Abstract
Microplastic contamination in terrestrial ecosystem is emerging as a global threat due to rapid production of plastic waste and its mismanagement. It affects all living organisms including plants. Hence, the current study aims at understanding the effect of polyethylene microplastics (PE-MPs) at different concentrations (0, 0.25, 0.50, 0.75, and 1.00% w/w) on the plant growth and yield attributes. With blackgram as a test crop, results revealed that a maximum reduction in physiological traits like photosynthetic rate; chlorophyll a, b; and total chlorophyll by 5, 14, 10, and 13% at flowering stage; and an increase in biochemical traits like ascorbic acid, malondialdehyde, proline, superoxide dismutase, and catalase by 11, 29.7, 16, 22, and 30% during vegetative stage was observed with 1% PE-MP application. Moreover, a reduction in growth and yield attributes was also observed with increasing concentration of microplastics. Additionally, application of 1% PE-MPs decreased the soil bulk density, available phosphorus, and potassium, whereas the EC, organic carbon, microbial biomass carbon, NO3-N, and NH4-N significantly increased. Moreover, the presence of PE-MPs in soil also had a significant influence on the soil enzyme activities. Metagenomic analysis (16 s) reveals that at genus level, Bacillus (19%) was predominant in control, while in 1% PE-MPs, Rubrobacter (28%) genus was dominant. Microvirga was found exclusively in T5, while the relative abundance of Gemmatimonas declined from T1 to T5. This study thus confirms that microplastics exert a dose-dependent effect on soil and plant characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics have now become an essential and inseparable component of human life. The exponential population growth exerts enormous pressure on plastic production, which is evident from the ever growing consumption of the plastics. As per the report of Plastics Europe 2021, there is a huge leap in the plastic production from 1950s to 2020 (1.5 to 367 million tons). According to estimates, 76% of plastic wastes is dumped in landfills or released into the soil, air, and water environment (Geyer 2020). These plastics are further broken down and enter into the environment as macroplastics (> 20 mm), mesoplastics (10–20 mm), and microplastics (< 5 mm) (Cole et al. 2011; Zhang et al. 2021a). With continuous breakdown of plastic particles, it is predicted that microplastic(MP) emission would increase by 1.3 to 2.5 times within 2040, amounting to roughly 3 million pieces (Lau et al. 2020).
Effect of microplastics (MPs) on aquatic ecosystem was reported by many researchers (Auta et al. 2017; Lambert and Wagner 2018); however, the studies highlighting their effect on terrestrial ecosystem are negligible. In recent years, many studies validated the increasing occurrence of MPs in the terrestrial environment (Lohmann 2017; de Souza Machado et al. 2018). Modern agricultural technologies like greenhouse covers, plastic mulch films (van Schothorst et al. 2021; Zhang et al. 2021b), plastic seed coatings (Gündoğdu et al. 2018), wastewater irrigation (Gündoğdu et al. 2018; He et al. 2018), landfills and leachates from landfills (He et al. 2019; Su et al. 2019; Silva et al. 2021a, b), bio-solid application to agricultural fields (Nizzetto et al. 2016; Mahon et al. 2017; He et al. 2018), soil conditioner application (Zubris and Richards 2005), application of compost and organic fertilizer (Weithmann et al. 2018), and atmospheric deposition (Klein and Fischer 2019) are the potential sources of MP pollution in soil, and thereby significantly affecting the soil biodiversity (Rillig et al. 2017; He et al. 2018) and crop growth (De Silva et al. 2021a, b).
Amongst various agricultural sources, mulching film made of polyethylene (PE) forms the major source of MPs in agricultural soil. It has been estimated that the plastic demand for mulching, silage films, and green house would rise by 50%, i.e., from 6.1 million tons in 2018 to 9.5 million tons in 2030 (Geyer 2020). Owing to their tedious recovery from soil, these MPs buildup in soil, thereby leading to accumulation in the field. This MP accumulation in agricultural soils has undesirable effect on soil properties such as altered pH (Boots et al. 2019; Zhou et al. 2021b); changes in bulk density (de Souza Machado et al. 2018; Zhang et al. 2019a); nutrient mobility (Guo et al. 2020; Dong et al. 2021; Ya et al. 2021); rise in dissolved organic carbon content (Meng et al. 2022); release of the additives like dioxins and furans (Li et al. 2021a; Yan et al. 2021a); and the upregulation or downregulation of a particular group of microorganisms, which in turn causes changes in composition of microbial community (Zhang et al. 2019b; Rong et al. 2021; Yan et al. 2021b). As a consequence, the soil ecosystem and agricultural productivity gets severely affected.
As far as plants are concerned, MP interferes in number of ways including nutrient uptake by blocking pores on the cell surface or transport pathways between the cells (Ma et al. 2016; Qi et al. 2018; Bosker et al. 2019; Yu et al. 2021a), reducing or delaying seed germination, and altered root and shoot growth (Qi et al. 2018; Bosker et al. 2019; Yu et al. 2021b).
The presence of MPs on the surface of plant roots prevents other contaminants from physically reaching the roots; however, they are more likely to cause phytotoxicity. Blackgram, a predominantly cultivated crop in tropical countries contains 56.6% carbohydrate, 26.2% crude protein, and 1.2% fat (Statista 2021). Although plastic mulch has not been used for blackgram cultivation, the left-over film residues from previous crop or the application of organic manures, sewage sludge, and littering of polyethylene plastic waste might be a source of MPs in soil (Dhevagi et al. 2022b). Alterations in the soil properties due to polyethylene microplastics may inhibit the growth and dry weight, since the crop is known for its sensitiveness to changes in the soil properties.
Although numerous researchers have examined the effect of MPs on cereals (Qi et al. 2018, Wang et al. 2020, Dong et al. 2021; Zhou et al. 2023; Iqbal et al. 2023), and horticultural crops (Mateos-Cárdenas et al. 2019; Bosker et al. 2019; Rillig et al. 2019; Yang et al. 2021a; Sahasa et al. 2023), only very few studies have been reported on the toxicity effect of microplastics on pulse crops, especially blackgram (In broad bean by Jiang et al. 2019; soybean by Wang et al. 2021 and mung bean by Soundarya and Sujatha 2023). Hence, the present study was formulated with the hypothesis that exposure to PE-MPs may influence the rhizospheric soil properties which in turn affect the growth and development of blackgram.
Materials and methods
Collection and characterization of materials
The polyethylene microplastics (PE-MPs) used for the experimental study were acquired from a private recycling business industry (M/s. Arunachal Polymer Industries (11.07469°N, 76.91207°E), located in Coimbatore, Tamil Nadu. Scanning electron microscope (SEM) was used to determine the size and shapes and EDAX (Quanta 250 (FEI, Netherlands)) to determine the composition of PE-MPs.
Blackgram (Vigna mungo) seeds (variety CO6) were obtained from NPRC (National Pulses Research Centre), Vamban. Viable seeds with a germination rate of 95% were chosen and steps have been taken to avoid microbial contamination. Uniform sized seeds were soaked in 2% sodium hypochlorite solution for 30 min and rinsed with sterile deionized water thrice before subjecting to various seed treatments.
The experimental soil (loam — top 0 to 20 cm) was collected from the crop field of wet land, Tamil Nadu Agricultural University, Coimbatore. The soils were shade dried, sieved through 2 mm to get rid of plant residues, large rocks, and gravel. Water holding capacity (Margesin and Schinner 2005), bulk density (Bashour and Sayegh 2007), porosity (Reynolds et al. 2008), pH and EC (Jackson 2005), soil organic carbon (Walkley and Black 1934), available nitrogen (Subbiah and Asija 1956), phosphorus (Olsen 1954), and potassium (Hanway and Heidal 1952) of the soil were characterized as per the standard procedures.
Experimental details
The current study was carried out at Tamil Nadu Agricultural University farm land, namely, Eastern Block. The study was conducted under greenhouse conditions and temperature during the study period ranged from 33.3 to 36.2 °C, while the minimum temperature was 22.7 to 30.8 °C and an average precipitation of 22.7 mm. Ten kilograms of processed farm soil were taken in pots to which different concentrations of microplastics having the same size (T1 (0%), T2 (0.25%), T3 (0.50%), T4 (0.75%), and T5 (1.0%) on dry weight basis (w/w)) were added. The concentrations were fixed based on the previous studies that quantified microplastics in different soils (Fuller and Gautam 2016; Huang et al. 2019; Lian et al. 2021). Before being mixed with the soil, the polyethylene microplastic particles were sonicated at 25 °C for 2 h to avoid aggregation. Microplastics were uniformly mixed with soil and three replications for each treatment for three different stages of plant growth were fixed (t = 5, s = 3, r = 3, and n = 15 for each stage of crop). Additionally, three plants per replication was maintained. The soil was maintained to 50% moisture and incubated 2 weeks for stabilization (de Souza Machado et al. 2019). Then, the blackgram seeds were sown, watered regularly, and steps were taken to maintain the plant density throughout the study period.
