Abstract
Pathological obstruction in lungs leads to severe decreases in muscle strength and mobility in patients suffering from chronic obstructive pulmonary disease. The purpose of this study was to investigate the interdependency between muscle strength, spirometric pulmonary functions and mobility outcomes in healthy older men and women, where skeletal muscle and pulmonary function decline without interference of overt disease. A total of 135 69- to 81-year-old participants were recruited into the cross-sectional study, which was performed as a part of European study MyoAge. Full, partial and no mediation models were constructed to assess the interdependency between muscle strength (handgrip strength, knee extension torque, lower extremity muscle power), spirometric pulmonary function (FVC, FEV1 and FEF50) and mobility (6-min walk and Timed Up and Go tests). The models were adjusted for age, sex, total fat mass, body height and site of enrolment. Partial mediation models, indicating both direct and pulmonary function mediated associations between muscle strength and mobility, fitted best to the data. Greater handgrip strength was significantly associated with higher FVC, FEV1 and FEF50 (p < 0.05). Greater muscle power was significantly associated with better performance in mobility tests. Results suggest that decline in mobility with aging may be caused by decreases in both muscle strength and power but also mediated through decreases in spirometric pulmonary function. Future longitudinal studies are warranted to better understand how loss of function and mass of the respiratory muscles will affect pulmonary function among older people and how these changes are linked to mobility decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with a loss of skeletal muscle mass and function (Häkkinen and Häkkinen 1991; Lexell et al. 1988; Porter et al. 1995; Vandervoort 2002). Decreases especially in muscle strength and power are related to reduced functional capacity and mobility and to adverse health outcomes (Bassey and Short 1990; Brown et al. 1995; Dela and Kjaer 2006; Park et al. 2006). These age-related changes are accelerated in chronic obstructive pulmonary disease (COPD) with progressive airflow limitation usually caused by cigarette smoking and/or external pollutants. A number of studies have confirmed that the losses in muscle strength and mass are more severe among COPD patients than those in healthy older adults and that disease progression, i.e. increased airflow limitation, contributes to loss of muscle mass, decreased functional capacity and eventually loss of independence (Eisner et al. 2008; Hamilton et al. 1995; Schols et al. 1993).
The interrelationship between muscle strength, pulmonary function and mobility among healthy older people has received less attention. Pulmonary function declines during aging even in the absence of extrinsic pollutants (Degens et al. 2012; Fletcher and Peto 1977; Quanjer et al. 2012). Reductions in pulmonary functions can be caused by several factors related to the lung tissue itself, such as fewer alveoli and capillaries or reduced diffusing capacity and increased residual volumes. These lung-level changes may further decrease exercise capacity and contribute to loss in muscle strength and power as well as mobility similarly as in COPD.
During healthy aging, however, musculoskeletal changes occur concurrently to changes in pulmonary function. It has been suggested that a decline in muscle strength initiates a chain of events which leads to reduced pulmonary function and low physical performance and mobility disability (Buchman et al. 2008). Reduced chest wall compliance, kyphosis and decreased respiratory muscle strength and endurance are examples of age-related musculoskeletal changes that may have an effect on pulmonary functions (Meyer 2005; Stanojevic et al. 2008).
Finally, it is possible that reduced muscle strength contributes to reduced pulmonary function though other factors such as decreased physical activity or mobility. Several studies have shown that physical activity may attenuate age-related decline in pulmonary function (Amara et al. 2001; Degens et al. 2012; Pelkonen et al. 2003).
The coinciding decrease in several functions of different body systems during aging such as decreases in spirometric pulmonary function, muscle strength and power and mobility raises the possibility of common underlying mechanisms. So far, these associations have been investigated mainly in COPD patients and less in healthy older adults. This study investigated the associations between muscle strength, spirometric pulmonary function and mobility in healthy older adults aged 69–81 years, without interference of overt disease. Full, partial mediation and no mediation path models were compared to distinguish possible direct and mediated associations between study outcomes.
