Abstract
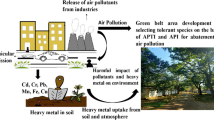
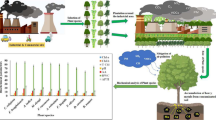
Green vegetation enrichment is a cost-effective technique for reducing atmospheric pollution. Fifteen common tropical plant species were assessed for identifying their air pollution tolerance, anticipated performance, and metal accumulation capacity at Jharia Coalfield and Reference (JCF) site using Air Pollution Tolerance Index (APTI), Anticipated Performance Index (API), and Metal Accumulation Index (MAI). Metal accumulation efficiencies were observed to be highest for Ficus benghalensis L. (12.67mg/kg) and Ficus religiosa L. (10.71 mg/kg). The values of APTI were found to be highest at JCF for F. benghalensis (APTI: 25.21 ± 0.95), F. religiosa (APTI: 23.02 ± 0.21), Alstonia scholaris (L.) R. Br. (APTI: 18.50 ± 0.43), Mangifera indica L. (APTI: 16.88 ± 0.65), Azadirachta indica A. Juss. (APTI: 15.87 ± 0.21), and Moringa oleifera Lam. (APTI: 16.32 ± 0.66). F. benghalensis and F. religiosa were found to be excellent performers to mitigate air pollution at JCF as per their API score. Values of MAI, APTI, and API were observed to be lowest at reference sites for all the studied plant species due to absence of any air polluting sources. The findings revealed that air pollution played a significant impact in influencing the biochemical and physiological parameters of plants in a contaminated coal mining area. The species with the maximum MAI and APTI values might be employed in developing a green belt to minimize the levels of pollutants into the atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, air quality in metropolitan cities of developed nations has degraded significantly due to massive rise in vehicular transportation, fossil fuel combustion in industries, coal mining activities, and the loss of vegetation cover (Shrivastava et al. 2013; Pandey et al. 2014; Amegah and Agyei-Mensah 2017; Jena et al. 2019; Mondal et al. 2020). Vehicular emissions and mining activities are regarded as significant sources of air pollution such as particulate matter, CO, SOx, NOx, trace elements, and polycyclic aromatic hydrocarbons (PAHs) (Jena and Singh 2017; Mondal and Singh 2021a, 2021b). The exposure of those contaminants into the environment not only degrades the quality of the atmospheric air, but also poses danger to humans causing asthma, bronchitis, chronic obstructive pulmonary disease (COPD), cardiac arrest, ventricular fibrillation, lung cancer, and several other cardiovascular diseases (Pope 2002; IARC 2016; Cadelis et al. 2014; Goudarzi et al. 2017; Al-hemoud et al. 2018; Pope et al. 2020).
In addition to this, numerous experts have investigated the negative impacts of air pollution on plants all over the world (Sadeghian and Mortazaienezhad 2012; Govindaraju et al. 2012; Pandey et al. 2014; Zhang et al. 2016). As green belts are efficient means to mitigate air emissions by trapping particulate matter and capturing gaseous contaminants, environmental activists and policymakers therefore stressed the importance of a permanent green belt in and near urban areas, and along highways, to decrease the consequences of poisonous fine particulates (Sharma et al. 2017; Karmakar and Padhy 2019; Alotaibi et al. 2020) and to use as bioindicator of air pollution (Gupta et al. 2011). Plants can eradicate air pollution by three mechanisms: leaf absorption, particulate accumulation on leaf surfaces, and particulate deposition on the leeward side of the leaf due to the wide surface area of the leaves, which acts as a sink (Prajapati and Tripathi 2008a, b; Roy et al. 2020; Javanmard et al. 2020). After penetration, the pollutants block the stomata and causes a decrease in the foliar pH due to the prevalence of sulfate (SO42-) and nitrate (NO3-) ion contents in the dust fall (Gupta et al. 2016). Leaves of plants serve as an environmental sink as they provide wide surface area for trapping and accumulation of air pollutants (Rai 2016). Leaves are one of the most effective pollutant-trapping systems through absorption and diffusion of particulates and gaseous pollutants (HF, SO2, NOx, and other trace elements) (Balasubramanian et al. 2018). However, air pollutants, on the other hand, may have a negative impact on plant growth by affecting the biochemical parameters, photosynthetic activity, morphological characteristics, and seed germination (Kaur and Nagpal 2017). Many plants can adapt to the changing environments through adaptations in biochemical parameters, especially in chlorophyll, ascorbic acid, leaf pH, and relative water content. The changes in the above biochemical parameters can be taken into account in estimating the Air Pollution Tolerance Index (APTI) of plant species while classifying the plant species as sensitive, intermediate, or tolerant to air pollutants (Rai and Panda 2014a, b; Molnár et al. 2020).
The Air Pollution Tolerance Index (APTI) is regarded as an effective method for identifying bioindicator plants, which is calculated by four biochemical parameters such as ascorbic acid (AA), total chlorophyll content (TCH), leaf extract pH (pH), and relative water content (RWC) (Pandey et al. 2015; Kaur and Nagpal 2017). AA serves as a coenzyme in the metabolism of carbohydrates, fats, and proteins, as well as the production of nucleic acid in RNA, in photosynthetic activity, and growth of plants (Mazher et al. 2011). TCH, as one of the most important components of energy generation in green plants, clearly impacts plant health, which is greatly influenced by several environmental factors (Agbaire and Akporhonor 2014). RWC is a key determinant of plant protoplasmic permeability. As a result, plants with greater RWC levels could be more tolerant to air pollutants (Nayak et al. 2015). The pH of plants is strongly related to air pollution, particularly sulfur dioxide. Plants having lower pH seem to be more sensitive and with pH approaching 7 are more tolerant (Swami and Chauhan 2015).