Analysis
The plants were harvested through destructive method at the end of each growth stage; i.e., vegetative, flowering and harvest, and their physiological, biochemical, growth, and yield attributes were determined. Similarly, the changes in physicochemical properties of the rhizospheric soil were also examined at the end of each growth stage.
Plant traits
With each treatment and growth stage, the third fully developed leaf from three randomly selected plants were subjected to physiological measurements through non-destructive method. Plant physiological parameters such as photosynthetic rate, transpiration rate, and stomatal conductance were measured using portable photosynthetic system (ADC Bio Scientific LCpro-SD System, UK) between 9.00 a.m. to 12.00 p.m. (Ramya et al. 2021). Plant biochemical traits like malondialdehyde (Heath and Packer 1968), proline (Bates et al. 1973), ascorbic acid (Keller and Schwager 1977), catalase, peroxidase, and superoxide dismutase (Kar and Mishra 1976) were quantified using standard procedures. Furthermore, plant height, root length, pod length, number of nodules, flowers, pods per plant, seeds per pod, total, and 100 grain weight were measured (Dhevagi et al. 2021). The changes in the root morphology were also assessed through Gia Roots software. The roots of blackgram were imaged using a destructive approach, rotating for 360° and capturing 20 snap shots at 18° intervals. These images were then examined for phenotypic characteristics such as maximum number of roots (MNR), average root width (diameter) (ARW), network surface area (NSA), specific root length (SRL), network volume (NV), depth (ND), width (NW), and perimeter (NP) using the GiA Roots Software Framework (Galkovskyi et al. 2012). All the parameters except yield traits were measured at 30 (vegetative), 45 (flowering), and 75th (harvest) day after sowing.
Soil properties
Similar to plant parameters, physico-chemical (water holding capacity, porosity, pH, EC, organic carbon, microbial biomass carbon, NO3-N, NH4-N, available P, and K) and biological properties (β-Glucosidase (Eivazi and Tabatabai 1988), dehydrogenase (Casida et al. 1964), urease, asparaginase (Hoffmann and Teicher 1961), and phosphatase (Tabatabai and Bremner 1972)) of rhizosphere soil were characterized at different plant growth stages using standard procedures.
Additionally, the rhizosphere soil samples (control and 1% PE-MPs) were collected at the flower initiation stage by following the method given by Chen et al. (2019) and subjected to 16 s meta-genomic analysis through destructive method. The DNA was extracted using QIAZEN kit, and the DNA was then tested using GEL check and Nano Drop (260/280 at ~ value of 1.8 to 2). Using primers (16sF:—5′ AGAGTTTGATGMTGGCTCAG3′ and 16sR:—5′ TTACCGCGGCMGCSGGCAC3′) in a thermal cycler with initial denaturation temperature of 95 °C for 3 min, the purified DNA (40 ng) was amplified. Twenty-five cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 15 s, elongation at 72 °C for 2 min, and final extension at 72 °C for 10 min were then performed followed by at 4 °C. Amplified DNA were purified using Ampure beads and quantified using a sensitivity assay kit (QubitdsDNA). Illumina Miseq with 2 × 300PE V3 sequencing kit was used to perform sequencing, and raw data QC was done using FASTQC and MULTIQC. Trimming of adapters and low-quality reads were done using TRIMGALORE. The trimmed reads were further taken for processing steps like merging of paired end reads; chimeria removal and OUT abundance calculation and estimation corrections were achieved by QIIME 2.
Statistical analysis
Factorial CRD was performed for all the experiments, to analyze the differences between the factors and their interactions. The SPSS software was used for computation of results, and the OriginPro 2021 software (origin, Northampton, MA, USA) was used to draw the graphs. The results of each treatment were documented and processed using Microsoft Excel 2016 and were represented as mean ± SD (standard deviation). The differences and similarities among various treatment and their interaction were analyzed through principle component analysis (PCA) using the R software.
Results and discussion
Characteristics of experimental soil and PE-MPs
The experimental soil had a water holding capacity of 34%, while the bulk density was 1.08 g/cc. The porosity was 41.78% with a pH of 8.68 and an electrical conductivity of 0.34 dSm−1. The organic carbon, available nitrogen, phosphorus, and potassium were 0.32%, 267, 25.0, and 323 kg ha−1. Scanning electron microscope with EDAX was used to determine the particle size of MPs, and it was found that the microplastics under study was irregular in shape. The particle size ranged from 6 to 600 µm with oxygen, carbon, potassium, and phosphorus content of 9.09, 90.88, 0.01, and 0.01%, respectively (Supplementary Figure S1). The concentrations and size were fixed based on the previous studies that quantified microplastics in different soils (Fuller and Gautam 2016; Huang et al. 2019; Lian et al. 2021; Sahasa et al. 2023).
Effect on plant traits
The effects of application of PE-MPs at 0.25, 0.50, 0.75, and 1% on various plant parameters at vegetative, flowering, and harvest stages of blackgram are summarized in Table 1.
Physiological traits
In the present study, physiological traits like photosynthetic rate, stomatal conductance, and chlorophyll content significantly declined upon exposure to different concentrations of microplastics. It was observed that compared to control, the maximum reduction in photosynthetic rate was observed at the rate of 8, 5, and 3% during vegetative, flowering, and harvest stages, respectively, when blackgram were grown in soil having 1% PE-MPs (Fig. 1a).
Similarly, the presence of PE-MPs had reduced stomatal conductance at all the growth stages of blackgram (Fig. 1b). The stomatal conductance was lowest during harvest stage, and irrespective of growth stages, the highest impact was observed in T5 (1.00% PE-MPs) exhibiting 36, 33, and 34% reduction during vegetative, flowering, and harvest stages respectively. However, exposure to different concentrations of microplastics did not significantly influence transpiration rate of blackgram, though there were significant differences among various crop growth stages (Fig. 1c).
A significant impact on chlorophyll a content of blackgram leaves was observed at all stages of crop growth owing to PE-MP exposure. The maximum reduction in chlorophyll a and b content of 14 and 11%, respectively, was observed when blackgram was grown in soil having 1.00% PE-MPs compared to control (Fig. 2 a and b). Similar results as like chlorophyll a and b were also observed in total chlorophyll content, wherein a maximum of 13% reduction was observed in T5 (1.00% PE-MPs) (Fig. 2c). In line with the present findings, a reduction in photosynthetic pigments with the addition of 0.3–1.0 g kg−1 PS-NH2 and PS-SO3H in Arabidopsis thaliana (Sun et al. 2020), 0.5% LDPE in common bean (Phaseolus vulgaris L.) (Meng et al. 2021), 0. 1 and 0.01 mg L−1 PSNPs in wheat (Triticum aestivum L.) (Zong et al. 2021), and 7% PE and PVC in soybean (Li et al. 2023) have also been reported. The reason for decrease in photosynthetic activity as observed in the present study might be due to decrease in the quantum yield of PS II reaction centres by MPs (Wu et al. 2019; Li et al. 2021b). Contradictory to the present study, MPs have also been shown to assist the plant photosynthesis by enhancing the alpha-amylase activity and breakdown of starch granules thereby increasing the amount of soluble sugars in seedlings (Bosker et al. 2019; Pignattelli et al. 2020; Qi et al. 2018).
Carotenoids in leaves have recorded 3% increase in case of treatment added with 1.00% PE-MPs compared to control, though being non-significant (Fig. 2d). These results corroborate with the findings of Li et al. (2020), wherein PVC (0.5, 1 and 2% w/w; 100 nm–18 μm) exposure promotes the production of carotenoids in lettuce (Lactua sativa L.). Upon MP exposure, the ROS accumulation in cells might have increased which in turn would have hindered the proteases activity involved in chlorophyll synthesis, thereby disturbing the photosynthetic electron transfer and uptake of nutrient and water by the plants (Gao et al. 2019).
Biochemical traits
Exposure to microplastics in plants generates excess ROS like hydrogen peroxide (H2O2), superoxide anions, singlet oxygen (O−), and hydroxyl radicals (OH−), thereby causing a permanent damage to the plants (Li et al. 2021c; Maity et al. 2020; Zhang et al. 2021a). It has been established that the microplastic’s polymer type and size are mostly responsible for the circumstances that lead to microplastic-induced ROS generation in blackgram.
