Abstract
The kinetic study of the gas-phase reactions of hydroxyl (OH) radicals and chlorine (Cl) atoms with CF3CHFCF2OCH3 (HFE-356mec3) and CHF2CHFOCF3 (HFE-236ea1) was performed by the pulsed laser photolysis/laser-induced fluorescence technique and a relative method by using Fourier Transform infrared (FTIR) spectroscopy as detection technique. The temperature dependences of the OH-rate coefficients (kOH(T) in cm3s−1) between 263 and 353 K are well described by the following expressions: 9.93 × 10–13exp{-(988 ± 35)/T}for HFE-356mec3 and 4.75 × 10−13exp{-(1285 ± 22)/T} for HFE-236ea1. Under NOx-free conditions, the rate coefficients kCl at 298 K and 1013 mbar (760 Torr) of air were determined to be (2.30 ± 1.08) × 10–13 cm3s−1and (1.19 ± 0.10) × 10–15 cm3s–1, for HFE-356mec3 and HFE-236ea1, respectively. Additionally, the relative kinetic study of the Cl + CH2ClCHCl2 reaction was investigated at 298 K, as it was used as a reference reaction in the kinetic study of the Cl-reaction with HFE-356mec3 and discrepant rate coefficients were found in the literature. The global atmospheric lifetimes were estimated relative to CH3CCl3 at the tropospheric mean temperature (272 K) as 1.4 and 8.6 years for HFE-356mec3 and HFE-236ea1, respectively. These values combined with the radiative efficiencies for HFE-356mec3 and HFE-236ea1 derived from the measured IR absorption cross sections (0.27 and 0.41 W m−2 ppv−1) yield global warming potentials at a 100-yrs time horizon of 143 and 1473, respectively. The contribution of HFE-356mec3 and HFE-236ea1 to global warming of the atmosphere would be large if they become widespread increasing their atmospheric concentration.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrofluoroethers (HFEs) have been presented in the last decades as potential substitutes of chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) in many industrial applications (Tsai 2005). To evaluate the environmental impact produced by their potential widespread emissions, it is necessary to know a priori the atmospheric lifetime due to gas-phase homogeneous reactions with oxidants (e.g., OH radicals, Cl atoms, ozone, etc.) and UV photolysis in the actinic region. In addition, it is important to know its contribution to the global warming of the atmosphere, since HFEs strongly absorb the infrared (IR) radiation within the so-called “IR atmospheric window” (700–1250 cm−1).
In this work, we present the gas-phase kinetics of the reactions of 1,1,2,3,3,3-hexafluoropropyl methyl ether (CF3CHFCF2OCH3, HFE-356mec3) and 1,2,2-trifluoroethyl trifluoromethyl ether (CHF2CHFOCF3, HFE-236ea1) with OH radicals as a function of temperature (263–353 K) and at 66.66 and 133.32 mbar (i.e., 50 and 100 Torr) of helium using the pulsed laser photolysis-laser induced fluorescence (PLP-LIF) technique.
with respect to the kinetics of reaction (R1), Urata et al. (2003) reported an estimate for the rate coefficient at room temperature, kOH(298 K), but no measurement has been reported yet. Therefore, an experimental study is needed to confirm the estimation of Urata et al. (2003). On the other hand, for reaction (R2), there is only a relative measurement of kOH(298 K) reported by Oyaro et al. (2005) at 1013 mbar of air using gas chromatography-mass spectrometry (GC–MS) as detection technique.
There are also limited literature data on the kinetic study of the Cl-reaction of HFE-356mec3 (R3) and HFE-236ea1 (R4).
As far as we know, there is only an absolute kinetic study on reaction (R3) as a function of temperature (263–363 K) using a very low-pressure reactor (< 4 mbar) and a quadrupole mass spectrometry for monitoring HFE-356mec3 (Papadimitriou et al. 2004). No data at tropospheric pressures are available to date. Regarding reaction (R4), Oyaro et al. (2005) employed a relative kinetic method using GC–MS to monitor HFE-236ea1 and the reference compound to measure kCl at room temperature, kCl(298 K), and 1013 mbar of air. These authors reported an average value of kCl(298 K) of (1.2 ± 2.0) × 10–15 cm3s−1, with extremely large uncertainties (ca. 200%). Hence, an additional measurement of kCl(298 K) is desired to clarify this point and to reduce the uncertainty.
The present study presents (i) the first absolute measurement of kOH(T) for reactions (R1) and (R2) as a function of T (263–353 K) and between 66.66 and 133.32 mbar using PLP-LIF, from which the Arrhenius parameters were derived; (ii) a revision of the relative kinetics of reactions (R3) and (R4) using different reference compounds; and (iii) an estimation of the atmospheric lifetime of HFE-356mec3 and HFE-236ea1 from kOH(272 K) and kCl(298 K) for global atmosphere, and from kOH(298 K) and kCl(298 K) for a coastal atmosphere at dawn.
Although HFEs are not expected to absorb radiation at λ > 200 nm (Orkin et al. 1999), the UV spectra of HFE-236ea1 and HFE-356mec3 were measured in this work between 190 and 400 nm. The absorption cross section (\({\sigma }_{\lambda }\)) at 248 nm was determined for HFE-356mec3 and HFE-236ea1 to assess their potential photolysis during the absolute kinetic experiments. Regarding the IR absorption cross sections (\({\sigma }_{\widetilde{\nu }}\)) in the mid-infrared region, Le Bris et al. (2020) determined this parameter for HFE-356mec3 between 550 and 3500 cm−1. Oyaro et al. (2005) reported \({\sigma }_{\widetilde{\nu }}\) for HFE-236ea1 in a similar wavenumber range (400–3200 cm−1). These authors also provided global warming potential (GWP) calculations (Le Bris et al. 2020; Oyaro et al. 2005). In the present work, Fourier transform infrared (FTIR) spectroscopy was used to reinvestigate the IR spectra of the titled HFEs between 500 and 4000 cm−1. Based on the results obtained in this work, the lifetime-corrected radiative efficiencies (REs) and GWPs relative to CO2 for both HFEs were reexamined.
