Abstract
Microplastics (MPs) are emerging persistent pollutants, and heavy metals are typical environmental pollutants, with their coexistence potentially compounding pollution and ecological risks. However, the interactive impacts and the relevant mechanisms of heavy metal and different types of MPs in plant-soil systems are still unclear. This study investigated the differential impacts of polyethylene MPs (PE MPs) and biodegradable polybutylene adipate MPs (PBAT MPs) on chromium (Cr) uptake in peanuts, focusing on plant performance and rhizosphere soil microenvironment. Compared with nondegradable PE-MPs, biodegradable PBAT MPs produced less significant influences on plant phytotoxicity, soil Cr bioavailability, and soil properties such as pH, CEC, DOC, and MBC, with the exception of MBN in Cr-contaminated soils. Compared to the control, soil pH and cation exchange capacity (CEC) decreased by MPs, while soil-soluble carbon (DOC), microbial biomass carbon, and nitrogen (MBC and MBN) increased by MPs. Compared to the control, soil-bioavailable Cr increased by 11.8–177.8% under PE MPs treatments, while increased by 5.1–156.9% under PBAT MPs treatments. The highest Cr content in shoots and roots was observed at 500.0 mg·kg−1 Cr level, which increased by 53.1% and 79.2% under 5% PE MPs treatments, respectively, as well as increased by 38.3% and 60.4% under 5% PBAT MPs treatments, respectively, compared with the control. The regression path analysis indicated that pH, MBC, MBN, and soil-bioavailable Cr played a vital role in the changes of soil properties and Cr uptake by peanuts induced by MPs. Soil bacterial community analysis revealed that Nocardioides, Proteobacteria, and Sphingomonas were reduced by the inhibition of MPs, which affected Cr uptake by peanuts. These results indicated that the peanut soil microenvironment was affected by PBAT and PE MPs, altering the Cr bioavailability and plant Cr uptake in Cr-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural soil covered with plastic film can retain heat, hold moisture, and promote production (Zhao et al. 2023a, b). However, the long-term large-area mulching production and its low recycling rate have led to the storage of large amounts of plastic debris in agricultural soil, causing “white pollution” (Liu et al. 2014). These plastic fragments in the soil also form microplastics (MPs) through hydrolysis, photodegradation, and abrasion (Liu et al. 2022b). Several studies indicate that soil heavy metal absorption and bioavailability are affected by MPs through altering soil pH, soil cation exchange (CEC), and soil soluble carbon (DOC) (An et al. 2023; Sajjad et al. 2022; Wang et al. 2021a, b), which poses more serious ecological risks to agricultural soils.
Polyethylene plastic (PE) is used widely in agriculture as the most common plastic film. However, considering the risks associated with the use of PE films for agricultural production, biodegradable plastic films such as polybutylene adipate (PBAT) have been developed as alternative materials (Qin et al. 2021). Biodegradable plastics are decomposed into CO2 and H2O through naturally occurring microbial mineralization (Paço et al. 2019). It is noted that biodegradable materials do not fully degrade in the natural environment and give rise to novel MPs (Sintim et al. 2020). In fact, due to its strong degradation properties, PBAT is more likely to break down from large to small pieces. Therefore, biodegradable MPs may have a different accumulation potential compared to conventional plastics. Previous studies have revealed that in comparison to PE films, PBAT disintegrated more quickly in the environment and absorbed more heavy metals (HM) (Li et al. 2020). Because biodegradable MPs age and degrade more readily in the environment, more heavy metals can be absorbed due to their specific surface area and rougher surface (Fan et al. 2021). However, the different impact on HM accumulations in plant and the relevant mechanisms between PE and biodegradable PBAT MPs were still unclear.
MPs broadly coexist with HMs in soil (Pang et al. 2023). As a prevalence of HM, Cr in agricultural fields can threaten human health, inhibit plant growth, and affect soil ecological security (Dotaniya et al. 2014; Tumolo et al. 2020). And Cr has a certain genotoxicity, and it affects growth and biological cells division and also induces many mitosis aberrations (Akhavan et al. 2016; Bouhadi et al. 2024). Because of their diverse morphology and susceptibility to external disturbances, the soil environment Cr pollution was affected by MPs and even caused serious compound pollution in soil (Li et al. 2022). It has been shown that the morphology of heavy metals was affected by MPs in soil (An et al. 2023). Previous studies have indicated that the bioavailability of heavy metals was increased by PE MPs (An et al. 2023; Li et al. 2021; Wang et al. 2020), while some studies indicated that both PE and PBAT MPs could reduce soil-bioavailable Cd, with PBAT MPs having a more significant reduction effect (Li et al. 2023). In addition, MPs can further affect peanut uptake of heavy metals through their effects on soil properties (Song et al. 2023b). However, PE and PABT MPs in Cr-contaminated soil on soil Cr morphology and Cr uptake by plants have not been clarified. Therefore, it is necessary to explore the mechanism that PE and PBAT MPs affect Cr accumulation by changing soil properties under soil Cr-contaminated environments.
Thus, this study aimed to investigate the different performance of peanut Cr uptake affected by PE MPs and PBAT MPs, which was revealed by plant performance and rhizosphere soil microenvironment. The objectives of this research are as follows: (1) investigate the different performance of PE and PBAT MPs on Cr accumulation in peanuts in Cr-contaminated soils; (2) reveal the mechanism of difference impact between PE and PBAT MPs using a regression path analysis (RPA) with respect to soil pH, DOC, CEC, MBC, MBN, and soil DTPA-extractable Cr; and (3) reveal the difference impact between PE and PBAT MPs on soil microbial-bacterial community by high-throughput sequencing method. Understanding the differences between PE and PBAT MPs impacts on soil Cr toxicity and bioavailability, as well as the environmental risks associated with their coexistence.
