Abstract
The principle of Fenton reagent is to produce ·OH by mixing H2O2 and Fe2+ to realize the oxidation of organic pollutants, although Fenton reagent has the advantages of non-toxicity and short reaction time, but there are its related defects. The Fenton-like technology has been widely studied because of its various forms and better results than the traditional Fenton technology in terms of pollutant degradation efficiency. This paper reviews the electro-Fenton technology among the Fenton-like technologies and provides an overview of the homogeneous electro-Fenton. It also focuses on summarizing the effects of factors such as H2O2, reactant concentration, reactor volume and electrode quality, reaction time and voltage (potential) on the efficiency of electro-Fenton process. It is shown that appropriate enhancement of H2O2 concentration, voltage (potential) and reaction volume can help to improve the process efficiency; the process efficiency also can be improved by increasing the reaction time and electrode quality. Feeding modes of H2O2 have different effects on process efficiency. Finally, a considerable number of experimental studies have shown that the combination of electro-Fenton with ultrasound, anodic oxidation and electrocoagulation technologies is superior to the single electro-Fenton process in terms of pollutant degradation.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fenton's reagent is a mixture of H2O2 and Fe2+, through the combination of these two substances produces ·OH in order to achieve the oxidation of organic pollutants for degradation and removal. The use of this reagent dates back to the late 19 century when Fenton (1894, 1896) catalyzed the oxidation of H2O2 through the use of FeSO4 salt and was used to degrade tartaric acid. In 1934, Haber and Weiss (1934) formalized the reaction mechanism of Fenton's reagent, which included the involvement of hydroxyl radicals (·OH). The ·OH is the most reactive oxygen species (ROS) known, with a standard oxidation potential of up to 2.8 V, and therefore has a low selectivity for substances. From the reaction mechanism, Fenton technology is in acidic conditions, H2O2 in the presence of Fe2+ to generate a strong oxidizing capacity of the hydroxyl radical and trigger more other reactive oxygen, in order to achieve the degradation of organic matter, the oxidation process for the chain reaction. The relevant reaction equations are as follows:
Chain reaction initiation phase:
Chain reaction delivery phase:
Chain reaction termination phase:
In the above reaction, the reaction of Fe3+ with H2O2 produces perhydroxyl radicals (·HO2), which is also known as a "Fenton-like" reaction. The oxidizing ability of ·HO2 is lower than that of other ROS, but ·HO2 is involved in the reduction of Fe3+ to Fe2+ (Equation (5)), which maintains the generation of ·OH and thus the reaction (Equation (2)). (Salgado et al. 2013).
Although the Fenton reagent has the advantages of non-toxicity, short reaction time and low cost, it also has the disadvantages of adding too much reagent and easy to produce secondary pollution. Fenton-like technology is a method of adding other technologies on the basis of traditional Fenton technology to achieve efficient degradation of pollutants. Since the Fenton-like technology has obvious improvement compared with the traditional Fenton technology, the research on Fenton-like technology has gradually become a hot spot. For example, Possetto et al. (2018) investigated the effect of degradation of Bioallethrin insecticide by using modified photo-Fenton process, and the experimental results showed that modified photo-Fenton process could achieve the complete mineralization of this insecticide in less than one hour. Hung et al. (2019) used a bacterial enzyme, dihydrolipoamide dehydrogenase, extracted from strain TX1 to form a Fenton's reagent for the degradation of the organic matter octylphenol polyethoxylate (OPEOn) by the bio-Fenton process in conjunction with its bacterial enzyme in the presence of excess metal mixtures, and the experimental results showed that the process combined with its bacterial enzyme allowed for the breakage of ethoxylate chains, which led to the efficient degradation of ethoxylates. Wang et al. (2020) carried out a study related to the de-complexation of the organic substance Ni-EDTA by microwave-assisted Fenton process, and the experimental results showed that microwave irradiation could promote the cleavage of refractory bonds in the substance under certain conditions, and the de-complexation efficiency of 94% could be achieved in 10 minutes of the reaction. Li et al. (2013b) carried out a study related to the treatment of wastewater collected from the munitions processing plant by using the ultrasound and Fenton process, and the results showed that: under certain conditions of pH value, temperature, and reaction time, increasing the ultrasonic intensity can accelerate the removal rate of TOC, COD and colored substances in the wastewater.
Electro-Fenton, as a kind of Fenton-like technology, its principle is to add an external power source on the basis of its Fenton technology to continuously generate H2O2 through electrochemical reaction, so as to degrade organic substances and pollutants. The principle of electro-Fenton technology in the electric field and acidic solution conditions, electro-Fenton system of the cathode surface of the electronic reaction of oxygen to generate H2O2, at the same time Fe3+ get electrons to reduce to Fe2+, through the strength of the electric field to control the rate of generation of H2O2 and Fe2+; in the anodic surface, the water loses electrons to be oxidized, generating oxygen and H+; the solution of the H2O2 and Fe2+ reaction to generate ·OH, used for oxidative degradation of organic matter, pollutants. Electro-Fenton does not require or only need to add a small amount of chemical reagents, and does not produce secondary pollution, in the electrolysis process parameters can be freely controlled, electro-Fenton process compared to the traditional Fenton reagent an advantage is that the reaction (Equation (1)) is electrocatalytic, because Fe2+ can be reduced by Fe3+ at the cathode and quickly generate (Brillas et al. 2009; Labiadh et al. 2015).
This review outlines the study of electro-Fenton related influencing factors and their related combined technologies, with the first part outlining the principle of homogeneous electro-Fenton reaction and their reaction forms. In the second part, the literature analysis is summarized for H2O2, reactant concentration, reaction volume and electrode quality, reaction time and voltage (potential) among the electro-Fenton influencing factors. The third part describes the combined application of electro-Fenton with ultrasound, anodic oxidation and electrocoagulation technology.
Homogeneous electro-fenton
The homogeneous electro-Fenton reaction, as a form of electro-Fenton reaction, occurs in the same phase, i.e., all chemical reactions take place in the liquid phase; oxidation and mineralization of the target pollutants are achieved through the use of H2O2 generated at the cathode, and catalyzed by soluble Fe2+ in solution. The advantages of this method, in addition to the fact that the Fe3+ produced after the reaction can be reduced to Fe2+ at the cathode, are as follows (Martínez-Huitle and Brillas 2009):
-
(i)
Since H2O2 is generated in situ, it effectively avoids transportation and storage risks;
-
(ii)
The addition of Fe2+ is much smaller than conventional Fenton reagents and the degradation rate of organic matter is faster than that of conventional Fenton;
-
(iii)
Potential total mineralization with relatively low power consumption.
However, there are corresponding disadvantages to the homogeneous electro-Fenton (Brillas 2021):
-
(i)
There is a great limitation of the reaction pH;
-
(ii)
Acidification of wastewater with large quantities of chemicals prior to treatment or neutralization of treated solutions prior to treatment;
-
(iii)
Sludge treatment after neutralization will increase the operating cost.
In disadvantage (i), since the application of homogeneous electro-Fenton is limited by strongly acidic pH conditions (pH: 2.8-3.5), post-treatment neutralization of the treated water is required from an environmental point of view (Ganiyu et al. 2018; Poza-Nogueiras et al. 2018). Figure 1 shows the mechanism of pollutant degradation by homogeneous electro-Fenton.
The homogeneous electro-Fenton process can have two different configurations. In the first configuration the Fenton reagent is added externally to the reactor and an inert electrode with high catalytic activity is used as an anode material, while in the second configuration only hydrogen peroxide is added externally and Fe2+ is supplied by a sacrificial cast iron anode (Govindan et al. 2014b, 2015a, 2020b). Depending on the addition or formation of Fenton reagents, the homogeneous electro-Fenton process can be divided into four electro-Fenton processes: Cathode-Fe2+ cycle method (EF-H2O2-Fere), Cathode-electro-Fenton (EF-H2O2), Sacrificial anode electro-Fenton (EF-Feox), Fe2+ cyclic method (EF-Fere) (Jiang and Zhang 2007; Kumar et al. 2019):
-
(1)
Cathode-Fe2+ cycle method (EF-H2O2-Fere)
This reaction configuration does not require the addition of H2O2 solution and Fe2+ solution, and H2O2 is produced by exposing air or O2 near the cathode, and under acidic conditions O2 undergoes a 2-electron reduction reaction at the cathode. While the anode material is iron, Fe loses electrons by oxidation to produce Fe2+. In this configuration, the electrode reaction is as shown in Fig. 2, and the related electrode reaction equation is as follows:
Anodic reaction:
Cathodic reaction:
-
(2)
Cathode-electro-Fenton method (EF-H2O2)
In this reaction configuration, H2O2 is still produced in situ by an electrode reaction, but Fe2+ needs to be added externally to participate in the system reaction to form the Fenton reagent. The reaction mechanism is that H2O2 is generated by exposing O2 or air near the cathode under acidic solution conditions and the reduction reaction occurs at the cathode, and H2O2 undergoes a Fenton reaction with the added Fe2+ to generate ·OH, and at the same time, oxidized Fe3+ is obtained, and Fe3+ can be reduced again at the cathode to form Fe2+. The reaction schematic diagram of the present configuration is shown in Fig. 3, and the relevant electrode reactions of this reaction configuration are as follows:
Anodic reaction:
Cathodic reaction:
Sometimes this reaction configuration can also be achieved by direct addition of Fe3+, which is reduced to Fe2+ by gaining electrons at the cathode, resulting in a Fenton's reagent reaction with H2O2.