Experimental set-ups and techniques
Absolute gas-phase kinetics of OH-reactions
The experimental system and procedure to determine the second-order rate coefficient kOH(T) has been explained elsewhere (Albaladejo et al. 2002; Antiñolo et al. 2012; Blázquez et al. 2017). Briefly, the pulsed laser photolysis at 248 nm of hydrogen peroxide (H2O2) was employed to generate in situ OH radicals in a 200 cm3 jacketed Pyrex® cell. The monitoring of the electronic ground state OH was performed by laser induced fluorescence at 310 nm, emitted from excited OH radicals at 282 nm. Under pseudo-first order conditions (i.e. HFE in large excess with respect to OH), the LIF signal (ILIF) follows a single exponential function. Some examples of the temporal evolution of ILIF are shown in Figure S1 of the supporting information (SI). From the analysis of such decays, the pseudo-first order coefficient (k’) for a given HFE concentration, temperature, and pressure (pT) is obtained. The loss of OH in the absence of HFE is due to reaction with H2O2 or diffusion out of the detection zone (k’0), as shown in Eq. E1.
The second-order rate coefficient kOH(T) for reactions (R1) and (R2) is obtained from k’-k’0 versus [HFE] plots. pT was set to 133.32 mbar of helium except for the experiments carried out at 298 K, where experiments were also performed at 66.66 mbar. All gases were introduced into the reactor by calibrated mass flow controllers (MFCs), particularly important for the HFE/He mixtures. An example of the calibration of the mass rate flow (FR) of the relatively concentrated HFE mixtures (9.8–10.4% for HFE-356mec3 and 25.5% for HFE-236ea1) from the storage bulb is shown in Figure S2. As shown, the “real” mass flow rate is much lower than the set one (50% for HFE-356mec3 and 66% for HFE-236ea1). By changing FR and the total flow rate, [HFE] in the reactor was varied ([HFE-356mec3] = (0.36–8.12) × 1016 cm−3 and [HFE-236ea1] = (0.06–2.08) × 1017 cm−3).
Relative kinetics for Cl-reactions
The experimental system and procedure to determine kCl(298 K) has been described elsewhere (Antiñolo et al. 2020; Asensio et al. 2022, 2023; Ballesteros et al. 2017; Ceacero-Vega et al. 2012). Briefly, it consists of a White-type Pyrex gas cell (V = 16 L) with an optical pathlength of (59.0 ± 4.9) m, which is surrounded by 3 actinic lamps (Philips Actinic BL TL 40W/10 1SL/25, λ = 340–400 nm) to generate Cl atoms in situ by photolysis of Cl2. The cell is coupled to a FTIR spectrometer (Thermo Nicolet, Nexus 870) with a liquid N2-cooled MCT (Mercury Cadmium Telluride) detector. In the kinetic study of reaction (R3), methane (CH4) and 1,2,2-trichloroethane (CH2ClCHCl2) were used as reference compounds, while for reaction (R4) due to the difficulty of finding a suitable reference compound, only 1,1,1-trichloroethane (CH3CCl3) was used. As discrepant kCl(298 K) values for CH2ClCHCl2 (reaction (R5)) were found in the literature, we decided to determine it before using CH2ClCHCl2 as a reference compound to study reaction (R3).
The reference compounds used in these preliminary kinetic experiments were CH4 and dichloromethane (CH2Cl2).
Considering all loss processes for the HFE and the reference compound, the integrated rate equation is given by:
kref refers to the rate coefficient for the Cl-reaction with the reference compound at 298 K and 1013 mbar; [HFE]0, [HFE]t, [Ref]0 and [Ref]t are the concentrations of the HFE and the reference compound at the beginning of the reaction and at a reaction time t, respectively. kloss and kRef,loss are the first-order rate coefficients for the secondary losses for the HFE and the reference compound, respectively. [HFE] and [Ref] were measured by FTIR spectroscopy. [HFE]0 and [Ref]0 are listed in Table S3. The IR spectra were recorded in the 650–4000 cm−1 range under static conditions and at an instrumental resolution of 2 cm–1. They were recorded every 2 min during 130 min for HFE-356mec3, 150 min for HFE-236ea1 and 90 min for CH2ClCHCl2. kloss and kRef,loss (Table S4) were measured prior to each experiment and include heterogeneous losses, photolysis and/or reaction with Cl2, as described in previous works (Antiñolo et al. 2020; Asensio et al. 2022).
UV and IR absorption spectroscopy in the gas-phase
The UV absorption spectroscopy system has been described in detail elsewhere (Asensio et al. 2022; Blázquez et al. 2020). It consists of a deuterium lamp (StellarNet, SL4 DT-200) placed at the entrance of a jacketed Pyrex® cell (optical path length, \({\ell}\)=(107.0 ± 0.5)cm), connected by an optical fiber to a f/2 spectrograph (StellarNet, BLK-C) that possesses a concave holographic grating (590 grooves/mm) and a slit of 100 µm The transmitted intensity was detected in a charge-coupled device detector (2048 pixels). The UV cell was filled with pure HFE-356mec3 (38–71 mbar ≡ 28.67–53.35 Torr), and HFE-236ea1 (92–133 mbar ≡ 68.68–99.76 Torr), resulting in [HFE] ranges of (0.93–1.74) × 1018 cm−3 and (2.24–3.25) × 1018 cm−3, respectively. The UV spectra were recorded at room temperature between 190 and 400 nm under static conditions by accumulating 6000 scans at an instrumental resolution of 3 nm.
The IR absorption spectra of the HFEs were recorded between 4000 and 500 cm1 using a FTIR spectrometer (Bruker, Tensor 27) with a liquid nitrogen cooled mercury cadmium telluride detector (Blázquez et al. 2022). The instrumental resolution was set to the highest one available with the FITR spectrometer, 0.5 cm−1. A single path (\({\ell}\)=10 cm) stainless steel cell, sealed with ZnSe windows, was used to measure the absorbance at each wavenumber (\({A}_{\widetilde{\upsilon }}\)) under static conditions. The cell was filled with a pure sample of HFE-356mec3 (total pressure in the cell, Pcell = 1.79–12.95 mbar (≡1.34–9.70 Torr)) or HFE-236ea1, (Pcell = 0.96–6.64 mbar ≡ 0.72–4.98 Torr). The HFE concentration ranges were [HFE-356mec3] = (0.43–3.15) × 1017 cm–3 and [HFE-236ea1] = (0.23–1.61) × 1016 cm−3.
A total of 3–9 spectra were used to obtain σλ, and \({\sigma }_{\widetilde{\upsilon }}\) from the slope of the Beer-Lambert’s plots as shown in the examples presented in Figure S3. In addition, the integrated IR absorption cross section of a band, Sint (in base e), defined by Eq. E3, was determined from the Beer-Lambert’s law (Eq. ES2) expressed in terms of the integrated absorbance, Aint (Eq. ES3), for comparison purposes.
