
Freter S, Dunbar M, Koller K, MacKnight C, Rockwood K. Risk of pre- and post-operative delirium and the delirium elderly at risk (DEAR) tool in hip fracture patients. Can Geriatr J. 2015;18(4):212–6. https://doi.org/10.5770/cgj.18.185.
Zhao S, Sun T, Zhang J, Chen X, Wang X. Risk factors and prognosis of postoperative delirium in nonagenarians with hip fracture. Sci Rep. 2023;13(1):1–7. https://doi.org/10.1038/s41598-023-27829-4.
Smith TO, Cooper A, Peryer G, Griffiths R, Fox C, Cross J. Factors predicting incidence of post-operative delirium in older people following hip fracture surgery: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2017;32(4):386–96. https://doi.org/10.1002/gps.4655.
Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatrics. 1997;9(SUPPL. 1):167–71. https://doi.org/10.1017/S1041610297004869.
Barthel M. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:56–61.
Lawton M, Brody E. Assessment of older people: selfmaintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Woo J, Yu R, Wong M, Yeung F, Wong M, Lum C. Frailty screening in the community using the FRAIL scale. J Am Med Dir Assoc. 2015;16(5):412–9. https://doi.org/10.1016/j.jamda.2015.01.087.
Kaiser MJ, Bauer JM, Ramsh C, Uter W, Guigoz Y, Cederlorm T, et al. Validation of the mini nutritional assessment short-form (MNA®-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782–8https://doi.org/10.1007/s12603-009-0214-7.
Herdman M, Badia X, Berra S. EuroQol-5D: a simple alternative for measuring health-related quality of life in primary care. Aten Primaria. 2001;28(6):425–30. https://doi.org/10.1016/s0212-6567(01)70406-4.
Yesavage JA. Geriatric depression scale. Psychopharmacol Bull. 1988;24(4):709–10. https://doi.org/10.1007/978-3-319-69892-2_736-1.
Tieges Z, Maclullich AMJ, Anand A, Brookes C, Cassarino M, O’connor M, et al. Diagnostic accuracy of the 4AT for delirium detection in older adults: systematic review and meta-analysis. Age Ageing. 2021;50(3):733–43. https://doi.org/10.1093/ageing/afaa224.
Delgado-Parada E, Morillo-Cuadrado D, Saiz-Ruiz J, Cebollada-Gracia A, Ayuso-Mateos JL, Cruz-Jentoft AJ. Diagnostic accuracy of the Spanish version of the 4AT scale (4AT-ES) for delirium screening in older inpatients. Eur J Psychiatry. 2022;36(3):182–90. https://doi.org/10.1016/j.ejpsy.2022.01.003.
Barahona E, Pinhao R, Galindo V, Noguera A. The diagnostic sensitivity of the memorial delirium assessment scale—Spanish version. J Pain Symptom Manage. 2018;55(3):968–72. https://doi.org/10.1016/j.jpainsymman.2017.11.013.
Meagher D, Adamis D, Leonard M, Trzepacz P, Grover S, Jabbar F, et al. Development of an abbreviated version of the delirium motor subtyping scale (DMSS-4). Int Psychogeriatrics. 2014;26(4):693–702. https://doi.org/10.1017/S1041610213002585.
Burton JK, Stott DJ, McShane R, Noel-Storr AH, Swann-Price RS, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the early detection of dementia across a variety of healthcare settings. Cochrane Database Syst Rev. 2021;7(7). https://doi.org/10.1002/14651858.CD011333.pub3.
Blandfort S, Gregersen M, Rahbek K, Juul S, Damsgaard EM. The short IQCODE as a predictor for delirium in hospitalized geriatric patients. Aging Clin Exp Res. 2020;32(10):1969–76. https://doi.org/10.1007/s40520-019-01412-2.
Luo J, Frisken S, Machado I, Zhang M, Pieper S, Golland P, et al. Using the variogram for vector outlier screening: application to feature-based image registration. Int J Comput Assist Radiol Surg. 2018;13(12):1871–80. https://doi.org/10.1007/s11548-018-1840-5.
Metsalu T, Vilo J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566–70. https://doi.org/10.1093/nar/gkv468.