-
(3)
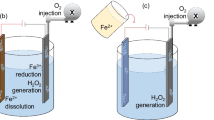
Sacrificial anode electro-Fenton method (EF-Feox)
In this reaction configuration, the anode material is iron electrode, Fe2+ is generated by Fe oxidation reaction at the anode, i.e., Fe loses two electrons to generate Fe2+, then Fe2+ reacts with the externally added H2O2 to generate ·OH, and Fe2+ generates Fe3+ through oxidation. In addition to the factor of ·OH in the process of degradation of organics and pollutants in the system, there are also sometimes flocculation effects of Fe(OH)2 and Fe(OH)3. Figure 4 shows the schematic diagram of this reaction configuration, and its related electrode reaction formula is as follows:
Anodic reaction:
Cathodic reaction:
-
(4)
Fe2+ cyclic Method (EF-Fere)
In this reaction configuration, H2O2 and Fe2+ are added externally to participate in the electro-Fenton system reaction, and there is no need to expose air or O2. During the electro-Fenton reaction, Fe2+ can be oxidized to produce Fe3+, and Fe3+ can be formed through the cathode reduction reaction to form Fe2+, which realizes the reuse of Fe2+. Moreover, this reaction does not produce a large amount of sludge, which causes secondary pollution. In addition, the reaction will not produce a large amount of sludge and cause secondary pollution. Figure 5 shows the schematic diagram of the reaction configuration, and its related electrode reaction equation is as follows:
Anodic reaction:
Cathodic reaction:
Similar to the reaction configuration in (2), sometimes this reaction configuration can be furthered by direct addition of Fe3+, by cathodic reduction, which results in a Fenton's reagent reaction.
Influencing factor on electro-Fenton
Effect of H2O2
H2O2 as an important component of the Fenton reagent in the electro-Fenton reaction has a significant impact on the degradation of pollutants or organic matter. H2O2 reagent can provide ·OH for the Fenton reaction and is used to perform the mineralization mechanism of the generated sludge (Rahmani et al. 2015; Moussavi et al. 2012). Related studies have shown that the concentration (dosage) and method of addition of H2O2 reagents can have an impact on the electro-Fenton process; the appropriate method of addition of H2O2 and the concentration (dosage) of the reagents can optimize the degradation efficiency of the electro-Fenton process to achieve efficient degradation of organic matter or pollutants, but if they are added inappropriately, the electro-Fenton reaction will be negatively impacted, especially for the economic cost of the H2O2 and the possible negative impact on the performance of the electro-Fenton (Akyol et al. 2013).
-
(1)
Additive concentration (dosage)
In the electro-Fenton reaction, the addition of H2O2 plays an important role in the degradation of substances. However, the concentration (dosage) of H2O2 does not have a positive relationship with the removal of substances in water. Related studies have shown that too high concentration (dosage) of H2O2 does not result in a higher removal efficiency of COD, and at the same time reduces the efficiency of the electro-Fenton in removing organic matter or pollutants (Zhang et al. 2021; Ghosh and Samanta 2011; Dolatabadi and Ahmadzadeh 2019).
The main reason why the addition of H2O2 enhances COD removal is the possible production of ·OH, which plays an important role in the decomposition of organic matter (Babuponnusami and Muthukumar 2012; Moayerikashani and Soltani 2013). The concentration of ·OH is determined by the concentration (dosage) of H2O2 added until the optimal H2O2 concentration (dosage) of the target pollutant is reached. However, when the concentration (dosage) of H2O2 exceeds the optimal value for the degradation of the pollutant, the degradation rate decreases. Relevant studies have analyzed that at too high concentration (dosage) of H2O2, the oxidation potential of the electro-Fenton process is lowered due to the scavenging effect of H2O2 on ·OH (Equation (24)(25)) (Sevimli et al. 2014; Mohajeri et al. 2010); in addition, at too high concentration (dosage) of H2O2, the combination of the two ·OHs to form H2O2 also results in the decrease in the efficiency of pollutant's degradation (Equation (26)) (Muruganandham and Swaminathan 2004), and the relevant reaction equations are as follows. In addition to the above reasons, it has been suggested that excess H2O2 leads to the generation of short-chain organic acids during pollutant degradation, which hinders further oxidation of the pollutant (Umar et al. 2010; Dargahi et al. 2021b).
$${\mathrm{H}}_2{\mathrm{O}}_2+\cdot\mathrm{OH}\longrightarrow {\mathrm{H}}_2\mathrm{O}+{\cdot\mathrm{H}\mathrm{O}}_2$$(24)$${\cdot\mathrm{H}\mathrm{O}}_2+\cdot\mathrm{OH}\longrightarrow {\mathrm{H}}_2\mathrm{O}+{\mathrm{O}}_2$$(25)$$2\cdot \mathrm{OH}\longrightarrow {\mathrm{H}}_2{\mathrm{O}}_2$$(26)In addition to this, the addition of H2O2 also increases the pH of the reaction solution (Zhao et al. 2012). Although electro-Fenton has a high removal efficiency, the consumption of H2O2 also increases the cost of consumption in addition to the electrode consumption (Can 2013). Therefore, the initial concentration (dosage) of H2O2 plays a very important role in the electro-Fenton reaction (Ting et al. 2009). Figure 6 also shows the influence of H2O2 concentration (dosage) on the electro-Fenton process in some studies.
-
(2)
Feeding mode
The feeding mode of H2O2 is an important parameter for the performance of an electro-Fenton system, and increasing H2O2 during the initial or reaction process will result in changes in the ratios of [H2O2]/[COD] and [H2O2]/[Fe2+]to achieve the final removal of COD (Primo et al. 2008). Back in 2005, Zhang et al. (2005) found that increasing the concentration of H2O2 in multiple steps in the Fenton system improved the treatment of waste leachate compared to increasing it in a single stage. In a subsequent study, they applied the electro-Fenton method to the treatment of waste leachate by using an electrolytic batch reactor containing 200 mL of solution; the results showed that COD removal increased with the gradation of dosage, with the highest removal (79%) when using a continuous addition mode (Zhang et al. 2006) (Fig. 7a). In recent years, Alavi et al. (2019) (Fig. 7b) compared the effect of one-step injection H2O2 versus four-step injection H2O2 on COD removal efficiency in the electro-Fenton process when treating leachate from a composting plant through the electro-Fenton process; the results showed that the minimum value of COD removal was associated with one-step injection and the maximum value was associated with four-step injection, and this finding exists in agreement with the trend of the results of the study conducted by Zhang et al. (2005, 2006).
Fig. 7 The reason for this experimental result is that the gradual addition of H2O2 keeps H2O2 at a low value, minimizing the negative effect of ·OH removal (Zhang et al. 2006). On the other hand, the addition of H2O2 in the initial steps of each stage of the experiment leads to a rapid production of ·OH, which makes the reaction of ·OH with H2O2 and Fe2+ more prominent; the relevant reaction equation is as follows (Alavi et al. 2019):
$$\cdot\mathrm{HO}+{\mathrm{H}}_2{\mathrm{O}}_2\longrightarrow {\cdot\mathrm{H}\mathrm{O}}_2+{\mathrm{H}}_2\mathrm{O}$$(27)$$\cdot\mathrm{HO}+{\mathrm{Fe}}^{2+}\longrightarrow {\mathrm{Fe}}^{3+}+{\mathrm{OH}}^{-}$$(28)In addition, Zhang et al. (2007) further investigated the effect of gradually shortening the feeding time on the degradation rate of pollutants on the basis of multiple dosing of H2O2. The results showed that appropriately shortening the feeding time can increase the content of H2O2, which can produce more ·OH by reacting with Fe2+, so as to enhance the pollutant removal efficiency; however, the further shortening of the feeding time will lead to the scavenging effect of the ·OH by the excess H2O2, resulting in a lower removal efficiency of the pollutants. However, Anotai et al. (2010) (Fig. 7c) found that the one-step and two-step additions of H2O2 resulted in similar degradation rates without significant differences in the degradation of aniline by the electro-Fenton process. During the degradation process, aniline was rapidly degraded in the first two minutes of the reaction, while the rate of aniline degradation slowed down in 2-30 minutes.
Finally, Table 1 lists relevant literature studies on the effect of H2O2 on the electro-Fenton process.
Table 1 Examples of effect of H2O2 by Electro-Fenton reaction process Table 1 Examples of effect of H2O2 by Electro-Fenton reaction process
Effect of reactant concentration
The concentration of reactive substances, as an important variable in the electro-Fenton reaction, can be analyzed to elucidate where the ability of the oxidation of substances in the electro-Fenton process lies. And the change of reaction concentration will also affect the degradation efficiency and degradation process of electro-Fenton reaction.
