Abstract
The current study evaluated the effects of air pollution on selected street trees in the National Capital Territory during the pre- and post-monsoon seasons to identify the optimally suitable tree for green belt development in Delhi. The identification was performed by measuring the air pollution tolerance index (APTI), anticipated performance index (API), dust-capturing capacity (DCC) and proline content on the trees. The APTI of street trees of Delhi varied significantly among different tree species (F11,88.91 = 47.18, p < 0.05), experimental sites (F3,12.52 = 6.65, p < 0.001) and between seasons (F1,31.12 = 16.51, p < 0.001), emphasizing the relationships between trees and other types of variables such as the climate and level of pollution, among other factors. This variability emphasizes the need to choose trees to use for urban greening in the improvement of air quality in different environments within cities. Ascorbic acid (AA) concentration and relative water content (RWC) had a strong influence on APTI with an extremely significant moderate positive correlation between AA concentration and APTI (r = 0.65, p < 0.001) along with RWC and APTI (r = 0.52, p < 0.001), indicating that higher levels of AA concentration and RWC are linked to increased air pollution tolerance. The PCA bi-plot indicates AA has poor positive loading coefficients with PC1 explaining 29.49% of the total variance in the dataset. The highest APTI was recorded in Azadirachta indica (22.01), Leucaena leucocephala (20.65), Morus alba (20.62), Ficus religiosa (20.61) and Ficus benghalensis (19.61), irrespective of sites and seasons. Similarly, based on API grading, F. religiosa and F. benghalensis were identified as excellent API grade 6 (81–90%), A. indica and Alstonia scholaris as very good API grade 5 (71–80%), M. alba, Pongamia pinnata and Monoon longifolium as good API grade 4 (61–70%) and Plumeria alba as moderate API grade 3 (51–60%) in different streets of Delhi. As these plants are indigenous to the region and hold significant socio-economic and aesthetic significance in Indian societies, they are advisable for avenue plantations as part of various government initiatives to support environmental sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
India recorded an astounding average particulate matter (PM) 2.5 concentration of 51.90%, making it the third most polluted country in the world (Malik et al. 2022). Alarmingly, within the top 30 list of the most polluted cities worldwide, a striking 21 cities are nestled in India with Delhi, the capital city on the top, which grapples with severe air pollution stemming from various sources such as biomass burning in the neighbouring states, vehicular emissions, industrial activities, rapid urbanization and deforestation (Katoch and Kulshrestha 2022). Air pollution leads to biodiversity loss, reduced agricultural productivity, economic proficiency (Chaudhary and Rathore 2019) and a variety of human health problems, including pulmonary, cardiac, vascular and neurological disorders, chronic respiratory symptoms and diseases in the elderly (Hossain et al. 2021; Zhang et al. 2019). Due to increased urbanization, infrastructure development and associated human activity, dust pollution in metropolitan regions has been rising gradually (Patel et al. 2023). Dust particles affect the biochemical properties of the leaf, causing some morphological abnormalities (Prajapati and Tripathi 2008).
Urban green spaces are recognized as nature-based alternatives for reducing air pollution in highly populated urbanized areas (Junior et al. 2022). Plant biochemical parameters such as ascorbic acid (AA), leaf extract pH, relative water content (RWC) and total chlorophyll (TC) help plants mitigate air pollution by enhancing tolerance (Patel et al. 2023). The World Health Organization (WHO) has suggested 9 m2 per individual green space to enhance the quality of life of urban residents (WHO 2017). In addition to providing several essential ecosystem services, vegetation in urban and peri-urban areas—such as parks, gardens, farms, grasslands, urban forests and street trees—also significantly contributes to reducing the adverse effects of urbanization (Bodnaruk et al. 2017). Nature-based solutions (NbS) are cost-effective and multi-functional, with additional benefits such as lower energy costs, improved public health and biodiversity conservation (Menon and Sharma 2021). Urban trees contribute significantly to reducing air pollution and mitigating climate change through their ability to filter air pollutants (Sicard et al. 2018), assimilate CO2 (Aly et al. 2020), regulate air temperature, mitigate stormwater runoff (Livesley et al. 2016) and provide recreational, social, psychological and aesthetic benefits (Sen and Guchhait 2021). Tree leaves and stomata used to be smaller in polluted areas compared to non-polluted ones (Dadkhah-Aghdash et al. 2022). Reactive oxygen species (ROS) develop when air pollutants react with leaf mesophyll cells after entering through the stomata of leaves (Skrynetska et al. 2019). Under stressful environmental conditions, ROSs—small, very reactive molecules—can cause structural damage to cells (Xie et al. 2019). The production of antioxidant compounds from leaves, such as ascorbic acid (AA), may be a useful strategy for shielding thylakoid membranes from oxidative damage when the environment is stressed (Tambussi et al. 2000). Moreover, AA regulates cell division and growth prevents ROS accumulation in cells and organelles and plays a role in photosynthesis, hormone synthesis and antioxidant development (Lisko et al. 2014; Smirnoff 1996). It can be found in large quantities in mature leaves, meristems, photosynthetic cells and fruits (Akram et al. 2017). It is now widely accepted that plants improve the quality of air in cities due to their ability to absorb, accumulate and metabolize air pollutants using their leaves and roots (Shrestha et al. 2021). Some of the plants are more resistant to air pollution as they can accumulate higher amounts of ascorbic acid, chlorophyll and relative water content and therefore can uptake air pollution through phytoaccumulation (Bharti et al. 2018).
Green infrastructure can limit exposure to air pollution and particulate matter by up to 50%, with hedges interspersed with trees (Hewitt et al. 2020). However, heavy dust load and dust depositions often alter the concentration of AA, leaf extract pH, total chlorophyll (TC), relative water content (RWC) and carotenoids on the foliage (Tripathi and Nema 2023). On the other hand, the leaf extract pH is significant in plant physiological activities as the majority of the enzymes involved in different biochemical activities in plants require varied pH ranges to function properly (Wahab et al. 2022). Plants with lower pH appear to be more sensitive to air pollutants, specifically SO2, whereas those with pH near 7 or above appear to be more tolerant as alkalinity is used to demonstrate the development of the detoxifying system (Swami and Chauhan 2015). Plant protoplasmic permeability is largely influenced by RWC, and plants with greater RWC levels are more resilient to air pollution (Nayak et al. 2015). Proline is an amino acid that functions as a signalling molecule, an osmolyte, a metal chelator and an antioxidant defence (Mattioli et al. 2009). To adapt to severe environments, many plants produce proline, which neutralizes ROS and modifies cellular redox potential to assist in stabilising subcellular structures (Hayat et al. 2012). Dust is the main contributor to PM pollution in the atmosphere, making up around 30–40% of the PM mass in Delhi (Tripathi and Nema 2023). However, dust accumulation on the upper side of the leaf, which functions as a sink because of the enormous surface area of the leaf, and dust building on leaf surfaces are all ways that plants might reduce air pollution (Roy et al. 2020). In recent years, it has become widely accepted that improving air quality by removing suspended PM can be accomplished with the assistance of appropriate plantation (Patel et al. 2023; Hariram et al. 2018), and plants are an environmentally sustainable treatment for improving air quality (Chaudhary and Rathore 2019; Prajapati and Tripathi 2008). Therefore, the present research attempted to identify the best air pollution-tolerant tree species among the urban street trees for efficient green belt development in Delhi using the air pollution tolerance index (APTI), anticipated performance index (API), dust capturing capacity (DCC) and proline content.
Materials and methods
Study site
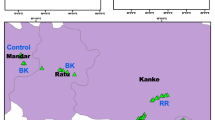
The present research was carried out in four selected areas, viz. Kamala Nehru Ridge (KNR), Guru Tegh Bahadur Nagar (GTB), Kashmere Gate (KG) and Bawana (BW) in the National Capital Territory (NCT) of Delhi. KNR is the least polluted site and represents the control due to its ample greenery, while GTB is characterized as a commercial area with moderate pollution levels due to more commercial activities. Similarly, KG represents a heavy traffic and highly polluted site due to heavy vehicular load at the inter-state bus terminal, Kashmere Gate, and BW is characterized as an industrial site with the highest level of pollution due to the presence of numerous industries along with heavy traffic loads (Fig. 1). The NCT, Delhi covers an area of 1483 km2 having an altitude of 216 m above mean sea level. Delhi experiences a semi-arid climate with hot and dry summers in April and May, followed by significant rainfall events in July and August.