Materials and methods
Participant recruitment
The data used in this study was collected as a part of the MyoAge project, which is a multicentric cross-sectional European study. Study rationale and design have been described in detail elsewhere (McPhee et al. 2013). Participants were recruited and data collected according to the same standard operational procedures in the University of Jyväskylä, Finland, Manchester Metropolitan University, UK, and in the Institute of Myology, Paris, France. All participants were socially active, i.e. they attended regularly to social activities to improve their knowledge or skills (e.g. university of third age, further learning, history, teaching children, church sessions, arts and crafts). Both physically inactive and active participants were recruited, but people engaged in athletic competitions were excluded. All participants were medically stable and community-dwelling individuals free from major diseases. Exclusion criteria included the following: dependent living situation, unable to walk a distance of 250 m, presence of morbidity (neurologic disorders, metabolic diseases, rheumatic diseases, recent malignancy, heart failure, severe COPD defined as GOLD stages 3 and 4), haemocoagulative syndromes, use of medication (immunosuppressive drugs, insulin), immobilization for 1 week during the previous 3 months and orthopedic surgery during the past 2 years or still causing pain or functional limitation. In total, 145 participants were recruited. However, participants with missing or incomplete results on spirometry (n = 8) or muscle power (n = 2) were excluded. Therefore, the final analyses were performed on 135 subjects, 34 men and 34 women from Jyväskylä, 29 men and 29 women from Manchester and 19 men and 10 women from Paris. Measurements were performed according to the good scientific practice, and the local medical ethical committees of the respective universities approved the study. Written informed consent was obtained from all participants, and data were handled without personal identification information.
Measurements
Health status
Information about diseases, medication and smoking were self-reported both using a questionnaire and verbally during a medical examination. Diseases were categorized into cardiovascular disease (including cardiovascular events, arterial surgery and hypertension), non-insulin-dependent diabetes mellitus, mild COPD, thyroid disease and osteoarthritis. Sum score including these diseases was calculated. The sum score of all oral and inhaled medication was calculated as well as pulmonary medication separately. Cognitive function was measured by use of the Mini Mental State Examination (MMSE), and depressive symptoms were measured by using the Geriatric Depression Scale (GDS). Excessive alcohol use was defined as more than 21 units per week for men or more than 14 units per week for women.
Level of physical activity
Preliminary information about lifestyle factors such as physical activity was collected during telephone interviews and completed during the measurements with standardized questionnaires. Participants were defined as physically active if they were involved in moderate or vigorous activities lasting ≥30 min per session, for ≥3 sessions per week, and had consistently maintained such activities for the majority of the past 3 years or more. Less active participants had completed moderate or vigorous physical activities no more than once per week over the previous 3 months and were rarely active in their daily lives.
Muscle strength
Muscle strength measurements included handgrip strength, knee extension torque and lower extremity muscle power.
Handgrip strength (kg) was measured using a handgrip dynamometer (Jamar, Sammons Preston Inc., Bolingbrook, IL) with participants in a standing position. Handgrip strength measurement can be used as an indicator of general muscle strength among older people (Rantanen et al. 1994). The handle of the dynamometer was adjusted to fit the participants’ hand with the second phalanx resting against the inner stirrup of the handle. The arm was fully extended along the side of the body with a gap of around 2 cm between the arm and the body. The participant was instructed to squeeze the handle as hard as possible for 3 s, and the maximum contraction force (in kg) was recorded. The tests were performed three times for each hand, alternating between right and left and with 30-s rest between trials. The highest value was used in analysis.
The maximal voluntary knee extension torque (Nm) of the dominant leg was tested (Finland and UK custom-made device; France Biodex system 3 Pro isokinetic dynamometer, Biodex Medical Systems, Shirley, NY, USA). Contraction was performed with the participant sitting on the testing chair with the hip and knee flexed at 90°. Upper body and hip straps were securely fastened to hold the participant firmly in place. The force transducer was set to 2 cm above the ankle malleolus. After a careful, standardized warm-up, maximal knee extension torque was measured at least three times, but trials were continued until there was less than 10 % difference between values for the two best efforts. A 90-s rest was allowed between the efforts. The highest value was selected as maximal knee extension torque and used for analysis.