Plants with higher APTI values are regarded as the most tolerant of polluted environments and are ideal for the establishment of green belts, while plants with lower APTI values are the most sensitive and serve as bioindicator species of air pollution (Roy et al. 2020). However, many studies have used a more comprehensive index called Anticipated Performance Index (API) which is used for quantifying environmental performance of plant species using APTI and socio-economic values of the specific plant species (Pandey et al. 2015) to indicate the suitability of plants for green belt development (Patel and Kumar 2018; Banerjee et al. 2021).
In recent times, Jharia Coalfield (JCF) is facing elevated levels of atmospheric pollution due to increased vehicular load, unscientific mining activities, loading and unloading of coal in the mines, lack of proper air pollution mitigation techniques, and loss of natural vegetation throughout the city of Dhanbad. This paper attempts to study the rate of bioaccumulation of trace elements (Cd, Cr, Fe, Mn, Zn, Ni, Pb, and Zn) on fifteen selected plant species at JCF using Metal Accumulation Index. Air Pollution Tolerance Index (APTI) and Anticipated Performance Index (API) were calculated to determine air pollution–tolerant plant species for developing efficient green belt in urbanized areas to improve the air quality for landscaping and urban planning (Kaur and Nagpal 2017; Sharma et al. 2019).
Materials and methods
Description of the study area
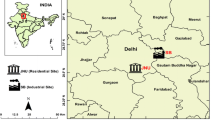
The present study was carried out at JCF, one of India’s largest coalfields, located in the heart of the Damodar River Valley, which encompasses an area of 450 m2 (Fig. 1). Drilling, blasting, associated mining activities, mine fire, and vehicular load at the coal mines contribute significantly to a higher concentration of air pollutants around JCF. A total of nine locations were selected around the coal mining areas of JCF for sampling of leaves, viz., Tetulmari PS (S1), Loyabad PS (S2), Lodhna PS (S3), Kenduadih PS (S4), Bastacolla Colliery (S5), Sijua Stadium (S6), Jogta 14 pit (S7), Katras (S8), and Kujama Colliery (S9). A reference site, IIT(ISM) Dhanbad (S10), was selected for comparing the biochemical and physiological changes that occur in vegetation community structure. Elevated concentration of primary air pollutants like PM10, PM2.5, SO2, and NOx (exceeds the permissible limit of National Ambient Air Quality Standard) was observed at JCF, as a result of which environmental quality gets deteriorated. The concentration of particulate matter appears highest during winter owing to lower wind speed (on average 3 km/h), lower temperature (on average 17̊ C), and lower pollutant scattering, while it remains lowest throughout post-monsoon owing to higher moisture content and rainfall during the monsoon season (Mondal et al. 2020).
Selection of plant species
Leaf samples of fifteen plant species, namely, Ficus religiosa L. (P1), Mangifera indica L. (P2), Alstonia scholaris (L.) R. Br. (P3), Azadirachta indica A. Juss. (P4), Moringa oleifera Lam. (P5), Ficus benghalensis L. (P6), Carica papaya L. (P7), Psidium guajava L. (P8), Ziziphus jujuba Mill. (P9), Hibiscus rosa sinensis L. (P10), Lantana indica Roxb. (P11), Bougainvillea spectabilis Willd. (P12), Rosa indica L. (P13), Calotropis gigantea (L.) W.T. Aiton (P14), and Catharanthus roseus (L.) G. Don. (P15), were collected during January 2019 to January 2020 by ensuring rainfall and temperature conditions. These fifteen species are found at both the study area, i.e., the coal mining areas of JCF and reference site, IIT(ISM) Dhanbad. Using polyethylene gloves and stainless steel scissors, samples of fully grown (mature) leaves were obtained in three replicates from different sides of the individual plant species (Alotaibi et al. 2020). The samples were then instantly carried to the lab in a heatproof box and washed with tap water and distilled water and stored at − 20 °C for further analysis.
Experimental analysis
Leaf area measurement
Fully mature fresh leaves after collection from the plant species were cleansed delicately. The estimated leaf area (cm2) of ten leaves per tree was calculated in triplicate with a leaf area meter, as explained by Leghari and Zaidi (2013).
Analysis of trace elements
The crushed leaf samples were steeped in a 5:1:1 mixture of tri acids, namely, HNO3, HClO4, and H2SO4, using the method of Allen et al. (1986). The blank was also prepared without the sample. The trace elements (Fe, Mn, Cu, Zn, Cd, Cr, and Pb) were estimated using Atomic Absorption Spectroscopy (GBC, Model Avanta).
Calculation of Metal Accumulation Index (MAI)
To optimize the relative performance of the plants in terms of metal accumulation, the Metal Accumulation Index (MAI) was formulated as follows:
where N is the total number of metals analyzed and Ij = x/dx is the sub-index for variable j, obtained by dividing the mean value (x) of each metal by its standard deviation (dx) (Liu et al. 2007).
Quality control and assurance
Glassware was immersed in 30% HCL and thoroughly cleaned with deionized water, followed by a precision and accuracy recovery investigation. Reagents used were of analytical grade. All tests were performed in triplicate, and the findings given are the average. For each trace element, the analytical accuracy was more than 5%, as measured by the relative standard deviation (RSD). The analytical accuracy and repeated sample analysis error for replicate samples were both within acceptable ranges. The percentage of recovery varied from 91.8to 97.2 percent.
Estimation of pH of leaf extract
Leaf samples of 0.25gms were homogenized in 10 ml double distilled water and centrifuged at 5000 rpm for 5 min, and the pH content of the leaf extract is estimated from the supernatant using pH meter (Karmakar and Padhy 2019).
Estimation of total chlorophyll content (TCH) of leaf extract
The total chlorophyll content of the leaf extract was estimated using the method introduced by Arnon (1949). Leaf samples of 0.25gms were crushed and homogenized with 10 ml of 80% acetone and centrifuged at 5000 rpm for 5 min. The Supernatant was volume makeup up to 25ml with 80% acetone. The optical density of the leaf extract was calculated at 645 nm and 663 nm using a UV-visible spectrophotometer. The total chlorophyll content (mg/g) of the leaves was calculated according to the following formulae:
Estimation of relative water content (RCH) of leaves
RCH of leaf samples were calculated by the given formula, explained by Henson et al. (1981).