Sample collection
Fresh leaf samples (10 to 30 leaves based on the size of leaf from 2 to 3 individual trees based on availability) were collected in zip-lock bags early in the morning (6:00–7:00 AM). Twelve commonly occurring urban street trees Azadirachta indica A. Juss., Alstonia scholaris (L.) R. Br., Cascabela thevetia (L.) Lippold, Cassia fistula L., Ficus religiosa L., Ficus benghalensis L., Leucaena leucocephala (Lam.) de Wit., Monoon longifolium (Sonn.) B.Xue & R.M.K.Saunders, Morus alba L., Neltuma juliflora (Sw.) Raf., Plumeria alba L. and Pongamia pinnata (L.) Pierre were selected (Ekka et al. 2024). The study was performed for two seasons: post-monsoon (November–December 2022) and pre-monsoon (May–June 2023) periods at the above-mentioned four different sites (KNR, GTB, KG and BW). All the samples were taken to a lab and stored at − 20 °C to determine the fresh weight, total chlorophyll (TC) content, carotenoids, ascorbic acid (AA) concentration, pH and RWC (Fig. 2). All the analyses were performed in triplicates to maintain precision and accuracy in results.
Biochemical analyses
The APTI, a useful tool for identifying bioindicator plants, is determined using AA concentration, leaf extract pH, RWC and TC (Kaur and Nagpal 2017). AA was determined using the 2,6-dichlorophenol indophenol method. The titrating media utilized was oxalic acid, 4%. 0.5 g of the material was extracted in 4% oxalic acid (10 ml). It was centrifuged for 5 min at 4500 rpm. Five millilitres of the supernatant was taken, and 10 ml of oxalic acid was added to it. It was then titrated against the dye until a pink colour emerged. For RWC, the weight of the freshly plucked leaves was taken to yield fresh weights (FW). The turgid weights (TW) were acquired by soaking the samples in deionized water overnight, while the dry weights (DW) were acquired by drying them at 105 °C in a hot air oven for 3 h (Liu and Ding 2008). TC and carotenoids were determined using the DMSO method given by Hiscox and Israelstam (1979); photosynthetic pigments were calculated following Wellburn (1994) where chlorophyll a, chlorophyll b, TC; and carotenoids expressed as mg/g fresh weight. Leaf extract pH was determined using 1 g of leaf sample which was ground with liquid nitrogen using mortar and pestle dissolved in 50 ml deionized water and measured the pH using a calibrated digital pH meter (Mettler Toledo) (Roy et al. 2020). Plants can be categorized into very sensitive (< 1), sensitive (1–17), intermediate (> 17–30) and tolerant (> 30–100) based on ATPI values (Table 1) (Patel et al. 2023). A total of 16 characters of plants including APTI and other biological (plant habit, canopy structure and type of plant), and socio-economical characters were used to determine API (Table 2). API (%) helps to classify plants as best (91–100: grade 7), excellent (81–90: grade 6), very good (71–80: grade 5), good (61–70: grade 4), moderate (51–60: grade 3), poor (41–50: grade 2) and very poor (31–40: grade 1) and not recommended (up to 30: grade 0) for green belt development (Prajapati and Tripathi 2008). To determine the DCC, filter paper was oven-dried and weighed (D1). Using a brush and double distilled water, the dust was removed from the leaf samples. The pre-weighed filter paper was used to filter the water containing dust, which was then eventually dried (at 85 °C for 4 h) and weighed. The dried weight of filter paper was taken (D2) using a sensitive weighing balance. A laser area meter was used to determine the area of leaf samples (S). DCC was calculated using the formula given by Manisha and Pal (2014). Proline was determined using the Bates et al. (1973) method where sulfosalicylic acid was used to extract proline from plant samples. Proline reacts with the acid ninhydrin to yield a red chromophore. This chromophore’s absorbance at 520 nm was then utilized to calculate the proline concentration μmol/g fresh weight (Bates et al. 1973). The biochemical characteristics of plants alter seasonally, indicating their ability to adapt to stress conditions, and it varies according to physiological characteristics, biotic, abiotic and physical variables. Therefore, the present research performs seasonal analyses (in both pre- and post-monsoon seasons) of APTI, API, proline, dust capturing capacity (DCC) and carotenoid content using standard methodologies, as explained in Table 3.
The statistical analysis was conducted using Origin (2019). The three-way ANOVA was applied for each parameter to assess the effect of seasons, sites and plant species, while separate two-way ANOVA analysis was performed to evaluate the impacts of sites for the pre- and post-monsoon seasons. On the other hand, Tukey’s post hoc test was used to see the effect sizes in ANOVA. Furthermore, correlations among various parameters were examined in terms of Pearson’s coefficient of correlation (PCC) and principal component analysis (PCA) in the Paleontological Statistics software (PAST, Version 4.11).
Results and discussion
Ascorbic acid (AA) concentration
The AA concentration of the street trees of Delhi varied significantly among different tree species (F11,38.8 = 24.10, p < 0.05), experimental sites (F3,5.54 = 3.44, p < 0.05) and between seasons (F1,174.45 = 108.43, p < 0.05). Similarly, it varied significantly between different sites (F3,3.53 = 4.66, p < 0.05) and street trees (F11,52.24 = 69.09, p < 0.001) in the post-monsoon season. There is evidence indicating that various species possess the physiological responses that enable them to reduce the effects of oxidative stress from pollutants in their environment. However, variation can also occur due to the quality of the soil, variations in microclimate conditions and changes in seasons that affect the amount and types of pollutants. This study established that urban trees are dynamic concerning their biochemical responses towards changing environmental stressors within different seasons. The highest AA concentration (mg/g) was observed in A. indica in all three sites (control site (KNR) 22.22 ± 0.44 followed by commercial site (GTB): 21.55 ± 0.22 and heavy traffic site (KG) 14.66 ± 0), but it was highest in Cassia fistula (19.77 ± 2.88) in the industrial site (BW) in the post-monsoon season. On the other hand, in the pre-monsoon season, it varied significantly among different tree species (F11,8.49 = 3.44, p < 0.001); however, the change was not significant among sites. The highest AA concentration (mg/g) was found in C. fistula (20.24 ± 2.02) in the heavy traffic site (KG), followed by Cascabela thevetia (17.03 ± 1.54) in the commercial site (GTB), M. alba (16.79 ± 2.02) in the industrial site (BW) and in A. indica (16.04 ± 1.78) in the control site (KNR) (Table 4). In the pre-monsoon season, the mean AA concentration (mg/g) during the pre-monsoon season in different plant species ranged from 16.37 (C. fistula) to 12.59 (P. pinnata), while in the post-monsoon season, it ranged from 17.48 (A. indica) to 11.04 (P. pinnata). AA is a lightweight antioxidant that helps protect plants against oxidative stress induced by air pollution, and its concentration rises to counteract the stressful conditions by scavenging free radicals produced in the plant cells (Tripathi and Nema 2023; Bui et al. 2023). Moreover, the reducing activity of AA increases with higher pH levels, potentially enhancing hexose sugar conversion to ascorbic acid, thereby relating to pollution tolerance (Pandit and Sharma 2020). The heavy traffic site (KG) in Delhi was found to be the most stressed experimental site in terms of AA concentration (mg/g) in both seasons (pre-monsoon: 14.67 ± 0.61 and post-monsoon: 12.97 ± 0.36), based on the findings of the current study. Reports from Delhi (Tripathi and Nema 2023) and Cheongju City, Korea (Bui et al. 2023) also indicated comparable patterns of greater AA concentrations during the pre-monsoon season as opposed to the post-monsoon season. Additionally, A. indica (15.96 mg/g), C. fistula (15.67 mg/g), C. thevetia (14.40 mg/g) and M. alba (13.83 mg/g) are the best street trees for green belt development in Delhi identified based on the AA concentration (> 13.48 mg/g, i.e. the mean of all trees, sites and seasons). Preferring these trees may reduce air pollution as well as increase biological diversity in urban environments.