Bedolla-Guzman et al. (2016) in investigating the effect of azo dye concentration on the degradation process found that the decolorization rate decreased as the dye concentration increased; Guelfi et al. (2018) in investigating the effect of herbicide concentration on the rate of degradation under certain conditions found that the time to reach complete removal increased when the concentration of herbicide increased. The reason for such an experimental phenomenon is that under certain reaction conditions, a certain amount of ·OH is produced per unit time. When the concentration of pollutant increases, the amount of ·OH is not sufficient to degrade the high concentration of pollutant, so the removal efficiency of pollutant decreases (Teymori et al. 2019). In contrast, the increase of organic molecules in solution requires more hydroxyl radicals for degradation (Annabi et al. 2016), so the time required to achieve a higher degradation rate increases when the concentration of reactants is higher. In addition, for the degradation of some organic substances or pollutants, higher reactive pollutants produce intermediates that are difficult to degrade during the degradation process, and this intermediate product is an obstacle to the degradation of the original target substance, and the intermediate product competes with the target substance for ·OH, which leads to a decrease in the rate of the reaction, which can be deduced from the experimental studies of El-Desoky et al. (2010) and Kadji et al. (2020) analysis.
On the other hand, changes in the concentration of reacting substances are also related to kinetics, and pseudo first-order and pseudo second-order reaction kinetics are commonly used to explore the relationship between the concentration of reacting substances and the rate of degradation with the following correlation equations (Equation (29)(30)):
Pseudo first-order reaction kinetics:
Pseudo second-order reaction kinetics:
where C0 and Ct denote the concentration of the substance at the beginning of the reaction and after the reaction time t, respectively, and K1 and K2 denote the pseudo first-order and pseudo second-order reaction constants, respectively. The values of K1 and K2 can be determined from the analysis of the slopes of lnCt versus t and 1/Ct versus t, respectively (Moussavi et al. 2011). From the experiments of Mansour et al. (2011), Teymori et al. (2019), it can be seen that the time required to reach the same ln(C0/Ct) is increasing with the increase in the concentration of the reactants and also due to the above analyzed reasons, the values of K1, K2 are eventually decreasing.
On the other hand, for the mineralization current efficiency (MCE), an increase in the concentration of reactive substances leads to an increase in the final MCE value (Flores et al. 2016a, 2017; Guelfi et al. 2016). This is due to the increase in organic loading, where a larger organic load favors a larger amount of oxidized substances to react with the intermediates (Flores et al. 2016b), thus reducing the occurrence of associated parasitic reactions. Common parasitic reactions are (i) the oxidation of BDD (·OH) to O2 (Equation (31)); (ii) the scavenging of ·OH by Fe2+ with H2O2 (Equation (32)(33)) (Sirés and Brillas 2012; Sirés et al. 2014); (iii) the isolation of S2O82- from SO42- with inhibition of emission of the reaction H2O (Equation (34)(36)); and (iv) the generation of other weaker oxidizing agents by reaction and at the anode (Equation (35)). The relevant reaction equations are given below:
However, Wang et al. (2010) in exploring the effect of concentration change of Acid Red 14 dye found that the MCE of the EF process increased to a maximum value when the initial AR14 concentration did not exceed a certain value, and thereafter, it gradually decreased over a longer electrolysis time. However, when the AR14 concentration exceeded a certain value, the MCE decreased to a minimum value and then increased with electrolysis time. This contradicts the results of some of the experiments, which they attribute to the ratio between the concentration of AR14 and the concentration of ·OH produced by the electro-Fenton reaction.
In addition to the degradation of substances, changes in the initial concentration of reactants can also have an impact on the reaction state and the synthesis of new substances in the conversion synthesis of related organic substances using the electro-Fenton process. For example, Galia et al. (2016), Lanzalaco et al. (2017) explored the effect of different PVP specific gravity on the synthesis of nanogels when polyvinylpyrrolidone (PVP) was synthesized using the electro-Fenton process and found that the degree of decay of the hydrodynamic radius (Rh) decreases as the specific gravity of the PVP is increased, which is attributed to the decrease in the average number of free radicals per chain due to a higher concentration of polymer chains and a fixed rate of generation of ·OH. Furthermore, in the experiments of Rekik et al. (2017) on the conversion of vanillic acid (VA) to protocatechuic acid (PCA), it was found by varying the concentration variable that: as the concentration of VA increased, the concentration of the converted PCA increased; in addition the energy consumption in the reaction decreased as the concentration of VA increased. This experimental phenomenon can be attributed to the reduction of ·OH loss in the secondary reaction (Equation (37)), which leads to an increase in process efficiency.
Figure 8 and Table 2 respectively show and list relevant literature studies on the effect of reactant concentration on the electro-Fenton process.
Table 2 Examples of effect of reactant concentration by Electro-Fenton reaction process
Effect of reaction volume and electrode quality
The current study shows that varying the reaction volume and the electrode quality as two geometrical parameters in the electro-Fenton reaction will have an effect on the yield of H2O2 in the reaction.
Xia et al. (2015) investigated the ability of polyacrylonitrile-based carbon fiber brush (PAN-CFB) cathodes to produce H2O2 in different volumes of Na2SO4 electrolyte. The experimental results showed that the electro-generated yield of H2O2 tended to increase and then decrease when the reaction volume was gradually decreased. However, their theoretical speculations showed that the yield of H2O2 should increase with decreasing reaction volume. They analyzed that the reason for this experimental deviation is that the reaction solution in a very small volume is not conducive to the accumulation of the yield of H2O2 and produces a larger decomposition of H2O2, which is reflected in the action of the anode on H2O2.
Chen et al. (2019), on the other hand, investigated the effect of different reactor volumes and electrode qualities on the yield of H2O2. They found that increasing the electrode quality of the cathode increases the yield of H2O2 while increasing the reactor volume decreases the yield of H2O2 but the current efficiency increases. For the electrode quality, increasing the electrode quality increases the sites where O2 reduction occurs, which in turn leads to an increase in the yield of H2O2. And increasing the reactor volume, although the concentration of H2O2 will decrease, the consumption of H2O2 will be weakened at low concentration of H2O2, and at the same time, the electron utilization efficiency is increased and the current efficiency is increased.
Effect of reaction time
As one of the influential parameters of the electro-Fenton process, the reaction time not only reflects the trend of the relevant parameters over time during the degradation or oxidation of pollutants or organics, but also provides an assessment and reference for finding the optimal degradation efficiency of pollutants or organics with the optimal process cost.
Flores et al. (2017) in the treatment of 4-hydroxyphenylacetic acid in olive oil mill wastewater by the electro-Fenton process under certain conditions, concluded that the TOC content decreased at a fast rate during the first 120 minutes, and then slowly with increasing time after 120 minutes. Mansour et al. (2015) in the treatment of sulphadiazine by using the electro-Fenton process under certain conditions, found that: electrolysis at mineralization was only 2.1% and 18.1% at 30 and 60 min and the oxidation level was very low, their experiments indicated that intermediates were produced during the degradation process. Typically, the rate of degradation or mineralization of a contaminant increases at a certain rate over time for a short period of time; after a certain degradation or mineralization value has been reached, the rate of mineralization or degradation increases slowly over a longer period of time. This is usually attributed to the formation of degradation by-products (e.g., short-chain carboxylic acids) with reduced reaction kinetics with hydroxyl radicals (Ouarda et al. 2020). On the one hand, an increase in electrolysis time is directly related to an increase in degradation efficiency due to certain reaction conditions. By increasing the electrolysis time, the production of ·OH is also enhanced and the increase in the amount of ·OH leads to a higher efficiency in the evaluation process. However, with the increase of electrolysis time, the content of target pollutants gradually decreased, which made the collision chance between ·OH and target pollutants decreased, and eventually led to a slow increase in the removal rate; on the other hand, since the target pollutants produce by-product intermediates during the degradation process, which are difficult to be mineralized, it takes a longer time for their degradation, which leads to an increase in the energy consumption of the process, and thus an increase in the operating cost.
In the electro-Fenton reaction, the mineralization current efficiency (MCE) usually exhibits a rapid increase at some time after the onset of degradation, after reaching a maximum value, followed by a gradual decrease and slow leveling off with time (Guelfi et al. 2016; Flores et al. 2016b). There are two main reasons for this phenomenon: (i) a decrease in organic matter content as degradation proceeds; and (ii) the formation of more difficult-to-degrade products (Sirés et al. 2014; Panizza and Cerisola 2009).
where F is Faraday's constant (96487 C mol−1), V is the volume of solution (L), Δ(TOC)exp is the experimental TOC reduction (mg L−1), 4.32×107 is the conversion factor (3600 s h−1 × 12000 mg C mol−2), m is the number of carbon atoms contained in the target pollutant or organic matter, and n is the number of electrons from the residual reaction during the mineralization process (which needs to be calculated from the reaction equation).
It is easy to see from the equation that when the conditions of the reaction are certain (i.e., n, F, V, m, and I are certain), the TOC reduction in the experiment increases in the pre-reaction as the time increases, which results in the rate of change of the numerator in the equation being greater than the rate of change of the denominator, which leads to an increase in the mineralization current efficiency. When the maximum value is passed, the rate of change of the denominator is greater than the rate of change of the numerator as time increases because the reduction of TOC tends to level off, so the mineralization current efficiency starts to decrease, and when it goes down to a certain value, the Δ(TOC)exp /t ratio tends to a constant value, so the mineralization current efficiency eventually levels off.