Torres KCL, de Rezende VB, Lima-Silva ML, de Sousa SLJ, Costa CG, de Melo MJV, et al. Immune senescence and biomarkers profile of Bambuí aged population-based cohort. Exp Gerontol. 2018;103:47–56. https://doi.org/10.1016/j.exger.2017.12.006.
Sayed N, Huang Y, Nguyen K, Krejciova-Rajaniemi Z, Grawe AP, Gao T, et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging. 2021;1(8):748. https://doi.org/10.1038/s43587-021-00102-x.
Phan QT, Chua KY, Jin A, Winkler C, Koh WP. CXCL9 predicts the risk of osteoporotic hip fracture in a prospective cohort of Chinese men—a matched case–control study. J Bone Miner Res. 2022;37(10):1843–9. https://doi.org/10.1002/jbmr.4646.
de Amorim JSC, Torres KCL, Teixeira-Carvalho A, Martins-Filho OA, Lima-Costa MF, Peixoto SV. Inflammatory markers and occurrence of falls: Bambuí cohort study of aging. Rev Saude Publica. 2019;53:1–11. https://doi.org/10.11606/S1518-8787.2019053000855.
de Amorim JSC, Torres KCL, Carvalho AT, Martins-Filho OA, Lima-Costa MF, Peixoto SV. Inflammatory markers associated with fall recurrence and severity: the Bambuí cohort study of aging. Exp Gerontol. 2020;132: 110837. https://doi.org/10.1016/j.exger.2020.110837.
Watson AES, Goodkey K, Footz T, Voronova A. Regulation of CNS precursor function by neuronal chemokines. Neurosci Lett. 2020;715: 134533. https://doi.org/10.1016/j.neulet.2019.134533.
Satarkar D, Patra C. Evolution, expression and functional analysis of CXCR3 in neuronal and cardiovascular diseases: a narrative review. Front Cell Dev Biol. 2022;10(June):1–17. https://doi.org/10.3389/fcell.2022.882017.
Lee KS, Chung JH, Lee KH, Shin MJ, Oh BH, Hong CH. Bioplex analysis of plasma cytokines in Alzheimer’s disease and mild cognitive impairment. Immunol Lett. 2008;121(2):105–9. https://doi.org/10.1016/j.imlet.2008.09.004.
Koper OM, Kaminska J, Sawicki K, Kemona H. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv Clin Exp Med. 2018;27(6):849–56. https://doi.org/10.17219/acem/68846.
Soares HD, Potter WZ, Pickering E, Kuhn M, Immermann FW, Shera DM, et al. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol. 2012;69(10):1310–7. https://doi.org/10.1001/archneurol.2012.1070.
Berchiche YA, Sakmar TP. CXC chemokine receptor 3 alternative splice variants selectively activate different signaling pathways. Mol Pharmacol. 2016;90(4):483–95. https://doi.org/10.1124/mol.116.105502.
Li H, Wang R. A focus on CXCR4 in Alzheimer’s disease. Brain Circ. 2017;3(4):199. https://doi.org/10.4103/bc.bc_13_17.
Pellecchia MT, Santangelo G, Picillo M, Pivonello R, Longo K, Pivonello C, et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson’s disease patients. J Neurol. 2013;260(2):438–44. https://doi.org/10.1007/s00415-012-6648-6.
Yasar Z, Elliott BT, Kyriakidou Y, Nwokoma CT, Postlethwaite RD, Gaffney CJ, et al. Sprint interval training (SIT) reduces serum epidermal growth factor (EGF), but not other inflammatory cytokines in trained older men. Eur J Appl Physiol. 2021;121(7):1909–19. https://doi.org/10.1007/s00421-021-04635-2.
Sanchez-Ramos J, Song S, Sava V. Granulocyte colony stimulating factor (G-CSF) decreases brain amyloid burden and reverses cognitive impairment in Alzheimer’s mice. Neuroscience. 2009;163(1):55–72. https://doi.org/10.1016/j.neuroscience.2009.05.071.
Tuo M, Xiao Y, Xu Y, Wang L, Wei X, Zhang L. Role of granulocyte-colony stimulating factor in the protection of cerebral vascular endothelium, white matter, and cognition. Curr Neurovasc Res. 2019;16(5):425–32. https://doi.org/10.2174/1567202616666191029115113.