Under abiotic stress condition, the essential amino acid, proline, is crucial for maintaining plant growth and metabolism. In the present study, a dose-dependent effect was observed in proline content at all the three growth stages (Fig. 3a). The presence of microplastics in soil increased the proline content in crop by approximately 16 and 12% during vegetative and flowering cum harvest stage, respectively, which might have been triggered due to abiotic stress caused by PE-MPs in soil (Rejeb et al. 2014). Superoxide dismutase (SOD), the first line of defence, catalyzes the conversion of harmful O2•- into O2 and H2O2, thereby shielding cells from possible damage. Moreover, it has been reported that different MPs have different effects on SOD enzymes based on their concentration, size, and type. In the present study, SOD activity has increased at the rate of 14 (T4), 16 (T5), and 22% (T4) during vegetative, flowering, and harvest stage, respectively (Fig. 3b), and, the highest activity was recorded in plants exposed to 0.75 and 1.00% PE-MPs. This significant increase is attributed as a result of the elevated ROS levels, which might upregulate the antioxidant producing genes (Li et al. 2013). The findings of the present study corroborate with the findings of Jiang et al. (2019), who described that Vicia faba root tips, compared to control, the SOD activity considerably increased after being exposed to 10, 50, and 100 mg L−1 of 5 µm and 100 nm PS-MPs. Additionally, PS-NPs at 50 and 100 mg L−1 significantly boosted the SOD activity in rice (Oryza sativa L.) roots (Zhou et al. 2021a). On contrary, a dose-dependent reduction in SOD was also reported in rice (Oryza sativa L.) (Wu et al. 2021).
One of the most prevalent water soluble antioxidants in plants is ascorbic acid (AsA), or vitamin C (Shao et al. 2008). The presence of PE-MPs in the current study significantly increased the ascorbic acid production at all the stages. This variation accounted for 11, 10, and 5% increase in T5 (1.00% PE-MPs) during vegetative, flowering, and harvest stages, respectively. The ascorbic acid production was highest during vegetative and flowering stages of crop in treatments with 0.50, 0.75, and 1.00% PE-MPs (Fig. 3c). Likewise, the increase in ascorbic acid due to MPs exposure have also been observed in other crops, including lettuce (Lactua sativa) (Gao et al. 2019), broad bean (Vicia faba) (Jiang et al. 2019), spring barley (Hordeum vulgae) (Li et al. 2021c), cucumber (Cucumis sativus) (Li et al. 2020), and rice (Oryza sativa L.) (Zhou et al. 2021a).
Malondialdehyde formed during the lipid peroxidation is frequently used as an indicator to measure the cell damage caused by stress in plants. The MDA content in the present study significantly inclined with PE-MP exposure at all crop growth stages (Fig. 3d) with the maximum effect in 1% PE-MPs (T5). Moreover, The MDA content was recorded the highest during vegetative stage at a rate of 29% increase in T5. This increase in MDA content is a sign of membrane lipid peroxidation in blackgram (Jiang et al. 2019; Dhevagi et al. 2022a) which might have been triggered by PE-MPs. Similar to the present findings, MDA was significantly affected in radish (Raphanus sativus) and broccoli (Brassica oleraceae var. italica) grown with seven different levels of low-density polyethylene (LDPE) (Lopez et al. 2022).
Peroxidase, in the presence of H2O2, oxidizes a wide range of compounds by converting H2O2 to oxygen and water. In the current study, PE-MP exposure had also increased the peroxidase (POD) activity in blackgram; however, the variations among the treatments were minimal (Fig. 3e). At all the three stages of crop growth, the overall increase in POD activity was observed to be 0.67 (T2), 1 (T3), and 4% (T4 and T5) compared to control. It was also observed that the addition of PE-MPs in soil increased the activity of catalase (Fig. 3f), an important antioxidant enzyme which helps to reduce the ROS damage. The highest catalase activity was recorded during flowering stage, and irrespective of growth stage, highest catalase activity was observed in T5 (1.00% PE-MPs). In contrast to the present findings, Jiang et al. (2019) reported decreased CAT activity in Vicia faba root tips with increasing concentrations of PS microplastics (5 µm).
Growth and yield attributes
It was observed that exposure to different concentrations of PE-MPs had a significant influence on the root length of blackgram at all the stages of crops (Fig. 4a). Maximum reduction in root length of about 15, 11, and 3% was observed during vegetative, flowering, and harvest stage. The root length was affected the most at vegetative stage due to microplastics and particularly in treatments with higher PE-MP concentrations (0.75% and 1.00% PE-MPs). The reduction in root length observed in the present study could be due to the adherence of microplastics which in turn inhibits the water imbibition as evidenced in Supplementary Figure S2 (Kalčíková et al. 2017). Similar results regarding altered root length were also reported in rye grass (Lolium perenne) (Boots et al. 2019), broad bean (Vicia faba) (Jiang et al. 2019), carrot (Daucus carota) (Lozano et al. 2021), wheat (Tritcum aestivum) (Qi et al. 2018), onion (Allium cepa) (Maity et al. 2020), spring barley (Hordeum vulgare) (Li et al. 2021b), and soybean (Glycine max) (Lian et al. 2022). Furthermore, Spanò et al. (2022) also observed a gradual decrease in rice (Oryza sativa L.) root length with increasing PSNPs (0.1 to 1 g L−1) concentration.
The shoot growth is hampered by MPs because they can interfere with the nutrient transfer to the shoots thereby impeding the cell connection in roots (Jiang et al. 2019). Furthermore, MPs might negatively regulate the plant growth-related genes, restrict their expression (Zhou et al. 2021a), and limit the activity of specific enzymes involved in glucose metabolism thereby impairing plant growth and development. Application of various concentrations of PE-MPs had a significant influence on shoot length (Fig. 4b). A maximum reduction in shoot length of about 22, 8, and 3% from the control (T1) was observed during vegetative, flowering, and harvest stages when exposed to PE-MPs at 1%. Nevertheless, unlike the trend in root length, the pattern of shoot length decline was undefined. The ratio of root to shoot was higher in treatments with microplastics than in control during vegetative and harvest stages by 8 (T5 — 1.00% PE-MPs) and 2% (T2 — 0.25% PE-MPs) respectively, while at flowering stage, a decreasing trend was recorded (by 7% when exposed to 1.00% PE-MPs). These findings corroborate with other results wherein, in agricultural soil, the reduction in shoot growth under microplastic exposure has been observed in garden cress (Lepidium sativum L.) at 0.02% (PE) and mixture of PVC and PE (Pignattelli et al. 2020), wheat (Triticum aestivum L.) at 1% (w/w) Bio-MPs (Qi et al. 2018), and rice (Oryza sativa L.) at 50–500 mg L−1 polystyrene (Wu et al. 2021). On contrary, MPs have also been reported to exhibit positive effects on plants like Calama grostis (Lozano and Rillig 2020), Allium fistulosum (De Souza Machado et al. 2019), and Daucus carota (Lozano et al. 2021). This indicates that since roots have direct contact with microplastics, they are more prone to being affected by microplastics than shoot.
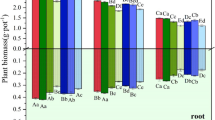
Moreover, PE-MPs in soil had a significant influence on the dry weight at all stages. A maximum reduction of about 13, 9, and 7% was observed during vegetative, flowering, and harvest stage (Fig. 4c). Similar to the trend observed in root length, plant dry weight was also found to be most affected at vegetative stage, followed by flowering stage particularly in treatments with higher PE-MP concentrations (0.75 and 1.00% PE-MPs). In line with the present findings, microplastics were also reported to reduce biomass in maize (Zea mays L.) (Lian et al. 2021), duckweed (Lemna minor L.) (Mateos-Cárdenas et al. 2019), plantain (Plantago lanceolata) (Van Kleunen et al. 2020), broad bean (Vicia faba) (Jiang et al. 2019), and cress (Lepidium sativum L.) (Pignattelli et al. 2021).The number of nodules per plant was significantly affected by the presence of PE-MPs in soil at various concentrations and in particular during vegetative stage (Fig. 4d). The root nodule declined by 29, 27, and 5% (T5 — 1% PE-MPs) during vegetative, flowering, and harvest stage of crop, and this was found to be in consistent with the findings of Bouaicha et al. (2022). On contrary, Meng et al. (2021) reported that root nodule was enhanced at higher concentrations of LDPE-MPs.
The effect of microplastics on soil–plant system differs based on its size, shape, concentration, and polymer type (Bosker et al. 2019; de Souza Machado et al. 2019; Lozano et al. 2021; Wang et al. 2021, 2022). On the other hand, the presence of microplastics at different concentrations had no significant impact on number of flowers per plant, pod weight, and 100 grain weight of blackgram. However, pod length and number of seeds per pod were observed to decrease at higher doses of microplastics in soil (Table 2); nevertheless, the variations were unpredictable and irregular. The morphological traits of roots from all the treatments were imaged at different stages of blackgram and analysed through GiaRoots software. The initial results yielded 20 parameters, out of which 12 parameters (diameter, Ndepth, MaxR, MedR, NwA, Perim, SRL, Nsurf, NWDR, Nvol, Nlen, and Nwidth) were selected to study the effect of polyethylene microplastics on root morphology. The presence of PE-MPs in soil had significant impact on all the 12 GiaRoots-derived root parameters except network depth (Table 3).