In the absence of Fe2+, the production of H2O2 may be produced faster for a short period of time, followed by a stabilization of the yield over a longer period of time. This phenomenon may be due to the increasing decomposition rate of H2O2, and when the H2O2 production is in equilibrium with the decomposition rate, the yield of H2O2 in solution tends to stabilize (Mounia and Djilali 2012). In the traditional electrochemical reaction system, the H2O2 content shows a tendency of increasing and then decreasing. The main reason may be that as the reaction proceeds, a large amount of Fe(OH)3 precipitates in the solution, which hinders the transfer of electrolyte in the solution. In contrast, for the electro-Fenton reaction using a composite cathode, the content of H2O2 is continuously increasing with time (Li et al. 2019a).
In summary, too short reaction time will make the process ineffective treatment; too long a reaction time will increase the operating cost of the process. As an important parameter, the reaction time is of great significance for the electro-Fenton process reaction. Figure 9 and Table 3 respectively show and list relevant literature studies on the effect of reaction time on the electro-Fenton process.
Table 3 Examples of effect of reaction time by Electro-Fenton reaction process
Effect of voltage (potential)
Voltage (potential), as an influential parameter in the electro-Fenton reaction, bears a strong resemblance to current. Related studies also illustrate that varying the voltage (potential) can also have an effect on Fenton reagents and the degradation of organic pollutants.
First of all, in terms of the production of H2O2, researchers such as Li et al. (2019b), Diouf et al. (2018) and Zheng et al. (2021) have investigated the effect of different voltages (potentials) on the production of H2O2. Their experimental studies all show the same trend, i.e., the maximum yield of H2O2 is reached at a certain potential or voltage, while the yield of H2O2 decreases beyond that potential or voltage. The applied voltage will directly affect the production of H2O2 (Haider et al. 2019) , on the one hand, according to Faraday's law, the current density increases with the increase of voltage, and this leads to an increase in the production rate of H2O2. On the other hand, when the voltage is too high, the cathode produces some side reactions, such as hydrogen precipitation reaction, H2O2 decomposition, or O2 undergoes four-electron reduction to H2O (Li et al. 2018; Xu et al. 2016; Özcan et al. 2008; Kuang et al. 2008), which prevents the generation of H2O2, thus resulting in a decrease in the yield of H2O2. Secondly, in terms of Fe2+/Fe3+, the experiments of Kim et al. (2018) showed that the concentration of Fe2+ increased with increasing potential, which indicated that the rate of conversion of Fe3+ to Fe2+ was accelerated. Li et al. (2019a, b, c) obtained the same experimental phenomenon, but they additionally concluded that the increasing voltage resulted in a decrease in the concentration of the total ferric ions in the solution; they concluded that the localized alkalinization of the cathode affected the pH near the cathode with the increase of the voltage (Petrucci et al. 2016), which led to the sedimentation of iron ions and decay of the rate of reduction of iron ions, resulting in a gradual decrease in the concentration of total iron ions.
Finally, voltage can also have an effect on the degradation efficiency of organic pollutants. Malakootian and Moridi (2017) investigated the effect of applied voltage on the degradation of Acid Red 18 dye. They found a significant increase in the dye removal efficiency by increasing the voltage from 10 V to 30 V and a decrease in the dye removal efficiency when the voltage was further increased to 40 V. George et al. (2013) studied the effect of applied voltage on the oxidation of Salicylic Acid and also came up with the same experimental pattern. Since voltage affects Fenton's reagent, which is a key factor in the degradation of organic pollutants, changing the voltage also directly affects the degradation efficiency of the pollutant. Ren et al. (2016) also found in the degradation effect of tartrazine that the degradation efficiency of the pollutant is close to 100% when the applied voltage is of a certain value and that the degradation efficiency of the pollutant reached a steady state when the applied voltage was higher than this voltage value. Cruz del Álamo et al. (2020) also showed that an increase in voltage led to a decrease in the efficiency of the mineralization current, which was also attributed to the generation of parasitic reactions. However, Lim et al. (2017) in the electro-Fenton treatment of palm oil mill wastewater found that the trend of COD removal efficiency was opposite to the trend of increasing applied voltage, with the highest COD removal efficiency being reached at the lowest voltage. They analyzed that the decrease in COD removal efficiency could be due to the oxidation of Fe2+ to Fe3+ at an oxidation potential of 0.771 V and the decomposition of H2O2 to H2O in an acidic solution at a reduction potential of 1.77 V, which would lead to a decrease in the concentration of the components that make up the Fenton reagent, which would lead to a decrease in the concentration of COD.
In addition, Li et al. (2011) also removed phenol wastewater by using a three-dimensional electro-Fenton system, and they found that when the voltage was elevated, the degradation of organic pollutants was accelerated, the level of polarization of the particle electrodes increased, and the number of working electrodes increased. They analyzed that the increase in voltage makes the main potential difference between the electrode and the conductive particles and electrolyte increase improves the impact of the electrochemical oxidation reaction, accelerates the direct and indirect oxidation rate, and makes the system more effective in treatment. Figures 10 and Table 4 respectively show and list relevant literature studies on the influence of voltage (potential) on the electro-Fenton process.
Table 4 Examples of effect of voltage (potential) by Electro-Fenton reaction process
Combination technology
Combined with ultrasound technology
Studies have shown that ultrasound technology has demonstrated excellent capabilities in numerous applications, such as in chemical processing (Kiss et al. 2018), chemical synthesis (Sancheti and Gogate 2017), cleaning technology (Tiwari 2015), water treatment (Goncharuk et al. 2008) and others. The application of ultrasound at frequencies higher than 20 kHz leads to the growth of cavitation bubbles that become stable after a high number of cycles; each bubble can be seen as a hot spot and collapses, thus generating energy to increase the temperature (up to 5000 K) and the pressure (up to 500 atm), while the bubbles cool down at a rate of up to 109 K/s (Nazari et al. 2018).
The ultrasound process in the degradation of pollutants in the mechanism embodied in the physical decomposition of the water acoustic cavitation generated cavities or micro bubbles (micro jets and shock waves) and ultrasound through the propagation of the liquid medium thereby causing this physical effect (Hasani et al. 2020; Wu et al. 2013). In the event of surface instability, the cavity collapse can be very asymmetric and generate high velocity liquid jets (microjets), thus increasing the mass transfer rate (Liang et al. 2007; Li et al. 2013a; Pokhrel et al. 2016). Ultrasound decomposition then occurs through three reaction zones, namely the gas phase region inside the bubble (pyrolysis reactions), the interfacial region (reactions occurring in the pressure/temperature gradient in the aqueous phase) and the native solution (Riesz and Kondo 1992). Although ultrasound technology has been shown to be effective in treating pollutants, there are many parameters related to cavitation and bubble breakup that affect the process of performing ultrasound, such as pressure amplitude, acoustic frequency, type of signal, and other factors such as liquid temperature, which affect the effectiveness of ultrasound in treating pollutants in the water column (Wood et al. 2017).
Ultrasound-electro-Fenton technology is a hybrid oxidation technology that combines ultrasound and electro-Fenton technology. In a system that combines electro-Fenton and ultrasound, the physical degradation of pollutants still occurs through the cavities or microbubbles generated by acoustic cavitation in the water, which are mixed and cleaned at high speeds on the surface of the electrodes through the solvation inhibition layer, leading to the enhanced transfer between the electrodes and the solution (Babuponnusami and Muthukumar 2012). The chemical effect, on the other hand, is due to the dramatic collapse of the microbubbles, which or cavities concentrate the ultrasound energy into the microreactor and reach the desired conditions in a short time (<1ns), where the microbubbles produce oxidized substances through homolytic cleavage of molecules (Trabelsi et al. 1996), and these oxidizing radicals enable pyrolysis of the organic matter and an increase in the rate of the reaction (Cai et al. 2014; Cai et al. 2016). In this case, the cavities are involved in the generation of hydroxyl radicals and free hydrogen, while the hydrogen radicals react with oxygen to generate hydroperoxide radicals (Li et al. 2013b). The relevant reaction equations are as follows (Equation (39)(40)):
In summary, ultrasound-electro-Fenton technology is a combination of physical and chemical mechanisms for effective pollutant degradation. This combination of technologies improves the cleanliness of the electrode surface, reduces process time, lowers energy consumption and improves environmental suitability and compatibility, and ultimately improves the pollutant removal efficiency through the resulting chemical and physical reactions (Hasani et al. 2020; Ranjit et al. 2008). Studies have also shown that ultrasound-electro-Fenton technology is more effective in the treatment of contaminants than electro-Fenton treatment alone. Hasani et al. (2020) utilized ultrasound-electro-Fenton for the removal of cefixime antibiotics from aqueous solutions and found that the maximum removal efficiency of the process under optimal conditions could reach 97.5%. Whereas, also under optimal conditions, the removal efficiency of the electro-Fenton process and ultrasound only reached 81.7% and 9%, respectively; Mahmoudi et al. (2021) compared the removal efficiencies of the ultrasound-electro-Fenton and electro-Fenton processes for Acid Black 172 (AB172) and Dispersed Blue 56 (DB56), and found that the maximal removal efficiency of the electro-Fenton was 82-88%, whereas the ultrasound-electro-Fenton could reach 89.5-91%; Babuponnusami and Muthukumar (2012) compared the effect of electro-Fenton and ultrasound-electro-Fenton on the degradation performance of phenol, and the results showed that the ultrasound-electro-Fenton method could achieve complete phenol removal in a shorter period of time compared to the electro-Fenton process.