To assess lower extremity muscle power, a maximal effort countermovement vertical jump was performed on a force platform (Finland custom-built force platform; UK Leonardo, Novotec Medical, Pforzheim, Germany; France AMTI, Watertown, MA, USA). Muscle power describes the capability to produce high force and velocity of movement during a dynamic action, reflecting the ability to generate muscular work per unit of time. Lower extremity muscle power correlates strongly with mobility among older people (Runge et al. 2004) and has been shown to decline to a greater extent than muscle strength during aging (Bean et al. 2003). Participants were asked to jump as high as possible while keeping hands on their hips. Emphasis was given to perform a fast countermovement and to push against the floor as hard as possible during the push-off phase. After instruction and demonstration by the researcher, participants performed a warm-up trial and three maximal efforts with a rest interval of 60 s. The maximum power (W) of the concentric phase was measured using the vertical component of the ground reaction force (Caserotti et al. 2001). The greatest value of three trials was taken for analysis.
Spirometric pulmonary function
Prior to measuring spirometric pulmonary function, the equipment was properly calibrated following the manufacturer guidelines. Spirometry was carried out with participants in a sitting position with the knees and hip flexed at around 90° and wearing a noseclip. The participant was asked to blow into the mouthpiece of the spirometer (Finland SpiroStar DX, Medikro and Spiro2000 software; UK and France Micro Medical Spiro USB spirometer and Spida 5 software, Cardinal Health) as forcefully and quickly as possible and to continue blowing until all of the air was expelled from the lungs. FVC (L), FEV1 (L/s) and forced expiratory flow at 50 % (FEF50, L/s) were analyzed. Measurements were repeated until the two highest values for FEV1 or FVC were within 0.15 L of one another (Miller et al. 2005). The highest value was used in the analysis. FEV1/FVC ratio was calculated by using the highest values of FEV1 and FVC.
Mobility
Mobility was estimated by 6-minute (6-min) walk test and Timed Up and Go (TUG) test. Subjects performed a 6-min walk test attempting to walk as far as possible in the allotted time (Enright 2003). The 6-min walk test is a performance-based measure of functional exercise capacity, and it can be used as an indicator of exercise tolerance or aerobic capacity. The test was performed on a 20-m track, and subjects were instructed to complete as many laps as possible within 6 min. The distance (m) covered in the 6-min walk was recorded and used in the analysis. Standardized verbal instructions were given at the end of each minute to inform of progress and the amount of time remaining.
A standardized TUG test is a commonly used functional test that estimates static and dynamic balance, lower extremity muscle power and walking ability (Podsiadlo and Richardson 1991). Participants were instructed to stand up from a chair without using their arms for support, to walk around a cone placed 3 m in front of the chair moving as quickly as possible whilst taking care not to run and to remain safe, and to return to the original sitting position. Time (s) needed to complete the task was recorded. Participants were allowed three trials with 1-min resting period between attempts, and the fastest attempt was used for analysis.
Anthropometry
Standing height (m) was measured to the nearest millimetre. Body mass (kg) was measured using calibrated weighing scales to one decimal place accuracy. Body mass index (BMI) was calculated as the following: body mass/height2 (kg/m). Body composition was estimated by dual-energy X-ray absorptiometry (DXA) (Finland Lunar Prodigy, version EnCore 9.30; UK Lunar Prodigy Advance, version EnCore 10.50.086; France Lunar Prodigy, version EnCore 12.30). Measurements were performed after a 12-h overnight fast to avoid conflicting effects of hydration status. During measurements, participants wore standard light cotton clothing, and all jewelry was removed. Estimation of total body fat mass (kg) was used in analysis.
Statistical analyses
Predicted pulmonary variables in Table 1 were calculated according to the current European equations (Quanjer et al. 2012).