Fresh leaves after taking to the laboratory were washed properly with distilled water. After removing excess water, fresh weights of the leaves are taken. After that, turgid weights of the leaves were taken by immersing them in distilled water overnight. Then, the leaves were blotted and dried at 115 °C for 2 h, to calculate the dry weight
Estimation of ascorbic acid content (AA) of leaf extract
Ascorbic acid content of the leaf samples was estimated using the method explained by Keller and Schwager (1977). Fresh leaves were homogenized with a mixture of oxalic acid and EDTA, and the supernatant was collected. Titration was then done with 2,6-dichlorophenol indophenol until the solution turns pink. The optical density of the solution was measured at 520 nm before (Es) and after adding one drop of ascorbic acid to the solution (Et). At the same time, the optical density of 2,6-dichlorophenol indophenol was also measured at the same wavelength (Eo). The concentration of ascorbic acid was calculated by the given formula after preparing a standard curve with the same method.
where W = weight of the fresh leaves, V = total volume of the mixture, and the value of [Eo – (Es – Et)] was evaluated by the standard curve.
Calculation of Air Pollution Tolerance Index (APTI)
APTI was calculated by the following equation, as explained by Singh and Rao (1983).
where A = ascorbic acid content of the leaves, T = total chlorophyll content, P = pH of leaf extract, and R = relative water content.
Calculation of Anticipated Performance Index (API)
API determines the performance of plant species, with respect to APTI values, and different biological attributes of the plant species like plant habitat, plant type, leaf size, canopy structure, and structure of laminar and economic values (Supplementary Table 1). Each plant is assigned a grade (+ or −) based on these characteristics, and they are scored (%) accordingly. Apart from that, plant species classification was done using the API score, which was calculated using the formula:
According to Govindaraju et al. (2012), plants with API score (%) ≤ 30 and 0 grade are classified under “not recommended” category, whereas plants with API score (%) 31–40 and grade 1 are categorized under “very poor” category. Similarly, plants scoring API (%) within 41–50 and grade 2 are categorized under “poor” category, and plants with API score (%) within 51–60 and grade 3 are categorized under “moderate” category. API score (%) of grade 4 is equal to 61–70, plants falling within this range come under “good” category, API (%) of grade 5 is equal to 71–80 and category of “very good,” API (%) of grade 6 is equal to 81–90 and category of “excellent,” and API (%) of grade7 is equal to 91–100 and comes under “best” category (Supplementary Table 2).
Results and discussion
Leaf area reduction
Figure 2 depicted the % of reduction change in affected leaf area (cm2) for leaves of selected plant species in the coal mining areas of JCF when compared with the reference site, IIT(ISM) Dhanbad. Leaves from the sampling sites were significantly smaller than the leaves of the reference site, indicating significant influence of atmospheric pollutants on the growth of plants. Among all the studied plant species, the lowest leaf area reduction was observed for F. benghalensis (10.56%), while the highest reduction was observed for C. roseus (34.12%). Similar findings like reduction in leaf area were also observed at Riyadh City, Saudi Arabia, for Ziziphus spina-christi and Prosopis juliflora (Alotaibi et al. 2020), indicating a significant impact of air pollutants on the leaf growth of sensitive species. Reduced leaf surface and photosynthetic activity were caused due to absorbance of atmospheric radiation resulting in decreased stomatal conductance, and chlorophyll content (Honour et al., 2009; Tiwari et al., 2006).
Concentration of heavy metal within the leaves
Mean concentration of trace elements along with their standard error is depicted in Table 1. Among all other metals, Mn and Fe concentrations were greater in the leaves of all the fifteen plants studied. It has been identified that plant foliage in the plant species of Jharia Coalfield (JCF) was richer in Fe, Mn, and Pb than Cd, Cr, Cu, and Zn. All the studied trace elements were observed higher at JCF than the reference site in the order of Mn >Fe > Pb >Cu > Cr >Zn > Cd. Two-way ANOVA revealed a statistically significant difference (p < 0.05) in all the seven trace element concentrations between JCF and reference sites for the studied plant species.
The concentration of trace elements in plant leaves varies depending on the plant type and specific location. Metal absorption and accumulation by plant species are affected by a variety of characteristics, including species, metal present, and ecotype. Specimens of the same ecotype that live in the same region might sometimes act differently. Thus, in the study of polymetallic pollution, studies of the entire plant communities in the polluted region are more trustworthy than utilizing a single species representative (Karmakar and Padhy 2019). Reference values are required for interpreting the field data and the assessment of pollution (Remon et al. 2013). The International Commission on Radiological Protection (ICRP) developed the term “Reference Man,” which has been adopted to assess the human body’s exposure to radiation via extrinsic or intrinsic origins (Markert 1992). In the same way, “Reference Plant” represents the average concentration of the inorganic elements present in plants. As per Markert (1992), the preceding values might be regarded for concentration of trace elements present in plants from an unpolluted environment: 0.05 mg/kg for Cd, 1.50 mg/kg for Cr, 10mg/kg for Cu, 150mg/kg for Fe, 200mg/kg for Mn, 1mg/kg for Pb, and 50 mg/kg for Zn.
Trace elements were emitted into the urbanized environment directly or indirectly by coal mine fire and other mining activity (Dubey et al. 2012; Roy et al. 2019). The primary causes of Cd and Cr in polluted air at JCF include spontaneous combustion of coal seams in the mines and adjacent coal-fired power plants (Mondal et al. 2020), traffic congestion, and burning of fossil fuels (Gope et al. 2017). Mn, Cu, and Zn were released into the atmosphere due to mining activities like drilling, blasting, and substantial transportation load at the mining sectors (Liu et al. 2016; Pang et al. 2016). Re-suspension of Pb-rich dust from heavy vehicles in the mining areas of JCF was the major reason for a higher concentration of Pb in the environment, whereas Fe (one of the crustal element) was emitted into the atmosphere due to the mining of coal at the sampling sites of JCF (Mondal and Singh 2021a, b).