Plots of the Beer-Lambert’s law for Sint are presented in Figure S4 of SI.
Chemicals
Helium (99.999%, Nippon Gases), synthetic air (99.999%, Air Liquide), HFE-236ea1 (95%, Apollo Scientific and 95%, Chemspace), and CH4 (99.995%, Sigma-Aldrich) were gases used as supplied. Aqueous solution of H2O2 (> 50% v/v, Scharlab) was pre-concentrated as described in (Albaladejo et al. 2002). Liquid HFE-356mec3 (> 98%, Chemspace), CH2Cl2 (99.8%, Sigma-Aldrich), CH2ClCHCl2 (> 98%, Cymit), and CH3CCl3 (95.10%, Cymit) were used after degasification by several freeze–pump–thaw cycles.
Results and discussion
Kinetic study of the OH- and Cl-reactions with HFEs
Temperature dependence of kOH(T)
The individual kOH(T) for HFE-356mec3 and HFE-236ea1 are listed as a function of temperature and total pressure in Tables S1 and S2 of the SI. The rate coefficients at a single temperature provided in Table 1 are the average of the rate coefficients obtained from the slope of the plot of k’-k’0 vs [HFE]. As it can be seen in Table 1, over the temperature range investigated kOH(T) are on the order of 10–14 cm3s−1 for HFE-356mec3 and 10–15-10–14 cm3s−1.
The rate coefficient kOH(T) increases when temperature increases. For HFE-356mec3, kOH(353 K) is around three times higher than kOH(263 K), while for HFE-236ea1 kOH(353 K) is four times higher than kOH(263 K). In Fig. 1, k’-k’0 vs [HFE] plots are shown for both HFEs at 263 K and 353 K together with those at 298 K at 66.66 and 133.32 mbar.
When the kinetic data were fitted to an Arrhenius expression, the uncertainty (± 2σ, only statistical) in A and Ea/R parameters was very large due to the scattering of the data, as shown by Eqns. E4 and E5.
By fixing the A factor, the uncertainty in Ea/R (± 2σ, only statistical) is reduced and the Arrhenius expressions that describe the observed T-dependence of kOH(T) (solid red line in Fig. 2) between 263 and 353 K are given by:
The dashed blue lines in Fig. 2 represent the confidence bands at a 95% level. The observed temperature dependence of kOH(T) is positive in both cases, yielding (Ea ± 2σ) of (8.2 ± 0.3) and (10.7 ± 0.2) kJ/mol−1 for reactions (R1) and (R2), respectively. Blázquez et al. (2022) reported an expression that relates Ea with log(kOH(298 K)) for a series of OH + HFE reactions. For HFE-356mec3 and HFE-236ea1, the estimated Ea using that expression are 10.3 kJ/mol−1 and 13.3 kJ/mol−1, respectively, which are in both cases 20% higher than the experimental value determined in this work.
Comparison of kOH(298 K) for HFE-356mec3 and HFE-236ea1 with previous studies
Table 2 summarizes kOH(298 K) for reactions (R1) and (R2) and those previously reported in the literature. For HFE-356mec3, Urata et al. (2003) estimated kOH(298 K) (1.77×10–14 cm3 s−1) using the modified Heicklen equation, which is half of the one reported in this work. For reaction (R2), there is only one relative kinetic measurement of kOH(298 K), which was performed by Oyaro et al. (2005) using GC–MS as a detection method for HFE-236ea1 and the reference compounds used (CH3CCl3, CH3CN, and CHF2CH2F). The reported individual kOH(298 K) obtained with each reference compound were: (6.9 ± 1.3)×10–15 cm3 s−1, (5.3 ± 2.7)×10–15 cm3s−1 and (8.3 ± 3.6)×10–15 cm3 s−1, respectively. Even though the scattering in the individual kOH(298 K), Oyaro et al. (2005) reported a weighted average of (6.8 ± 1.1)×10–15 cm3s–1, which agrees, within the uncertainty limits, with kOH(298 K) obtained in this work by an absolute kinetic method. The large uncertainty in the individual kOH(298 K) reported by Oyaro et al. (2005) is greatly influenced by uncertainties in recommended kref, although they are well-known and currently recommended by the JPL-NASA panel (Burkholder et al. 2020).
Relative kinetics of the Cl + CHCl2CH2Cl reaction
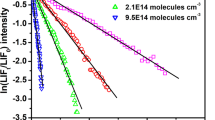
In Figure S5a, a relative plot according to Eq. E2 is shown for reaction (R5) for each reference compound used. The kCl(298 K)/kref ratio was obtained from the slope of such plots, as shown in Table S5. Individual kCl(298 K) obtained with both reference compounds are in good agreement, despite the large data scattering observed with CH2Cl2 (Figure S5b). A weighted average of (3.08 ± 0.36)×10–13 cm3 s–1 is reported. The weighting factor was 1/σ2, where σ is the standard deviation in the individual kCl(298 K).
Comparison of kCl(298 K) for CH2ClCHCl2 with previous studies
As shown in Table 3, kCl(298 K) reported here is in very good agreement, within the error limits, with the absolute value reported by Wine and Semmes (1983). The relative kinetic studies of Cillien et al. (1967) and Tschuikow‐Roux et al. (1986) yield a much lower kCl(298 K). Note that the complex relative method used by these authors determines independently the rate coefficient for each H-abstraction channel and the values listed in Table 3 are the overall rate coefficient. Tschuikow‐Roux et al. (1986) recalculated kCl(298 K) for reaction (R5) reported by Cillien et al. (1967) using the recommended value of kref for CH4, finding an even lower value than originally reported. The reported uncertainties in kCl(298 K) for (R5) are very large (see Table IV of Tschuikow-Roux et al.’s work). Note that Cillien et al. (1967) reported kCl(323 K), the lowest temperature they measured, so kCl(298 K) should be somehow lower, according to our observations. In conclusion, we have elucidated the discrepancy among previous relative studies and kCl(298 K) for CH2ClCHCl2 determined in this work was used as kref in the kinetic study of the Cl + HFE-236ea1 reaction.