Materials and methods
Experimental materials
The topsoil (0–20 cm) of Qingdao city, Shandong province’s Chengyang District (39°19′02″N, 120°23′20″E), was used to gather the experimental soil. Vegetables, corn, and peanuts were the primary crops farmed in the region. Each of the three soils had a mild acidity. To examine their texture and prepare for planting experiments, the soil samples were dried, crushed, and sieved through a 100-mesh screen. K2Cr2O7 was dissolved in deionized water. After mixing well, slowly pour into the soil. Two levels of Cr soil were obtained, 250 and 500 mg·kg−1, in which the original soil Cr concentration was 52.4 mg·kg−1.
Polybutylene terephthalate (PBAT) MPs (1–300 μm) and polyethylene (PE) MPs (8.68–500 μm) were bought from the Dongming Business. A 0.5-mm sieve was used to grind and filter the PE and PBAT materials. Before used, the sieved PE and PBAT particles were washed and rinsed with deionized water and 0.1 mol·L−1 HNO3. Luhua 14 variety was chosen as peanut seed and provided by Qingdao Maoyuan Seed Company. After 10 min of sterilizing with a 2% sodium hypochlorite solution, the seeds were rinsed with deionized water. The fundamental properties of the soils are shown in Table 1.
Experimental design
Seven treatments were used in the pot experiment at soil Cr concentrations of 52.4, 250.0, and 500.0 mg·kg−1, which had a perfectly randomized design: control, 0.1% PE, 1% PE, 5% PE, 0.1% PBAT, 1% PBAT, and 5% PBAT. The pots were divided into 21 groups, each with 3 replications, and were organized in a randomized complete block design. The pot trial was served as the site in a greenhouse at Qingdao Agriculture University during the growing season from mid-March to mid-August. The average temperature of day and night were 28 ± 6 °C and 22 ± 6 °C in complete test period, respectively. And the relative humidity ranges of day and night were 56 ± 10% and 65 ± 13%, respectively.
The soil was fully mixed with various MP types and doses. After being carefully blended, the soil-MPs combination was weighed into the ceramic pots (8 kg each pot) and maintained 3 months before the experiment. After sterilizing, seeds were submerged in water for 24 h at temperature of 20 ~ 25 °C greenhouse and then placed in ceramic pots, covered with 1 cm of treated soil. Each container included three seedlings of the same size once the peanuts emerged. Each ceramic pot was arranged in the growth chamber at random. After 45 days, the plants were taken and examined.
Sampling and chemical analysis
Following a brief shake, rhizosphere soils were extracted from soils attached to peanut roots (Lynch and Whipps 1990). After the soil naturally dried, it was powdered through a sieve (0.15 mm) and put away. These plants’ dry weight was ascertained. Following grinding, the samples of peanut plants were digested using a 5:1 HNO3–HClO4 mixture. Inductively coupled plasma emission spectrometry (ICP-OES, Perkin-Elmer Optimam 8 × 00) was used to measure the Cr content of the plants. Diethylenetriaminepentaacetic acid (DTPA) (soil-liquid ratio: 1:5) was used to extract the effective state of Cr in the soil, and ICP-OES (Agilent 5900 ICP-OES, USA) was used to measure the content (Soltanpour and Schwab 1997). Soil pH was measured using a combination electrode at a ratio of 1:5 (soil/water, m/v). A total organic carbon analyzer was used to calculate the DOC of the soil at a ratio of 1:10 (soil/0.01-M CaCl2, m/v). The ammonium acetate exchange method was used to calculate the CEC of the soil (Schollenberger and Simon 1945). Microbiomes carbon and nitrogen were determined by chloroform fumigation-K2SO4 extraction method (Wang et al. 2007). Soil microbial-bacterial community was determined with the high-throughput sequencing method (Rong et al. 2021).
Statistical analysis
Statistical analysis and plotting were performed using SPSS v.26.0 and Origin v.2021. Analysis of variance (ANOVA) was performed using Duncan’s multiple tests, which was statistically different (p < 0.05). Regression path analysis (RPA) was used to determine the relationship of soil trait indicators (pH, DOC, CEC, MBC, and MBN) with shoot and root Cr concentration.
Result
Plant biomass
Both shoot and root dry biomasses were significantly affected by the levels of Cr and MPs according to the ANOVA results (p < 0.05). In Fig. 1, plant biomass was significantly inhibited by PE MPs both at the doses of 5.0% and 1.0% by 6.6 ~ 12.5% and 16.2 ~ 30.5% while decreased by 3.9 ~ 11.1% and 11.7 ~ 30.5% under PBAT MPs treatments. Specially, there was no significant difference of shoot and root biomass inhibition between PBAT and PE MPs with the dose of 5.0%.
Effect of different MPs types and levels and Cr concentrations on peanut plant biomass. Note: Capital letters mean significant variation among the Cr concentrations at the same MPs level and type (p < 0.05). Lowercase letters mean significant variation among the MPs level treatments at the same Cr concentrations (p < 0.05)
Cr accumulation in plants
Compared to CK, the PE MPs significantly increased shoot and root Cr contents by 5.50 ~ 74.9% and 16.4 ~ 79.2% under all MPs levels (p < 0.05), while those for PBAT MPs were 10.3 ~ 59.5% and 3.0 ~ 60.4%, respectively (Fig. 2). Across all three Cr levels, there was a discernible change in the shoot or root Cr concentrations between any PE and PBAT MPs levels. The MPs levels generated increases in plant Cr accumulation that varied with the Cr levels, similar to the differences in plant Cr concentrations. The largest plant Cr accumulations were observed at the 5% PE MPs treatment across the three soils. PE MPs induced more increases than PBAT MPs for Cr uptake at the three doses of MPs across all three Cr levels. At the soil Cr level of 250.0 mg·kg−1, three PE MPs levels significantly increased the shoot Cr accumulation by 17.8%, 40.2%, and 74.9%, respectively, compared with CK, while the PBAT were 13.0%, 25.1%, and 59.6%, respectively. The soil Cr content and MPs levels had a major impact on plant Cr accumulation, and their combination had a major impact on peanut Cr accumulation.