With the in-depth study of ultrasound-electro-Fenton, the reaction system using three-dimensional electro-Fenton in ultrasound-electro-Fenton has begun to emerge, but there are still very few techniques on ultrasound-electro-Fenton that include three-dimensional electro-Fenton. Currently, Dargahi et al. (2021a) evaluated the degradation of the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) by three-dimensional ultrasound-electro-Fenton using PAC/Fe3O4 as a particle electrode, and explored the relevant influencing factor variables in the degradation process. The experimental results showed that the removal of 2,4-D, COD and TOC was 96.2%, 92.31% and 86.5%, respectively, under the optimal reaction conditions. In addition, a significant reduction in the biotoxicity of the outlet effluent from the 3D/SEF process was detected. Finally, the 2,4-D herbicide was completely degraded by hydroxyl radicals generated by the electrocatalytic process and converted to CO2 and H2O products. In summary, as an enhanced technology of electro-Fenton, ultrasound-electro-Fenton showed better results in degrading pollutants compared to the single electro-Fenton system. Table 5 lists the relevant literature research on the combination of electro-Fenton process and ultrasound technology.
Table 5 Research on the combination of Electro-Fenton and ultrasonic technology
Combined with anodic oxidation technology
As a type of electrochemical advanced oxidation processes (EAOP), anodic oxidation (AO) is probably the simplest and most effective alternative for the direct or indirect generation of reactive substances at the anode (Hu et al. 2020). In the anodic oxidation process, high current densities are used for the anodic oxidation of pollutants by applying high current densities to the anode and generating oxidizing physisorbed hydroxyl radicals, M(·OH), which are intermediates of water discharged to O2 at the anode surface by reaction (Equation (41)) (Panizza and Cerisola 2009; Marselli et al. 2003; Martínez-Huitle and Ferro 2006; Brillas and Martínez-Huitle 2015):
In terms of materials used for anodizing, the use of inactive anodes such as Ti4O7 or boron doped diamond (BDD) instead of active anodes such as mixed metal oxides (MMOs) and dimensionally stabilized anodes (DSAs) allows for a better degradation of organic matter and improved mineralization of the solution (Ouarda et al. 2020). Many works have demonstrated that some inactive anodes, such as BDD, SnO2 and PbO2, are ideal anodes for mineralization of organic pollutants into CO2 and aqueous end products (Sirés et al. 2014). And as an effective technology for treating organic pollutants, anodic oxidation can be realized in combination with physical, chemical or biological processes (Becerril-Estrada et al. 2020; Chu et al. 2012; Ouarda et al. 2018) to obtain higher removal efficiency of pollutants. The electro-Fenton process, as a chemical process, can be combined with anodic oxidation to enhance the removal efficiency of pollutants. Ouarda et al. (2020) compared the removal of TOC from acetaminophen synthetic solutions by EF, AO-H2O2 and EF-AO techniques, respectively. It was found that after 8 h of reaction, the removal of TOC from the solution using a single EF process was 76 ± 5%, the removal efficiency achieved by the AO-H2O2 method was 81.0 ± 3.4%, while the combined EF-AO technique achieved a removal efficiency of 87 ± 2.7%. They concluded that the ability of the EF-AO process to achieve higher TOC removal rates can be explained by the generation of hydroxyl radicals on the anode surface and in the native solution of the Fenton reaction. However, different experimental results were obtained by Vasconcelos et al. (2016), whom, when comparing the removal of the dye Reactive Black 5 (RB-5) through the use of the AO, EF, and AO-EF processes, respectively, found that the removal efficiency of the dye color and TOC was higher than that of the combined AO-EF process when using either the AO or EF process alone. Through their analysis, they concluded that RB-5 was oxidized by heterogeneous BDD (·OH) on the BDD surface and also by homogeneous ·OH radicals formed by the Fenton reaction that electrically generates hydrogen peroxide. However, it is also possible that the RB-5 dye is reduced at the cathode surface, ultimately leading to a decrease in color removal efficiency.
However, in general, anodic oxidation combined with electro-Fenton are efficient and significant in terms of degradation on organic pollutants, and repreparation of substances. For example, Olvera-Vargas et al. (2018) proposed an integrated dynamic cross flow electro-Fenton (DCF-EF) during which the anodic oxidation of acetaminophen was performed using a Ti4O7 anode. The experimental results showed that under optimal reaction conditions, complete degradation of the drug and 44% mineralization efficiency could be achieved, and that Ti4O7 rods as anode contributed significantly to the mineralization of acetaminophen. Labiadh et al. (2016) treated the concentrate obtained from sanitary waste leachate during reverse osmosis by such a combined process, where boron-doped diamond was used as an anode electrode and pretreated, and finally electro-Fenton treatment was carried out, and the experiments showed that this combined process could be effective for the treatment of organic loads and nitrogenous substances in the reverse osmosis concentrate. In this combined process, anodic oxidation dominates, and the anodic oxidation process removes nitrogen-containing compounds more effectively than electro-Fenton, and the combined process also enhances the biodegradability index of the effluent. Ou et al. (2019) treated polyaniline (PANI) wastewater and re-preparation of PANI by this process. The experimental results showed that the COD removal in PANI wastewater could reach 89% under certain reaction conditions. Moreover, the yield of PANI-T can reach 92.1% using treated PANI wastewater, which indicates that it is efficient and environmentally friendly to treat PANI wastewater and re-prepare PANI using this process.
Currently, boron-doped diamond (BDD) has a higher oxidizing capacity than other common anodes in AO (Hamza et al. 2009; Ciríaco et al. 2009; Rodrigo et al. 2010; Cavalcanti et al. 2013). This is due to the fact that the BDD anode O2 overpotential is larger and the interaction between the surface and ·OH is very low, thus favoring the reaction of BDD (·OH) with organics, which allows the BDD anode to achieve the best performance in destroying organics (Rocha et al. 2011), which makes this type of electrode the electrode of choice for anodic oxidation. Table 6 lists the relevant literature research on the combination of electro-Fenton process and anodic oxidation technology.
Table 6 Research on the combination of Electro-Fenton and anodic oxidation technology
Combined with electrocoagulation technology
Electrocoagulation (EC) is one of the most widely used electrochemical methods in wastewater treatment (Khaled et al. 2015), a well-established wastewater treatment method that has been widely explored for treating a wide range of wastewater pollutants due to its ease of operation, versatility, environmental friendliness and low footprint (Das et al. 2022). In terms of the mechanism of treating pollutants in wastewater, electrocoagulation involves the production of coagulants by electrochemical oxidation in situ at a sacrificial anode. By releasing metal ions and further converting them to hydroxides, these hydroxides can neutralize charges or act as scavenging flocs with large surface areas to promote aggregation or precipitation into sludge and adsorption of dissolved pollutants (Ghernaout 2013; Ghernaout and Ghernaout 2012). During electrocoagulation, pollutants can be removed by adsorption, precipitation, electrostatic attraction, anodic oxidation, and cathodic reduction (Thiam et al. 2014); however, the process of removal that occurs can be affected by the type and concentration of the electrolyte medium in solution (Govindan et al. 2014a, 2015b, 2020a). As for the choice of anode materials, Fe and Al are the traditional anode soluble metals used in electrocoagulation process due to their low cost and high valence (Ilhan et al. 2008; Chafi et al. 2011).
When the anode electrode is Fe, Fe is oxidized to form Fe2+, while at the same time H2O at the cathode undergoes reduction and produces OH- , and the combination of Fe2+ and OH- produces insoluble Fe(OH)n (n=2,3). These Fe(OH)n will act as a coagulant to the suspended solids, producing dense flocs and precipitation. In addition, the production of H2 at the cathode leads to flotation of particles at the surface, and the production of insoluble particles of different densities and sizes during the re-precipitation process can be eliminated by filtration (Hosny 1996; Chen et al. 2002; Kyzas and Matis 2016). Two production mechanisms have been proposed for the production of iron hydroxide material (Mollah et al. 2001):
Mechanism 1
Anode.
Cathode.
Overall.
Mechanism 2
Anode.
Cathode.
Overall.