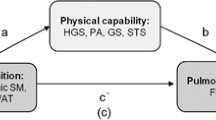
Path models were constructed to assess the interdependence between muscle strength, spirometric pulmonary function and mobility outcomes. Alternative models were constructed for the three separate causal orderings of the sets of muscle strength (MS), pulmonary function (PF) and mobility (M) variables (PF → M → MS, MS → M → PF and MS → PF → M). For each of these model combinations, three additional models, full, partial and no mediation models, were constructed and compared to assess the mediating association of the second variable in the chain. The first model was specified as the full mediation model shown in Fig. 1 using only the associations shown with dotted and solid arrows. In the second model, direct paths (dashed arrows in Fig. 1) were added to the model. In the third model, only direct associations between muscle strength and mobility as well as association between spirometric pulmonary functions and mobility variables were tested (dashed and solid arrows in Fig. 1). This model corresponds to a multiple regression model with correlations among predictors. Separate models were constructed for each pulmonary function variable yielding a total of 36 separate models. The best fitting model for each pulmonary function variable was identified by the Akaike information criterion (AIC). Since the mediation models were nested, the parsimony of these models was compared using the likelihood ratio test (LRT). All models were adjusted for age, sex, total fat mass and body height. The multicentre design was accounted for by multigroup analysis, where model parameters of interest were set equal across centres while intercept and residual parameters were allowed to vary across centres. Model coefficients were estimated with Mplus version 7 (Muthén 1998–2012).
Conceptual models for full mediation (dotted and solid arrows), partial mediation (dotted, solid and dashed arrows) and no mediation (solid and dashed arrows). All model variables were adjusted for age, gender, height, total body fat and site of enrolment. Variable-specific error variances (e) are shown for the model variables. FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, FEF50 maximal expiratory flow at 50 %, TUG Timed Up and Go test, Power lower extremity power
Results
Characteristics of the study population are shown in Table 1. The mean age of the participants was 75 years, and 55 % of them were women. Overall, men were taller, had higher total body mass, lean mass and lower percentage of body fat. Men performed better in all muscle strength and power and physical performance tests, and they had greater FVC, FEV1 and FEF50 values.
When all alternative models for the three separate causal orderings were compared, model AIC estimates for FVC (243, AIC range for other models 244–267), FEV1 (222, AIC range for other models 224–246) and FEF50 (474, AIC range for other models 476–498) suggested that the best fitting models were the partial mediation models with the MS → PF → M arm of model as the best fitting one. Because the difference in AIC estimates between the best and second best fitting models was rather small, the three mediation models within the MS → PF → M arm of models were further compared using the LRT suggesting that the partial mediation model was favoured over the other two for FVC (min p = 0.011), FEV1 (min p = 0.002) and FEF50 (min p = 0.009).
Although analysis showed that partial mediation models fitted the data best, none of the paths were statistically significantly mediated through pulmonary function. However, greater handgrip strength was significantly associated with higher FVC, FEV1 and FEF50 (all p < 0.05) (Table 2). Greater muscle power was statistically significantly associated with better performance in 6-min walk (p = 0.001) and TUG (p < 0.001) tests. Handgrip strength and knee extension torque were not significantly associated with 6-min walk test or TUG.
Discussion
The aim of the present study was to assess associations between muscle strength, spirometric pulmonary function and mobility in apparently healthy older participants. Several models were constructed to assess causal orderings and possible direct and mediated associations between study variables. Results of this study showed that causal, partially mediated model from muscle strength to pulmonary function and further on to mobility fitted best to the data. Results may indicate that decline in mobility is regulated directly through decreases in muscle strength and power but also partly mediated through decreases in spirometric pulmonary function in healthy participants. Although individual paths between muscle strength and mobility through pulmonary function were not statistically significant, significant associations were observed between handgrip strength and FVC, FEV1 and FEF50. Muscle power also had a direct effect on mobility, as assessed by 6-min walk and TUG tests.
Incidence in COPD increases with chronological age through extrinsic effects of the environment and intrinsic effects of aging (Vonbank et al. 2012). It is uncertain how age-related changes in pulmonary function should be separated from COPD (Maciewicz et al. 2009). A better understanding of functional mechanisms that are behind the aging-related deterioration of respiratory function will help to distinguish respiratory pathologies and better understand the aging process. It is generally suggested that smoking and external pollutants are the most common cause of decline in pulmonary function with aging and that decline in pulmonary function leads to decreases in muscle strength and mobility. However, results of this study suggest that in healthy older participants of whom a minority suffers from low-level decline in pulmonary function, associations between muscle strength, pulmonary function and mobility also exist but are somewhat different than those in COPD patients.