Potential available Cr in soils
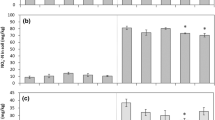
Soil DTPA-extractable Cr contents were significantly increased by 11.9 ~ 110.4%, 21.4 ~ 162.1%, and 20.6 ~ 177.8% under PE MPs treatments at the Cr levels of 54.2, 250.0, and 500.0 mg·kg−1, respectively, while increased by 5.1 ~ 98.3%, 5.9 ~ 135.0%, and 10.6 ~ 156.9% under PBAT MPs, respectively, compared with CK (p < 0.05). There were notable variations in the soil DTPA-extractable Cr concentrations between the three Cr levels, MPs types, and levels in Fig. 3.
Soil properties
Figure 4 showed the effects of different MPs types and levels on soil properties. The CEC and DOC dropped as soil Cr contamination increased, in contrast to soil pH and MBC. PE and PBAT MPs significantly reduced soil pH and CEC (p < 0.05), and PE reduced more than the corresponding concentration of PBAT. pH and CEC under 5% PE MPs treatments were reduced by 3.1% and 16.5% at the Cr level of 250.0 mg·kg−1, while PBAT treatment was 2.3% and 13.9%. PE and PBAT MPs significantly increased DOC, MBC, and MBN (p < 0.05), and PE MPs increased DOC and MBC more than PBAT MPs. DOC and MBC under 5% PE MPs treatments were increased by 21.4% and 95.3% at the Cr level of 500.0 mg·kg−1 while increased by 18.6% and 71.3% under PBAT MPs treatments, respectively. PBAT MPs had a greater impact on MBN than PE MPs. MBN were increased by 43.0% at the Cr level of 500.0 mg·kg−1 under 5% PE treatments while increased by 167.9% under 5% PBAT MPs treatments. Among all the soil physicochemical properties, MPs had a significant effect on soil physicochemical properties, with nonsignificant differences between PE and PBAT MPs at 0.1% dose and significant differences at 1% and 5%.
Relationship between the soil properties and the accumulation of Cr in plants
The pH, soil DTPA-extractable Cr, DOC, MBN, and MBC were significant factors influencing Cr levels in the roots and shoots, according to the RPA of the Cr content in plants and the fundamental soil characteristics (Fig. 5). Among them, there was a negative correlation between Cr accumulation in peanuts and pH, DOC, MBC, and MBC (p < 0.001). Meanwhile, the negative impact values were − 0.367, − 0.317, − 0.338, and − 0.354 for aboveground and − 0.400, − 0.312, − 0.142, and − 0.336 for belowground, respectively. Soil DTPA-extractable Cr had the greatest impact on plant Cr accumulation, and the impact parameters for shoot and root were 0.928 and 0.891, respectively. The analysis showed that different MPs species and contents changed the Cr concentration of peanuts by influencing soil properties, which in turn changed peanut growth in the soil either directly or indirectly. The main factors influencing this were soil pH, DOC, MBC, MBN, and soil DTPA-extractable Cr.
Microbial bacterial community
The effects of soil Cr contamination and the MPs on the relative abundance of bacteria in soil fractions are shown in Fig. 6. In CK, Nocardioides (19.43%) is the predominant genus, followed by Proteobacteria (17.49%), Nitrososphaera (9.01%), Sphingomonas (7.15%), Acidobacteria (4.04%), Mycobacterium (3.37%), Bacteroidetes (3.36%), Actinobacteria (3.06%), Bacillus (1.53%), Lysobacter (1.44%), Microlunatus (1.31%), Sinorhizobium (1.27%), Skermanella (1.23%), Devosia (1.08%), Microvirga (0.69%), Flavobacterium (0.43%), Gaiella (0.35%), and Streptomyces (0.30%) at the Cr level of 250.0 mg·kg−1. Soil Cr levels affect soil microbial community changes. With increasing the soil Cr content, the proportion of Nitrososphaera and Sphingomonas was increased. In contrast, the proportion of Nocardioides and Proteobacteria was decreased. MPs doses and types affect soil microbial community changes. Compared with CK, with increasing the PE MPs, the proportion of Nitrososphaera and Sphingomonas were increased by 6.15 ~ 60.13% and 2.36 ~ 26.28%, respectively. And the PBAT MPs were increased by 21.74 ~ 73.91% and 28.09 ~ 32.49%, respectively. However, in 0.1% PE, 0.1% PBAT, and 5% PBAT, Sphingomonas was decreased by 11.50 ~ 58.32%. Compared with CK, with increasing the PE MPs, the proportion of Nocardioides and Proteobacteria were decreased by 18.11 ~ 51.04% and 6.60 ~ 27.44%, respectively. And the PBAT MPs were decreased by 18.76 ~ 53.50% and 7.65 ~ 37.99%, respectively.