TC content
The total chlorophyll (TC) content of street trees of Delhi varied significantly among different tree species (F11,6.47 = 74.23, p < 0.05), experimental sites (F3,2.51 = 28.82, p < 0.001) and seasons (F1,4.54 = 52.16, p < 0.001). On the other hand, in the post-monsoon season, it varied significantly among different tree species (F11,2.49 = 20.96, p < 0.001) and sites (F3,2.47 = 20.72, p < 0.001). A high chlorophyll concentration is connected with rapid growth and tolerance to air pollution (Yarnvudhi et al. 2022), as chlorophyll aids in the formation of antioxidants such as AA (Shrestha et al. 2021). The highest TC content (mg/g) was observed in M. longifolium in the commercial site (GTB) (3.21 ± 0.06) and also in the control site (KNR) (2.83 ± 0.08), while it was highest in the industrial site (BW) in C. fistula (2.86 ± 0.34) and the heavy traffic site (KG) in L. leucocephala (2.41 ± 0.22) in the post-monsoon season (Table 4). On the contrary, in the pre-monsoon season, it varied significantly among different tree species (F11,6.42 = 116.46, p < 0.001) and sites (F3,1.34 = 24.29, p < 0.001). The highest TC content (mg/g) was observed in M. alba (4.87 ± 0.18) in the commercial site (GTB), followed by L. leococephala (4.22 ± 0.33) in the industrial site (BW), C. fistula (3.76 ± 0.14) in the control site (KNR) and L. leococephala (2.66 ± 0.28) in the heavy traffic site (KG) in the pre-monsoon season. Air pollutants such as PM, SO2 and NO2, settle down on leaves, clogging stomata thereby reducing transpiration rate and chlorophyll content in urban and semi-urban areas (Bharti et al. 2018). The mean TC content (mg/g) in the pre-monsoon season in different street trees ranged from 3.72 (L. Leucoceplala) to 0.92 (F. benghalensis), while in the post-monsoon season, it ranged from 2.72 (L. Leucocephala) to 1.51 (A. scholaris). Dust deposition on the leaf surface obstructs light flow, lowering the amount and intensity of light that reaches chloroplasts and raising leaf temperature, leading to a decrease in photosynthetic pigments (Shah et al. 2017). The study found that the heavy traffic site (KG) was the most stressed experimental site in Delhi, with lower TC concentration (mg/g) in pre-monsoon season (1.74 ± 0.13) and control site (KNR) in post-monsoon season (1.51 ± 0.21). Additionally, L. leucolephala (3.22 mg/g), M. alba (2.63 mg/g), M. longifolium (2.19 mg/g) and N. juliflora (1.94 mg/g) are the best street trees for the green belt development in Delhi identified based on the TC content (> 1.88 mg/g, i.e. the mean of all trees, sites and seasons).
RWC
The relative water content (RWC) content of street trees of Delhi varied significantly among different tree species (F11,1271.36 = 22.65, p < 0.05), experimental sites (F3,2223.84 = 39.62, p < 0.001) and seasons (F1,769.45 = 13.71, p < 0.05). Similarly, it varied significantly among different sites (F3,4298.62 = 75.02, p < 0.001) and tree species (F11,689.85 = 12.04, p < 0.001) in the post-monsoon season. Plants with higher RWC show more tolerance to air pollution; however, plants grown in high PM conditions show lower RWC (Bui et al. 2023). The highest RWC content (%) was observed in C. fistula (94.82 ± 0.51) in the industrial site (BW) followed by M. alba (92.04 ± 0.21) in the commercial site (GTB), M. longifolium (89.43 ± 2.04) in the control site (KNR) and F. religiosa (85.06 ± 1.14) in the heavy traffic site (KG) in the post-monsoon season (Table 4). On the contrary, in the pre-monsoon season, it varied significantly among different tree species (F11,1010.72 = 18.39, p < 0.001); however, the change was not significant among sites. The highest RWC content (%) was observed in M. alba in both the commercial site (GTB) (93.98 ± 2.36) and control site (KNR) (87.16 ± 3.69), while it was highest both in the heavy traffic site (KG) (91.85 ± 0.89) and in the industrial site (BW) (91.55 ± 0.45) in A. indica. The mean RWC content (%) in the pre-monsoon season in different street trees ranged from 91.67 (A. indica) to 60.79 (C. fistula), while in the post-monsoon season, it ranged from 87.74 (F. religiosa) to 64.22 (A. scholaris). According to the results of this investigation, the heavy traffic site (KG) was the most stressed in the post-monsoon season with the lowest RWC content (62.96%), while the control site (KNR) was the most stressed experimental site in Delhi during the pre-monsoon season with the lowest RWC content (75.31%). Generally, PM buildup on leaves causes a loss of dissolved nutrients and water (Mei et al. 2021; Shrestha et al. 2021), and a higher RWC (%) leads to a higher hydration level (Patanè et al. 2022). Because RWC and protoplasmic permeability are directly correlated, plants with higher RWC levels are more resilient and able to withstand the stresses caused by polluted air (Gupta et al. 2016). Additionally, F. religiosa (86.32%), A. indica (85.62%), M. alba (81.61%), P. alba (80.78%), F. benghalensis (79.21%) and N. juliflora (76.82%) are the best street trees for the green belt development in Delhi identified based on the RWC content (> 75.94%, i.e. the mean of all trees, sites and seasons). With a few exceptions, most crops have a normal leaf RWC of ~ 60% to 70% at the beginning of wilting. Depending on the plant species, this percentage might vary from 98% in completely turgid transpiring leaves to ~ 30–40% in extremely dried and dying leaves (Zhang et al. 2015). The present research recorded a minimum of 63.09% (C. fistula) to a maximum of 86.32% (F. religiosa) RWC in different street trees irrespective of sites and seasons which is showing initial wilting in some species like in C. fistula (63.09%) and P. pinnata (68.40%).