The principle component analysis (PCA) is used to determine the association in growth patterns among the treatments and its interactions. The PCA was performed for growth, physiological, biochemical, and root traits at vegetative, flowering, and harvest stage of blackgram to assess the variables which contributed to maximum variance. The analysis resulted in four principle components (PC1, PC2, PC3, and PC4) and the PCs that contributed to > 70% variance was plotted in the biplot. During vegetative stage (Fig. 5a), the proportion of variance (%) of PC1 (Eigenvalue 11.00), PC2 (Eigenvalue 3.044), PC3 (Eigenvalue 1.349), and PC4 (eigenvalue 0.6118) was 68.72, 19.02, 8.43, and 3.82%, respectively. The variance (%) however was observed to be decreased during flowering stage (PC1 (67.55%), PC2 (16.67%), PC3 (12.08%), and PC4 (3.70%)) and harvest stage (PC1 (55.12%), PC2 (20.06%), PC3 (16.38), and PC4 (8.44%)). Treatments with 0.75 and 1% PE-MPs were significantly different from other treatments during all the plant stages (Fig. 5a, b, c). From the results of PCA analysis, it can be observed that occurrence of microplastics in soil had induced stress regulating chemicals (catalase, peroxidase, SOD, MDA, ascorbic acid, proline, and carotenoids) in plants at 0.75 and 1% concentrations. Furthermore, at 0.75 and 1% PE-MPs levels, the growth and physiological parameters of plant are significantly influenced than at 0.25 and 0.50% PE-MPs. Regarding root traits, T1, T2, and T3 contributed most of the weightage during vegetative stage as observed from the biplot graphs, while during flowering and harvest stage the differences among the treatments is lesser.
Effect on soil characteristics
After entering the soil, MPs coalesce with soil organic matter (SOM) and microbial secretions and thereby become embedded in the soil microstructure (Rillig et al. 2017). This causes changes in the soil physicochemical properties through increasing the water-holding capacity and soil porosity, decreasing the moisture permeability, bulk density, altering soil pH, and thereby disturbing the integrity of soil structure (Lwanga et al. 2017; Zhao et al. 2021).
Physico-chemical properties
In the current study, the effect of PE-MPs on soil was examined at different growth stages. The effects of application of PE-MPs at 0.25, 0.50, 0.75, and 1% PE-MPs on physico-chemical and biological properties of rhizosphere soil at vegetative, flowering, and harvest stage of blackgram are given in Table 4. Bulk density and porosity of the soil are inversely proportional to each other and the effect of different concentrations of polyethylene microplastics (PE-MPs) on bulk density and porosity is shown in Fig. 6a. The presence of microplastics in soil reduced the bulk density up to 7%, and as a consequence, the pore space percentage in soil increased up to 15%. de Souza Machado et al. (2019) reported that soil bulk density was decreased by PS, PP, PET, PES, and PEHD at 2.0% w/w. Similarly, Zhang et al. (2019a) indicated that higher concentration of MPs with more than 30 µm size significantly decreased the bulk density. However, the PE-MPs had no significant effect on soil pH (Fig. 6b), though a maximum increase of 2% was recorded in T5 (1.00% PE-MPs) at different blackgram growth stages. These findings were in correspondence with the results of Jian et al. (2022) who reported an increase in soil pH at 0.1 and 1% microplastics and Zhao et al. (2021) who also reported a significant increase in pH with the addition of polyamide, polyester, polyurethane, and polycarbonate microplastics. Similarly, it was reported that PS-MPs and PTFE can lower soil pH (Dong et al. 2021), while PA-MPs and HDPE-MPs could increase it (Yang et al. 2021a). On contrary, Boots et al. (2019) indicated a decline in soil pH with the addition of synthetic fibers, polylactic acid, and high-density polyethylene. Meanwhile, the soil EC was also found to increase with increasing microplastics dosage and the increased was from 0.49, 0.42, and 0.33 dS m−1 in control (T1) to 0.53, 0.47, and 0.37 dS m−1 in the treatments with 1% PE-MPs during vegetative, flowering, and harvest stages of blackgram (Fig. 6b). The present findings are in accordance with the results of Jian et al. (2022), wherein an increase in soil EC was observed in the presence of 0.1 and 1% microplastics.
In addition, the soil organic carbon was observed to have increased after the addition of microplastics to the soil (Fig. 6c). The rate of increase in soil organic carbon ranged from 6 to 15, 18 to 21, and 23 to 26% in treatments with microplastics. Additionally, it was also recorded that the soil microbial biomass carbon (MBC) had increased with increase in microplastics concentrations in the soil (Fig. 6c). The soil MBC content increased at the rate of 6, 7, and 8% during vegetative, flowering, and harvest stages in T5 (1.00% PE-MPs). This might be attributed to the hike in soil organic carbon, which in turn promoted soil enzyme activities by triggering the microbial community growth. These results corroborate with the findings of Liu et al. (2017) who reported an increase in dissolved organic carbon with the addition of polypropylene powder. Similar findings were also reported by Rillig (2018), Zumstein et al. (2018), Zhang et al. (2019a), and Ren et al. (2021). Contrastingly, Zhang et al. (2020) indicated that though there are no significant variations in soil total organic carbon due to the addition of polyester microfibers, the organic carbon content in the large macro-aggregates significantly reduced, while contrasting changes were observed in small macro-aggregates. Yu et al. (2021b) reported that after 150 days of incubation with polyethylene, the total organic carbon, and dissolved organic carbon significantly declined.
The changes in soil available nutrients due to PE-MPs at different growth stages of blackgram are graphically represented in Fig. 6d. During different growth stages of blackgram, the available NO3-N was highest in T5 (1.00% PE-MPs) which accounted for 17, 22, and 22% increase at consecutive stages, while NH4-N increased by 10, 14, and 23% in T5 at respective crop growth stages compared to control (T1). Contrastingly, the soil available P and K decreased with increasing concentrations of PE-MPs in soil. The maximum reduction in available P was recorded in T5 (1%) showing 20, 25, and 26% reduction during vegetative, flowering, and harvest stages, respectively. Likewise, the available K was lowest in treatments with high PE-MPs levels (T4 and T5) recording 10, 14, and 16% reduction in T5 at vegetative, flowering, and harvest stages, respectively. Chen et al. (2020) observed a significant reduction in NH4-N with the exposure to polylactic acid, while the NO3-N and NO2-N increased significantly. Furthermore, Yan et al. (2021b) indicated a significant reduction in NO3-N and available phosphorus in paddy soil with exposure to polyvinyl chloride microplastics. Several other studies have also reported similar results (Liu et al. 2017; Yu et al. 2020; Yang et al. 2021b).
Biological properties
In soil, during the biochemical conversion of organic matter, soil enzymes play a vital role, and the activities of these enzymes are strongly correlated with soil organic matter (SOM), soil physicochemical characteristics, and microbial activity and/or biomass. Hence, studies on how soil microplastics influence soil enzymes are essential. In the present study, it was observed that the presence of PE-MPs in soil had positively influenced enzymes involved in C and N cycles like dehydrogenase (DHA), β-glucosidase (BG), urease (URE), and asparaginase (ASP) activity, while enzymes involved in P cycle like acid (ACP) and alkaline phosphatase (ALP) were negatively affected (Fig. 6e).
The soil dehydrogenase activity was highest during the vegetative stage (23% increase from control), while at flowering and harvest stages of blackgram, 15 and 17% increase was recorded with 1% PE-MP application. The β-glucosidase activity was also enhanced by PE-MPs by maximum of 7, 10, and 13% at vegetative, flowering, and harvest stages of blackgram, respectively, with the application of 1% PE-MPs. The increase in soil organic carbon in treatment with microplastics might be due to MPs-enhanced dehydrogenase and β-glucosidase activities in soil. Furthermore, soil urease activity was highest at harvest stage (37%) with 1% PE-MP application. Similar to the current findings, Lian et al. (2021) observed 8% increase in the soil urease activity with maize as the test crop. Contrastingly, Fu et al. (2022) reported that PE-MPs (0.2%) had no significant effect on dehydrogenase and urease activity of maize rhizosphere under acidic soil (pH 5.17 ± 0.03) condition. The soil used in the current experimental study was alkaline, and PE-MP dosage was fixed up to 1% which would have attributed for the contrasting results. Similarly, asparaginase activity was also improved by PE-MPs up to 30% with 1% PE-MPs (T5) at all the crop growth stages. The end product of soil urease is ammonium and CO2 (Rao et al. 2014), and as discussed above, the increase in urease and asparaginase activity of soil triggered by PE-MPs might have led to increase in ammoniacal nitrogen in soil.