Similarly, in a study done at Oleśnica, Poland, a higher concentration of Fe (92mg/kg) and Mn (146mg/kg) was reported for plant species Robinia pseudoacacia (Samecka-Cymerman et al. 2009). Likewise, Nadgórska-Socha et al. (2017) also observed higher concentrations of Pb, Fe, and Mn in the leaves of B. pendula (16.02mg/kg) and R. pseudoacacia (11.08mg/kg). Contrastingly, Hu et al. (2014) observed a much lower concentration of Cr than that found during the present study for certain plant species like Ailanthus altissima (0.24mg/kg), Salix matsudana var. matsudana (0.22mg/kg), Picea asperata (0.21mg/kg), Robinia pseudoacacia (0.09mg/kg), and Ulmus pumila (0.12mg/kg) in Yan'an, China.
Metal Accumulation Index (MAI)
The Metal Accumulation Index (MAI) is a standardized method used to measure the heavy metal accumulating efficiency in plants (Hu et al. 2014; Khalid et al. 2019) and is presented in Table 2. The index is governed by a range of parameters, including local air quality, microclimate, sample altitude (tree), sampling time, and plant features (Hu et al. 2014). In the present study, the MAI value was highest in F. benghalensis (12.67) at JCF followed by F. religiosa (10.71 mg/kg), M. indica (7.32 mg/kg), A. scholaris (7.10 mg/kg), A. indica (6.74 mg/kg), and M. oleifera (6.58 mg/kg) whereas lower MAI values were reported for C. roseus (5.29mg/kg) and C. gigantea (5.40mg/kg). Similarly, Roy et al. (2020) also reported higher MAI values for F. benghalensis and F. religiosa at Jharkhand indicating their higher metal accumulating capacity. The plant species with higher MAI values act as good phyto-extractors and therefore may be used as barriers between polluted and vulnerable areas (Hu et al. 2014).
pH
Variability in pH values was noted among species (Table 2), which signifies the differences in responses among each plant species for a particular pollutant in a polluted environment (Kaur and Nagpal 2017). Comparison in pH values was estimated between the coal mining areas of JCF and the reference site. The values of pH at reference sites were significantly lower than the sites at coal mining areas of JCF for all the plant species. pH values of all the plant species at JCF ranged between 5.00 and 9.00, with the lowest value observed for C. roseus (5.07 ± 0.43), whereas the highest value was observed for F. religiosa (8.68 ± 0.43). Similarly, higher pH values for plant species were also observed by Govindaraju et al. (2012) (8.49; Ficus benjamina), as well as by Karmakar and Padhy (2019) (6.31; Azadirachta indica). In the study area, higher pH values of F. religiosa (8.68), F. benghalensis (8.60), A. scholaris (7.85), C. papaya (6.76), M. oleifera (6.60), and A. indica (6.50), indicated more tolerance towards air pollution even in a polluted atmosphere. However, plants with lower pH values are adversely affected by air pollution due to their higher stomatal sensitivity (Dhankar et al. 2015).
Relative water content (%)
The results obtained revealed that the values of RWC (as a percentage) varied across 15 different plant species (Table 2), the highest being observed at the reference site when compared to the sites of JCF. Among all the species, RWC was found to be highest for F. benghalensis and lowest for C. roseus at sites of JCF and reference, respectively. Apart from these, RWC of plants like F. religiosa, M. indica, A. scholaris, and M. oleifera were found to be above 90% in the study area. Leaf water content is responsible for growth, transpiration, respiration, and maintenance of physiological balance (Dhankhar et al. 2015; Kaur and Nagpal 2017); as a result, higher RWC enhance the resistivity of the plants during drought (Geravandi et al. 2011). As RWC is directly related to protoplasmic permeability, a higher proportion of RWC in plants improves resistance and enables the plant more tolerant of stress caused by polluted air (Jyothi and Jaya 2010; Gupta et al. 2016). Similar findings were also observed in previous studies including those of Krishnaveni (2018) and Rathore et al. (2018).
Total chlorophyll content
The variation in chlorophyll content of leaves of 15 different plant species at the sites of JCF and the reference is depicted in Table 2. The chlorophyll content of leaves observed in the plants of reference site was found to be much higher than the sites of JCF for all the studied plant species. The findings of this study were consistent with the findings of a recent study done by Alotaibi et al. (2020), who reported that the TCH contents of five plant species (A. lebbeck, E. camaldulensis, F. altissima, P. juliflora, and Z. spinachristi) decreased as the concentration of pollution increased. Gaseous pollutants (SO2, NO2, and particulate matter), high temperature, drought, particulate matter deposition, and soil pollution can all lower chlorophyll concentration in plants (Karmakar et al. 2016). Chlorophyll, present in leaves, trap sunlight from the atmosphere and convert it into chemical energy. A decrease in TCH content is related to accumulation of particulate matter in the leaf surface, which ultimately leads to blockage of stomatal pores and decrease in photosynthetic productivity (Rai 2016).