Leaf extract pH
The leaf extract pH of the street trees of Delhi varied significantly among different tree species (F11,12.81 = 394.43, p < 0.05), experimental sites (F3,5.75 = 177.01, p < 0.05) and between seasons (F1,89.15 = 2749.29, p < 0.05). Similarly, it varied significantly among different experimental sites (F3,14.93 = 298.30, p < 0.001) and street trees (F11,8.31 = 166.15, p < 0.001) in the post-monsoon season. Seasonal variations concerning the pH of the collected leaf extracts are significant, as has also been established in this study. Earlier studies noted that temperature and humidity impact on biochemical changes in plants exposed to air pollution (Husen 2021). This seems to imply that trees could use various defensive strategies depending on current levels of pollution at specific seasons (Husen 2021). The highest leaf extract pH was observed in F. benghalensis in the industrial site (BW) (8.99 ± 0.04), followed by F. religiosa in all three sites with 8.98 ± 0.04 in the heavy traffic site (KG), 8.7 ± 0.02 in the commercial site (GTB) and 8.39 ± 0.02 in the control site (KNR) during post-monsoon season. On the contrary, in the pre-monsoon season, it varied significantly among different street trees (F115.92 = 396.20, p < 0.001) and different experimental sites (F3,0.49 = 37.20, p < 0.001). The highest leaf extract pH was found in F. religiosa in the commercial site (GTB) (8.57 ± 0.06), followed by M. alba both in the industrial site (BW) (7.84 ± 0.03) and control site (KNR) (7.62 ± 0.02) and F. benghalensis (7.53 ± 0.06) in the heavy traffic site (KG) (Table 4). The mean leaf extract pH in the pre-monsoon season in different street trees ranged from 7.65 (M. alba) to 5.16 (N. Juliflora), while in the post-monsoon season, it ranged from 8.95 (F. religiosa) to 6.17 (N. Juliflora). The trees at the control location in Delhi demonstrated greater vulnerability to stress in terms of low leaf extract pH in both seasons (pre-monsoon 6.21 and post-monsoon 6.72), as per the findings of the current study. Increased pH of leaf extract in areas under stress could be a result of plant defence mechanisms activating to lessen pollution stress in areas with high traffic and industry. Plants’ tolerance to air pollution is influenced by the leaf extract pH (Shrestha et al. 2021), and plants with a greater leaf extract pH (> 7.00) are more resistant to air pollution than plants with a lower leaf extract pH (Roy et al. 2020; Najafi et al. 2023). Additionally, F. religiosa (8.05), M. alba (8.03), F. benghalensis (7.8), C. fistula (7.14) and P. pinnata (7.05) are the best street trees for the green belt development in Delhi identified based on the leaf extract pH (> 6.94, i.e. the mean of all trees, sites and seasons). Earlier studies also reported F. benghalensis and F. religiosa as some of the best pollution-tolerating tree species with the highest leaf extract pH in the Jharia coalfield region of Jharkhand (Mondal and Singh 2022). However, acidic gases such as SO2, NO2 or CO2 in the ambient air form acid radicals in the leaf tissue causing stress by creating a highly acidic (low pH) environment in the cell sap (Yarnvudhi et al. 2022). Since low pH causes greater sensitivity to air pollution, plants with lower leaf extract pH are highly impacted by pollution (Dhankar et al. 2015). In this study, N. juliflora showed the lowest leaf extract pH (5.67), followed by P. alba (6.05), L. leucocephala (6.40), A. scholaris (6.48), A. indica (6.67), C. thevetia (6.71) and M. longifolium (6.78), indicating their greater sensitivity towards pollution thereby must be avoided in green belt development in a heavily polluted city like Delhi.
APTI
The air pollution tolerance index (APTI) of the street trees of Delhi varied significantly among different tree species (F11,88.91 = 47.18, p < 0.05), experimental sites (F3,12.52 = 6.65, p < 0.001) and between seasons (F1,31.12 = 16.51, p < 0.001), emphasizing the relationships between trees and other types of variables such as the climate and level of pollution among other factors. This variability emphasizes the need to choose trees to use for urban greening in the improvement of air quality in different environments within cities. Similarly, it varied significantly among different sites (F3,27.80 = 17.95, p < 0.001) and street trees (F11,44.95 = 29.01, p < 0.001) during the post-monsoon season. The highest APTI was observed in A. indica in both the control site (KNR) (27.19) and commercial site (GTB) (25.03), while it was highest in F. religiosa (27.13) in the industrial site (BW) and in N. juliflora (21.05) at the heavy traffic site (KG) during post-monsoon season. On the contrary, in the pre-monsoon season, it varied significantly among different tree species (F11,72.73 = 32.76, p < 0.001); however, the variation was insignificant among different experimental sites. The highest APTI was found in F. benghalensis (27.33) industrial site (BW), followed by M. alba both in the commercial site (GTB 26.42) and the control site (KNR 25.83) and the lowest in C. fistula (24.11) in the heavy traffic site (KG) (Table 4). The mean APTI in the pre-monsoon season in different street trees ranged from 22.71 (M. alba) to 16.70 (N. Juliflora), while in the post-monsoon season, it ranged from 23.38 (A. indica) to 16.00 (C. fistula). The street trees at the commercial site (GTB) during pre-monsoon (19.58) and in the industrial site (BW) (19.92) during post-monsoon (19.92) showed greater resistance against air pollution in Delhi, as indicated by the highest APTI values reported in the current study. Furthermore, A. indica (22.01), L. leucocephala (20.65), M. alba (20.62), F. religiosa (20.61) and F. benghalensis (19.61) are the best street trees for the green belt development in Delhi identified based on the APTI (> 19.08, i.e. the mean of all trees, sites and seasons). The APTI of street trees varies based on their biochemical composition and all the selected street trees of Delhi (except N. juliflora with APTI 16.94 as a sensitive species) were found under the intermediate category having APTI > 17 to 30. Previous studies also reported the majority of the street trees in the intermediate category in various Indian cities including Kanpur, Uttar Pradesh (Yadav et al. 2023), Santiniketan campus, West Bengal (Karmakar et al. 2021) and Lucknow, Uttar Pradesh (Bharti et al. 2018) (Fig. 3). The changes in the leaf extract pH of street trees in Delhi draw attention towards the fact that plants possess a delicate balance of response to various stress factors in the environment. Future studies should therefore identify the mechanisms that control these biochemical responses as well as examine the possibilities of adopting tree species, with suitable pH ranges to improve on the green practices of urbanization to improve the air quality and embrace a sustainable environment.
APTI of twelve selected tree species (Azadirachta indica, Alstonia scholaris, Cascabela thevetia, Cassia fistula, Ficus religiosa, Ficus benghalensis, Leucaena leucocephala, Monoon longifolium, Morus alba, Neltuma juliflora, Plumeria alba and Pongamia pinnata) at four sites (control, commercial, heavy traffic and industrial) during pre- and post-monsoon seasons
The correlation analysis among the biochemical parameters of the APTI reveals noteworthy connections between different parameters (Fig. 4). Notably, a strong extremely significant positive correlation was observed between AA concentration and APTI (r = 0.65, p < 0.001), indicating that higher levels of AA concentration are linked with increased air pollution tolerance. Similarly, RWC (%) also has a strong influence on APTI with a strong extremely significant positive correlation between RWC and APTI (r = 0.52, p < 0.001). On the other hand, TC content showed comparatively moderate influence with an extremely significant moderate positive correlation with APTI (r = 0.40, p < 0.001), suggesting that elevated TC levels correspond to higher air pollution tolerance. However, a significantly weak positive correlation was observed between leaf extract pH and APTI (r = 0.28, p < 0.001), indicating poor influence of leaf extract pH on APTI. Based on this correlation analysis, it can be summarized that AA concentration has the highest influence on APTI followed by RWC, TC content and leaf extract pH (Table 5). The activity of AA, a key electron donor in photosynthesis, is pH-dependent and leaf extract pH has a considerable influence on the overall photosynthetic activity of plants (Shrestha et al. 2021). In the present research, a significantly weak inverse relationship was observed between AA concentration and leaf extract pH (r = − 0.17, p < 0.01) with the increase in AA concentration in the cell sap leading to decreased leaf extract pH.
API
Based on anticipated performance index (API) grading given by Prajapati and Tripathi (2008), F. religiosa and F. benghalensis were categorized as excellent API grade 6 (81–90%), followed by A. indica and A. scholaris as very good API grade 5 (71–80%), M. alba, P. pinnata and M. longifolium as good API grade 4 (61–70%) and P. alba as moderate API grade 3 (51–60%) (Table 4). These street trees API grade 3 and above are highly suitable for developing green belts in urban areas (Prajapati and Tripathi 2008), while C. thevetia, N. juliflora and L. leucocephala were categorized as poor API grade 2 (41–50%) and should be avoided for plantation in cities. F. religiosa and F. benghalensis were the most recommended species for urban green belt development by previous researchers in different Indian cities (Singh et al. 2023, Mondal and Singh 2022). In conformity with the present report, earlier studies also similarly categorized street trees in Indian cities (Patel et al. 2023; Roy et al. 2020).