Relative kinetics of Cl + HFE reactions
In Fig. 3A, the plots of Eq. E2 for the Cl-reaction with HFE-356mec3 are depicted for each reference compound. The good linearity and the zero intercept of these plots indicate the absence of interfering secondary chemistry that can affect the determination of kCl(298 K). The used kref for CH4 and CH2ClCHCl2 were those recommended by Atkinson et al. (2006) and the obtained in this work, respectively. As shown in Fig. 3B, where both datasets are combined, there is some scattering. Nevertheless, the individual kCl(298 K) (Table S6) lies within the stated uncertainties and, thus, a weighted average is reported in Table 4. For HFE-236ea1, kref for CH3CCl3 was taken from the JPL-NASA evaluation panel (Burkholder et al. 2020).
Relative plots obtained for the Cl-reaction with HFE-356mec3 (A) and HFE-236ea1 (B). kref for CH4 and CH2ClCHCl2 are listed in Table S6
Comparison of kCl(298 K) for HFE-356mec3 and HFE-236ea1 with previous studies
In Table 4, kCl(298 K) obtained in this work for reactions (R3) and (R4) are compared with those reported in the literature (Oyaro et al. 2005; Papadimitriou et al. 2004). Papadimitriou et al. (2004) reported kCl(298 K) = (2.09 ± 1.48)×10–13 cm3 s−1 for HFE-356mec3 at ca. 4 mbar. In this work kCl(298 K) has been determined for the first time at sea level pressure. kCl(298 K) determined by Papadimitriou et al. (2004) is in good agreement, within their large error limits, with that reported here. This means that no pressure dependence of kCl(298 K) is observed in that wide pressure range. Oyaro et al. (2005) used CH3CCl3 and CHF2CH2F as reference compounds to study the kinetics of the Cl + HFE-236ea1 reaction. These authors reported individual kCl(298 K) with three times the standard deviation (± 3σ) uncertainties. Despite that, these authors reported a weighted average of kCl(298 K). Even considering ± 2σ, as in our work, the individual kCl(298 K) still present large uncertainties: (1.1 ± 1.4)×10–15 and (2.0 ± 4.6)×10–15 cm3 s−1. Current recommendations of kref for the Cl-reactions of CH3CCl3 and CHF2CH2F (Burkholder et al. 2020) present lower uncertainty factors, f(298 K) (see Table 4). Hence, considering these values in kref and f(298 K), we recalculated the weighted average kCl(298 K) from Oyaro et al.’s results to be (1.34 ± 0.14) × 10–15 cm3s−1 (see Table 4). This manifests the importance of reporting accurate absolute rate coefficients to reduce the uncertainty in the recommended kref which will be further used in relative measurements of kCl(298 K).
Comparison of the OH- and Cl-reactivity of HFEs with their structurally analogous HFCs
The equivalent HFC to CF3CHFCF2OCH3 (HFE-356mec3) is CF3CHFCF2CH3 (HFC-356mec) but no information is available, as far as we know. When comparing kOH(298 K) and kCl(298 K) determined in this work for HFE-356mec3 with those reported by Barry et al. (1997) for the analogue HFC, HFC-365mfc (Table S7), it is clear that the presence of the ether group increases both rate coefficients. Particularly for the Cl-reaction, kCl(298 K) increases more than two orders of magnitude with respect to the HFC. Concerning the temperature dependence of kOH(T), Ea for the OH-reactions of HFE-356mec3 and its HFC analogue are similar, within the experimental uncertainties. For CHF2CHFOCF3 (HFE-236ea1), the equivalent HFC is CHF2CHFCF3 (HFC-236ea). The decrease in kOH(298 K) for HFC-236ea reported by Atkinson et al. (2006) is less pronounced than that for HFE-356mec3. However, the same trend is observed in Ea. No kinetic data has been found in the literature for the Cl + HFC-236ea reaction.
Absorption cross sections in the UV and IR region
HFEs weakly absorb at wavelengths longer than 200 nm (Figures S6 and S7), especially HFE-236ea1 which presents σλ lower than 1 × 10–23 cm2 molecule−1 at λ > 210 nm. In contrast, HFE-356mec3 presents higher absorption at λ > 210 nm, σ210nm = (1.08 ± 0.04) × 10–21 cm2. σλ are listed at 0.5 nm intervals in the Excel spreadsheet provided in the SI. Considering σλ at the photolysis wavelength (σ248nm = (1.17 ± 0.35) × 10–22 cm2), the laser fluences and [HFE] employed in this work, HFE-356mec3 and HFE-236ea1 are not photolyzed during the kinetic experiments. A photolysis quantum yield for HFEs of 1 was assumed.
In the mid-IR region, the obtained \({\sigma }_{\widetilde{v}}\) for HFE-356mec3 and HFE-236ea1 are listed in the Excel spreadsheet provided in the SI between 500 and 4000 cm–1 and depicted in Figure S8. In the 2700–1600 cm−1 range, both HFEs present negligible absorption, and a very weak band in the 3100–2700 cm−1 range. The obtained IR spectra between 500 and 1500 cm−1 in terms of \({\sigma }_{\widetilde{v}}\) for both HFEs are depicted as black lines in Figure S9, while IR spectra reported in the literature are plotted as blue lines.
In Table S8, the value and the position of the absorption peak (\({\sigma }_{\widetilde{v}, max}\)) are listed together with those reported in the literature. \({\sigma }_{\widetilde{v}, max}\) for HFE-356mec3 and HFE-236ea1 is located at 1211.9 cm−1 and 1136 cm−1, respectively. For HFE-356mec3, the position of \({\sigma }_{\widetilde{v}, max}\) is in excellent agreement with the high-resolution study of Le Bris et al. (2020) at 305 K (1211.9 cm−1), which reports \({\sigma }_{\widetilde{v}, max}\) 7% higher than ours. For HFE-236ea1, Oyaro et al. (2005) reported that \({\sigma }_{\widetilde{v}, max}\) was located at 1136 cm−1 which is in excellent agreement with \({\sigma }_{\widetilde{v}, max}\) determined in this work although \({\sigma }_{\widetilde{v}, max}\) at 1136 cm−1 is 21% lower than ours. With respect to Sint, it is summarized in Table S8 to compare with previous works. For HFE-356mec3, Sint(1650–660 cm−1) is 7% lower than that of Le Bris et al. (2020). For HFE-236ea1, Sint(1600–465 cm–1) reported by Oyaro et al. (2005) is around 22% lower than the reported in this work, consequence of their lower IR absorption cross sections in the 1100–1300 cm−1 region. Additional experiments carried out at an instrumental resolution of 1 cm−1 provide the same IR absorption cross section for HFE-236ea1 (See Figure S10). Thus, the instrumental resolution of the FTIR spectrometer used is not the source of the observed discrepancy.