Impaired muscle strength is associated with reduced pulmonary functions. This association was found between handgrip strength and pulmonary functions FVC, FEV1 and FEF50, but not with knee extension torque or muscle power and pulmonary function. Relationships between muscle aging and other physiological changes are dependent on the muscles under investigation and measurements used. For example, differences in muscle loading (e.g. in weight-bearing muscles) between individuals may interfere the association between muscle strength and spirometric pulmonary function. Handgrip strength is less influenced by the physical activity level than, for example, knee extension torque. The fact that handgrip strength also strongly predicts the development of functional disabilities, longevity and mortality (Ling et al. 2010; Rantanen et al. 1999, 2003, 2012; Taekema et al. 2010), supports the idea that systemic factors, such as inflammation and hormonal changes, and musculature changes that affect both spirometric pulmonary function and muscle strength, may manifest most clearly in handgrip strength decline (Stenholm et al. 2008).
Major intrinsic reasons for declining pulmonary function with age are suggested to be loss of lung elasticity, aggravated by increasing stiffness of the chest wall and reduced strength of respiratory muscles controlling exhalation (Dyer 2012). Muscle strength and mass decrease gradually during aging (Porter et al. 1995; Vandervoort. 2002). Whether the muscles involved in respiration and those involved in mobility are affected similarly with aging is not clear, but the majority of the information available suggests that age-related decline in muscle strength does not spare the muscles of respiration (Enright et al. 1994; Harik-Khan et al. 1998; Summerhill et al. 2007). Advancing age is independently associated with reductions of maximal inspiratory and expiratory pressure, which can be used as surrogates for respiratory muscle strength (Chen and Kuo 1989; Enright et al. 1994; Sachs et al. 2009). Maximal inspiratory pressure has been shown to correlate well with handgrip strength in a large cohort study with older adults (Enright et al. 1994) suggesting similar aging effects on both muscle groups.
Our results support the hypothesis that muscle strength loss initiates the causal chain that contributes to decreased pulmonary function and mobility limitations (Buchman et al. 2008). The mechanisms behind age-related changes in muscle tissue are most likely a combination of systemic physiological mechanisms (hormonal and neural changes, increased inflammation, reduced protein synthesis, increased muscle proteolysis, decreased regenerative capacity, motor neuron loss and reduced excitability, decreased central drive, and increased muscle/fat content etc.) and decreased physical loading through reduced physical activity and exercise training with increasing age. In addition to its role in motor function, skeletal muscle has roles in other functions such as in systemic metabolism. Skeletal muscles are vulnerable to a wide range of systemic disorders and chronic diseases that can lead to impaired strength and mobility disability (Gosker et al. 2000).
In this study, direct paths between muscle power and mobility were statistically significant indicating that higher muscle power was associated with better walking ability. Although muscle strength is generally thought to limit physical performance, individual paths between handgrip strength and knee extension torque and mobility were not statistically significant. These results are, however, in line with previous studies hypothesizing that decreased lower extremity muscle power may be the key factor in age-related decline of physical performance (Runge et al. 2004) and functional capacity (Bassey et al. 1992; Bean et al. 2003; Yamauchi et al. 2010). Muscle power is lower in older individuals, the decline in maximal power takes place before failure in muscle strength (Skelton et al. 1995) and is greater than in muscle strength (Häkkinen et al. 1998; Macaluso and De Vito 2004). Lower extremity muscle power has also been shown to be a performance-based function, which is assumed to be caused by the fact that daily living tasks such as walking or rising from a chair are dynamic movements that require power production (Aalund et al. 2013).
This study was performed in three European research laboratories as a part of multicentre research co-operation. Similar strict inclusion/exclusion criteria were used in all laboratories, and all measurements were performed according to detailed standard operation procedures, described in detail elsewhere (McPhee et al. 2013). The study population included a group of socially active and relatively healthy participants which minimizes the influence of diseases and cognitive impairment on relationships between muscle strength, spirometric pulmonary function and mobility. Therefore, the results cannot be generalized for the entire elderly population, but they reflect the associations between these variables in healthy older subjects. Although we adjusted models with confounders known to influence muscle strength, pulmonary function and mobility, it is possible that the associations are explained by other factors not identified and/or controlled in the analysis. Strengths of the present study include healthy older participants and several carefully performed functional tests in laboratory settings.