Proline
The proline concentration (µmol/g) of the street trees of Delhi varied significantly among different tree species (F11,4.71 = 135.03, p < 0.05), experimental sites (F3,2.65 = 75.96, p < 0.05) and between seasons (F1,11.75 = 336.87, p < 0.05). Similarly, it varied significantly among different sites (F3,3.34 = 55.96, p < 0.001) and street trees (F11,3.79 = 63.51, p < 0.001) in the post-monsoon season. This study demonstrates that proline is an important biochemical that should be used to evaluate the stress response in plants given that most of the plants are grown in polluted environments within urban areas. This huge change in proline concentration also indicates that trees belonging to different species can withstand some stress factors in the environment depending on their physiological and genetic mechanism. Also, fluctuations of these levels during different seasons prove that plants have responsive changes to unpredictable environmental conditions like temperature and humidity related to monsoon seasons (Ashraf and Foolad 2007). These fluctuations indicate that proline buildup affords trees with enhanced stress tolerance during specific stages of their life and, as a result, improves their ability to endure air pollution. The highest proline concentration (µmol/g) was observed in M. alba in the commercial site (GTB) (6.59 ± 0.51), followed by N. Juliflora in both the industrial site (6.33 ± 0.02) and control site (KNR) (3.79 ± 0.12) and A. indica (5.06 ± 0.15) in the heavy traffic site (KG) in the post-monsoon season. On the other hand, in the pre-monsoon season, it varied significantly among different tree species (F11,1.98 = 196.70, p < 0.001) and experimental sites (F3,0.31 = 30.47, p < 0.001). The highest proline concentration (µmol/g) was found in A. indica in both the industrial (BW) (5.09 ± 0.01) and control (KNR) (4.82 ± 0.28) sites, followed by C. fistula in the commercial site (GTB) (4.92 ± 0.1) and M. alba in the heavy traffic site (KG) (3.44 ± 0.03) (Table 4). The mean proline concentration (µmol/g) in the pre-monsoon season in different plant species ranged from 2.90 (A. scholaris) to 3.91 (A. indica), while during the post-monsoon season, it ranged from 2.98 (F. benghalensis) to 4.56 (N. juliflora). Proline levels in plants are affected by ambient air pollution; it increases under stress (Sanaeirad et al. 2017). Under abiotic stress, such as air pollution, proline acts as an osmolyte and accumulates in leaves to shield tissues from oxidative damage (Gupta et al 2016). However, proline accumulation in plants is a symptom rather than an adaptive stress response (Agbaire 2016). In terms of high proline content (µmol/g) in both seasons (pre-monsoon: 3.35 ± 0.81 and post-monsoon: 3.98 ± 0.29), the industrial site (BW) was found to be the most stressed experimental site in Delhi based on the findings of the current study. In general, proline concentrations in plant tissues ranged from 0.5 (unstressed) to 50 (stressed) μmol/g (Carillo and Gibon 2011). Further, F. benghalensis (2.94 µmol/g), P. alba (2.98 µmol/g), P. pinnata (3.06 µmol/g), F. religiosa (3.09 µmol/g), A. scholaris (3.14 µmol/g) and C. thevetia (3.17 µmol/g) are the best street trees for the green belt development in Delhi identified based on the proline concentration (< 3.37 µmol/g, i.e. the mean of all trees, sites and seasons) (Fig. 5).
Proline (µmol/g) levels of twelve selected tree species (Azadirachta indica, Alstonia scholaris, Cascabela thevetia, Cassia fistula, Ficus religiosa, Ficus benghalensis, Leucaena leucocephala, Monoon longifolium, Morus alba, Neltuma juliflora, Plumeria alba and Pongamia pinnata) at four sites (control, commercial, heavy traffic and industrial) during pre- and post-monsoon seasons
Dust-capturing capacity
The DCC of the street trees of Delhi varied significantly among different tree species (F11,1.39 = 85.70, p < 0.05), experimental sites (F3,3.70 = 228.35, p < 0.05) and between seasons (F1,6.86 = 423.04, p < 0.05). Similarly, it varied significantly among different sites (F3,3.01 = 127.64, p < 0.001) and street trees (F11,1.244 = 52.73, p < 0.001) in the post-monsoon season. Plants under abiotic stress from dust load produce ROS, which damage plant cell membranes (Demidchik et al. 2014). The amount of such effects is determined by plant tolerance to dust particles and its chemical composition. The highest DCC (mg/cm2) was observed in P. alba in the industrial site (1.76 ± 0.139), followed by C. fistula in the heavy traffic area (KG) (1.27 ± 0.14), F. benghalensis in the commercial site (GTB) (1.20 ± 0.08) and M. longifolium in the control site (KNR) (0.374 ± 0.105) (Table 4). On the other hand, in the pre-monsoon season, it varied significantly among different tree species (F11,0.40 = 44.89, p < 0.001) and sites (F3,1.34 = 151.74, p < 0.001). The highest DCC (mg/cm2) was found in F. religiosa (1.24 ± 0.05) in the industrial site (BW), followed by P. alba (1.01 ± 0.09) in both the heavy traffic (KG) and control (0.30 ± 0.03) sites and A. indica (0.27 ± 0.01) in the commercial site (GTB) (Table 4). The mean DCC (mg/cm2) during pre-monsoon season in different street trees ranged from 0.074 (P. alba) to 0.06 (C. thevetia), while during post-monsoon season, it ranged from 1.27 (F. benghalensis) to 0.08 (C. thevetia). The study found that street trees at an industrial site in Delhi experience higher levels of DCC (mg/cm2) during both seasons (pre-monsoon 0.55 and post-monsoon 0.78). Height, tree canopy, phyllotaxy and the external features of the foliage (hairs, cuticles, etc.) all have an impact on the capacity of the foliage to accumulate dust (Prusty et al. 2005). Lanceolate leaves, which are wider below the middle and hence have less surface-specific drag and fluttering than leaves with narrow bases, retain more PM than other shaped leaves. This is another important characteristic linked to PM deposition on the leaf surface (Vogel 1989). Similarly, leaves with smaller surface areas and shorter petioles accumulate more PM than those with larger areas and longer petioles (Leonard et al. 2016). Additionally, F. religiosa (0.90), P. alba (0.86), F. benghalensis (0.85), A. indica (0.64) and C. fistula (0.49) are the best street trees for the green belt development in Delhi identified based on the DCC (> 0.47 mg/cm2, i.e. the mean of all trees, sites and seasons) may be due to their larger leaf surfaces and favoured leaf shapes compared to the others. Similarly, in previous studies, the highest DCC was recorded in F. benghalensis and F. religiosa (Mondal and Singh 2022). F. benghalensis has rough, large surfaces, dense canopy and higher surface areas, potentially retaining higher dust volume (Roy et al. 2020) (Fig. 6).
Dust capturing capacity of twelve selected tree species (Azadirachta indica, Alstonia scholaris, Cascabela thevetia, Cassia fistula, Ficus religiosa, Ficus benghalensis, Leucaena leucocephala, Monoon longifolium, Morus alba, Neltuma juliflora, Plumeria alba and Pongamia pinnata) at four sites (control, commercial, heavy traffic and industrial) during pre- and post-monsoon seasons
Carotenoids
The carotenoid content of street trees of Delhi varied significantly among different tree species (F11,0.59 = 105.64, p < 0.05), experimental sites (F3,0.34 = 60.75, p < 0.05) and seasons (F1,0.53 = 96.81, p < 0.05). On the other hand, in the post-monsoon season, it varied significantly among different tree species (F11,0.22 = 32.57, p < 0.001) and sites (F3,0.41 = 61.37, p < 0.001). Carotenoids have a similar production route to chlorophyll and protect plants from photooxidation and oxidative stress by boosting their concentration under abiotic stress (Dadkhah-Aghdash et al. 2022; Swapnil et al. 2021). Plants exposed to air pollution alter their carotenoid concentrations, which affects the photosynthetic efficiency of plants impacting their overall growth and development (Joshi and Swami 2009). The highest carotenoid content (mg/g) was observed in L. leucocephala in the commercial site (GTB) (1.1 ± 0.01), followed by industrial site (BW) (0.75 ± 0.02) and the heavy traffic site (KG) (0.63 ± 0.05), while it was (0.5 ± 0.01) in M. longifolium in the control site (KNR) in the post-monsoon season (Table 4). Conversely, during the pre-monsoon season, it varied significantly among different tree species (F11,0.57 = 87.41, p < 0.001) and sites (F3,0.14 = 21.78, p < 0.001). The highest carotenoid content (mg/g) was observed in M. alba (1.31 ± 0.08) in the commercial site (GTB), followed by L. leucocephala (1.1 ± 0.08) in the industrial site (BW) and C. fistula in the control (KNR) (1.05 ± 0.02) and heavy traffic (KG) (0.64 ± 0.09) sites during pre-monsoon season. The mean carotenoid content (mg/g) in both pre- and post-monsoon seasons was highest in L. leucocephala (0.83 and 0.97, respectively), while it was lowest in F. benghalensis (0.27 in each season). The study indicated that the commercial site (GTB) was the most stressed experimental site in Delhi, with comparatively greater carotenoid concentrations (mg/g) in both pre-monsoon (0.64 ± 0.12) and post-monsoon seasons (0.55 ± 0.08). Additionally, L. leucocephala (0.90 mg/g), M. alba (0.71 mg/g), C. fistula (0.59 mg/g) and M. longifolium (0.57 mg/g) are the best street trees for the green belt development in Delhi identified based on the carotenoid content (> 0.51 mg/g, i.e. the mean of all trees, sites and seasons) (Fig. 7). The decrease in pigment levels may be due to reduced pigment synthesis as a result of dust shadowing, an alkaline environment brought on by dust particles dissolving in the cell sap, which can result in pigment degradation through photobleaching and/or the inhibition of enzymes essential for pigment biosynthesis (Li and Mu 2021).