Atmospheric implications
Tropospheric lifetimes, τtropos
As shown in Figure S7, the investigated HFEs do not appreciably absorb in the solar actinic UV region (λ > 290 nm), which may indicate that their degradation by UV photolysis in the troposphere can be negligible, especially if their photolysis quantum yield is very low in the actinic region. Further studies would be necessary to confirm this assertion. Therefore, the tropospheric lifetime of HFE-356mec3 and HFE-236ea1, τtropos, mainly depends on their reactivity towards the atmospheric oxidants (OH, Cl, O3 or NO3).
τOx is the lifetime due to the reaction of HFE-356mec3 and HFE-236ea1 with the oxidant, Ox. To our knowledge, the rate coefficients for the reactions of O3 and NO3 with the HFEs are not known, although they are expected to be very low compared to the reaction with OH and Cl. Thus, here we consider only the removal by OH and Cl, and τOx is denoted as τOH and τCl.
Two scenarios were considered to estimate the tropospheric lifetime of HFEs due to homogeneous reactions: a global atmosphere (scenario i) and a coastal atmosphere at dawn (scenario ii), where the Cl chemistry can play a significant role. The atmospheric lifetime due to the reaction of HFEs with OH determines the degree of homogeneity in their distribution in the atmosphere. For long-lived gases (τOH greater than a few months), the atmospheric lifetime is usually estimated relative to CH3CCl3 at the tropospheric mean temperature, 272 K (Spivakovsky et al. 2000):
where \({\tau }_{OH}\left({\text{CH}}_{3}{\text{CCl}}_{3}\right)\) is the atmospheric lifetime of CH3CCl3 due to reaction with OH (5.99 years) and \({k}_{{\text{OH}+\text{CH}}_{3}{\text{CCl}}_{3}}(272\text{ K})\) is 6.03 × 10–15 cm3 s−1 (Hodnebrog et al. 2020). The rate coefficient for (R1) and (R2) at 272 K, \({k}_{\text{OH}}(272\text{ K})\), were obtained from equations E5 and E6, respectively. For HFE-356mec3, \({k}_{\text{OH}}(272\text{ K})\)= 2.63 × 10–14 cm3 s−1 and for HFE-236ea1, \({k}_{\text{OH}}(272\text{ K})\)= 4.22 × 10–15 cm3 s−1. The estimated \({\tau }_{\text{OH}}\) for HFE-356mec3 and HFE-236ea1 is 1.4 and 8.6 years, respectively.
To evaluate the relative importance of the degradation of the HFEs by Cl with respect to the OH reaction, the estimation of the atmospheric lifetime of HFEs due to Cl-reaction, \({\tau }_{Cl}(\text{HFE})\), was carried out using Eq. E9, the rate coefficients obtained in this work at 298 K and the 24-h average Cl concentration (see Table S9).
Estimates of \({\tau }_{Cl}\) for HFE-356mec3 and HFE-236ea1 are 138 years and 26605 years, respectively. Although in this estimation the temperature conditions of the surface were used instead of the tropospheric mean temperature, the global removal of these HFEs is dominated by the reaction with OH radicals. Therefore, the global atmospheric lifetime can be taken as \({\tau }_{OH}\) and were considered in the calculation of GWP (see “Other potential atmospheric sinks of HFE-236ea1 and HFE-356mec3” section).
In a coastal atmosphere at dawn, \({\tau }_{Cl}(\text{HFE})\) and \({\tau }_{OH}(\text{HFE})\) were estimated using Eq. E9 and E10, respectively, where [Cl] and [OH] are the concentrations of the oxidants at the first hours of the day (see Table S9).
Even though \({\tau }_{Cl}(\text{HFE})\) are reduced two orders of magnitude (see Table S9), the OH reaction is still the main degradation route for HFE-356mec3 and HFE-236ea1 at the first hours of the day.
Other potential atmospheric sinks of HFE-236ea1 and HFE-356mec3
Other potential removal processes for these HFEs in the troposphere may be the direct dissolution of the gaseous pollutant into seawater or transfer from rainwater. Fluorinated ethers are expected to be very soluble in water, according to the estimation of the water solubility from US Environmental Protection Agency’s EPISuite™. For HFE-356mec3, the water solubility is estimated to be 522.2 mg/L at 25 ºC, while for HFE-236ea1 it is estimated to be 5946 mg/L. These values are in fresh water and can be taken as upper limits, since the ocean salinity may decrease the water solubility of the HFEs, as estimated for several HFCs by Li et al. (2019). Therefore, if these HFEs are uptaken onto the ocean and dissolved they could affect their atmospheric residence time (Wang et al. 2023) since it involves not only atmospheric loss rates (i.e., reaction with OH) but also ocean processes (such as ocean uptake and outgassing) and other processes (such as stratospheric photochemistry).
If these HFEs were rainout, these pollutants may be transferred into the ocean. However, calculations made to assess the contribution of wet deposition to the atmospheric removal of these HFEs show that it is negligible compared to chemical removal by OH radicals. Based on the EPISuite estimated Henry’s law constants for CF3CHFCF2OCH3 and CHF2CHFOCF3, kpc = 0.3487 and 0.02826 atm m3/mole, respectively, at 25 ºC, their lifetime due to wet deposition is on the order of thousands of years. This lifetime was estimated from Eqn. (E11) (Jiménez et al. 2009).
where kcp = 0.0029 M atm−1 for CF3CHFCF2OCH3 and 0.035 M atm−1 for CHF2CHFOCF3 at 25 ºC and the annual average precipitation rate (vprecip) was taken for Spain over the 1991–2020 period (636 mm/yr). The individual lifetime for CF3CHFCF2OCH3 and CHF2CHFOCF3 due to wet deposition is extremely long (11,213 yrs and 909 at 0.5 km altitude and 25ºC) to affect the atmospheric overall lifetime.
On the other hand, as the transport time from troposphere to stratosphere is of several years, these long-lived HFEs can be then transported to the stratosphere. Thus, the dynamical coupling between stratosphere and troposphere cannot be dismissed. The potential photolysis rate (J) of HFE-356mec3 and HFE-236ea1 in the stratosphere strongly depends on the photolysis quantum yield. Considering the UV absorption cross sections determined in this work between 190 and 400 nm and the spectral actinic flux between 180 and 400 nm at an altitude of 50 km (Demore et al. 1997), in the most favorable scenario (a photolysis quantum yield of 1) J would be 1.7 × 10–6 s−1 and 4.8 × 10–7 s−1, respectively. These J values translate in a stratospheric lifetime for HFE-356mec3 and HFE-236ea1 due to photolysis of around 7 and 24 days. But these numbers may not be realistic if the photolysis quantum yield is much lower than unity, and have to be taken cautiously. Further photochemical studies would be needed to determine the photolysis quantum yield of these HFEs as a function of wavelength.