When the material is Al, Al is first oxidized in solution to produce Al3+, which is subsequently converted to Al(OH)3 and then polymerized to Aln(OH)3n at a suitable pH value. The relevant reaction equation is (Chen 2004):
Although electrocoagulation is very effective for the treatment of pollutants in wastewater, on the one hand, electrocoagulation constantly consumes the electrode plates as they pass through the electrolytic reaction, and therefore needs to be replaced frequently; secondly, oxidized films and precipitates are formed on the surface of the plates, which leads to a decrease in the efficiency of electrocoagulation; and thirdly, the colloids produced in the process of electrocoagulation are sometimes re-dissolved. In order to solve the above problems, the combination of electrocoagulation with another process is a promising method to improve the removal efficiency of water pollutants. For example, free radical-assisted electrocoagulation is a new combination that shows higher performance (Al-Qodah et al. 2018). While electro-Fenton, an advanced oxidation technology capable of generating reactive free radicals, is used in combination with the electrocoagulation process, usually with Fe electrodes as sacrificial anodes and H2O2 externally added to degrade the pollutants. When electrocoagulation occurs and Fe2+ is electrochemically generated, Fe2+ activates H2O2 and generates reactive free radicals that accelerate the degradation of the contaminants (Govindan et al. 2014b, 2015a). Figure 11 shows a diagram of the H2O2 assisted EC process and the mechanism of pollutant degradation by the Fe electrode.
The advantages of the electro-Fenton process for the removal of organic matter became apparent when it was combined with the electrocoagulation process. Zhao et al. (2012) tested the removal efficiency of plugboard wastewater under a single electrocoagulation process versus a combined electrocoagulation-electro-Fenton process, and it was found that, with a single electro-coagulation process, only nearly 30% of the COD could be removed, but with the addition of H2O2, the removal of COD in the wastewater was dramatically increased to 76%. This is due to the fact that the in situ generation of Fe2+ can react with H2O2 to produce ·OH after the addition of H2O2 to the solution, resulting in an increase in COD removal. Anfruns-Estrada et al. (2017), on the other hand, compared the ability of two electrochemical techniques, electrocoagulation and electro-Fenton, to disinfect both primary and secondary effluents from municipal wastewater treatment plants. It was shown that the EF process at pH = 3.0 achieved substantial or even complete microbial inactivation within 30 min compared to single EC, but was inferior to the EC process in TOC removal, whereas in continuous combined EC/EF treatment, the bacterial membranes were weakened due to interactions with Fe(OH)n floc and oxidants, and subsequently further subjected to rapid oxidant inactivation, higher TOC removal than the single EC and EF processes, achieving more effective effluent treatment of the effluent.
Related studies have also shown that the combined process of EC and EF not only has a higher removal efficiency in treating organic pollutants compared to a single EC process, but this combined process is also more powerful than EC combined with other processes. Afanga et al. (2020) tested a hybrid electrochemical process that included electrocoagulation (EC) alone and combined with electro-Fenton (EF), anodic oxidation (AO), and peroxic coagulation (PC) for the treatment of textile industry wastewater using an intermittent reactor; the experimental results showed that the treatment effectiveness of textile industry wastewater decreased in the order of EC-EF>EC-AO>EC-PC>EC. The study also showed that the energy consumption using the integrated process was lower than that of EC alone; where the energy consumption required for TOC removal was 3 kWh·kg-1 when the EC process was used alone, whereas when EC was combined with EF, the energy consumption decreased to 0.45 kWh·kg-1. Similarly, Zazou et al. (2019) treated a textile industrial wastewater containing reactive dyestuff mixtures by electrocoagulation combined with different electrochemical advanced oxidation processes (PC, AO and EF) to treat the textile industrial wastewater containing a mixture of reactive dyes; the experimental results showed that among the tested electrochemical advanced oxidation processes, the sequential EC-EF treatment was preferred under optimal reaction conditions and was very effective because it provided electrically generated hydroxyl radicals ·OH. By using the combined EC-EF process, 97%, 100%, and 100% of the TOC, turbidity, and color removal rates. In contrast, TOC removal was only 92% and 68% for the EC-AO and EC-PC processes, respectively. In terms of energy consumption, the single EC process required 3 kWh to remove 1 kg of TOC while treating wastewater under optimal conditions, while the EC-EF process required only 0.1 kWh.
It is concluded that EC-EF as a combined process is not only more significant in treating organic pollutants compared to a single EC process, requires less energy than a single EC process, but is also more effective than the combination of EC with other processes. In addition, studies related to the treatment of other pollutants using the EC-EF process in addition to the above studies are also listed in Table 7.
Table 7 Research on the combination of Electro-Fenton and electrocoagulation technology
Conclusion
This paper lists and analyzes the influencing factors and combination technologies related to the electro-Fenton process; among the influencing factors analyzed above, the addition of H2O2 can improve the removal efficiency of the electro-Fenton process, but the excessive addition will have a scavenging effect on the ·OH, and in addition, it will also increase the pH of the solution and the operating cost of the reaction. In terms of feeding mode, the removal efficiency of multiple additions is better than a single addition. An increase in reactant concentration decreases the reaction constant, which in turn has an effect on the degradation efficiency of the pollutant. The two geometric parameters, reaction volume and electrode quality, are less studied, but from the only studies, increasing the electrode quality favors the reaction, while increasing the reaction volume hinders the generation of H2O2. The reaction time not only reflects the trend of the relevant parameters with time during the degradation or oxidation of pollutants or organics, but also provides an assessment and reference for finding the optimal degradation efficiency and optimal process cost of pollutants or organics. Finally, there is a similarity between the effects of voltage (potential) and current, and excessive voltage (potential) can also produce the occurrence of side reactions such as hydrogen precipitation reaction in solution and H2O2 decomposition. With the synergistic effect of electro-Fenton with ultrasound, anodic oxidation, and electrocoagulation technology, the efficiency of pollutant degradation is better than the efficiency of single electro-Fenton process.
Data availability
All data and charts in this article are cited from publicly available and published literature.
References
Afanga H, Zazou H, Titchou FE et al (2020) Integrated electrochemical processes for textile industry wastewater treatment: system performances and sludge settling characteristics. Sustain Environ Res:30(1). https://doi.org/10.1186/s42834-019-0043-2
Akyol A, Can OT, Demirbas E et al (2013) A comparative study of electrocoagulation and electro-Fenton for treatment of wastewater from liquid organic fertilizer plant. Sep Purif Technol 112:11–19. https://doi.org/10.1016/j.seppur.2013.03.036
Alavi N, Dehvari M, Alekhamis G et al (2019) Application of electro-Fenton process for treatment of composting plant leachate: kinetics, operational parameters and modeling. J Environ Health Sci Eng. https://doi.org/10.1007/s40201-019-00361-2
Al-Qodah Z, Al-Shannag M, Bani-Melhem K et al (2018) Free radical-assisted electrocoagulation processes for wastewater treatment. Environ Chem Lett 16(3):695–714. https://doi.org/10.1007/s10311-018-0711-1
Anfruns-Estrada E, Bruguera-Casamada C, Salvadó H et al (2017) Inactivation of microbiota from urban wastewater by single and sequential electrocoagulation and electro-Fenton treatments. Water Res 126:450–459. https://doi.org/10.1016/j.watres.2017.09.056
Annabi C, Fourcade F, Soutrel I et al (2016) Degradation of enoxacin antibiotic by the electro-Fenton process: Optimization, biodegradability improvement and degradation mechanism. J Environ Manag 165:96–105. https://doi.org/10.1016/j.jenvman.2015.09.018
Anotai J, Su C-C, Tsai Y-C et al (2010) Effect of hydrogen peroxide on aniline oxidation by electro-Fenton and fluidized-bed Fenton processes. J Hazard Mater 183(1-3):888–893. https://doi.org/10.1016/j.jhazmat.2010.07.112