Discussion
Effect of microplastics on peanut growth and Cr uptake in peanuts under the Cr-contaminated soil
This study found that PE and PBAT MPs negatively affected shoot and root growth of peanut plants. Han et al. (2024) showed similar findings that soil MPs could significantly inhibit branch growth in terms of biomass and length of pak choi. It has been shown that MPs affect soil bulkiness, porosity, water-holding capacity, and water-stabilizing aggregates (de Souza-Machado et al. 2018; Qi et al. 2020). This alters the dynamics of soil water storage, and consequently crop water and nutrient uptake, leading to a reduction in biomass accumulation during the plant growth stage. Furthermore, compared to nondegradable PE MPs, biodegradable PBAT MPs show higher negative consequences. Adipic acid, terephthalic acid, and butanediol, which are potential PBAT breakdown products, have been demonstrated in earlier research to impede plant growth (Liu et al. 2022a; Martin-Closas et al. 2014). The toxicity of PBAT breakdown products may be the cause of the shoot and root that PBAT MPs generate. Conversely, harmful chemicals that support plant growth might not have been formed in PE MPs treatments during exposure, considering the environmental stability of PE MPs. In this study, MPs promoted peanut Cr uptake and accumulation and increased soil bioavailable Cr concentration which was maximum at 5%. Zhou et al. (2019) reported that MPs enhanced plant Cr uptake. This might be because heavy metals can absorbed on the high specific surface area of PE and PBAT MPs. The accumulated heavy metals can be easily released into the solution, which improves the bioavailability of heavy metals and thus enhances Cr uptake by peanuts (Zhou et al. 2019). Plant Cr uptake is influenced by the interaction between MPs and Cr. The MPs might have the adsorption or chelation capacity for soluble heavy metals in soil, which might lead to a more notable impact on the chemical speciation of heavy metals (Wen et al. 2022). Meanwhile, microbial communities are affected by Cr contamination, which alters the degradation of MPs (Cervantes et al. 2001). Root secretions are altered by MPs while affecting the soil environment and plant uptake of Cr. Previous studies have shown that the content of low-molecular-weight organic acids in tomato root exudates was significantly increased after exposure to MPs (Shi et al. 2023). Zhao et al. (2023a, b) found that cinnamon soil was affected by MPs increasing the production and release of soil metabolites and rhizosphere amino acids, which in turn increased the absorption of Cd by maize roots (Zhao et al. 2023a, b). Soil physicochemical properties such as soil pH and organic matter are altered by inter-root secretions (Yang et al. 2024). Microbial communities are altered through the soil environment by inter-root secretions. The microbial-mediated degradation of MPs is influenced by microorganisms, and this may be their secretion of enzymes that catalyzes the cleavage of MP chains into monomers. (Yuan et al. 2020). The enzymes are secreted under the control of different microorganisms in their coding, while there is a correlation between the degradation of different types of microplastics and the types of enzymes (Yuan et al. 2020). Thus, microbial enzymes are influenced by microbial community structure, which ultimately affects MPs decomposition (Paramdeep et al.2022). The degradation of MPs was affected, which altered their surface area and adsorption capacity, and in turn modified the bioavailability of soil heavy metals. The effect of PE MPs on Cr uptake by peanuts and soil DTPA-extractable Cr was greater than that of PBAT MPs. Similar to the research of Sun et al (2023), this could be the result of the PBAT MPs’ degradation decreasing the amount of heavy metal adsorption sites. Meanwhile, PBAT MPs increased the water content due to its high water retention in the soil (Huang et al. 2024). Salt-based ions in the soil are leached or lost with water, soil salt-based saturation and buffering properties are reduced, and hydrogen ion saturation is increased, causing a decrease in soil pH. Thus, the effectiveness of Cr and the promotion of Cr uptake by peanuts were improved.
Effects of microplastics on soil properties in Cr-contaminated soil
Numerous studies showed that MPs affect soil pH through a variety of pathways. Studies have indicated that MPs may modify the quantity of cations exchanged in the soil and permit free proton exchange capacity (CEC) across a wide surface area, thereby influencing changes in the pH of the soil (Ma et al. 2023b). The CEC content of PBAT MPs was higher than that of PE MPs, which was compatible with variations in soil pH and similar to the findings of this investigation. Due to PBAT MPs easily degradable characteristics, this would result in a larger specific surface area, providing better exchange sites and therefore increasing the CEC content (Zuo et al. 2019). In addition, PE MPs is hydrophobic, which improves soil leaching and lowers soil pH, whereas PBAT MPs is water absorbent, which weakens the leaching effect (Gao et al. 2021). Bioavailable Cr in soil is affected by pH, and the lower the pH, the higher the bioavailable state Cr content (Shahid et al. 2017). Therefore, in this study, soil pH was affected by MPs to alter bioavailable Cr content and consequently affect plant uptake of Cr.
MPs significantly increased DOC content, and the increments of PE MPs treatments were higher than PBAT MPs treatments. This is because the dynamic alterations of the two MPs to the dissolved organic matter (DOM) in the soil, which rely on the imbalance between in situ mineralization and the generation of water-soluble organic matter, would exert an influence on it (Liu et al. 2017). By incorporating MPs, the soil’s stored organic carbon, nitrogen, and phosphorus are activated, and the release buildup of nutrients into the soil solution is encouraged (Liu et al. 2017; Wang et al. 2022b). MPs can affect DOC by lowering soil pH. This is because soil pH can change soil properties, and soil properties affect DOC content (Li et al. 2019; Wang et al. 2022a). In addition, as DOC content increased, MBC content also increased. This is because MPs can release DOC by acting as organic carbon either directly or through their intermediates (Lee et al. 2020; Rillig et al. 2021). Meanwhile, MPs can be changed into soil DOC by soil bacteria (Wang et al. 2021d). In contrast, the release of harmful substances from PBAT MPs after degradation inhibits microorganisms, which in turn reduces soil DOC. Soil Cr mobility, bioavailability, adsorption, and desorption in soil are influenced by the presence of soil organisms (Zeng et al. 2011). Organic matter usually acts as a carrier of Cr in soil and exhibits coupled storage of metal and OM (Quenea et al. 2009), and then peanut Cr uptake was influenced.
In this study, PE and PBAT MPs as exogenous substances had a greater effect on soil microbial biomass. This may be due to the fact that MPs alter the internal porosity of the soil, which in turn has an effect on soil aggregates and soil water content. This alters soil physicochemical properties as well as soil material cycling, which ultimately affects the survival of soil microorganisms (Blagodatsky et al. 2000; Wang et al. 2023). This suggests that MPs can potentially have an indirect impact on soil bacteria by altering soil pH. PE MPs provide a more suitable environment for microorganisms to survive due to their water absorption, which improves microbial decomposition of organic matter and increases microbial carbon (Tang et al. 2023). The degradation of PBAT MPs provided bioavailable carbon for microorganisms and increased microbial carbon (Sun et al. 2022; Zumstein et al. 2018). Meanwhile, the degradation of MPs in soil may induce cycling of other macro- and micronutrients (Song et al. 2023b, c). For example, high microbial C:N ratios may promote microbial N immobilization and enhance MBN (Brown et al. 2022). In this study, MBC and MBN were raised by MPs, while MBC was affected by PE MPs more than PBAT MPs, and MBN is the opposite. This may be due to the difference in the structure of PE and PBAT which release different substances under natural conditions. In contrast, PBAT MPs releases terephthalic acid (TPA), which has an inhibitory effect on microorganisms to reduce the total microbial population (Ma et al. 2023a, b). However, as PBAT MPs can provide sufficient carbon source, it increases the individual C content of microorganisms leading to a high C:N ratio and improving microbial N conversion. In addition, the entry of PE and PBAT MPs into the soil and the different microbial populations that degrade them due to their different compositions also contributed to the changes (Liang et al. 2023). Bioavailable Cr is affected by soil microorganisms which lead to changes in its form and content depending on the microbial population as well as the microbial community (Shahid et al. 2017; Song et al. 2023a; Wani et al. 2022).