Ascorbic acid content
Significant variation in ascorbic acid content was observed among the studied plant species at JCF and reference sites (Table 2). Higher ascorbic acid content was observed at the sites of JCF when compared with reference site. From the results, it is observed that F. benghalensis showed the highest ascorbic acid content (14.11 ± 0.32), followed by F. religiosa (12.32 ± 0.54), L. indica (9.56 ± 0.42), A. scholaris (9.34 ± 0.28), and M. indica (9.08 ± 0.23), whereas the lowest was found for C. roseus (5.89 ± 0.52) and C. gigantea (6.09 ± 0.32). The higher ascorbic acid content recorded in leaves of polluted locations showed the tolerance of plant species to their developing environment’s heightened air pollution levels (Zhang et al. 2016; Yadav and Pandey 2020). Similarly, a study conducted at Riyadh City, Saudi Arabia, by Alotaibi et al. (2020) identified higher ascorbic acid content for Ziziphus. spinachristi (84.6mg/g), Albizia. lebbeck (47mg/g), Eucalyptus camaldulensis (29.1mg/g), Ficus altissima (24.8mg/g), and Prosopis. juliflora (16mg/g) due to elevated environmental stress (sites adjacent to cement factory). According to Palit et al. (2013), in stressful environmental conditions, ascorbic acid acts as a strong, low-molecular-weight antioxidant and maintains the integrity of cell division as well as cell membranes in plants. Furthermore, ascorbic acid can mitigate the detrimental effects of air pollution; hence, it serves an important role in establishing plant tolerance to air pollution.
Air Pollution Tolerance Index (APTI)
Four biochemical parameters, viz., total chlorophyll, pH, ascorbic acid, and relative water content, were used to calculate the APTI of an individual plant species (Table 2). APTI investigates the vulnerability of particular plant species to air pollution (Singh et al. 1991). Higher values of APTI of the plant species were found at sites of JCF, whereas lower APTI values were found at the reference site. This finding is confirmed by equivalent research published in the literature (Karmakar and Padhy 2019). The potential of a plant species to withstand air pollution may be assessed using the APTI. Plants having a higher APTI value are regarded tolerant to air pollution and may be utilized to reduce pollution levels. The species with a lower index (sensitive species) on the other hand might be utilized as an indicator of air pollution (Singh and Rao 1983). According to the values of APTI, in the present study, F. benghalensis (25.21 ± 0.95) was found to be the most tolerant, followed by F. religiosa (23.02 ± 0.21). Similar findings were also reported at various cities in India by Yadav and Pandey (2020) (Bathinda City, Punjab), Roy and Bhattacharya (2020) (Dhanbad and Bokaro City, Jharkhand), Sahu et al. (2020) (Sambalpur, Odisha), Rai and Panda (2014a, b) (Aizawl, Mizoram), Prajapati and Tripathi (2008a, b) (Varanasi, Uttar Pradesh), and Mondal et al. (2011) (Burdwan, West Bengal) indicating high pollution tolerance of F. benghalensis and F. religiosa due to their higher APTI scores. Contrastingly, C. roseus (11.63 ± 0.87) and C. gigantea (11.87 ± 0.34) were found to be sensitive to air pollution due to their lower APTI scores.
As plants with high pollution tolerance can aid with pollutant accumulation, planting pollution-tolerant species to preserve contaminated areas is a long-term solution to satisfy industrial and commercial expansion (Mok et al. 2013). Depending on the geographical region, limited volatile organic compound emission, least pollen production, and soil properties, the same plant species might react differently, such as being sensitive, intermediate, or tolerant (Raza et al. 1985; Beckett et al. 1998; Yang et al. 2005).
Anticipated Performance Index (API)
Plants react differently to various contaminants. The classification of plants as tolerant or sensitive based on a single parameter (such as total chlorophyll, leaf extract pH, ascorbic acid, or RWC) may not offer a clear perspective. As a result, calculating APTI based on those factors is more useful in determining the tolerance level of plant species (Ogunkunle et al. 2015; Nadgórska-Socha et al. 2017). However, APTI alone is insufficient for the selection of tolerant species required to establish a green belt. API is computed by combining APTI and biological and socioeconomic characteristics. Table 3 shows the API values for the current study. The study identified F. religiosa and F. benghalensis as excellent performers on the basis of all parameters. Plant species like M. indica, A. scholaris, A. indica, and L. indica also exhibited good performance. Species that are not recommended for plantation at JCF due to their lower API score are R. indica and C. roseus. Similar findings were also reported by Govindaraju et al. (2012); Banerjee et al. (2018), and Roy et al. (2020), where they identified F. religiosa, F. benghalensis, M. indica, A. scholaris, A. indica, and L. indica as good performers for air pollution abatement at the polluted environment. Plants with API ratings of excellent, very good, or good are recommended for green belt development. As a result, the combined effect of APTI and API is an important and useful tool for selecting suitable plants that may be used in the building of a green belt from the existing vegetation in the region. By supporting the growth of green belts in polluted regions, the ambient air quality of metropolitan areas can be considerably enhanced. Except for the study we performed, there is absolutely no similar study undertaken at JCF that has investigated this issue. As a result, more research on the plant species should be done to identify their various degrees of tolerance and sensitivity and their applicability in reducing air pollution.
Conclusions
As this study demonstrates, individual tree species react differently to air pollution. According to our findings, polluted metropolitan areas led to a decrease in leaf area, total chlorophyll content, and leaf relative water content. As suggested by the data, suitable tree species should be selected depending on their tolerance to pollution load and utilizing MAI, APTI, and API indicators. In the present study, the highest MAI values were observed for F. benghalensis (12.67mg/kg) at JCF followed by F. religiosa (10.71 mg/kg), M. indica (7.32 mg/kg), A. scholaris (7.10 mg/kg), A. indica (6.74 mg/kg), and M. oleifera (6.58 mg/kg), indicating higher metal accumulating potential of the plants from the polluted atmosphere. Plants with lower APTI and API values (C. roseus and C. gigantea) act as sensitive species which can be used as a bioindicator, whereas plant species exhibiting higher APTI and API values (F. benghalensis, F. religiosa, M. indica, A. scholaris, A. indica, and M. oleifera) are tolerant to air pollution, which can be proposed for planting programs in polluted areas to reduce pollution levels. Significantly, the tolerant plant species can be implemented into a green belt design of Jharia Coalfield, to aid in the long-term control of air pollution effectively.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon request.