Contrasting to the effect of PE-MPs on other soil enzymes, acid and alkaline phosphatase activity in soil was observed to decrease from control (T1). The acid phosphatase activity in soil declined at the rate of 3–25, 2–23, and 7–27%, and as for alkaline phosphatase activity, the range of decline varied from 4 to 10, 9 to 14, and 7 to 31% at vegetative, flowering, and harvest stages, respectively. Parallel results were also reported by Rao et al. (2014), Lian et al. (2021), and Fu et al. (2022). The activities of four out of six enzymes analyzed were observed to have increased in soil with different microplastics levels, which might be the cause for enhanced soil microbial biomass carbon. Similarly, since the enzymes involved in P-cycling (acid and alkaline phosphatase) were down-regulated, available phosphorus in soil also decreased in the presence of PE-MPs. The decrease in acid and alkaline phosphatase enzyme activity could be a result of decrease in the abundance of Bacillus genus in soil where microplastics are employed as evidenced from the results of 16 s metagenomics analysis.
Metagenomic analysis
The results of comparative analysis revealed that the soils had 253 common elements and 57 and 21 unique microbial groups were found in both T1 and T5 rhizosphere soil, respectively (Fig. 6f). The percent composition of each phylum in control (T1) and 1% PE-MPs (T5) is represented in Fig. 7. In both the soil samples, the dominant phylum was Proteobacteria (38.78–41.86%), followed by Actinobacteria (23.18–27.49%), Firmicutes (19.85–26.12%), Acidobacteria (2.27–4.20%), Bacteroidetes (2.19–2.27%), Chloroflexi (1.12–1.94%), Gemmatimonadetes (1.23–1.67%), Plantomycetes (0.62–1.79%), Nitrospirae (0.73–1.27%), Cyanobacteria (0.27–0.31%), and others (0.33–0.52%). The addition of 1% PE-MPs resulted in higher abundance of Proteobacteria, Actinobacteria, and Firmicutes compared to control. The results of statistical analysis suggested no significant differences in the bacterial community composition at phylum level between control and soil with 1% PE-MPs, except Proteobacteria and Firmicutes.
At genus level, Bacillus (19%) was predominant in control (T1), while in 1% PE-MPs (T5), Rubrobacter (28%) community was dominant. Rubrobacter is a radio-tolerant group and is involved in organic matter decomposition. Similar results regarding its increase in abundance was also reported when exposed to polypropylene carbonate incorporated soil (Liang et al. 2022). However, the reason for increase in its abundance is not yet clearly known. Microvirga was found exclusively in T5, while the relative abundance of Gemmatimonas declined from T1 to T5 (Fig. 8). With Microvirga being root nodulating bacteria, the root nodulation frequency must have increased in plants grown in T5 soil; however, the opposite effect was observed in the current study. The cause for this has to be explored further in future through studies with specific focus on root nodulating microbial groups and microplastics. On further examination, it was observed that the relative abundance of Streptomyces genus also declined from T1 to T5. This might be due to oxidative damage caused by microplastics in Streptomyces which is a gram-positive bacterium with no extracellular polymeric substances surrounding plasma membrane to protect against microplastics (Liu et al. 2021). The community heterogeneity (α-diversity) in each treatment was measured using Shannon–Wiener H index and Simpson D index. The results suggest that control (T1) had higher relative genus richness (H = 2.73; D = 0.243), compared to rhizosphere soil with 1% PE-MPs (H = 2.42; D = 0.277). Whereas, the beta-diversity (measured by Sorenson’s co-efficient) was 0.867 which suggests that the communities overlap by more than 80% with slight differences.
Microplastics can influence soil microbial community by acting as a distinct habitat for microbial enrichment and colonization (Zhang et al. 2019b; Ren et al. 2020; Seeley et al. 2020; Qiang et al. 2023). In the current study, minor significant differences in alpha-diversity (Shannon index, T1 — 2.72 and T5 — 2.43) and community structure were observed between control and 1% PE-MPs as discussed above. These results were similar to the observations of Qi et al. (2020) who suggested that the effect of LDPE-MPs on bacterial community composition was relatively lower than bio-degradable microplastics. This is because bio-degradable microplastics after their degradation in soil act as carbon or in some cases nutrient sources for rhizosphere microbial communities (Qi et al. 2020); while PE-MPs although add to soil organic carbon, the carbon is inert and might not be available for microbes for their metabolism. The minor changes observed between control and 1% PE-MPs could be either as a result of changes in other soil parameters like soil bulk density and porosity brought about by PE-MPs or PE-MPs acting as habitat for specific community of bacteria (i.e., Proteobacteria and Firmicutes). Furthermore, rhizosphere soil from 1% PE-MPs was observed to have Escherichia and Salmonella which are pathogenic organisms as confirmed in previous studies (Wu et al. 2019; Kaur et al. 2021) which suggested that microplastics act as carrier of pathogenic microbes. Also, control soil had 57 unique microbial groups, while T5 had 21 exclusive microbial groups. Therefore, the hypothesis that microplastics in soil could alter microbial composition holds true as results of the metagenomics analysis suggest that relative richness of genus was affected by addition of PE-MPs, while the abundance of specific groups of microbes was observed to increase.
Conclusion
Microplastics have been reported to significantly alter soil properties thereby affecting the growth and yield of agricultural crops. The outcomes of current study suggest that constant contact between plant and microplastics in soil would inevitably alter the plant growth either directly or indirectly. Amongst various concentrations under study, the maximum phytotoxic effect was observed when exposed to 1% PE-MPs. Hence, it is imperative to understand that the sensitivity of plants to microplastics might be dependent on crop growth stage as observed in the current study; wherein, higher impact was found during vegetative stage in most of the plant traits. Moreover, the effects of microplastics on different plant traits could be insignificant, mild, significant, or very severe. For instance, in this study, the impact of PE-MPs on root nodulation was significant, while the impact on other plant growth traits were mild and yield was unaffected. Although the yield was not affected as much as physiological and biochemical functions of blackgram, there could be compromise in the seed quality. Hence, in the future studies, along with plant growth traits, the changes in yield quality parameters due to microplastics have to be examined. Furthermore, it is also essential to compare the sensitivity of every crop to microplastics in soil, establish sensitivity index which would aid in crop selection if an agriculture land is heavily polluted with microplastics.
Data availability
Not applicable.