Radiative efficiencies and GWPs
Using the same methodology as in our previous works (Blázquez et al. 2022, 2017), REs (in W m−2 ppbv−1) and GWPs relative to CO2 of HFEs were calculated for a time horizon of 100 years, GWP(100 yrs). In this work, the instantaneous REs were corrected with \({\tau }_{OH}(\text{HFE})\) as explained by Shine and Myhre (2020). The fractional correction factors were calculated from \({\tau }_{OH}(\text{HFE})\) in scenario i of Table S9 to be 0.793 for HFE-356mec3 and 0.948 for HFE-236ea1. The obtained lifetime-corrected REs are summarized in Table 5. As shown, RE for HFE-236ea1 is higher (34%) than that for HFE-356mec3, mainly due to the smaller correction of the instantaneous RE (5% versus 21%).
For HFE-356mec3, as \({\tau }_{OH}(\text{HFE})\) is shorter than previously reported, the lifetime-corrected RE is lower than those reported (0.29–0.31 W m−2 ppbv−1) (Burkholder et al. 2018; Hodnebrog et al. 2020; Le Bris et al. 2020). The overestimation of RE for HFE-356mec3 previously reported (7–13% higher) is due to the consideration of the atmospheric lifetime of CF3CHFCF2OCH3 to be equal to that of CHF2CF2OCH2 because no experimental data were available. In addition, Burkholder et al. (2018), considered the RE for HFE-356mec3 given by Hodnebrog et al. (2013). For HFE-236ea1, Oyaro et al. (2005) reported an instantaneous RE (0.35 W m−2 ppbv−1) according to the procedure given by Pinnock et al. (1995) Applying the fractional correction factor to the instantaneous RE reported by Oyaro et al. (2005) the resulting RE (0.40 W m−2 ppbv−1) is in excellent agreement with the value determined in this work (0.41 W m−2 ppbv−1).
The GWP(100 yrs) values in Table 5 were obtained using the lifetime-corrected REs and \({\tau }_{OH}(\text{HFE})\) from scenario i of Table S9. As shown, GWP(100 yrs) for HFE-236ea1 is much higher than that for HFE-356mec3, because both RE and \({\tau }_{OH}(\text{HFE})\) are higher. GWP(100 yrs) for HFE-356mce3 calculated in this work is 143, around 45% lower than the values from the literature (Burkholder et al. 2018; Hodnebrog et al. 2020; Le Bris et al. 2020). This is a consequence of the combined effect of a higher \({\tau }_{OH}(\text{HFE})\) and RE. For HFE-236ea1, GWP(100 yrs) from corrected results from Oyaro et al. (2005) is still 9% higher than that from this work. When compared GWP(100 yrs) of HFE-236ea1 with that of its structurally analogue HFC, CHF2CHFCF3 (HFC-236ea), it is reduced 6%. However, using the results from Oyaro et al. (2005) would GWP(100 yrs) of HFE-236ea1 would increase 3% with respect to HFC-236ea. This indicates that accurate lifetimes and REs are necessary to establish the impact of a pollutant on global warming. Similarly, the impact on the global warming of the structurally analogue HFC for HFE-356mec3, CF3CHFCF2CH3 (HFC-356mec) has to be evaluated, since no information is available, as far as we know. Nevertheless, as shown in Table 5, GWP(100 yrs) for an HFC with the same number of C-F bonds as HFC-356mec, i. e. CF3CH2CF2CH3 (HFC-365mfc), is ca. 6 times higher than that for HFE-356mec3.
Conclusions
This work reports the first determination of the rate coefficient kOH(T) for the OH-reaction with CF3CHFCF2OCH3 (HFE-356mec3) and CHF2CHFOCF3 (HFE-236ea1) as a function of temperature (263–353 K). A positive temperature dependence of kOH(T) was observed with activation energies of (8.2 ± 0.3) and (10.7 ± 0.2) kJ/mol−1 for HFE-356mec3 and HFE-236ea1, respectively. At room temperature, kOH(298 K) is (3.68 ± 0.95)×10–14 cm3s−1 for HFE-356mec3 and (6.6 ± 1.3)×10–15 cm3s−1 for HFE-236ea1. No pressure dependence of kOH(T) was observed in the 66.66–133.32 mbar range. In addition, the rate coefficient for the corresponding Cl-reactions at 298 K and 1013 mbar (760 Torr), kCl(298 K), was determined to be (2.30 ± 1.08)×10–13 cm3s−1 for HFE-356mec3 and (1.19 ± 0.10) × 10–15 cm3s−1 for HFE-236ea1. At 298 K, HFE-356mec3 reacts 6 times faster with Cl atoms than with OH radicals. However, HFE-236ea1 reacts more than 5 times faster with OH than with Cl.
Concerning the UV photolysis in the troposphere of the investigated HFEs, as they do not appreciably absorb at wavelengths longer than 200 nm (σλ < 1 × 10–21 cm2 for HFE-356mec3 and < 2.5 × 10–22 cm2 for HFE-236ea1), it is expected that this removal process is not important. However, the photolysis rate of these HFEs would depend on the photolysis quantum yield which is not known. The tropospheric lifetimes for HFE-356mec3 and HFE-236ea1, estimated relative to CH3CCl3, were 1.4 and 8.6 years, respectively, being the main removal pathway for these HFEs the reaction with OH radicals. From the IR absorption cross sections determined in this work between 4000 and 500 cm−1 for HFE-356mec3 and HFE-236ea1, their lifetime-corrected radiative efficiency was calculated to be 0.27 and 0.41 W m−2 ppbv−1, respectively. At a time horizon of 100 years, HFE-356mec3 and HFE-236ea1 present GWPs relative to CO2 of 143 and 1473, respectively, which implies that for the same amount of CO2 and HFE emitted (1 kg) to the atmosphere, the contribution to global warming of these HFEs would be more than 100 times higher than CO2 effect for HFE-356mec3 and more than 1000 times higher for HFE-236ea1. Therefore, HFE-356mec3 and HFE-236ea1 would largely impact on the radiative forcing of the atmosphere, if they become widespread increasing their atmospheric concentration.
Data availability
The manuscript has data included as electronic supplementary material.