Code availability
Not applicable.
References
Agbaire PO, Akporhonor EE (2014) The effects of air pollution on plants around the vicinity of the Delta Steel Company, Ovwian-Aladja, Delta State, Nigeria. IOSR J Environ Sci Toxicol Food Technol 8(7):61–65
Al-Hemoud A, Al-Dousari A, Al-Shatti A, Al-Khayat A, Behbehani W, Malak M (2018) Health impact assessment associated with exposure to PM10 and dust storms in Kuwait. Atmosphere 9(1):6
Alotaibi MD, Alharbi BH, Al-Shamsi MA, Alshahrani TS, Al-Namazi AA, Alharbi SF, … Qian Y (2020) Assessing the response of five tree species to air pollution in Riyadh City, Saudi Arabia, for potential green belt application. Environ Sci Pollut Res 27:29156-29170
Amegah AK, Agyei-Mensah S (2017) Urban air pollution in Sub-Saharan Africa: Time for action. Environ Pollut 220:738–743
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Balasubramanian A, Prasath CH, Gobalakrishnan K, Radhakrishnan S (2018) Air pollution tolerance index (APTI) assessment in tree species of Coimbatore urban city, Tamil Nadu, India. Int J Environ Clim Change 8:27–38
Banerjee S, Banerjee A, Palit D, Roy P (2019) Assessment of vegetation under air pollution stress in urban industrial area for greenbelt development. Int J Environ Sci Technol 16(10):5857–5870
Banerjee S, Palit D, Banerjee A (2021) Variation of tree biochemical and physiological characters under different air pollution stresses. Environ Sci Pollut Res 28(14):17960–17980
Beckett KP, Freer-Smith PH, Taylor G (1998) Urban woodlands: their role in reducing the effects of particulate pollution. Environ Pollut 99(3):347–360
Cadelis G, Tourres R, Molinie J (2014) Short-term effects of the particulate pollutants contained in Saharan dust on the visits of children to the emergency department due to asthmatic conditions in Guadeloupe (French Archipelago of the Caribbean). PloS One 9(3):e91136
Dubey B, Pal AK, Singh G (2012) Trace metal composition of airborne particulate matter in the coal mining and non–mining areas of Dhanbad Region, Jharkhand, India. Atmos Pollut Res 3:238–246. https://doi.org/10.5094/APR.2012.026
Geravandi M, Farshadfar E, Kahrizi D (2011) Evaluation of some physiological traits as indicators of drought tolerance in bread wheat genotypes. Russ J Plant Physiol 58(1):69–75
Gope M, Masto RE, George J, Hoque RR, Balachandran S (2017) Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol, India. Ecotoxicol Environ Saf 138:231–241
Goudarzi G, Daryanoosh S M, Godini H, Hopke P K, Sicard P, De Marco A., … Khaniabadi Y O (2017) Health risk assessment of exposure to the Middle-Eastern Dust storms in the Iranian megacity of Kermanshah. Public Health 148:109-116
Govindaraju M, Ganeshkumar RS, Muthukumaran VR, Visvanathan P (2012) Identification and evaluation of air-pollution-tolerant plants around lignite-based thermal power station for greenbelt development. Environ Sci Pollut Res 19(4):1210–1223
Gupta GP, Kumar B, Kulshrestha UC (2016) Impact and pollution indices of urban dust on selected plant species for green belt development: mitigation of the air pollution in NCR Delhi, India. Arab J Geosci 9(2):136
Gupta S, Nayek S, Bhattacharya P (2011) Effect of air-borne heavy metals on the biochemical signature of tree species in an industrial region, with an emphasis on anticipated performance index. Chem Ecol 27(4):381–392
Henson IE, Mahalakshmi V, Bidinger FR, Alagarswamy G (1981) Genotypic variation in pearl millet (Pennisetum americanum (L.) Leeke), in the ability to accumulate abscisic acid in response to water stress. J Exp Bot 899–910
Honour SL, Bell JNB, Ashenden TW, Cape JN, Power SA (2009) Responses of herbaceous plants to urban air pollution: effects on growth, phenology and leaf surface characteristics. Environ Pollut 157(4):1279–1286
Hu Y, Wang D, Wei L, Zhang X, Song B (2014) Bioaccumulation of heavy metals in plant leaves from Yan′ an city of the Loess Plateau, China. Ecotoxicol Environ Saf 110:82–88
International Agency for Research on Cancer (2016) Outdoor air pollution.IARC Monographs on the evaluation of carcinogenic risks to humans 109:1-448
Javanmard Z, Kouchaksaraei MT, Hosseini SM, Pandey AK (2020) Assessment of anticipated performance index of some deciduous plant species under dust air pollution. Environ Sci Pollut Res 27(31):38987–38994
Jena S, Perwez A, Singh G (2019) Trace element characterization of fine particulate matter and assessment of associated health risk in mining area, transportation routes and institutional area of Dhanbad, India. Environ Geochem Health 41:2731–2747. https://doi.org/10.1007/s10653-019-00329-z
Jena S, Singh G (2017) Human health risk assessment of airborne trace elements in Dhanbad, India. Atmos Pollut Res 8:490–502. https://doi.org/10.1016/j.apr.2016.12.003
Jyothi SJ, Jaya DS (2010) Evaluation of air pollution tolerance index of selected plant species along roadsides in Thiruvananthapuram, Kerala. J Environ Biol 31(3):379–386
Karmakar D, Malik N, Padhy PK (2016) Effects of industrial air pollution on biochemical parameters of Shorea robusta and Acacia auriculiformis. Res J Recent Sci 2277:2502
Karmakar D, Padhy PK (2019) Air pollution tolerance, anticipated performance, and metal accumulation indices of plant species for greenbelt development in urban industrial area. Chemosphere 237:124522
Kaur M, Nagpal AK (2017) Evaluation of air pollution tolerance index and anticipated performance index of plants and their application in development of green space along the urban areas. Environ Sci Pollut Res 24(23):18881–18895