References
Auta HS, Emenike C, Fauziah S (2017) Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ Int 102:165–176. https://doi.org/10.1016/j.envint.2017.02.013
Bashour II, Sayegh AH (2007) Methods of analysis for soils of arid and semi-arid regions. FAO
Bates LS, Waldren RP, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207. https://doi.org/10.1007/BF00018060
Boots B, Russell CW, Green DS (2019) Effects of microplastics in soil ecosystems: above and below ground. Environ Sci Technol 53(19):11496–11506. https://doi.org/10.1021/acs.est.9b03304
Bosker T, Bouwman LJ, Brun NR, Behrens P, Vijver MG (2019) Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 226:774–781. https://doi.org/10.1016/j.chemosphere.2019.03.163
Bouaicha O, Tiziani R, Maver M, Lucini L, Miras-Moreno B, Zhang L, Trevisan M, Cesco S, Borruso L, Mimmo T (2022) Plant species-specific impact of polyethylene microspheres on seedling growth and the metabolome. Sci Total Environ 840:156678. https://doi.org/10.1016/j.scitotenv.2022.156678
Casida LE Jr, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98(6):371–376
Chen Z, Maltz MR, Cao J, Yu H, Shang H, Aronson E (2019) Elevated O3 alters soil bacterial and fungal communities and the dynamics of carbon and nitrogen. Sci Total Environ 677:272–280. https://doi.org/10.1016/j.scitotenv.2019.04.310
Chen H, Wang Y, Sun X, Peng Y, Xiao L (2020) Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 243:125271. https://doi.org/10.1016/j.chemosphere.2019.125271
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62(12):2588–2597. https://doi.org/10.1016/j.marpolbul.2011.09.025
De Silva Y, Rajagopalan U, Kadono H (2021) Microplastics on the growth of plants and seed germination in aquatic and terrestrial ecosystems. Glob J Environ Sci Manag 7(3):347–368. https://doi.org/10.22034/GJESM.2021.03.03
de Souza Machado AA, Kloas W, Zarfl C, Hempel S, Rillig MC (2018) Microplastics as an emerging threat to terrestrial ecosystems. Glob Change Biol 24(4):1405–1416. https://doi.org/10.1111/gcb.14020
de Souza Machado AA, Lau CW, Kloas W, Bergmann J, Bachelier JB, Faltin E, Becker R, Görlich AS, Rillig MC (2019) Microplastics can change soil properties and affect plant performance. Environ Sci Technol 53(10):6044–6052. https://doi.org/10.1021/acs.est.9b01339
Dhevagi P, Ramya A, Priyatharshini S, Poornima R (2021) Effect of elevated tropospheric ozone on Vigna Mungo L. varieties. Ozone: Sci Eng 44(6):566–586. https://doi.org/10.1080/01919512.2021.2009332
Dhevagi P, Poornima R, Keerthi Sahasa RG, Ramya A, Karthika S, Sivasubramanian K (2022a) The crux of microplastics in soil—a review. Int J Environ Anal Chem 1–33
Dhevagi P, Ramya A, Poornima R, Chandrakumar K (2022b) Effectiveness of ethylene diurea in ameliorating ozone stress in blackgram varieties (Vigna mungo L.). Arch Agron Soil Sci 1–16. https://doi.org/10.1080/03650340.2022.2099542
Dong Y, Gao M, Qiu W, Song Z (2021) Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol Environ Saf 211:111899. https://doi.org/10.1016/j.ecoenv.2021.111899
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soils. Soil Biol Biochem 20(5):601–606
Fu Q, Lai J-L, Ji X-H, Luo Z-X, Wu G, Luo X-G (2022) Alterations of the rhizosphere soil microbial community composition and metabolite profiles of Zea mays by polyethylene-particles of different molecular weights. J Hazard Mater 423:127062. https://doi.org/10.1016/j.jhazmat.2021.127062
Fuller S, Gautam A (2016) A procedure for measuring microplastics using pressurized fluid extraction. Environ Sci Technol 50(11):5774–5780. https://doi.org/10.1021/acs.est.6b00816
Galkovskyi T, Mileyko Y, Bucksch A, Moore B, Symonova O, Price CA, Topp CN, Iyer-Pascuzzi AS, Zurek PR, Fang S (2012) GiA Roots: software for the high throughput analysis of plant root system architecture. BMC Plant Biol 12(1):1–12. https://doi.org/10.1186/1471-2229-12-116
Gao M, Liu Y, Song Z (2019) Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosaHort). Chemosphere 237:124482. https://doi.org/10.1016/j.chemosphere.2019.124482
Geyer R (2020) A Brief History of Plastics. In: Streit-Bianchi M, Cimadevila M, Trettnak W (eds) Mare Plasticum — the plastic sea. Springer, Cham. https://doi.org/10.1007/978-3-030-38945-1_2
Gündoğdu S, Çevik C, Güzel E, Kilercioğlu S (2018) Microplastics in municipal wastewater treatment plants in Turkey: a comparison of the influent and secondary effluent concentrations. Environ Monit Assess 190(11):1–10. https://doi.org/10.1007/s10661-018-7010-y
Guo J-J, Huang X-P, Xiang L, Wang Y-Z, Li Y-W, Li H, Cai Q-Y, Mo C-H, Wong M-H (2020) Source, migration and toxicology of microplastics in soil. Environ Int 137:105263. https://doi.org/10.1016/j.envint.2019.105263
Hanway JJ, Heidal H (1952) Soil analysis methods as used in Iowa State College Soil Testing Laboratory. Iowa State College of Agriculture Bulletin 57:1–31. https://doi.org/10.12691/ajwr-7-1-5
He D, Luo Y, Lu S, Liu M, Song Y, Lei L (2018) Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC, Trends Anal Chem 109:163–172. https://doi.org/10.1016/j.trac.2018.10.006
He P, Chen L, Shao L, Zhang H, Lü F (2019) Municipal solid waste (MSW) landfill: a source of microplastics?-Evidence of microplastics in landfill leachate. Water Res 159:38–45. https://doi.org/10.1016/j.watres.2019.04.060
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hoffmann GG, Teicher K (1961) Ein kolorimetrisches verfahren zur bestimmung der ureaseaktivität in Böden. Z Pflanzenernähr Bodenkd 95(1):55–63
Huang Y, Zhao Y, Wang J, Zhang M, Jia W, Qin X (2019) LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ Pollut 254:112983. https://doi.org/10.1016/j.envpol.2019.112983
Iqbal B, Javed Q, Khan I, Tariq M, Ahmad N, Elansary HO., ... Du D (2023) Influence of soil microplastic contamination and cadmium toxicity on the growth, physiology, and root growth traits of Triticum aestivum L. S Afr J Bot 160;369–375. https://doi.org/10.1016/j.sajb.2023.07.025
Jackson ML (2005) Soil chemical analysis: advanced course. UW-Madison Libraries parallel press
Jian M, Niu J, Li W, Huang Y, Yu H, Lai Z, Liu S, Xu EG (2022) How do microplastics adsorb metals? a preliminary study under simulated wetland conditions. Chemosphere 309:136547. https://doi.org/10.1016/j.chemosphere.2022.136547
Jiang X, Chen H, Liao Y, Ye Z, Li M, Klobučar G (2019) Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ Pollut 250:831–838. https://doi.org/10.1016/j.envpol.2019.04.055
Kalčíková G, Gotvajn AŽ, Kladnik A, Jemec A (2017) Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ Pollut 230:1108–1115. https://doi.org/10.1016/j.envpol.2017.07.050
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57(2):315–319. https://doi.org/10.1104/pp.57.2.315
Kaur K, Reddy S, Barathe P, Oak U, Shriram V, Kharat SS., Govarthanan M, Kumar V (2021) Microplastic-associated pathogens and antimicrobial resistance in environment. Chemosphere 133005. https://doi.org/10.1016/j.chemosphere.2021.133005
Keller T, Schwager H (1977) Air pollution and ascorbic acid. Eur J for Pathol 7(6):338–350. https://doi.org/10.1111/j.1439-0329.1977.tb00603.x
Klein M, Fischer EK (2019) Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci Total Environ 685:96–103. https://doi.org/10.1016/j.scitotenv.2019.05.405
Lambert S., Wagner M. (2018). Microplastics are contaminants of emerging concern in freshwater environments: an overview (pp 1–23). Springer International Publishing. https://doi.org/10.1007/978-3-319-61615-5_1,
Lau WW, Shiran Y, Bailey RM, Cook E, Stuchtey MR, Koskella J, Velis CA, Godfrey L, Boucher J, Murphy MB (2020) Evaluating scenarios toward zero plastic pollution. Science 369(6510):1455–1461. https://doi.org/10.1126/science.aba9475
Li S, Li S-K, Gan R-Y, Song F-L, Kuang L, Li H-B (2013) Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind Crops Prod 51:289–298. https://doi.org/10.1016/j.indcrop.2013.09.017
Li Z, Li Q, Li R, Zhao Y, Geng J, Wang G (2020) Physiological responses of lettuce (Lactuca sativa L.) to microplastic pollution. Environmental Science and Pollution Research 27(24):30306–30314. https://doi.org/10.1007/s11356-020-09349-0
Li J, Ouyang Z, Liu P, Zhao X, Wu R, Zhang C, Lin C, Li Y, Guo X (2021) Distribution and characteristics of microplastics in the basin of Chishui River in Renhuai, China. Sci Total Environ 773:145591. https://doi.org/10.1016/j.scitotenv.2021.145591
Li Q, Zeng A, Jiang X, Gu X (2021) Are microplastics correlated to phthalates in facility agriculture soil? J Hazard Mater 412:125164. https://doi.org/10.1016/j.jhazmat.2021.125164