In conclusion, results of this study suggest that mobility decline in older age is mediated by decreases in muscle strength, and especially by decreases in muscle power, but also partly mediated by decreases in spirometric pulmonary function. Decrease in skeletal muscle strength was associated with decreases in spirometric pulmonary function, but associations linking pulmonary function and mobility are less clear in older healthy subjects. Further work, especially longitudinal studies, is needed to identify factors that link muscle strength and pulmonary function with mobility disability in healthy older subjects.
References
Aalund PK, Larsen K, Hansen TB, Bandholm T (2013) Normalized knee-extension strength or leg-press power after fast-track total knee arthroplasty: which measure is most closely associated with performance-based and self-reported function? Arch Phys Med Rehabil 94:384–390
Amara CE, Koval JJ, Paterson DH, Cunningham DA (2001) Lung function in older humans: the contribution of body composition, physical activity and smoking. Ann Hum Biol 28:522–536
Bassey EJ, Short AH (1990) A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol 60:385–390
Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA (1992) Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 82:321–327
Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L (2003) A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci 58:728–733
Brown M, Sinacore DR, Host HH (1995) The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci 50 Spec No:55−59
Buchman AS, Boyle PA, Wilson RS, Gu L, Bienias JL, Bennett DA (2008) Pulmonary function, muscle strength and mortality in old age. Mech Ageing Dev 129:625–631. doi:10.1016/j.mad.2008.07.003
Caserotti P, Aagaard P, Simonsen EB, Puggaard L (2001) Contraction-specific differences in maximal muscle power during stretch-shortening cycle movements in elderly males and females. Eur J Appl Physiol 84:206–212. doi:10.1007/s004210170006
Chen HI, Kuo CS (1989) Relationship between respiratory muscle function and age, sex, and other factors. J Appl Physiol 66:943–948
Degens H, Maden-Wilkinson TM, Ireland A et al (2012) Relationship between ventilatory function and age in master athletes and a sedentary reference population. Age (Dordr). doi:10.1007/s11357-012-9409-7
Dela F, Kjaer M (2006) Resistance training, insulin sensitivity and muscle function in the elderly. Essays Biochem 42:75–88
Dyer C (2012) The interaction of ageing and lung disease. Chron Respir Dis 9:63–67. doi:10.1177/1479972311433766
Eisner MD, Blanc PD, Yelin EH et al (2008) COPD as a systemic disease: impact on physical functional limitations. Am J Med 121:789–796. doi:10.1016/j.amjmed.2008.04.030
Enright PL (2003) The six-minute walk test. Respir Care 48:783–785
Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE (1994) Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular Health Study Research Group. Am J Respir Crit Care Med 149:430–438. doi:10.1164/ajrccm.149.2.8306041
Fletcher C, Peto R (1977) The natural history of chronic airflow obstruction. Br Med J 1:1645–1648
Gosker HR, Wouters EF, van der Vusse GJ, Schols AM (2000) Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr 71:1033–1047
Häkkinen K, Häkkinen A (1991) Muscle cross-sectional area, force production and relaxation characteristics in women at different ages. Eur J Appl Physiol Occup Physiol 62:410–414
Häkkinen K, Kallinen M, Izquierdo M et al (1998) Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol 84:1341–1349
Hamilton AL, Killian KJ, Summers E, Jones NL (1995) Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med 152:2021–2031
Harik-Khan RI, Wise RA, Fozard JL (1998) Determinants of maximal inspiratory pressure. The Baltimore Longitudinal Study of Aging. Am J Respir Crit Care Med 158:1459–1464. doi:10.1164/ajrccm.158.5.9712006
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84:275–294
Ling CH, Taekema D, de Craen AJ, Gussekloo J, Westendorp RG, Maier AB (2010) Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ 182:429–435. doi:10.1503/cmaj.091278
Macaluso A, De Vito G (2004) Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol 91:450–472. doi:10.1007/s00421-003-0991-3
Maciewicz RA, Warburton D, Rennard SI (2009) Can increased understanding of the role of lung development and aging drive new advances in chronic obstructive pulmonary disease? Proc Am Thorac Soc 6:614–617. doi:10.1513/pats.200908-094RM