Aval AE, Hasani AH, Omrani GA et al (2017) Removal of Landfill Leachate's Organic load by modified Electro-Fenton process. Int J Electrochem Sci:9348–9363. https://doi.org/10.20964/2017.10.65
Babuponnusami A, Muthukumar K (2012) Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem Eng J 183:1–9. https://doi.org/10.1016/j.cej.2011.12.010
Becerril-Estrada V, Robles I, Martínez-Sánchez C et al (2020) Study of TiO2/Ti4O7 photo-anodes inserted in an activated carbon packed bed cathode: Towards the development of 3D-type photo-electro-Fenton reactors for water treatment. Electrochim Acta 135972. https://doi.org/10.1016/j.electacta.2020.135972
Bedolla-Guzman A, Sirés I, Thiam A et al (2016) Application of anodic oxidation, electro-Fenton and UVA photoelectro-Fenton to decolorize and mineralize acidic solutions of Reactive Yellow 160 azo dye. Electrochim Acta 206:307–316. https://doi.org/10.1016/j.electacta.2016.04.166
Benhadji A, Ahmed MT (2020) Yellow 2G dye degradation by electro-Fenton process using steel electrode as catalysis and its phytotoxicity effect. Water Sci Technol. https://doi.org/10.2166/wst.2020.361
Bocos E, Brillas E, Sanromán MÁ et al (2016) Electrocoagulation: Simply a Phase Separation Technology? The Case of Bronopol Compared to Its Treatment by EAOPs. Environ Sci Technol 50(14):7679–7686. https://doi.org/10.1021/acs.est.6b02057
Brillas E (2021) Recent development of electrochemical advanced oxidation of herbicides. A review on its application to wastewater treatment and soil remediation. J Clean Prod 290:125841. https://doi.org/10.1016/j.jclepro.2021.125841
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B Environ 166-167:603–643. https://doi.org/10.1016/j.apcatb.2014.11.016
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem Rev 109(12):6570–6631. https://doi.org/10.1021/cr900136g
Cai C, Wang L, Gao H et al (2014) Ultrasound enhanced heterogeneous activation of peroxydisulfate by bimetallic Fe-Co/GAC catalyst for the degradation of Acid Orange 7 in water. J Environ Sci 26(6):1267–1273. https://doi.org/10.1016/s1001-0742(13)60598-7
Cai J, Zhou M, Pan Y et al (2019) Degradation of 2,4-dichlorophenoxyacetic acid by anodic oxidation and electro-Fenton using BDD anode: influencing factors and mechanism. Sep Purif Technol 115867. https://doi.org/10.1016/j.seppur.2019.115867
Cai M, Su J, Lian G et al (2016) Sono-advanced Fenton decolorization of azo dye Orange G: Analysis of synergistic effect and mechanisms. Ultrason Sonochem 31:193–200. https://doi.org/10.1016/j.ultsonch.2015.12.017
Can OT (2013) COD removal from fruit-juice production wastewater by electrooxidation electrocoagulation and electro-Fenton processes. Desalin Water Treat 52(1-3):65–73. https://doi.org/10.1080/19443994.2013.781545
Cavalcanti EB, Garcia-Segura S, Centellas F et al (2013) Electrochemical incineration of omeprazole in neutral aqueous medium using a platinum or boron-doped diamond anode: degradation kinetics and oxidation products. Water Res 47(5):1803–1815. https://doi.org/10.1016/j.watres.2013.01.002
Chafi M, Gourich B, Essadki AH et al (2011) Comparison of electrocoagulation using iron and aluminium electrodes with chemical coagulation for the removal of a highly soluble acid dye. Desalination 281:285–292. https://doi.org/10.1016/j.desal.2011.08.004
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38(1):11–41. https://doi.org/10.1016/j.seppur.2003.10.006
Chen L, Pinto A, Alshawabkeh AN (2019) Activated Carbon as a Cathode for water disinfection through the Electro-Fenton process. Catalysts 9(7):601. https://doi.org/10.3390/catal9070601
Chen X, Chen G, Yue PL (2002) Novel electrode system for electroflotation of wastewater. Environ Sci Technol 36(4):778–783. https://doi.org/10.1021/es011003u
Chu YY, Qian Y, Wang WJ et al (2012) A dual-cathode electro-Fenton oxidation coupled with anodic oxidation system used for 4-nitrophenol degradation. J Hazard Mater 199-200:179–185. https://doi.org/10.1016/j.jhazmat.2011.10.079
Ciríaco L, Anjo C, Correia J et al (2009) Electrochemical degradation of Ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Electrochim Acta 54(5):1464–1472. https://doi.org/10.1016/j.electacta.2008.09.022
Cruz del Álamo A, Zou R, Pariente MI et al (2020) Catalytic activity of LaCu0.5Mn0.5O3 perovskite at circumneutral/basic pH conditions in electro-Fenton processes. Catal Today. https://doi.org/10.1016/j.cattod.2020.03.027
Daghrir R, Drogui P (2012) Coupled electrocoagulation–electro-Fenton for efficient domestic wastewater treatment. Environ Chem Lett 11(2):151–156. https://doi.org/10.1007/s10311-012-0390-2
Dargahi A, Hasani K, Mokhtari SA et al (2021a) Highly effective degradation of 2,4-Dichlorophenoxyacetic acid herbicide in a three-dimensional sono-electro-Fenton (3D/SEF) system using powder activated carbon (PAC)/Fe3O4 as magnetic particle electrode. J Environ Chem Eng 9(5):105889. https://doi.org/10.1016/j.jece.2021.105889
Dargahi A, Moradi M, Marafat R et al (2021b) Applications of advanced oxidation processes (electro-Fenton and sono-electro-Fenton) for degradation of diazinon insecticide from aqueous solutions: optimization and modeling using RSM-CCD, influencing factors, evaluation of toxicity, and degradation pathway. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01753-x
Das P, Sharma M, Purkait MK (2022) Recent progress on electrocoagulation process for wastewater treatment: A review. Sep Purif Technol 292:121058. https://doi.org/10.1016/j.seppur.2022.121058
Dindaş GB, Çalışkan Y, Çelebi EE et al (2018) Sequential treatment of food industry wastewater by electro- fenton and electrocoagulation processes. Int J Electrochem Sci:12349–12359. https://doi.org/10.20964/2018.12.82
Diouf I, Dia O, Diedhiou MB et al (2018) Electro-generation of hydrogen peroxide using a graphite cathode from exhausted batteries: Study of influential parameters on electro-Fenton process. Environ Technol:1–26. https://doi.org/10.1080/09593330.2018.1537309
Do TM, Byun JY, Kim SH (2017) An electro-Fenton system using magnetite coated metallic foams as cathode for dye degradation. Catal Today 295:48–55. https://doi.org/10.1016/j.cattod.2017.05.016
Dolatabadi M, Ahmadzadeh S (2019) A rapid and efficient removal approach for degradation of metformin in pharmaceutical wastewater using electro-fenton process; optimization by response surface methodology. Water Sci Technol. https://doi.org/10.2166/wst.2019.312
El-Desoky HS, Ghoneim MM, Zidan NM (2010) Decolorization and degradation of Ponceau S azo-dye in aqueous solutions by the electrochemical advanced Fenton oxidation. Desalination 264(1-2):143–150. https://doi.org/10.1016/j.desal.2010.07.018
Fenton HJH (1894) LXXIII.—Oxidation of tartaric acid in presence of iron. J Chem Soc Trans 65:899–910. https://doi.org/10.1039/ct8946500899
Fenton HJH (1896) XLI.—The constitution of a new dibasic acid, resulting from the oxidation of tartaric acid. J Chem Soc Trans 69:546–562. https://doi.org/10.1039/ct8966900546
Flores N, Cabot PL, Centellas F et al (2017) 4-Hydroxyphenylacetic acid oxidation in sulfate and real olive oil mill wastewater by electrochemical advanced processes with a boron-doped diamond anode. J Hazard Mater 321:566–575. https://doi.org/10.1016/j.jhazmat.2016.09.057
Flores N, Sharif F, Yasri N et al (2018) Removal of tyrosol from water by adsorption on carbonaceous materials and electrochemical advanced oxidation processes. Chemosphere 201:807–815. https://doi.org/10.1016/j.chemosphere.2018.03.028
Flores N, Sirés I, Garrido JA et al (2016a) Degradation of trans-ferulic acid in acidic aqueous medium by anodic oxidation, electro-Fenton and photoelectro-Fenton. J Hazard Mater 319:3–12. https://doi.org/10.1016/j.jhazmat.2015.11.040
Flores N, Thiam A, Rodríguez RM et al (2016b) Electrochemical destruction of trans-cinnamic acid by advanced oxidation processes: kinetics, mineralization, and degradation route. Environ Sci Pollut Res 24(7):6071–6082. https://doi.org/10.1007/s11356-015-6035-9
Galia A, Lanzalaco S, Sabatino MA et al (2016) Crosslinking of poly(vinylpyrrolidone) activated by electrogenerated hydroxyl radicals: A first step towards a simple and cheap synthetic route of nanogel vectors. Electrochem Commun 62:64–68. https://doi.org/10.1016/j.elecom.2015.12.005
Ganiyu SO, Zhou M, Martínez-Huitle CA (2018) Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl Catal B Environ 235:103–129. https://doi.org/10.1016/j.apcatb.2018.04.044
George SJ, Gandhimathi R, Nidheesh PV et al (2013) Electro-Fenton Method Oxidation of Salicylic Acid in Aqueous Solution with Graphite Electrodes. Environ Eng Sci 30(12):750–756. https://doi.org/10.1089/ees.2013.0242
Ghernaout D (2013) Advanced oxidation phenomena in electrocoagulation process: a myth or a reality? Desalin Water Treat 51(40-42):7536–7554. https://doi.org/10.1080/19443994.2013.792520