Li S, Wang T, Guo J, Dong Y, Wang Z, Gong L, Li X (2021) Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J Hazard Mater 415:125614. https://doi.org/10.1016/j.jhazmat.2021.125614
Li H, Song F, Song X, Zhu K, Lin Q, Zhang J, Ning G (2023) Single and composite damage mechanisms of soil polyethylene/polyvinyl chloride microplastics to the photosynthetic performance of soybean (Glycine max [L.] merr.). Front Plant Sci 13:110. https://doi.org/10.3389/fpls.2022.1100291
Lian J, Liu W, Meng L, Wu J, Zeb A, Cheng L, Lian Y, Sun H (2021) Effects of microplastics derived from polymer-coated fertilizer on maize growth, rhizosphere, and soil properties. J Clean Prod 318:128571. https://doi.org/10.1016/j.jclepro.2021.128571
Lian Y, Liu W, Shi R, Zeb A, Wang Q, Li J, Zheng Z, Tang J (2022) Effects of polyethylene and polylactic acid microplastics on plant growth and bacterial community in the soil. J Hazard Mater 435:129057. https://doi.org/10.1016/j.jhazmat.2022.129057
Liang J, Zhang J, Yao Z, Luo S, Tian L, Tian C, Sun Y (2022) Preliminary findings of polypropylene carbonate (PPC) plastic film mulching effects on the soil microbial community. Agriculture 12(3):406. https://doi.org/10.3390/agriculture12030406
Liu H, Yang X, Liu G, Liang C, Xue S, Chen H, Ritsema CJ, Geissen V (2017) Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 185:907–917. https://doi.org/10.1016/j.chemosphere.2017.07.064
Liu X, Ma J, Yang C, Wang L, Tang J (2021) The toxicity effects of nano/microplastics on an antibiotic producing strain-Streptomyces coelicolor M145. Sci Total Environ 764:142804. https://doi.org/10.1016/j.scitotenv.2020.142804
Lohmann R (2017) Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans—but should microplastics be considered POPs themselves? Integr Environ Assess Manag 13(3):460–465. https://doi.org/10.1002/ieam.1914
López MD, Toro MT, Riveros G, Illanes M, Noriega F, Schoebitz M, Garcia - Viguera C, Moreno DA (2022) Brassica sprouts exposed to microplastics: effects on phytochemical constituents. Sci Total Environ 823;153796. https://doi.org/10.1016/j.scitotenv.2022.153796
Lozano YM, Rillig MC (2020) Effects of microplastic fibers and drought on plant communities. Environ Sci Technol 54(10):6166–6173. https://doi.org/10.1021/acs.est.0c01051
Lozano YM, Lehnert T, Linck LT, Lehmann A, Rillig MC (2021) Microplastic shape, polymer type, and concentration affect soil properties and plant biomass. Front Plant Sci 12:616645. https://doi.org/10.3389/fpls.2021.616645
Lwanga EH, Gertsen H, Gooren H, Peters P, Salánki T, van der Ploeg M., Besseling E, Koelmans AA, Geissen V (2017) Incorporation of microplastics from litter into burrows of Lumbricusterrestris. Environ Pollut 220:523–531. https://doi.org/10.1016/j.envpol.2016.09.096
Ma Y, Huang A, Cao S, Sun F, Wang L, Guo H, Ji R (2016) Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ Pollut 219:166–173. https://doi.org/10.1016/j.envpol.2016.10.061
Mahon AM, O’Connell B, Healy MG, O’Connor I, Officer R, Nash R, Morrison L (2017) Microplastics in sewage sludge: effects of treatment. Environ Sci Technol 51(2):810–818. https://doi.org/10.1021/acs.est.6b04048
Maity S, Chatterjee A, Guchhait R, De S, Pramanick K (2020) Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J Hazard Mater 385:121560. https://doi.org/10.1016/j.jhazmat.2019.121560
Margesin R, Schinner F (2005) Manual for soil analysis-monitoring and assessing soil bioremediation, Vol 5, Springer Science & Business Media 1–355
Mateos-Cárdenas A, Scott DT, Seitmaganbetova G, van Pelt Frank N, AK JM (2019) Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Sci Total Environ 689:413–421. https://doi.org/10.1016/j.scitotenv.2019.06.359
Meng F, Yang X, Riksen M, Xu M, Geissen V (2021) Response of common bean (Phaseolus vulgaris L.) growth to soil contaminated with microplastics. Sci Total Environ 755:142516. https://doi.org/10.1016/j.scitotenv.2020.142516
Meng F, Yang X, Riksen M, Geissen V (2022) Effect of different polymers of microplastics on soil organic carbon and nitrogen — a mesocosm experiment. Environ Res 204(Pt A):111938. https://doi.org/10.1016/j.envres.2021.111938
Nizzetto L, Bussi G, Futter MN, Butterfield D, Whitehead PG (2016) A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ Sci Process Impacts 18(8):1050–1059. https://doi.org/10.1039/c6em00206d
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture
Pignattelli S, Broccoli A, Renzi M (2020) Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci Total Environ 727:138609. https://doi.org/10.1016/j.scitotenv.2020.138609
Pignattelli S, Broccoli A, Piccardo M, Felline S, Terlizzi A, Renzi M (2021) Short-term physiological and biometrical responses of Lepidium sativum seedlings exposed to PET-made microplastics and acid rain. Ecotoxicol Environ Saf 208:111718. https://doi.org/10.1016/j.ecoenv.2020.111718
PlasticsEurope (2021) Plastics—the facts 2020. PlasticsEurope. Available at: https://www.plasticseurope.org/de. Accessed on Feb 2022
Qi Y, Yang X, Pelaez AM, Lwanga EH, Beriot N, Gertsen H, Garbeva P, Geissen V (2018) Macro-and micro-plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci Total Environ 645:1048–1056. https://doi.org/10.1016/j.scitotenv.2018.07.229
Qi Y, Ossowicki A, Yang X, Lwanga EH, Dini-Andreote F, Geissen V, Garbeva P (2020) Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J Hazard Mater 387:121711. https://doi.org/10.1016/j.jhazmat.2019.121711
Qiang L, Hu H, Li G, Xu J, Cheng J, Wang J, Zhang R (2023) Plastic mulching, and occurrence, incorporation, degradation, and impacts of polyethylene microplastics in agroecosystems. Ecotoxicol Environ Saf 263:115274. https://doi.org/10.1016/j.ecoenv.2023.115274
Ramya A, Dhevagi P, Priyatharshini S, Chandrasekhar C, Valliappan K, Venkataramani S (2021) Physiological and biochemical response of rice cultivars (Oryza sativa L.) to elevated ozone. Ozone: Sci Eng 43(4):363–377
Rao M, Scelza R, Gianfreda L (2014) Soil enzymes. Enzymes in agricultural sciences. Foster City: OMICS Group eBooks:10–43. https://doi.org/10.2136/sssabookser5.2.c37
Rejeb KB, Abdelly C, Savouré A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80:278–284. https://doi.org/10.1016/j.plaphy.2014.04.007
Ren X, Tang J, Liu X, Liu Q (2020) Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ Pollut 256:113347. https://doi.org/10.1016/j.envpol.2019.113347
Ren X, Tang J, Wang L, Liu Q (2021) Microplastics in soil-plant system: effects of nano/microplastics on plant photosynthesis, rhizosphere microbes and soil properties in soil with different residues. Plant Soil 462(1):561–576. https://doi.org/10.1007/s11104-021-04869-1
Reynolds W, Topp GC, Carter M, Gregorich E (2008) Soil water analyses: principles and parameters. Soil sampling and methods of analysis. 2nd ed. CRC Press, Boca Raton, FL:913–939.
Rillig MC (2018) Microplastic disguising as soil carbon storage. ACS Publications. https://doi.org/10.1021/acs.est.8b02338
Rillig MC, Ingraffia R, de Souza Machado AA (2017) Microplastic incorporation into soil in agroecosystems. Front Plant Sci 8:1805. https://doi.org/10.3389/fpls.2017.01805
Rillig MC, Lehmann A, de Souza Machado AA, Yang G (2019) Microplastic effects on plants. New Phytol 223(3):1066–1070. https://doi.org/10.1111/nph.15794
Rong L, Zhao L, Zhao L, Cheng Z, Yao Y, Yuan C, Wang L, Sun H (2021) LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci Total Environ 773:145640. https://doi.org/10.1016/j.scitotenv.2021.145640
Sahasa RGK, Dhevagi P, Poornima R, Ramya A, Moorthy PS, Alagirisamy B, Karthikeyan S (2023) Effect of polyethylene microplastics on seed germination of Blackgram (Vigna mungo L.) and Tomato (Solanum lycopersicum L.). Environ Adv 11:100349. https://doi.org/10.1016/j.envadv.2023.100349
Seeley ME, Song B, Passie R, Hale RC (2020) Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat Commun 11(1):1–10. https://doi.org/10.1038/s41467-020-16235-3
Shao HB, Chu LY, Jaleel CA, Zhao CX (2008) Water-deficit stress-induced anatomical changes in higher plants. CR Biol 331(3):215–225. https://doi.org/10.1016/j.crvi.2008.01.002
Silva AL, Prata JC, Duarte AC, Soares AM, Barceló D, Rocha-Santos T (2021) Microplastics in landfill leachates: the need for reconnaissance studies and remediation technologies. Case Stud Chem Environ Eng 3:100072. https://doi.org/10.1016/j.cscee.2020.100072
Soundarya M, Sujatha K (2023) A pilot-scale study on microplastics on the growth of Vigna radiata (mung bean). Biochem Cell Arch 23(1):163–167