Effects of microplastics on the bacterial community structure
The bacterial community structure was changed after the addition of Cr and MPs in the soil, which was also depended on the Cr content, MPs types, and doses. Previous studies have found that MPs changed the microbial community and had an impact on the genera of microorganisms (Rong et al. 2021). This study demonstrated that Sphingomonas and Nitrososphaera increased under both PE and PBAT MPs treatments, with PBAT MPs which showed a more strongly emphasized dominating genera than PE. Due to the escalating environmental pressures resulting from ecological alterations such as MPs, microorganisms are obliged to expedite their growth in order to obtain more favorable ecological niches (Steinberg 2012). This might be attributed to the fact that MPs furnish the nutrient environment demanded by microorganisms, thereby attracting microorganisms and modifying the microbial community (Sun et al. 2024). The current study also demonstrated that glycolysis and the TCA cycle were disrupted significantly by MPs (Yuan et al. 2023). This change might be attributed to the plasticizers and other detrimental additives released from the biodegradable PBAT MPs, which affect the ultimate carbohydrate metabolic pathways of macromolecular organic compounds, such as fatty acids and polysaccharides (Yuan et al. 2023). The generation of intracellular energy and electrons is influenced by the change of ultimate carbohydrate metabolic pathways, thereby resulting in implications for the growth, reproduction, and functionality of microbes (Yuan et al. 2023). Additionally, the amount of nitrogen metabolism bacteria increased due to the enhanced biodegradability of MPs (Sun et al. 2024). As an important dominant group species involved in ammonia oxidation, the growth and reproduction of Nitrososphaera are facilitated by MPs (Zheng et al. 2021). Huang et al. (2019) disclosed that the activities of soil catalase were stimulated by low-density polyethylene (LDPE) MPs and urease to different degrees, altered the composition of the soil bacterial community, and influenced the soil properties. The results of this study indicated that PE and PBAT MPs reduced soil pH. Nocardioides grows in neutral and alkaline environments, and a decrease in soil pH may force changes in its community (Srinivasan et al. 2014; Sultanpuram et al. 2015). The inhibition of Proteobacteria is possibly due to the inhibition of microbial production of soluble microbial product and extracellular polymeric substance by MPs (Wang et al. 2022c). Members of Sphingomonas are commonly related to the management of Cr-contaminated soil and the facilitation of plant growth (Okazaki et al. 2021; Siddika et al. 2022). This might be attributed to a considerable reduction in Cr-induced oxidative stress through regulating metal-responsive reductions and enzymatic antioxidants, thereby reducing oxidative stress (Bilal et al. 2018). The chemical components in soils could have played an important role in shaping the microbial communities in these soils (Cai et al. 2019). Similarly, Nocardioides are considered to be the bacterial species which promote plant growth and development (Okazaki et al. 2021). The formation of microbial communities and their survival activities are affected by soil chemistry in a considerable way. However, the effects of Cr contamination and MPs on soil physicochemical properties attenuated the bacterial promotion of Cr tolerance in peanut. Heavy metal Cr and MPs alter the soil microenvironment, and then the microbial living environment was changed, which in turn altered the microbial community (Li et al. 2017).
Overall, degradable PBAT MPs had greater beneficial effects on plant growth and plant Cr uptake than PE MPs, through altering soil characteristics and microbiological bacterial community structure. Therefore, biodegradable PBAT plastic as a substitute for PE plastic has certain practical significance, especially in higher Cr content agricultural land. However, the products of the degradation process of PBAT plastics need to be addressed, mainly including the production of MPs as well as other toxic substances that have a negative impact on plant growth and soil traits (Ma et al. 2023a, b). Thus, further research is required in the future, particularly in field settings, to fully understand the variability in the long-term consequence of biodegradable MPs in soil–plant systems. Furthermore, more thorough studies are needed to comprehend the intricate molecular mechanisms underlying the roles of soil and plant cells.
Conclusions
This study demonstrated that MPs exert significant effects on soil characteristics and the bioavailability of Cr, with the type and dosage of MPs exhibiting notable influence across all Cr levels. Comparatively, biodegradable PBAT MPs demonstrated a less impact on soil traits, plant phytotoxicity, and Cr availability when contrasted with PE MPs. RPA results underscored the role of MPs in enhancing Cr uptake by altering soil physical–chemical and microbiological properties. While soil pH, MBC, MBN, and soil DTPA-extractable Cr are important factors affecting the Cr absorption of peanut, it is worth noting that a single factor is rarely the only mechanism affecting the Cr absorption of peanut. Meanwhile, MPs and Cr altered the structure of soil microbial community and affected Cr uptake by peanut. Given the current knowledge gap, further supporting long-term and outdoor tests need to be warranted to assess the individual and combined potential effects of various MPs in soils contaminated by other pollutants on soil–plant systems.
Data availability
The authors are unable or have chosen not to specify which data has been used.