Carotenoid content (mg/g) levels of twelve selected tree species (Azadirachta indica, Alstonia scholaris, Cascabela thevetia, Cassia fistula, Ficus religiosa, Ficus benghalensis, Leucaena leucocephala, Monoon longifolium, Morus alba, Neltuma juliflora, Plumeria alba and Pongamia pinnata) at four sites (control, commercial, heavy traffic and industrial) during pre- and post-monsoon seasons
The Pearson correlation among different biochemical parameters found interesting patterns showing a statistically significant relationship between certain variables. Specifically, a strong extremely significant positive correlation was observed between carotenoids and TC content (r = 0.89, p < 0.001), suggesting that higher TC content is related to elevated carotenoid levels. On the other hand, carotenoids showed a statistically significant positive relationship with AA (r = 0.14, p < 0.05). Additionally, DCC showed a very weak but statistically significant negative relationship with AA (r = − 0.18, p < 0.01), TC (r = − 0.18, p < 0.001) and carotenoids (r = − 0.13, p < 0.05) but statistically extremely significant weak positive relationship with leaf extract pH (r = 0.27, p < 0.001). Similarly, proline showed a statistically significant positive correlation with AA (r = 0.14, p < 0.05) and carotenoids (r = 0.17, p < 0.01) (Fig. 8).
PCA-based multivariate analysis contributes to understanding the influence of plant biochemical parameters on air pollution tolerance in different plant species. In the present research, PCA was performed to identify the plant biochemical parameters that are affected by air pollution, thereby making plant pollution tolerant. Three principal components (PCs) have been generated based on the eigenvalue, and the significance of each was determined in the variables that have the strongest correlations. PC1 had a strong positive loading coefficient with total chlorophyll (0.90) and carotenoids (0.92); PC2 had a moderate positive loading coefficient with leaf extract pH (0.62) and DCC (0.68), while PC3 had a poor to moderate positive loading coefficients with AA concentration (0.48), proline (0.64) and RWC (0.60) signifying an increase in one parameter may lead to the increase in others. The PCA bi-plot indicates AA has poor positive loading coefficients with PC1 explaining 29.49% of the total variance in the dataset (Table 5; Fig. 9A), while TC and carotenoids are very strongly associated with PC2 which accounts for 22.80% of the total variance. The scree plot indicates that the PC1 and PC2 lie below the broken stick model, while PC1 lie above the elbow in terms of the eigenvalue (%), PC2 lie below the elbow, therefore, PC1 (AA) is the most significant component (Fig. 9B) for determining the overall contribution in the pollution tolerance in plants. Seasonal PCA-based analysis was also performed by the earlier researcher to identify the plant species with better pollution tolerance in various seasons in Durgapur City, West Bengal (Sarkar et al. 2021). The present research exhibited mixed results regarding its suitability for PCA or factor analysis, while Bartlett’s Sphericity Test indicated strong evidence against the null hypothesis of uncorrelated variables, with an extremely significant (p < 0.001) correlation among the variables, suggesting that the data contains meaningful relationships and justifying the use of PCA or factor analysis.
It should be noted that in using this approach we will be unable to generate definitive conclusions regarding the success (or otherwise) of these initiatives, guidelines or principles at reducing aggression, violence or crime within society. Nevertheless, it is hoped that by doing so we can offer a more balanced perspective on our findings and identify areas where future research can develop.
The major caveat of the present research was due to the small sample size and restricted diversity, which implies that our conclusion may not apply to other geographical areas or species of trees. Further, monitoring during pre-monsoon and post-monsoon seasons may not be sufficient enough to study all the environmental factors and variations in air pollution over an annual cycle. Additionally, it is worth noting that soil quality, water status and human management practices could also affect the health and tolerance of street trees. In future studies, more physiological and biochemical parameters can be considered to make the response to tree health and stress more holistic.
Conclusions
Vegetation in the form of urban forests, parks, green belts, green roofs and vertical gardens can help in mitigating air pollution and heat island effects in cities. Selecting pollution-tolerant trees with better phytoaccumulation capacity of dust is one of the prerequisites for green belt development in highly polluted cities like Delhi. The present study identified different native street trees based on their basic biochemical properties that contribute effectively to mitigating the ambient pollution levels in the rapidly urbanizing Delhi metropolitan city. The study highlighted a set of native species, viz. A. indica, L. leucocephala, M. alba, F. religiosa and F. benghalensis, as optimally appropriate for street tree plantation to strengthen the green belt development in Delhi identified based on APTI irrespective of sites and seasons. However, different street trees were also chosen for social forestry plantations and green-belt development, with recommended API grades of three or above based on plant habits, canopy and laminar structure and economic and social merits. It includes F. religiosa and F. benghalensis as excellent with API grade 6 (81–90%), A. indica and A. scholaris as very good with API grade 5 (71–80%), M. alba, P. pinnata and M. longifolium as good with API grade 4 (61–70%) and P. alba as moderate with API grade 3 (51–60%) in different streets of Delhi. Since these species are native to the region with vast socio-economic importance and aesthetic values in Indian societies, these species may be recommended for avenue plantations under different government programmes to promote environmental sustainability. Future research should comprise descriptive studies to consider the long-term effects of pollution on the physiological and biochemical responses of the selected tree species. Additionally, research about the genetic and molecular foundations of air pollution stress tolerance may be useful towards formulating ways of improving genotypes of urban trees. Urban policymakers need to incorporate ecologically sustainable native species into urban forestry initiatives, and the selection of trees should be made from the research findings on trees’ tolerance to pollution or their benefits to the environment. The implementation of this approach will also be effective in the enhancement of environmental quality, conservation of species and general sustainable utilization of cities. In the same way, the existing strategies for urban planning are enhanced by incorporating the evaluation of tree performance about the current levels of air pollution that allows better management of green urban areas.
Data availability
All the data generated or analyzed during this study are included in this article. The rest of the raw data may be available with proper request to the corresponding author.