Spanò C, Muccifora S, Castiglione MR, Bellani L, Bottega S, Giorgetti L (2022) Polystyrene nanoplastics affect seed germination, cell biology and physiology of rice seedlings in-short term treatments: evidence of their internalization and translocation. Plant Physiol Biochem 172:158–166. https://doi.org/10.1016/j.plaphy.2022.01.012
Statista (2021) Pulses in India — statistics and facts. Statista Research Department. https://www.statista.com/topics/5757/pulses-in-India/#dossierSummary__chapter2. Accessed March 2021
Su Y, Zhang Z, Wu D, Zhan L, Shi H, Xie B (2019) Occurrence of microplastics in landfill systems and their fate with landfill age. Water Res 164:114968. https://doi.org/10.1016/j.watres.2019.114968
Subbiah B, Asija G (1956) A rapid processor of determination of available nitrogen in nitrogen in soil. Curr Sci 25:259–260
Sun X-D, Yuan X-Z, Jia Y, Feng L-J, Zhu F-P, Dong S-S, Liu J, Kong X, Tian H, Duan J-L (2020) Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat Nanotechnol 15(9):755–760. https://doi.org/10.1038/s41565-020-0707-4
Tabatabai MA, Bremner JM (1972) Assay of urease activity in soils. Soil Biol Biochem 4(4):479–487
van Schothorst B, Beriot N, Huerta Lwanga E, Geissen V (2021) Sources of light density microplastic related to two agricultural practices: the use of compost and plastic mulch. Environments 8(4):36. https://doi.org/10.3390/environments8040036
vanKleunen M, Brumer A, Gutbrod L, Zhang Z (2020) A microplastic used as infill material in artificial sport turfs reduces plant growth. Plants, People, Planet 2(2):157–166. https://doi.org/10.1002/ppp3.10071
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Wang F, Zhang X, Zhang S, Zhang S, Adams CA, Sun Y (2020) Effects of co-contamination of microplastics and Cd on plant growth and Cd accumulation. Toxics 8(2):36. https://doi.org/10.3390/toxics8020036
Wang L, Liu Y, Kaur M, Yao Z, Chen T, Xu M (2021) Phytotoxic effects of polyethylene microplastics on the growth of food crops soybean (Glycine max) and mung bean (Vigna radiata). Int J Environ Res Public Health 18(20):10629. https://doi.org/10.3390/ijerph182010629
Wang F, Wang Q, Adams CA, Sun Y, Zhang S (2022) Effects of microplastics on soil properties: current knowledge and future perspectives. J Hazard Mater 424:127531. https://doi.org/10.1016/j.jhazmat.2021.127531
Weithmann N, Möller JN, Löder MG, Piehl S, Laforsch C, Freitag R (2018) Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci Adv 4(4):eaap8060. https://doi.org/10.1126/sciadv.aap8060
Wu Y, Guo P, Zhang X, Zhang Y, Xie S, Deng J (2019) Effect of microplastics exposure on the photosynthesis system of freshwater algae. J Hazard Mater 374:219–227. https://doi.org/10.1016/j.jhazmat.2019.04.039
Wu J, Liu W, Zeb A, Lian J, Sun Y, Sun H (2021) Polystyrene microplastic interaction with Oryza sativa: toxicity and metabolic mechanism. Environ Sci Nano 8(12):3699–3710. https://doi.org/10.1039/D1EN00636C
Ya H, Jiang B, Xing Y, Zhang T, Lv M, Wang X (2021) Recent advances on ecological effects of microplastics on soil environment. Sci Total Environ 798:149338. https://doi.org/10.1016/j.scitotenv.2021.149338
Yan Y, Zhu F, Zhu C, Chen Z, Liu S, Wang C, Gu C (2021) Dibutyl phthalate release from polyvinyl chloride microplastics: influence of plastic properties and environmental factors. Water Res 204:117597. https://doi.org/10.1016/j.watres.2021.117597
Yan Y, Chen Z, Zhu F, Zhu C, Wang C, Gu C (2021) Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. Bull Environ Contam Toxicol 107(4):602–609. https://doi.org/10.1007/s00128-020-02900-2
Yang M, Huang D-Y, Tian Y-B, Zhu Q-H, Zhang Q, Zhu H-H, Xu C (2021) Influences of different source microplastics with different particle sizes and application rates on soil properties and growth of Chinese cabbage (Brassica chinensis L.). Ecotoxicol Environ Saf 222:112480. https://doi.org/10.1016/j.ecoenv.2021.112480
Yang W, Cheng P, Adams CA, Zhang S, Sun Y, Yu H, Wang F (2021) Effects of microplastics on plant growth and arbuscular mycorrhizal fungal communities in a soil spiked with ZnO nanoparticles. Soil Biol Biochem 155:108179. https://doi.org/10.1016/j.soilbio.2021.108179
Yu H, Zhang X, Hu J, Peng J, Qu J (2020) Ecotoxicity of polystyrene microplastics to submerged carnivorous Utricularia vulgaris plants in freshwater ecosystems. Environ Pollut 265:114830. https://doi.org/10.1016/j.envpol.2020.114830
Yu H, Peng J, Cao X, Wang Y, Zhang Z, Xu Y, Qi W (2021) Effects of microplastics and glyphosate on growth rate, morphological plasticity, photosynthesis, and oxidative stress in the aquatic species Salvinia cucullata. Environ Pollut 279:116900. https://doi.org/10.1016/j.envpol.2021.116900
Yu H, Zhang Z, Zhang Y, Song Q, Fan P, Xi B, Tan W (2021) Effects of microplastics on soil organic carbon and greenhouse gas emissions in the context of straw incorporation: a comparison with different types of soil. Environ Pollut 288:117733. https://doi.org/10.1016/j.envpol.2021.117733
Zhang G, Zhang F, Li X (2019) Effects of polyester microfibers on soil physical properties: perception from a field and a pot experiment. Sci Total Environ 670:1–7. https://doi.org/10.1016/j.scitotenv.2019.03.149
Zhang M, Zhao Y, Qin X, Jia W, Chai L, Huang M, Huang Y (2019) Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci Total Environ 688:470–478. https://doi.org/10.1016/j.scitotenv.2019.06.108
Zhang S, Han B, Sun Y, Wang F (2020) Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J Hazard Mater 388:121775. https://doi.org/10.1016/j.jhazmat.2019.121775
Zhang K, Hamidian AH, Tubić A, Zhang Y, Fang JK, Wu C, Lam PK (2021) Understanding plastic degradation and microplastic formation in the environment: A review. Environ Pollut 274:116554. https://doi.org/10.1016/j.envpol.2021.116554
Zhang Z, Peng W, Duan C, Zhu X, Wu H, Zhang X, Fang L (2021) Microplastics pollution from different plastic mulching years accentuate soil microbial nutrient limitations. Gondwana Res 108:91–101. https://doi.org/10.1016/j.gr.2021.07.028
Zhao T, Lozano YM, Rillig MC (2021) Microplastics increase soil pH and decrease microbial activities as a function of microplastic shape, polymer type, and exposure time. Front Environ Sci 9:675803. https://doi.org/10.3389/fenvs.2021.675803
Zhou C-Q, Lu C-H, Mai L, Bao L-J, Liu L-Y, Zeng EY (2021) Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J Hazard Mater 401:123412. https://doi.org/10.1016/j.jhazmat.2020.123412
Zhou J, Wen Y, Marshall MR, Zhao J, Gui H, Yang Y, Zeng Z, Jones DL, Zang H (2021) Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci Total Environ 787:147444. https://doi.org/10.1016/j.scitotenv.2021.147444
Zhou W, Wang Q, Wei Z, Jiang J, Deng J (2023) Effects of microplastic type on growth and physiology of soil crops: implications for farmland yield and food quality. Environ Pollut 326:121512. https://doi.org/10.1016/j.envpol.2023.121512
Zong X, Zhang J, Zhu J, Zhang L, Jiang L, Yin Y, Guo H (2021) Effects of polystyrene microplastic on uptake and toxicity of copper and cadmium in hydroponic wheat seedlings (Triticum aestivum L.). Ecotoxicol Environ Saf 217:112217. https://doi.org/10.1016/j.ecoenv.2021.112217
Zubris KAV, Richards BK (2005) Synthetic fibers as an indicator of land application of sludge. Environ Pollut 138(2):201–211. https://doi.org/10.1016/j.envpol.2005.04.013
Zumstein MT, Schintlmeister A, Nelson TF, Baumgartner R, Woebken D, Wagner M, Kohler H-PE, McNeill K, Sander M (2018) Biodegradation of synthetic polymers in soils: tracking carbon into CO2 and micfsrobial biomass. Sci Adv 4(7):eaas9024. https://doi.org/10.1126/sciadv.aas9024
Acknowledgements
The authors would like to thank the Tamil Nadu Agricultural University for providing all support and facilities in conducting the study.
Author information
Authors and Affiliations
Contributions
Raveendra Gnana Keerthi Sahasa: writing original draft, conceptualization and editing; Periyasamy Dhevagi: conceptualization, methodology, supervision and editing; Ramesh Poornima: data curation, writing original draft and editing; Ambikapathi Ramya: conceptualization, data curation and editing; Subburamu Karthikeyan: conceptualization, supervision and editing; Sengottaiyan Priyadharshini: data curation and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahasa, R.G.K., Dhevagi, P., Poornima, R. et al. Dose-dependent toxicity of polyethylene microplastics (PE–MPs) on physiological and biochemical response of blackgram and its associated rhizospheric soil properties. Environ Sci Pollut Res 30, 119168–119186 (2023). https://doi.org/10.1007/s11356-023-30550-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30550-4