McPhee JS, Hogrel JY, Maier AB et al (2013) Physiological and functional evaluation of healthy young and older men and women: design of the European MyoAge study. Biogerontology. doi:10.1007/s10522-013-9434-7
Meyer KC (2005) Aging. Proc Am Thorac Soc 2:433–439. doi:10.1513/pats.200508-081JS
Miller MR, Hankinson J, Brusasco V et al (2005) Standardisation of spirometry. Eur Respir J 26:319–338. doi:10.1183/09031936.05.00034805
Muthén LKMB (1998–2012) Mplus. Statistical analysis with latent variables (Fifth edition). Muthén & Muthén, Los Angeles, CA
Park SW, Goodpaster BH, Strotmeyer ES et al (2006) Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 55:1813–1818
Pelkonen M, Notkola IL, Lakka T, Tukiainen HO, Kivinen P, Nissinen A (2003) Delaying decline in pulmonary function with physical activity: a 25-year follow-up. Am J Respir Crit Care Med 168:494–499. doi:10.1164/rccm.200208-954OC
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148
Porter MM, Vandervoort AA, Lexell J (1995) Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 5:129–142
Quanjer PH, Stanojevic S, Cole TJ et al (2012) Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 40:1324–1343. doi:10.1183/09031936.00080312
Rantanen T, Era P, Heikkinen E (1994) Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing 23:132–137
Rantanen T, Guralnik JM, Foley D et al (1999) Midlife hand grip strength as a predictor of old age disability. JAMA 281:558–560
Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM (2003) Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc 51:636–641
Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, White L (2012) Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr) 34:563–570. doi:10.1007/s11357-011-9256-y
Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D (2004) Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging 24:335–340. doi:10.1111/j.1475-097X.2004.00567.x
Sachs MC, Enright PL, Hinckley Stukovsky KD, Jiang R, Barr RG, Multi-Ethnic Study of Atherosclerosis Lung Study (2009) Performance of maximum inspiratory pressure tests and maximum inspiratory pressure reference equations for 4 race/ethnic groups. Respir Care 54:1321–1328
Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF (1993) Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 147:1151–1156
Skelton DA, Young A, Greig CA, Malbut KE (1995) Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc 43:1081–1087
Stanojevic S, Wade A, Stocks J et al (2008) Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med 177:253–260. doi:10.1164/rccm.200708-1248OC
Stenholm S, Rantanen T, Heliövaara M, Koskinen S (2008) The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc 56:462–469. doi:10.1111/j.1532-5415.2007.01567.x
Summerhill EM, Angov N, Garber C, McCool FD (2007) Respiratory muscle strength in the physically active elderly. Lung 185:315–320. doi:10.1007/s00408-007-9027-9
Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ (2010) Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing 39:331–337. doi:10.1093/ageing/afq022
Vandervoort AA (2002) Aging of the human neuromuscular system. Muscle Nerve 25:17–25
Vonbank K, Strasser B, Mondrzyk J et al (2012) Strength training increases maximum working capacity in patients with COPD—randomized clinical trial comparing three training modalities. Respir Med 106:557–563. doi:10.1016/j.rmed.2011.11.005
Yamauchi J, Mishima C, Nakayama S, Ishii N (2010) Aging-related differences in maximum force, unloaded velocity and power of human leg multi-joint movement. Gerontology 56:167–174. doi:10.1159/000235814
Acknowledgments
Gerontology Research Center is a joint effort between the University of Jyväskylä and the University of Tampere. The authors thank Tommi Seikkula, MSc., for the valuable and trustworthy work related to the data collection at the University of Jyväskylä, Finland.
Funding
This study was supported by an unrestricted grant from the seventh framework program MyoAge (HEALTH-2007-2.4.5-10), 050-060-810 Netherlands Consortium for Healthy Aging (NCHA)), Estonian Science Foundation (grants # 8736 and # 7823), the Estonian Ministry of Education and Research (grant SF01080114As08) and the Association Française Contre les Myopathies (AFM).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sillanpää, E., Stenroth, L., Bijlsma, A.Y. et al. Associations between muscle strength, spirometric pulmonary function and mobility in healthy older adults. AGE 36, 9667 (2014). https://doi.org/10.1007/s11357-014-9667-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11357-014-9667-7