Keller T, Schwager H (1977) Air pollution and ascorbic acid. Eur J For Pathol 7(6):338–350
Khalid N, Masood A, Noman A, Aqeel M, Qasim M (2019) Study of the responses of two biomonitor plant species (Datura alba & Ricinus communis) to roadside air pollution. Chemosphere 235:832–841
Krishnaveni G (2018) Air pollution tolerance index of selected plants in Vijayawada city, Andhra Pradesh. Int J Green Pharm (IJGP) 11(04)
Leghari SK, Zaidi MA (2013) Effect of air pollution on the leaf morphology of common plant species of Quetta city. Pak J Bot 45(S1):447–454
Liu S, Wu Q, Cao X, Wang J, Zhang L, Cai D et al (2016) Pollution assessment and spatial distribution characteristics of heavy metals in soils of coal mining area in Longkou City. Huan Jing Ke Xue= Huanjing Kexue 37(1):270–279
Liu YJ, Zhu YG, Ding H (2007) Lead and cadmium in leaves of deciduous trees in Beijing, China: development of a metal accumulation index (MAI). Environ Pollut 145(2):387–390
Markert B (1992) Establishing of ‘Reference Plant’for inorganic characterization of different plant species by chemical fingerprinting. Water Air Soil Pollut 64(3):533–538
Mazher AA, Zaghloul SM, Mahmoud SA, Siam HS (2011) Stimulatory effect of kinetin, ascorbic acid and glutamic acid on growth and chemical constituents of Codiaeum variegatum L plants. Am-Eurasian J Agric Environ Sci 10(3):318–323
Mok HF, Majumder R, Laidlaw WS, Gregory D, Baker AJ, Arndt SK (2013) Native Australian species are effective in extracting multiple heavy metals from biosolids. Int J Phytoremediation 15(7):615–632
Molnár VÉ, Simon E, Tóthmérész B, Ninsawat S, Szabó S (2020) Air pollution induced vegetation stress–the air pollution tolerance index as a quick tool for city health evaluation. Ecol Indic 113:106234
Mondal D, Gupta S, Datta JK (2011) Anticipated performance index of some tree species considered for green belt development in an urban area. Int Res J Plant Sci 2(4):99–106
Mondal S, Singh G (2021) PM 2.5-bound trace elements in a critically polluted industrial coal belt of India: seasonal patterns, source identification, and human health risk assessment. Environ Sci Pollut Res 1–14
Mondal S, Singh G (2021) Pollution evaluation, human health effect and tracing source of trace elements on road dust of Dhanbad, a highly polluted industrial coal belt of India. Environ Geochem Health 43(5):2081–2103
Mondal S, Singh G, Jain MK (2020) Spatio-temporal variation of air pollutants around the coal mining areas of Jharia Coalfield, India. Environ Monit Assess 192:1–17
Nadgórska-Socha A, Kandziora-Ciupa M, Trzęsicki M, Barczyk G (2017) Air pollution tolerance index and heavy metal bioaccumulation in selected plant species from urban biotopes. Chemosphere 183:471–482
Nayak D, Patel DP, Thakare HS, Satashiya K, Shrivastava PK (2015) Evaluation of air pollution tolerance index of trees. Res Environ Life Sci 8(1):7–10
Ogunkunle CO, Suleiman LB, Oyedeji S, Awotoye OO, Fatoba PO (2015) Assessing the air pollution tolerance index and anticipated performance index of some tree species for biomonitoring environmental health. Agrofor Syst 89(3):447–454
Palit D, Kar D, Misra P, Banerjee A (2013) Assessment of air quality using several bio monitors of selected sites of Durgapur, Burdwan district by air pollution tolerance index approach. Indian J Sci Res 4(1):149–152
Pandey AK, Pandey M, Mishra A, Tiwary SM, Tripathi BD (2015) Air pollution tolerance index and anticipated performance index of some plant species for development of urban forest. Urban For Urban Green 14(4):866–871
Pandey B, Agrawal M, Singh S (2014) Coal mining activities change plant community structure due to air pollution and soil degradation. Ecotoxicology 23(8):1474–1483
Pandey J, Kumar D, Singh VK, Mohalik NK (2016) Environmental and Socio-Economic Impacts of Fire in Jharia Coalfield, Jharkhand, India: An Appraisal. Curr Sci 110:1639. https://doi.org/10.18520/cs/v110/i9/1639-1650
Pang WP, Qin FX, Lyu YC et al (2016) Chemical speciations of heavy metals and their risk assessment in agricultural soils in a coal mining area from Xingren County, Guizhou Province, China. Ying Yong Sheng Tai Xue Bao. J Appl Ecol 27(5):1468–1478
Patel D, Kumar JN (2018) An evaluation of air pollution tolerance index and anticipated performance index of some tree species considered for green belt development: a case study of Nandesari industrial area, Vadodara, Gujarat, India. Open J Air Pollut 7(01):1
Pope CA III, Coleman N, Pond ZA, Burnett RT (2020) Fine particulate air pollution and human mortality: 25+ years of cohort studies. Environ Res 183:108924
Pope CA III (2002) Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287:1132. https://doi.org/10.1001/jama.287.9.1132
Prajapati SK, Tripathi BD (2008) Anticipated Performance Index of some tree species considered for green belt development in and around an urban area: a case study of Varanasi city, India. J Environ Manag 88(4):1343–1349
Prajapati SK, Tripathi BD (2008) Seasonal variation of leaf dust accumulation and pigment content in plant species exposed to urban particulates pollution. J Environ Qual 37(3):865–870
Rai PK (2016) Impacts of particulate matter pollution on plants: implications for environmental biomonitoring. Ecotoxicol Environ Saf 129:120–136