References
Albaladejo J, Ballesteros B, Jiménez E, Martín P, Martínez E (2002) A PLP–LIF kinetic study of the atmospheric reactivity of a series of C4–C7 saturated and unsaturated aliphatic aldehydes with OH. Atmos Environ 36:3231–3239. https://doi.org/10.1016/S1352-2310(02)00323-0
Antiñolo M, González S, Ballesteros B, Albaladejo J, Jiménez E (2012) Laboratory Studies of CHF2CF2CH2OH and CF3CF2CH2OH: UV and IR Absorption Cross Sections and OH Rate Coefficients between 263 and 358 K. J Phys Chem A 116:6041–6050. https://doi.org/10.1021/jp2111633
Antiñolo M, Asensio M, Albaladejo J, Jiménez E (2020) Gas-phase reaction of trans-2-Methyl-2-butenal with Cl: Kinetics, gaseous products, and SOA formation. Atmosphere 11. https://doi.org/10.3390/atmos11070715
Asensio M, Antiñolo M, Blázquez S, Albaladejo J, Jiménez E (2022) Evaluation of the daytime tropospheric loss of 2-methylbutanal. Atmos Chem Phys 22:2689–2701. https://doi.org/10.5194/acp-22-2689-2022
Asensio M, Blázquez S, Antiñolo M, Albaladejo J, Jiménez E (2023) Atmospheric impact of 2-methylpentanal emissions : Kinetics, photochemistry, and formation of secondary pollutants. Atmos Chem Phys 23:14115–14126. https://doi.org/10.5194/acp-23-14115-2023
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF, Hynes RG, Jenkin ME, Rossi MJ, Troe J (2006) Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II – gas phase reactions of organic species. Atmos Chem Phys 6:3625–4055. https://doi.org/10.5194/acp-6-3625-2006
Ballesteros B, Jiménez E, Moreno A, Soto A, Antiñolo M, Albaladejo J (2017) Atmospheric fate of hydrofluoroolefins, CxF2x+1CH=CH2 (x = 1,2,3,4 and 6): Kinetics with Cl atoms and products. Chemosphere 167:330–343. https://doi.org/10.1016/j.chemosphere.2016.09.156
Barry J, Locke G, Scollard D, Sidebottom H, Treacy J, Clerbaux C, Colin R, Franklin J (1997) 1,1,1,3,3,-pentafluorobutane (HFC-365mfc): Atmospheric degradation and contribution to radiative forcing. Int J Chem Kinet 29:607–617. https://doi.org/10.1002/(SICI)1097-4601(1997)29:8
Blázquez S, Antiñolo M, Nielsen OJ, Albaladejo J, Jiménez E (2017) Reaction kinetics of (CF3)2CFCN with OH radicals as a function of temperature (278–358 K): A good replacement for greenhouse SF6? Chem Phys Lett 687:297–302. https://doi.org/10.1016/j.cplett.2017.09.039
Blázquez S, González D, Neeman EM, Ballesteros B, Agúndez M, Canosa A, Albaladejo J, Cernicharo J, Jiménez E (2020) Gas-phase kinetics of CH3CHO with OH radicals between 11.7 and 177.5 K. Phys Chem Chem Phys 22:20562–20572. https://doi.org/10.1039/d0cp03203d
Blázquez S, Espinosa S, Antiñolo M, Albaladejo J, Jiménez E (2022) Kinetics of CF3CH2OCH3 (HFE-263fb2), CHF2CF2CH2OCH3 (HFE-374pcf), and CF3CF2CH2OCH2 (HFE-365mcf3) with OH radicals, IR absorption cros. Phys Chem Chem Phys 24:14354–14364. https://doi.org/10.1039/d2cp00160h
Burkholder JB (Lead Author), Hodnebrog Ø, Orkin VL (2018) Summary of abundances, lifetimes, Ozone Depletion Potentials (ODPs), Radiative Efficiencies (REs), Global Warming Potentials (GWPs), and Global Temperature Change Potentials (GTPs), Appendix A in Scientific assessment of ozone depletion: 2018. Global ozone research and monitoring project-report no. 58. world meteorological organization, Geneva, Switzerland
Burkholder JB, Sander SP, Abbatt JPD, Barker JR, Huie RE, Kolb CE, Kurylo MJ, Orkin VL, Wilmouth DM, Wine PH (2020) Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 19. JPL Publ 19–5:1–153
Ceacero-Vega AA, Ballesteros B, Bejan I, Barnes I, Jiménez E, Albaladejo J (2012) Kinetics and mechanisms of the tropospheric reactions of menthol, borneol, fenchol, camphor, and fenchone with hydroxyl radicals (OH) and chlorine atoms (Cl). J Phys Chem A 116:4097–4107. https://doi.org/10.1021/jp212076g
Cillien C, Goldfinger P, Huybrechts G, Martens G (1967) Hydrogen abstraction from chlorinated ethanes by chlorine atoms. Int J Chem Kinet 5:539–543. https://doi.org/10.1002/kin.550050404
Demore WB, Howard CJ, Sander SP, Ravishankara AR, Golden DM, Kolb CE, Hampson RF, Molina MJ, Kurylo MJ (1997) Chemical kinetics and photochemical data for use in stratospheric modeling. Evaluation No. 12 JPL Publ 97-4:1–269
Hodnebrog, Etminan M, Fuglestvedt JS, Marston G, Myhre G, Nielsen CJ, Shine KP, Wallington TJ (2013) Global warming potentials and radiative efficiencies of halocarbons and related compounds: A comprehensive review. Rev Geophys 51:300–378. https://doi.org/10.1002/rog.20013
Hodnebrog, Aamaas B, Fuglestvedt JS, Marston G, Myhre G, Nielsen CJ, Sandstad M, Shine KP, Wallington TJ (2020) Updated Global Warming Potentials and Radiative Efficiencies of Halocarbons and Other Weak Atmospheric Absorbers. Rev Geophys. https://doi.org/10.1029/2019RG000691
Jiménez E, Lanza B, Antiñolo M, Albaladejo J (2009) Photooxidation of leaf-wound oxygenated compounds, 1-penten-3-ol, (z)-3-hexen-1-ol, and 1-penten-3-one, initiated by OH radicals and sunlight. Environ Sci Technol 43:1831–1837. https://doi.org/10.1021/es8027814
Le Bris K, DeZeeuw J, Godin PJ, Strong K (2020) Radiative efficiency and global warming potential of the hydrofluoroether HFE-356mec3 (CH3OCF2CHFCF3) from experimental and theoretical infrared absorption cross-sections. J Mol Spectrosc 367:111241. https://doi.org/10.1016/j.jms.2019.111241