References
Akhavan O, Hashemi E, Zare H, Shamsara M, Taghavinia N, Heidari F (2016) Influence of heavy nanocrystals on spermatozoa and fertility of mammals. Mater Sci Eng, C 69:52–59
An Q, Zhou T, Wen C, Yan C (2023) The effects of microplastics on heavy metals bioavailability in soils: a meta-analysis. J Hazard Mater 460:132369
Bilal S, Khan AL, Shahzad R, Kim YH, Imran M, Khan MJ, Al-Harrasi A, Kim TH, Lee IJ (2018) Mechanisms of Cr (VI) resistance by endophytic Sphingomonas sp. LK11 and its Cr (VI) phytotoxic mitigating effects in soybean (Glycine max L.). Ecotoxicol Environ Saf 164:648–658
Blagodatsky SA, Heinemeyer O, Richter J (2000) Estimating the active and total soil microbial biomass by kinetic respiration analysis. Biol Fertil Soils 32:73–81
Bouhadi M, Abchir O, Yamari I, El Youbi AEH, Azgaoui A, Chtita S, El Hajjouji H, El Kouali M, Talbi M, Fougrach H (2024) Genotoxic effects and mitosis aberrations of chromium (VI) on root cells of Vicia faba and its molecular docking analysis. Plant Physiol Biochem 207:108361
Brown RW, Chadwick DR, Bending GD, Collins CD, Whelton HL, Daulton E, Covington JA, Bull ID, Jones DL (2022) Nutrient (C, N and P) enrichment induces significant changes in the soil metabolite profile and microbial carbon partitioning. Soil Biol Biochem 172:108779
Cai M, Hu C, Wang X, Zhao Y, Jia W, Sun X, Elyamine AM, Zhao X (2019) Selenium induces changes of rhizosphere bacterial characteristics and enzyme activities affecting chromium/selenium uptake by pak choi (Brassica campestris L. ssp. Chinensis Makino) in chromium contaminated soil. Environ Pollut 249:716–727
Cervantes C, Campos-García J, Devars S, Gutiérrez-Corona F, Loza-Tavera H, Torres-Guzmán JC, Moreno-Sánchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25(3):335–347
de Souza-Machado AA, Lau CW, Till J, Kloas W, Lehmann A, Becker R, Rillig MC (2018) Impacts of microplastics on the soil biophysical environment. Environ Sci Technol 52(17):9656–9665
Dotaniya ML, Thakur JK, Meena VD, Jajoria DK, Rathor G (2014) Chromium pollution: a threat to environment-a review. Agric Rev 35(2):153–157
Fan X, Gan R, Xie Y, Liu J, Li Y, Liu Q, Hou J (2021) Adsorption and desorption behavior of antibiotics on polylactic acid and polyethylene microplastics before and after aging. Res Environ Sci 34(7):1747–1756
Gao X, Xie D, Yang C (2021) Effects of a PLA/PBAT biodegradable film mulch as a replacement of polyethylene film and their residues on crop and soil environment. Agric Water Manag 255:107053
Han Y, Teng Y, Wang X, Wen D, Gao P, Yan D, Yang N (2024) Biodegradable PBAT microplastics adversely affect pakchoi (Brassica chinensis L.) growth and the rhizosphere ecology: focusing on rhizosphere microbial community composition, element metabolic potential, and root exudates. Sci Total Environ 912:169048
Huang Y, Zhao Y, Wang J, Zhang M, Jia W, Qin X (2019) LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut 254:112983
Huang W, Jiang G, Xie L, Chen X, Zhang R, Fan X (2024) Effect of oxygen-containing functional groups on the micromechanical behavior of biodegradable plastics and their formation of microplastics during aging. J Hazard Mater 463:132911
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38(5):991–999
Lee YK, Murphy KR, Hur J (2020) Fluorescence signatures of dissolved organic matter leached from microplastics: polymers and additives. Environ Sci Technol 54:11905–11914
Li X, Meng D, Li J, Yin H, Liu H, Liu X, Cheng C, Xiao Y, Liu Z, Yan M (2017) Response of soil microbial communities and microbial interactions to long-term heavy metal contamination. Environ Pollut 231:908–917
Li M, Wang J, Guo D, Yang R, Fu H (2019) Effect of land management practices on the concentration of dissolved organic matter in soil: a meta-analysis. Geoderma 344:74–81
Li R, Liu Y, Sheng Y, Xiang Q, Zhou Y, Cizdziel JV (2020) Effect of prothioconazole on the degradation of microplastics derived from mulching plastic film: apparent change and interaction with heavy metals in soil. Environ Pollut 260:113988
Li H, Wang F, Li J, Deng S, Zhang S (2021) Adsorption of three pesticides on polyethylene microplastics in aqueous solutions: kinetics, isotherms, thermodynamics, and molecular dynamics simulation. Chemosphere 264:128556
Li Y, Zhang Y, Su F, Wang Y, Peng L, Liu D (2022) Adsorption behaviour of microplastics on the heavy metal Cr (VI) before and after ageing. Chemosphere 302:134865
Li C, Sun H, Shi Y, Zhao Z, Zhang Z, Zhao P, Gao Q, Zhang X, Chen B, Li Y, He S (2023) Polyethylene and poly (butyleneadipate-co-terephthalate)-based biodegradable microplastics modulate the bioavailability and speciation of Cd and As in soil: insights into transformation mechanisms. J Hazard Mater 445:130638
Liang R, Sun F, Zhang C, Zhang R, Wang H, Wang X (2023) Research progress on the interaction between microplastics and microorganisms in soil environment. Chin J Biotechnol 39(2):500–515
Liu EK, He WQ, Yan CR (2014) ‘White revolution’to ‘white pollution’—agricultural plastic film mulch in China. Environ Res Lett 9(9):091001
Liu H, Yang X, Liu G, Liang C, Xue S, Chen H, Ritsema CJ, Geissen V (2017) Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 185:907–917
Liu J, Wang P, Wang Y, Zhang Y, Xu T, Zhang Y, Xu T, Zhang Y, Xi J, Hou L, Zhang Z, Lin Y (2022a) Negative effects of poly (butylene adipate-co-terephthalate) microplastics on Arabidopsis and its root-associated microbiome. J Hazard Mater 437:129294
Liu L, Xu M, Ye Y, Zhang B (2022b) On the degradation of (micro) plastics: degradation methods, influencing factors, environmental impacts. Sci Total Environ 806:151312
Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129:1–10