Khedr EM, Gomaa AMS, Ahmed OG, Sayed HMM, Gamea A. Cognitive impairment, P300, and transforming growth factor β1 in different forms of dementia. J Alzheimer’s Dis. 2020;78(2):837–45. https://doi.org/10.3233/JAD-200885.
Estrada LD, Oliveira-Cruz L, Cabrera D. Transforming growth factor beta type I role in neurodegeneration: implications for Alzheimer´s disease. Curr Protein Pept Sci. 2018;19(12):1180–8. https://doi.org/10.2174/1389203719666171129094937.
Khan SH, Lindroth H, Jawed Y, Wang S, Nasser J, Seyffert S, et al. Serum biomarkers in postoperative delirium after esophagectomy. Ann Thorac Surg. 2022;113(3):1000–7. https://doi.org/10.1016/j.athoracsur.2021.03.035.
Culjak M, Perkovic MN, Uzun S, Strac DS, Erjavec GN, Leko MB, et al. The association between TNF-alpha, IL-1 alpha and IL-10 with Alzheimer’s disease. Curr Alzheimer Res. 2020;17(11):972–84. https://doi.org/10.2174/1567205017666201130092427.
Ahmad MA, Kareem O, Khushtar M, Akbar M, Haque MR, Iqubal A, et al. Neuroinflammation: a potential risk for dementia. Int J Mol Sci. 2022;23(2):616. https://doi.org/10.3390/ijms23020616.
Lee WJ, Liao YC, Wang YF, Lin IF, Wang SJ, Fuh JL. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: a two-year follow-up study. Sci Rep. 2018;8(1):4–11. https://doi.org/10.1038/s41598-018-19807-y.
Smith RJ, Lachner C, Singh VP, Trivedi S, Khatua B, Cartin-Ceba R. Cytokine profiles in intensive care unit delirium. Acute Crit Care. 2022;37(3):415–28. https://doi.org/10.4266/acc.2021.01508.
Perna L, Trares K, Perneczky R, Tato M, Stocker H, Möllers T, et al. Risk of late-onset depression and cognitive decline: results from inflammatory proteome analyses in a prospective population-based cohort study. Am J Geriatr Psychiatry. 2022;30(6):689–700. https://doi.org/10.1016/j.jagp.2021.12.001.
Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, et al. Cerebrospinal fluid inflammatory markers in Parkinson’s disease - associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–9. https://doi.org/10.1016/j.bbi.2013.07.007.
Dogrul RT, Dogrul AB, Konan A, Caglar O, Sumer F, Caliskan H, et al. Does preoperative comprehensive geriatric assessment and frailty predict postoperative complications? World J Surg. 2020;44(11):3729–36. https://doi.org/10.1007/s00268-020-05715-8.
Yang Y, Zhao X, Gao L, Wang Y, Wang J. Incidence and associated factors of delirium after orthopedic surgery in elderly patients: a systematic review and meta-analysis. Aging Clin Exp Res. 2021;33(6):1493–506. https://doi.org/10.1007/s40520-020-01674-1.
Acknowledgements
We would like to thank the patients and staff at the Orthopedics and Anesthesiology Departments of Hospital Universitario de Navarra. We also thank the Clinical Neuroproteomics Unit of Navarrabiomed and the Navarrabiomed Biobank for supporting this study. We thank RRV, AGJ, and RRO for their assistance in statistics and data interpretation. The sponsors had no role in the design and conduct of the study, the collection, management, analysis, or interpretation of the data.
Funding
This work was supported by a grant from the Department of Economic and Business Development from the Government of Navarra (Ref. 0011–1411-2020–000028).
Author information
Authors and Affiliations
Contributions
LLV, NMV, JROGand BACV designed the study. RRV and AGJ performed statistical analyses and interpreted data. LLV acquired interpreted data and drafted the manuscript. MMV, RRV, MI, FZ, BACV, BVM, JROG, AMHO, AJMV, JFI, RRO, and ESM critically revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lozano-Vicario, L., Muñoz-Vázquez, Á.J., Ramírez-Vélez, R. et al. Association of postoperative delirium with serum and cerebrospinal fluid proteomic profiles: a prospective cohort study in older hip fracture patients. GeroScience 46, 3235–3247 (2024). https://doi.org/10.1007/s11357-024-01071-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-024-01071-w