Li P, Mühle J, Montzka SA, Oram DE, Miller BR, Weiss RF, Fraser PJ, Tanhua T (2019) Atmospheric histories, growth rates and solubilities in seawater and other natural waters of the potential transient tracers HCFC-22, HCFC-141b, HCFC-142b, HFC-134a, HFC-125, HFC-23, PFC-14 and PFC-116. Ocean Sci 15:33–60. https://doi.org/10.5194/os-15-33-2019
Orkin VL, Villenave E, Huie RE, Kurylo MJ (1999) Atmospheric lifetimes and global warming potentials of hydrofluoroethers: Reactivity toward OH, UV spectra, and IR absorption cross sections. J Phys Chem A 103:9770–9779. https://doi.org/10.1021/jp991741t
Oyaro N, Sellevåg SR, Nielsen CJ (2005) Atmospheric chemistry of hydrofluoroethers: Reaction of a series of hydrofluoroethers with OH radicals and Cl atoms, atmospheric lifetimes, and global warming potentials. J Phys Chem A 109:337–346. https://doi.org/10.1021/jp047860c
Papadimitriou VC, Kambanis KG, Lazarou YG, Papagiannakopoulos P (2004) Kinetic Study for the Reactions of Several Hydrofluoroethers with Chlorine Atoms. J Phys Chem A 108:2666–2674. https://doi.org/10.1021/jp031081z
Pinnock S, Hurley MD, Shine KP, Wallington TJ, Smyth TJ (1995) Radiative forcing of climate by hydrochlorofluorocarbons and hydrofluorocarbons. J Geophys Res 100:227–238. https://doi.org/10.1029/95jd02323
Shine KP, Myhre G (2020) The Spectral Nature of Stratospheric Temperature Adjustment and its Application to Halocarbon Radiative Forcing. J Adv Model Earth Syst 12:1–16. https://doi.org/10.1029/2019MS001951
Spivakovsky CM, Logan JA, Montzka SA, Balkanski YJ, Foreman-Fowler M, Jones DBA, Horowitz LW, Fusco AC, Brenninkmeijer CAM, Prather MJ, Wofsy SC, McElroy MB (2000) Three-dimensional climatological distribution of tropospheric OH: Update and evaluation. J Geophys Res Atmos 105:8931–8980. https://doi.org/10.1029/1999JD901006
Tsai W (2005) Environmental risk assessment of hydrofluoroethers (HFEs). J Hazard Mater 119:69–78. https://doi.org/10.1016/j.jhazmat.2004.12.018
Tschuikow-Roux E, Faraji F, Niedzielski J (1986) Rate constants and kinetic isotope effects for hydrogen/deuterium abstraction by chlorine atoms from the chloromethyl group in CH2ClCH2Cl, CD2ClCD2Cl, CH2ClCHCl2, and CH2ClCDCl<s. Int J Chem Kinet 18:513–527. https://doi.org/10.1002/kin.550180502
Urata S, Takada A, Uchimaru T, Chandra AK (2003) Rate constants estimation for the reaction of hydrofluorocarbons and hydrofluoroethers with OH radicals. Chem Phys Lett 368:215–223. https://doi.org/10.1016/S0009-2614(02)01718-9
Wang P, Solomon S, Lickley M, Scott JR, Weiss RF, Prinn RG (2023) On the Influence of Hydroxyl Radical Changes and Ocean Sinks on Estimated HCFC and HFC Emissions and Banks. Geophys Res Lett 50. https://doi.org/10.1029/2023GL105472
Wine PH, Semmes DH (1983) Kinetics of Cl(2PJ) Reactions with Chloroethanes CH3CH2Cl, CH3CHCl2, CH2ClCH2Cl, and CH2ClCHCl2. J Phys Chem 87:3572–3578
Acknowledgements
The authors would like to thank Prof. Claus J. Nielsen and Dr. Karine Le Bris for providing their IR absorption cross sections of HFE-356mec3 and HFE-236ea1. The authors also thank Prof. Juan Tejeda from UCLM for performing the purity analysis by NMR of HFE-356mec3. M. Asensio also acknowledges the regional government of Castilla-La Mancha through the CINEMOL project (Ref.: SBPLY/19/180501/000052) co-funded by the European Regional Development Fund (FEDER) and the University of Castilla-La Mancha (Ref.: 2022-GRIN-34143) for funding her contracts during the performance of these experiments.
Funding
This work has been supported by the regional government of Castilla-La Mancha through the CINEMOL project (Ref.: SBPLY/19/180501/000052) and INTERESFERA project (Ref.: SBPLY/23/180225/000054), both co-funded by the European Regional Development Fund (FEDER). We also acknowledge the University of Castilla-La Mancha for funding this work through the Ayudas para la financiación de actividades de investigación dirigidas a grupos (Ref.: 2022-GRIN-34143) co-funded by FEDER funds.
Author information
Authors and Affiliations
Contributions
Sara Espinosa and María Asensio carried out the experiments, made the analysis and interpretation of data and prepared the original draft. María Antiñolo contributed to the conceptualization and supervision of the experiments, and the preparation and review of the original draft. José Albaladejo reviewed and edited the original draft. Elena Jiménez contributed to the conceptualization and supervision of the experiments, reviewed and edited the original draft, and acquired the funds and managed the funded projects.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Gerhard Lammel
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• HFE-356mec3 and HFE-236ea1 react slowly with OH and Cl at 298 K.
• Activation energies for OH-reactions were 8.2 and 10.7 kJ mol–1 for HFE-356mec3 and HFE-236ea1.
• They absorb in the mid-IR (500-1500 cm–1) with peak absorption cross-sections ~ 10–18 cm2 molecule–1.
• Lifetime-corrected radiative efficiencies of the investigated HFEs were 0.27 and 0.41 W m–2 ppbv–1.
• Even the investigated HFEs still present relatively high GWP100-yrs, it is reduced with respect to analogue HFCs.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Espinosa, S., Asensio, M., Antiñolo, M. et al. Atmospheric chemistry of CF3CHFCF2OCH3 (HFE-356mec3) and CHF2CHFOCF3 (HFE-236ea1) initiated by OH and Cl and their contribution to global warming. Environ Sci Pollut Res 31, 50347–50358 (2024). https://doi.org/10.1007/s11356-024-34374-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34374-8