Ma J, Cao Y, Fan L, Xie Y, Zhou X, Ren Q, Yang X, Gao X, Feng Y (2023a) Degradation characteristics of polybutylene adipate terephthalic acid (PBAT) and its effect on soil physicochemical properties: a comparative study with several polyethylene (PE) mulch films. J Hazard Mater 456:131661
Ma J, Xu M, Wu J, Yang G, Zhang X, Song C, Long L, Chen C, Xu C, Wang Y (2023b) Effects of variable-sized polyethylene microplastics on soil chemical properties and functions and microbial communities in purple soil. Sci Total Environ 868:161642
Martin-Closas L, Botet R, Pelacho AM (2014) An in vitro crop plant ecotoxicity test for agricultural bioplastic constituents. Polym Degrad Stab 108:250–256
Okazaki K, Tsurumaru H, Hashimoto M, Takahashi H, Okubo T, Ohwada T, Minamisawa K, Ikeda S (2021) Community analysis-based screening of plant growth-promoting bacteria for sugar beet. Microbes Environ 36(2):ME20137
Paço A, Jacinto J, da Costa JP, Santos PS, Vitorino R, Duarte AC, Rocha-Santos T (2019) Biotechnological tools for the effective management of plastics in the environment. Crit Rev Environ Sci Technol 49:410–441
Pang X, Chen C, Sun J, Zhan H, Xiao Y, Cai J, Yu X, Liu Y, Yang G (2023) Effects of complex pollution by microplastics and heavy metals on soil physicochemical properties and microbial communities under alternate wetting and drying conditions. J Hazard Mater 458:131989
Paramdeep KAUR, Singh K, Singh B (2022) Microplastics in soil: impacts and microbial diversity and degradation. Pedosphere 32(1):49–60
Qi Y, Beriot N, Gort G, Lwanga EH, Gooren H, Yang X, Geissen V (2020) Impact of plastic mulch film debris on soil physicochemical and hydrological properties. Environ Pollut 266:115097
Qin M, Chen C, Song B, Shen M, Cao W, Yang H, Zeng G, Gong J (2021) A review of biodegradable plastics to biodegradable microplastics: another ecological threat to soil environment. J Clean Prod 312:127816
Quenea K, Lamy I, Winterton P, Bermond A, Dumat C (2009) Interactions between metals and soil organic matter in various particle size fractions of soil contaminated with waste water. Geoderma 149(3–4):217–223
Rillig MC, Leifheit E, Lehmann J (2021) Microplastic effects on carbon cycling processes in soils. PLoS Biol 19:e3001130
Rong L, Zhao L, Zhao L, Cheng Z, Yao Y, Yuan C, Wang L, Sun H (2021) LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci Total Environ 773:145640
Sajjad M, Huang Q, Khan S, Khan MA, Liu Y, Wang J, Lian F, Wang Q, Guo G (2022) Microplastics in the soil environment: a critical review. Environ Technol Innov 27:102408
Schollenberger CJ, Simon RH (1945) Determination of exchange capacity and exchangeable bases in soil-ammonium acetate method. Soil Sci 59:13–24
Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PM (2017) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241:73–137
Shi R, Liu W, Lian Y, Zeb A, Wang Q (2023) Type-dependent effects of microplastics on tomato (Lycopersicon esculentum L.): focus on root exudates and metabolic reprogramming. Sci Total Environ 859:160025
Siddika A, Islam MM, Parveen Z, Hossain MF (2022) Remediation of chromium (VI) from contaminated agricultural soil using modified biochars. Environ Manage 71(4):809–820
Sintim HY, Bary AI, Hayes DG, Wadsworth LC, Anunciado MB, English ME, Bandopadhyay S, Schaeffer SM, DeBruyn JM, Miles CA, Reganold JP, Flury M (2020) In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci Total Environ 727:138668
Soltanpour PN, Schwab AP (1997) A new soil test for simultaneous extraction of macro-and micro-nutrients in alkaline soils. Commun Soil Sci Plant Anal 8(3):195–207
Song N, Wang B, Liu J, Wang F, Wang X, Zong H (2023a) Is degradable plastic film alternative? Insights from crop productivity enhancement and soil environment improvement. Eur J Agron 149:126882
Song X, Jin J, Li H, Wang F, Liu J, Wang X, Huang X, Chai C, Song N, Zong H (2023b) Kaolinite reduced Cd accumulation in peanut and remediate soil contaminated with both microplastics and cadmium. Ecotoxicol Environ Saf 266:115580
Song X, Jin J, Yin H, Wang T, Zong H, Wang F, Liu J, Huang X, Wang B, Chai C, Li Z, Liu D, Wang X, Song N (2023c) Trichoderma viride F7 improves peanut performance while remedying cadmium-contaminated soil with microplastics. Pedosphere. https://doi.org/10.1016/j.pedsph.2023.06.010
Srinivasan S, Lee SS, Lee JJ, Kim MK (2014) Nocardioides soli sp. nov., a bacterium isolated from a mountain soil. Antonie van Leeuwenhoek 106(2):271–278
Steinberg CE (2012) Stress ecology: environmental stress as ecological driving force and key player in evolution. Springer Science & Business Media
Sultanpuram VR, Mothe T, Mohammed F (2015) Nocardioides solisilvae sp. nov., isolated from a forest soil. Antonie Van Leeuwenhoek 107(6):1599–1606
Sun Y, Li X, Cao N, Duan C, Ding C, Huang Y, Wang J (2022) Biodegradable microplastics enhance soil microbial network complexity and ecological stochasticity. J Hazard Mater 439:129610
Sun Y, Peng BY, Wang X, Li Y, Wang Y, Zhang Y, Xia S, Zhao J, Zhao J (2023) Adsorption and desorption mechanisms of oxytetracycline on poly (butylene adipate-co-terephthalate) microplastics after degradation: the effects of biofilms, Cu (II), water pH, and dissolved organic matter. Sci Total Environ 863:160866
Sun J, Zhang X, Gong X, Sun Y, Zhang S, Wang F (2024) Metagenomic analysis reveals gene taxonomic and functional diversity response to microplastics and cadmium in an agricultural soil. Environ Res 251:118673
Tang Y, Li G, Iqbal B, Tariq M, Rehman A, Khan I, Du D (2023) Soil nutrient levels regulate the effect of soil microplastic contamination on microbial element metabolism and carbon use efficiency. Ecotoxicol Environ Saf 267:115640