References
Agbaire OP (2016) Impact of air pollution on proline and soluble sugar content of selected plant species. Chem Mater Res 8(5):72–76
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci 8:613. https://doi.org/10.3389/fpls.2017.00613
Aly SH, Zakaria R, Kondorura CF (2020) The capability of green open space in absorbing carbon monoxide and carbon dioxide emissions in Balai Kota Makassar. IOP Conf Ser: Earth Environ Sci 419(1):012169. https://doi.org/10.1016/j.proeng.2017.07.074
Ashraf MFMR, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216
Bates LS, Waldren RPA, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bharti SK, Trivedi A, Kumar N (2018) Air pollution tolerance index of plants growing near an industrial site. Urban Clim 24:820–829. https://doi.org/10.1016/j.uclim.2017.10.007
Bodnaruk EW, Kroll CN, Yang Y, Hirabayashi S, Nowak DJ, Endreny TA (2017) Where to plant urban trees? A spatially explicit methodology to explore ecosystem service tradeoffs. Landsc Urban Plan 157:457–467. https://doi.org/10.1016/j.landurbplan.2016.08.016
Bui HT, Jeong NR, Park BJ (2023) Seasonal variations of particulate matter capture and the air pollution tolerance index of five roadside plant species. Atmosphere 14(1):138. https://doi.org/10.3390/atmos14010138
Carillo P, Gibon Y (2011) Protocol: extraction and determination of proline. PrometheusWiki 2011:1–5
Chaudhary IJ, Rathore D (2019) Dust pollution: its removal and effect on foliage physiology of urban trees. Sustain Cities Soc 51:101696. https://doi.org/10.1016/j.scs.2019.101696
Dadkhah-Aghdash H, Rasouli M, Rasouli K, Salimi A (2022) Detection of urban trees sensitivity to air pollution using physiological and biochemical leaf traits in Tehran, Iran. Sci Rep 12(1):15398. https://doi.org/10.1038/s41598-022-19865-3
Dhankar R, Mor V, Lilly S, Chopra K, Khokhar A (2015) Evaluation of anticipated performance index of some tree species of Rohtak city, Haryana, India. Int J Recent Sci Res 6(3):2890–2896
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65(5):1259–1270. https://doi.org/10.1093/jxb/eru004
Ekka P, Shilky, Baishya R, Saikia P (2024) Ecological analyses of street trees of Indian cities to achieve United Nations sustainable development goals. Ecol Front. https://doi.org/10.1016/j.ecofro.2024.03.003
Gupta GP, Kumar B, Kulshrestha UC (2016) Impact and pollution indices of urban dust on selected plant species for green belt development: mitigation of the air pollution in NCR Delhi, India. Arab J Geosci 9:1–15. https://doi.org/10.1007/s12517-015-2226-4
Hariram M, Sahu R, Elumalai SP (2018) Impact assessment of atmospheric dust on foliage pigments and pollution resistances of plants grown nearby coal based thermal power plants. Arch Environ Contam Toxicol 74:56–70. https://doi.org/10.1007/s00244-017-0446-1
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466. https://doi.org/10.4161/psb.21949
Hewitt CN, Ashworth K, MacKenzie AR (2020) Using green infrastructure to improve urban air quality (GI4AQ). Ambio 49:62–73. https://doi.org/10.1007/s13280-019-01164-3
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57(12):1332–1334. https://doi.org/10.1139/b79-163
Hossain MS, Frey HC, Louie PK, Lau AK (2021) Combined effects of increased O3 and reduced NO2 concentrations on short-term air pollution health risks in Hong Kong. Environ Pollut 270:116280. https://doi.org/10.1016/j.envpol.2020.116280
Husen A (2021) Morpho-anatomical, physiological, biochemical and molecular responses of plants to air pollution. Harsh Environment and Plant Resilience: Molecular and Functional Aspects, pp 203–234. https://doi.org/10.1007/978-3-030-65912-7_9
Joshi PC, Swami A (2009) Air pollution induced changes in the photosynthetic pigments of selected plant species. J Environ Biol 30(2):295–298
Junior DPM, Bueno C, da Silva CM (2022) The effect of urban green spaces on reduction of particulate matter concentration. Bull Environ Contam Toxicol 108(6):1104–1110
Karmakar D, Deb K, Padhy PK (2021) Ecophysiological responses of tree species due to air pollution for biomonitoring of environmental health in urban area. Urban Clim 35:100741. https://doi.org/10.1016/j.uclim.2020.100741
Katoch A, Kulshrestha UC (2022) Assessment of indoor air pollution through fine particle capturing potential and accumulation on plant foliage in Delhi, India. Aerosol and Air Quality Research 22(9):220014
Kaur M, Nagpal AK (2017) Evaluation of air pollution tolerance index and anticipated performance index of plants and their application in development of green space along the urban areas. Environ Sci Pollut Res 24:18881–18895. https://doi.org/10.1007/s11356-017-9500-9
Leonard RJ, McArthur C, Hochuli DF (2016) Particulate matter deposition on roadside plants and the importance of leaf trait combinations. Urban For Urban Green 20:249–253. https://doi.org/10.1016/j.ufug.2016.09.008
Li L, Mu G (2021) Similar effects as shade tolerance induced by dust accumulation and size penetration of particulates on cotton leaves. BMC Plant Biol 21:1–13. https://doi.org/10.1186/s12870-021-02926-6
Lisko KA, Aboobucker SI, Torres R, Lorence A (2014) Engineering elevated vitamin C in plants to improve their nutritional content, growth, and tolerance to abiotic stress. Phytochemicals-Biosynthesis Funct Appl 44:109–128. https://doi.org/10.1007/978-3-319-04045-5_6
Liu YJ, Ding HUI (2008) Variation in air pollution tolerance index of plants near a steel factory: implication for landscape-plant species selection for industrial areas. WSEAS Trans Environ Dev 4(1):24–32
Livesley SJ, McPherson EG, Calfapietra C (2016) The urban forest and ecosystem services: impacts on urban water, heat, and pollution cycles at the tree, street, and city scale. J Environ Qual 45(1):119–124. https://doi.org/10.2134/jeq2015.11.0567
Malik A, Aggarwal SG, Ohata S, Mori T, Kondo Y, Sinha PR, Patel P, Kumar B, Singh K, Soni D, Koike M (2022) Measurement of black carbon in Delhi: evidences of regional transport, meteorology and local sources for pollution episodes. Aerosol Air Qual Res 22(8):220128. https://doi.org/10.4209/aaqr.220128
Manisha ESP, Pal AK (2014) Dust arresting capacity and its impact on physiological parameter of the plants. Strategic technologies of complex environmental Issues-A sustainable approach, pp 111–115
Mattioli R, Costantino P, Trovato M (2009) Proline accumulation in plants: not only stress. Plant Signal Behav 4(11):1016–1018. https://doi.org/10.4161/psb.4.11.9797
Mei P, Malik V, Harper RW, Jiménez JM (2021) Air pollution, human health and the benefits of trees: a biomolecular and physiologic perspective. Arboricultural J 43(1):19–40. https://doi.org/10.1080/03071375.2020.1854995
Menon JS, Sharma R (2021) Nature-based solutions for co-mitigation of air pollution and urban heat in Indian cities. Front Sustain Cities 3:705185. https://doi.org/10.3389/frsc.2021.705185
Mondal S, Singh G (2022) Air pollution tolerance, anticipated performance, and metal accumulation capacity of common plant species for green belt development. Environ Sci Pollut Res 29(17):25507–25518. https://doi.org/10.1007/s11356-021-17716-8
Najafi ZM, Mosleh Arani A, Etesami H (2023) The importance of plant growth-promoting rhizobacteria to increase air pollution tolerance index (APTI) in the plants of green belt to control dust hazards. Front Plant Sci 14:1098368. https://doi.org/10.3389/fpls.2023.1098368
Nayak D, Patel DP, Thakare HS, Satashiya K, Shrivastava PK (2015) Evaluation of air pollution tolerance index of trees. Res Environ Life Sci 8(1):7–10
Origin V (2019) OriginLab Corporation. Northampton, MA, USA