Rai PK, Panda LL (2014) Dust capturing potential and air pollution tolerance index (APTI) of some road side tree veetation in Aizawl, Mizoram, India: an Indo-Burma hot spot region. Air Qual Atmos Health 7(1):93–101
Rai PK, Panda LL (2014) Dust capturing potential and air pollution tolerance index (APTI) of some road side tree vegetation in Aizawl, Mizoram, India: an Indo-Burma hot spot region. Air Qual Atmos Health 7(1):93–101
Rathore DS, Kain T, Gothalkar P (2018) A study of air pollution status by estimation of APTI of certain plant species around Pratapnagar circle in Udaipur city. Int J Agric Environ Biotechnol 11(1):33–38
Raza SN, Vijaykumari N, Murthy MS (1985) Air pollution tolerance index of certain plants of Hyderabad. In: Symp Biomonitoring State of Environment, New Delhi. Indian National Science Academy 243–245. https://scholar.google.com/scholar?cluster=2758462790716092871&hl=en&as_sdt=2005&sciodt=0,5
Remon E, Bouchardon JL, Le Guédard M, Bessoule JJ, Conord C, Faure O (2013) Are plants useful as accumulation indicators of metal bioavailability? Environ Pollut 175:1–7
Roy A, Bhattacharya T (2020) Air pollution tolerance, dust capturing capacity of native tropical trees for green belt development in Dhanbad and Bokaro city, Jharkhand, India. J Indian Chem Soc 97:635–643
Roy A, Bhattacharya T, Kumari M (2020) Air pollution tolerance, metal accumulation and dust capturing capacity of common tropical trees in commercial and industrial sites. Sci Total Environ 722:137622
Roy D, Singh G, Seo YC (2019) Carcinogenic and non-carcinogenic risks from PM10-and PM2. 5-Bound metals in a critically polluted coal mining area. Atmos Pollut Res 10(6):1964–1975. https://doi.org/10.1016/j.apr.2019.09.002
Sadeghian MM, Mortazaienezhad F (2012) Selection and identification of air pollution-tolerant plants by air pollution tolerance index (APTI) in urban parks of Isfahan, Iran. Afr j Biotechnol 11(55):11826–11829
Sahu C, Basti S, Sahu SK (2020) Air pollution tolerance index (APTI) and expected performance index (EPI) of trees in sambalpur town of India. SN Appl Sci 2(8):1–14
Samecka-Cymerman A, Stankiewicz A, Kolon K, Kempers AJ (2009) Self-organizing feature map (neural networks) as a tool to select the best indicator of road traffic pollution (soil, leaves or bark of Robinia pseudoacacia L.). Environ Pollut 157(7):2061–2065
Sharma B, Bhardwaj SK, Kaur L, Sharma A (2017) Evaluation of air pollution tolerance index (APTI) as a tool to monitor pollution and green belt development: a review. J Appl Natl Sci 9(3):1637–1643
Sharma B, Bhardwaj SK, Sharma S, Nautiyal R, Kaur L, Alam NM (2019) Pollution tolerance assessment of temperate woody vegetation growing along the National Highway-5 in Himachal Pradesh, India. Environ Monit Assess 191(3):1–14
Shrivastava RK, Neeta S, Geeta G (2013) Air pollution due to road transportation in India: a review on assessment and reduction strategies. J Environ Res Dev 8(1):69
Singh SK, Rao DN (1983) Evaluation of plants for their tolerance to air pollution. In:Proceedings Symposium on Air Pollution Control. Indian Association for Air Pollution Control. 1:218–224. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Singh+SK%2C+Rao+DN+%281983%29+Evaluation+of+plants+for+their+tolerance+to+airpollution.+In%3AProceedings+Symposium+on+Air+Pollution+Control.+Indian+Association+224.%C2%A0&btnG=
Singh SK, Rao DN, Agrawal M, Pandey J, Naryan D (1991) Air pollution tolerance index of plants. J Environ Manag 32(1):45–55
Swami, A., & Chauhan, D (2015) Impact of air pollution induced by automobile exhaust pollution on air pollution tolerance index (APTI) on few species of plants. International J Sci Res 4(3). ISSN No: 2277-8179. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Swami%2C+A.%2C+%26+Chauhan%2C+D+%282015%29+Impact+of+air+pollution+induced+by+automobile+exhaust+pollution+on+air+pollution+tolerance+index+%28APTI%817&btnG=
Tiwari S, Agrawal M, Marshall FM (2006) Evaluation of ambient air pollution impact on carrot plants at a sub urban site using open top chambers. Environ Monit Assess 119(1):15–30
Yadav R, Pandey P (2020) Assessment of Air Pollution Tolerance Index (APTI) and Anticipated Performance Index (API) of roadside plants for the development of greenbelt in urban area of Bathinda City, Punjab, India. Bull Environ Contam Toxicol 105(6):906–914
Yang J, McBride J, Zhou J, Sun Z (2005) The urban forest in Beijing and its role in air pollution reduction. Urban For Urban Green 3(2):65–78
Zhang PQ, Liu YJ, Chen X, Yang Z, Zhu MH, Li YP (2016) Pollution resistance assessment of existing landscape plants on Beijing streets based on air pollution tolerance index method. Ecotoxicol Environ Saf 132:212–223
Acknowledgments
The authors are grateful to the Indian Institute Technology (Indian School of Mines), Dhanbad, for providing all the necessary laboratory facilities during the research work.
Author information
Authors and Affiliations
Contributions
G.S. supervised the work. S.M. performed the analysis and calculations. S.M. wrote the manuscript in consultation with G.S.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies involving human participants performed by any of the authors.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mondal, S., Singh, G. Air pollution tolerance, anticipated performance, and metal accumulation capacity of common plant species for green belt development. Environ Sci Pollut Res 29, 25507–25518 (2022). https://doi.org/10.1007/s11356-021-17716-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17716-8