Tumolo M, Ancona V, De Paola D, Losacco D, Campanale C, Massarelli C, Uricchio VF (2020) Chromium pollution in European water, sources, health risk, and remediation strategies: an overview. Int J Environ Res Public Health 17(15):5438
Wang QR, Li YC, Klassen W (2007) Changes of soil microbial biomass carbon and nitrogen with cover crops and irrigation in a tomato field. J Plant Nutr 30(4):623–639
Wang F, Zhang X, Zhang S, Zhang S, Adams CA, Sun Y (2020) Effects of cocontamination of microplastics and Cd on plant growth and Cd accumulation. Toxics 8:36
Wang F, Wang X, Song N (2021) Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agr Ecosyst Environ 315:107425
Wang F, Wang X, Song N (2021) Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ 784:147133
Wang J, Peng C, Li H, Zhang P, liu Z, (2021c) The impact of microplastic-microbe interactions on animal health and biogeochemical cycles: a mini-review. Sci Total Environ 773:145697
Wang Y, Wang X, Li Y, Li J, Liu Y, Xia S, Zhao J (2021d) Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem Eng J 404:126412
Wang F, Liu Y, Liang B, Liu J, Zong H, Guo X, Wang X, Song N (2022a) Variations in soil aggregate distribution and associated organic carbon and nitrogen fractions in long-term continuous vegetable rotation soil by nitrogen fertilization and plastic film mulching. Sci Total Environ 835:155420
Wang F, Wang Q, Adams CA, Sun Y, Zhang S (2022b) Effects of microplastics on soil properties: current knowledge and future perspectives. J Hazard Mater 424:127531
Wang Q, Li Y, Liu Y, Zhou Z, Hu W, Lin L, Wu Z (2022c) Effects of microplastics accumulation on performance of membrane bioreactor for wastewater treatment. Chemosphere 287:131968
Wang Z, Li W, Li W, Yang W, Jing S (2023) Effects of microplastics on the water characteristic curve of soils with different textures. Chemosphere 317:137762
Wani KI, Naeem M, Aftab T (2022) Chromium in plant-soil nexus: speciation, uptake, transport and sustainable remediation techniques. Environ Pollut 315:120350
Wen X, Yin L, Zhou Z, Kang Z, Sun Q, Zhang Y, Long Y, Nie X, Wu Z, Jiang C (2022) Microplastics can affect soil properties and chemical speciation of metals in yellow-brown soil. Ecotoxicol Environ Saf 243:113958
Yang H, Ji S, Wu D, Zhu M, Lv G (2024) Effects of root–root interactions on the physiological characteristics of haloxylon ammodendron seedlings. Plants 13(5):683
Yuan J, Ma J, Sun Y, Zhou T, Zhao Y, Yu F (2020) Microbial degradation and other environmental aspects of microplastics/plastics. Sci Total Environ 715:136968
Yuan Y, Zu M, Li R, Zuo J, Tao J (2023) Soil properties, microbial diversity, and changes in the functionality of saline-alkali soil are driven by microplastics. J Hazard Mater 446:130712
Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, Zhang G (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159(1):84–91
Zhao M, Xu L, Wang X, Li C, Zhao Y, Cao B, Zhang C, Zhang J, Wang J, Chen Y, Zou G (2023a) Microplastics promoted cadmium accumulation in maize plants by improving active cadmium and amino acid synthesis. J Hazard Mater 447:130788
Zhao Y, Mao X, Li S, Huang X, Che J, Ma C (2023b) A review of plastic film mulching on water, heat, nitrogen balance, and crop growth in farmland in China. Agronomy 13(10):2515
Zheng M, He S, Feng Y, Wang M, Liu YX, Dang C, Wang J (2021) Active ammonia-oxidizing bacteria and archaea in wastewater treatment systems. J Environ Sci 102:273–282
Zhou Y, Liu X, Wang J (2019) Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci Total Environ 694:133798
Zumstein MT, Schintlmeister A, Nelson TF, Baumgartner R, Woebken D, Wagner M, Kohler HE, Mcneill K, Sander M (2018) Biodegradation of synthetic polymers in soils: tracking carbon into CO2 and microbial biomass. Sci Adv 4(7):eaas9024
Zuo L, Li H, Lin L, Sun Y, Diao Z, Liu S, Zhang Z, Xu X (2019) Sorption and desorption of phenanthrene on biodegradable poly (butylene adipate co-terephtalate) microplastics. Chemosphere 215:25–32
Funding
This work was supported by the Technical System of Ecological Agriculture of Modern Agricultural Technology System in Shandong Province (No. SDAIT-30–02), the Natural Science Foundation of Shandong Province (No. ZR2022MD050 and No. ZR2022MD118), the Beijing Academy of Agricultural and Forestry Sciences Science and Technology Innovation Capacity Construction Project (No. KJCX20240312), the Humanities and Social Science Foundation Project of Ministry of Education (No. 22YJCZH166), and the National Key Research and Development Program (No. 2023YFD1701901).
Author information
Authors and Affiliations
Contributions
Jianpeng Jin, conceptualization, methodology, validation, writing—reviewing and editing, visualization, and resources. Xuexia Wang, conceptualization, investigation, methodology, validation, writing—original draft, and resources. Ying Sha, methodology, validation, formal analysis, data curation, resources, and supervision. Fangli Wang, conceptualization, methodology, formal analysis, and data curation. Xiaoli Huang, methodology, investigation, and formal analysis. Haiying Zong, conceptualization, methodology, and formal analysis. Jun Liu, formal analysis and data curation. Ningning Song, conceptualization, investigation, methodology, formal analysis, writing—original draft, writing—reviewing and editing, visualization, resources, and supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Manyun Zhang
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, J., Wang, X., Sha, Y. et al. Changes in soil properties and microbial activity unveil the distinct impact of polyethylene and biodegradable microplastics on chromium uptake by peanuts. Environ Sci Pollut Res 31, 53369–53380 (2024). https://doi.org/10.1007/s11356-024-34743-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34743-3