Pandit J, Sharma AK (2020) A review of effects of air pollution on physical and biochemical characteristics of plants. Int J Chem Stud 8:1684–1688. https://doi.org/10.22271/chemi.2020.v8.i3w.9442
Patanè C, Cosentino SL, Romano D, Toscano S (2022) Relative water content, proline, and antioxidant enzymes in leaves of long shelf-life tomatoes under drought stress and rewatering. Plants 11(22):3045. https://doi.org/10.3390/plants11223045
Patel K, Chaurasia M, Rao KS (2023) Urban dust pollution tolerance indices of selected plant species for development of urban greenery in Delhi. Environ Monit Assess 195(1):16. https://doi.org/10.1007/s10661-022-10608-5
Prajapati SK, Tripathi BD (2008) Seasonal variation of leaf dust accumulation and pigment content in plant species exposed to urban particulates pollution. J Environ Qual 37(3):865–870. https://doi.org/10.2134/jeq2006.0511
Prusty BAK, Mishra PC, Azeez PA (2005) Dust accumulation and leaf pigment content in vegetation near the national highway at Sambalpur, Orissa, India. Ecotoxicol Environ Saf 60(2):228–235. https://doi.org/10.1016/j.ecoenv.2003.12.013
Roy A, Bhattacharya T, Kumari M (2020) Air pollution tolerance, metal accumulation and dust capturing capacity of common tropical trees in commercial and industrial sites. Sci Total Environ 722:137622. https://doi.org/10.1016/j.scitotenv.2020.137622
Sadasivam S, Balasubramanian T (1987) Practical manual in Biochemistry. TNAU, Coimbatore, p 14
Sanaeirad H, Majd A, Abbaspour H, Peyvandi M (2017) The effect of air pollution on proline and protein content and activity of nitrate reductase enzyme in Laurus nobilis L. plants. J Mol Biol Res 7(4):99–105. https://doi.org/10.5539/JMBR.V7N1P99
Sarkar S, Mondal K, Sanyal S, Chakrabarty M (2021) Study of biochemical factors in assessing air pollution tolerance index of selected plant species in and around Durgapur industrial belt, India. Environ Monit Assess 193(8):474. https://doi.org/10.1007/s10661-021-09253-1
Sen S, Guchhait SK (2021) Urban green space in India: perception of cultural ecosystem services and psychology of situatedness and connectedness. Ecol Ind 123:107338. https://doi.org/10.1016/j.ecolind.2021.107338
Shah K, Amin NU, Ahmad I, Shah S, Hussain K (2017) Dust particles induce stress, reduce various photosynthetic pigments and their derivatives in Ficus benjamina: a landscape plant. Int J Agric Biol 19(6):1469–1474
Shrestha S, Baral B, Dhital NB, Yang HH (2021) Assessing air pollution tolerance of plant species in vegetation traffic barriers in Kathmandu Valley, Nepal. Sustain Environ Res 31:1–9. https://doi.org/10.1186/s42834-020-00076-2
Sicard P, Agathokleous E, Araminiene V, Carrari E, Hoshika Y, De Marco A, Paoletti E (2018) Should we see urban trees as effective solutions to reduce increasing ozone levels in cities? Environ Pollut 243:163–176. https://doi.org/10.1016/j.envpol.2018.08.049
Singh SK, Rao DN, Agrawal M, Pandey J, Naryan D (1991) Air pollution tolerance index of plants. J Environ Manag 32(1):45–55. https://doi.org/10.1016/S0301-4797(05)80080-5
Singh AK, Kumar M, Bauddh K, Singh A, Singh P, Madhav S, Shukla SK (2023) Environmental impacts of air pollution and its abatement by plant species: acomprehensive review. Environmental Science and Pollution Research 30(33):79587–79616
Skrynetska I, Karcz J, Barczyk G, Kandziora-Ciupa M, Ciepał R, Nadgórska-Socha A (2019) Using Plantago major and Plantago lanceolata in environmental pollution research in an urban area of Southern Poland. Environ Sci Pollut Res 26:23359–23371. https://doi.org/10.1007/s11356-019-05535-x
Smirnoff N (1996) Botanical briefing: the function and metabolism of ascorbic acid in plants. Ann Bot 78(6):661–669. https://doi.org/10.1006/anbo.1996.0175
Swami A, Chauhan D (2015) Impact of air pollution induced by automobile exhaust pollution on air pollution tolerance index (APTI) on few species of plants. Science 4(3):342–343
Swapnil P, Meena M, Singh SK, Dhuldhaj UP, Marwal A (2021) Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr Plant Biol 26:100203. https://doi.org/10.1016/j.cpb.2021.100203
Tambussi EA, Bartoli CG, Beltrano J, Guiamet JJ, Araus JL (2000) Oxidative damage to thylakoid proteins in water-stressed leaves of wheat (Triticum aestivum). Physiol Plant 108(4):398–404. https://doi.org/10.1034/j.1399-3054.2000.t01-1-100409.x
Tripathi DP, Nema AK (2023) Seasonal variation of biochemical parameters and air pollution tolerance index (APTI) of selected plant species in Delhi city, and detailed meta-analysis from Indian metropolitan cities. Atmospheric Environ 309:119862. https://doi.org/10.1016/j.atmosenv.2023.119862
Vogel S (1989) Drag and reconfiguration of broad leaves in high winds. J Exp Bot 40(8):941–948. https://doi.org/10.1093/jxb/40.8.941
Wahab A, Abdi G, Saleem MH, Ali B, Ullah S, Shah W, Mumtaz S, Yasin G, Muresan CC, Marc RA (2022) Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: a comprehensive review. Plants 11(13):1620. https://doi.org/10.3390/plants11131620
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J Plant Physiol 144(3):307–313
WHO (2017) Urban Green Space Interventions and Health. [online] World Health Organization. Available at: https://cdn.who.int/media/docs/librariesprovider2/euro-health-topics/environment/urban-green-space-intervention.pdf?sfvrsn=a2e135f3_1&download=true. Accessed on 19 Mar. 2024
Xie X, He Z, Chen N, Tang Z, Wang Q, Cai Y (2019) The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res Int 2019. https://doi.org/10.1155/2019/9732325
Yadav A, Dixit A, Singh D (2023) Estimation of air pollution tolerance index of plants in selected locations in Kanpur City, India. Mater Today: Proc. https://doi.org/10.1016/j.matpr.2023.04.222
Yarnvudhi A, Leksungnoen N, Andriyas T, Tor-Ngern P, Premashthira A, Wachrinrat C, Marod D, Hermhuk S, Pattanakiat S, Nakashizuka T, Kjelgren R (2022) Assessing the cooling and air pollution tolerance among urban tree species in a tropical climate. Plants 11(22):3074. https://doi.org/10.3390/plants11223074
Zhang T, Yu LX, Zheng P, Li Y, Rivera M, Main D, Greene SL (2015) Identification of loci associated with drought resistance traits in heterozygous autotetraploid alfalfa (Medicago sativa L.) using genome-wide association studies with genotyping by sequencing. PLoS one 10(9):e0138931. https://doi.org/10.1371/journal.pone.01389
Zhang JJ, Adcock IM, Bai Z, Chung KF, Duan X, Fang Z, Gong J, Li F, Miller RK, Qiu X, Rich DQ (2019) Health effects of air pollution: what we need to know and to do in the next decade. J Thorac Dis 11(4):1727. https://doi.org/10.21037/jtd.2019.03.65
Acknowledgements
The authors express their sincere gratitude to the Department of Botany, University of Delhi for providing support for conducting experiments. The authors are also thankful to the DST-FIST Program of the Department of Environmental Sciences, Central University of Jharkhand (CUJ), Ranchi sponsored by DST, Govt. of India (Ref. No. SR/FST/ES-I/2019/55 (C) dated 25th March 2021).
Funding
Financial support in the form of an institutional fellowship was provided by the CUJ to Shilky to carry out her Ph.D. research.
Author information
Authors and Affiliations
Contributions
S participated in the field data collection, analyses and interpretation and drafted the manuscript under the supervision of RB and PS; PS conceived the study and also contributed to finalizing the manuscript; and RB contributed to finalizing the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shilky, Baishya, R. & Saikia, P. Identification of urban street trees for green belt development for optimizing pollution mitigation in Delhi, India. Environ Sci Pollut Res 31, 54962–54978 (2024). https://doi.org/10.1007/s11356-024-34802-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34802-9