Ghernaout D, Ghernaout B (2012) Sweep flocculation as a second form of charge neutralisation—a review. Desalin Water Treat 44(1-3):15–28. https://doi.org/10.1080/19443994.2012.691699
Ghosh P, Samanta AN (2011) Ray, S. Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 266(1-3):213–217. https://doi.org/10.1016/j.desal.2010.08.029
Gomathi E, Balraj B, Kumaraguru K (2018) Electrochemical degradation of scarlet red dye from aqueous environment by titanium-based dimensionally stable anodes with SS electrodes. Appl Biol Chem 61(3):289–293. https://doi.org/10.1007/s13765-018-0357-5
Goncharuk VV, Malyarenko VV, Yaremenko VA (2008) Use of ultrasound in water treatment. J Water Chem Technol 30(3):137–150. https://doi.org/10.3103/s1063455x08030028
Govindan K, Angelin A, Kalpana M et al (2020a) Electrocoagulants Characteristics and Application of Electrocoagulation for Micropollutant Removal and Transformation Mechanism. ACS Appl Mater Interfaces 12(1):1775–1788. https://doi.org/10.1021/acsami.9b16559
Govindan K, Oren Y, Noel M et al (2014a) Effect of dye molecules and electrode material on the settling behavior of flocs in an electrocoagulation induced settling tank reactor (EISTR). Sep Purif Technol 133:396–406. https://doi.org/10.1016/j.seppur.2014.04.046
Govindan K, Raja M, Noel M et al (2014b) Degradation of pentachlorophenol by hydroxyl radicals and sulfate radicals using electrochemical activation of peroxomonosulfate, peroxodisulfate and hydrogen peroxide. J Hazard Mater 272:42–51. https://doi.org/10.1016/j.jhazmat.2014.02.036
Govindan K, Raja M, Uma Maheshwari S et al (2015a) Analysis and understanding of amido black 10B dye degradation in aqueous solution by electrocoagulation with the conventional oxidants peroxomonosulfate, peroxodisulfate and hydrogen peroxide. Environ Sci: Water Res Technol 1(1):108–119. https://doi.org/10.1039/c4ew00030g
Govindan K, Raja M, Uma Maheshwari S et al (2015b) Comparison and understanding of fluoride removal mechanism in Ca2+, Mg2+ and Al3+ ion assisted electrocoagulation process using Fe and Al electrodes. J Environ Chem Eng 3(3):1784–1793. https://doi.org/10.1016/j.jece.2015.06.014
Govindan K, Sumanasekara VDW, Jang A (2020b) Mechanisms for degradation and transformation of β-blocker atenolol via electrocoagulation, electro-Fenton, and electro-Fenton-like processes. Environ Sci: Water Res Technol 6(5):1465–1481. https://doi.org/10.1039/d0ew00114g
Gozzi F, Sirés I, Thiam A et al (2017) Treatment of single and mixed pesticide formulations by solar photoelectro-Fenton using a flow plant. Chem Eng J 310:503–513. https://doi.org/10.1016/j.cej.2016.02.026
Guan W, Zhang B, Tian S et al (2018) The synergism between electro-Fenton and electrocoagulation process to remove Cu-EDTA. Appl Catal B Environ 227:252–257. https://doi.org/10.1016/j.apcatb.2017.12.036
Guelfi DRV, Gozzi F, Sirés I et al (2016) Degradation of the insecticide propoxur by electrochemical advanced oxidation processes using a boron-doped diamond/air-diffusion cell. Environ Sci Pollut Res 24(7):6083–6095. https://doi.org/10.1007/s11356-016-6416-8
Guelfi DRV, Ye Z, Gozzi F et al (2018) Ensuring the overall combustion of herbicide metribuzin by electrochemical advanced oxidation processes. Study of operation variables, kinetics and degradation routes. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2018.10.029
Haber F, Weiss J (1934) The Catalytic decomposition of Hydrogen Peroxide by Iorn Salts. Proceedings of the Royal Society of London. Series A. Math Phys Sci 147:332–351. https://doi.org/10.2307/96281
Haider MR, Jiang W-L, Han J-L et al (2019) In-situ electrode fabrication from polyaniline derived N-doped carbon nanofibers for metal-free electro-Fenton degradation of organic contaminants. Appl Catal B Environ 117774. https://doi.org/10.1016/j.apcatb.2019.117774
Hamza M, Abdelhedi R, Brillas E et al (2009) Comparative electrochemical degradation of the triphenylmethane dye Methyl Violet with boron-doped diamond and Pt anodes. J Electroanal Chem 627(1-2):41–50. https://doi.org/10.1016/j.jelechem.2008.12.017
Hasani K, Peyghami A, Moharrami A et al (2020) The efficacy of sono-electro-Fenton process for removal of Cefixime antibiotic from aqueous solutions by Response Surface Methodology (RSM) and evaluation of toxicity of effluent by microorganisms. Arab J Chem. https://doi.org/10.1016/j.arabjc.2020.05.012
Hoang NT, Holze R (2020) Degradation of pesticide Cartap in Padan 95SP by combined advanced oxidation and electro-Fenton process. J Solid State Electrochem. https://doi.org/10.1007/s10008-020-04581-7
Hosny AY (1996) Separating oil from oil-water emulsions by electroflotation technique. Sep Technol 6(1):9–17. https://doi.org/10.1016/0956-9618(95)00136-0
Hu Z, Cai J, Song G et al (2020) Anodic oxidation of organic pollutants: anode fabrication, process hybrid and environmental applications. Curr Opin Electrochem 100659. https://doi.org/10.1016/j.coelec.2020.100659
Hung K-C, Nguyen NT, Sun Y-L et al (2019) Bio-Fenton reaction involved in the cleavage of the ethoxylate chain of nonionic surfactants by dihydrolipoamide dehydrogenase from Pseudomonas nitroreducens TX1. Sci Rep 9(1). https://doi.org/10.1038/s41598-019-43266-8
Ilhan F, Kurt U, Apaydin O et al (2008) Treatment of leachate by electrocoagulation using aluminum and iron electrodes. J Hazard Mater 154(1-3):381–389. https://doi.org/10.1016/j.jhazmat.2007.10.035
Jiang C, Zhang J (2007) Progress and prospect in electro-Fenton process for wastewater treatment. J Zhejiang Univ (Sci) 8(7):1118–1125. https://doi.org/10.1631/jzus.2007.a1118
Kadji H, Yahiaoui I, Garti Z et al (2020) Kinetic degradation of amoxicillin by using the electro-Fenton process in the presence of a graphite rods from used batteries. Chin J Chem Eng. https://doi.org/10.1016/j.cjche.2020.08.032
Khaled B, Wided B, Béchir H et al (2015) Investigation of electrocoagulation reactor design parameters effect on the removal of cadmium from synthetic and phosphate industrial wastewater. Arab J Chem. https://doi.org/10.1016/j.arabjc.2014.12.012
Kim H-G, Ko Y-J, Lee S et al (2018) Degradation of organic compounds in actual wastewater by electro-fenton process and evaluation of energy consumption. Water Air Soil Pollut 229(10). https://doi.org/10.1007/s11270-018-3987-7
Kiss AA, Geertman R, Wierschem M et al (2018) Ultrasound-assisted emerging technologies for chemical processes. J Chem Technol Biotechnol 93(5):1219–1227. https://doi.org/10.1002/jctb.5555
Kuang F, Zhang D, Li Y et al (2008) Electrochemical impedance spectroscopy analysis for oxygen reduction reaction in 3.5% NaCl solution. J Solid State Electrochem 13(3):385–390. https://doi.org/10.1007/s10008-008-0570-y
Kumar A, Rana A, Sharma G et al (2019) Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J Mol Liq 111177. https://doi.org/10.1016/j.molliq.2019.111177
Kyzas GZ, Matis KA (2016) Electroflotation process: a review. J Mol Liq 220:657–664. https://doi.org/10.1016/j.molliq.2016.04.128
Labiadh L, Fernandes A, Ciríaco L et al (2016) Electrochemical treatment of concentrate from reverse osmosis of sanitary landfill leachate. J Environ Manag 181:515–521. https://doi.org/10.1016/j.jenvman.2016.06.069
Labiadh L, Oturan MA, Panizza M et al (2015) Complete removal of AHPS synthetic dye from water using new electro-fenton oxidation catalyzed by natural pyrite as heterogeneous catalyst. J Hazard Mater 297:34–41. https://doi.org/10.1016/j.jhazmat.2015.04.062
Lan H, He W, Wang A et al (2016) An activated carbon fiber cathode for the degradation of glyphosate in aqueous solutions by the Electro-Fenton mode: optimal operational conditions and the deposition of iron on cathode on electrode reusability. Water Res 105:575–582. https://doi.org/10.1016/j.watres.2016.09.036
Lanzalaco S, Sirés I, Sabatino MA et al (2017) Synthesis of polymer nanogels by electro-Fenton process: investigation of the effect of main operation parameters. Electrochim Acta 246:812–822. https://doi.org/10.1016/j.electacta.2017.06.097
Le TXH, Nguyen TV, Amadou Yacouba Z et al (2017) Correlation between degradation pathway and toxicity of acetaminophen and its by-products by using the electro-Fenton process in aqueous media. Chemosphere 172:1–9. https://doi.org/10.1016/j.chemosphere.2016.12.060
Li D, Sun T, Wang L et al (2018) Enhanced electro-catalytic generation of hydrogen peroxide and hydroxyl radical for degradation of phenol wastewater using MnO2/Nano-G|Foam-Ni/Pd composite cathode. Electrochim Acta 282:416–426. https://doi.org/10.1016/j.electacta.2018.06.075
Li H, Zhou M, Chen Y et al (2019a) Design of a high efficiency electro-Fenton system with Fe0@Fe3O4/ACF composite cathode for lignin wastewater treatment. IOP Conf Ser Earth Environ Sci 295:042076. https://doi.org/10.1088/1755-1315/295/4/042076
Li J, Song D, Du K et al (2019b) Performance of graphite felt as a cathode and anode in the electro-Fenton process. RSC Adv 9(66):38345–38354. https://doi.org/10.1039/c9ra07525a
Li J-T, Lan R-J, Bai B et al (2013a) Ultrasound-promoted degradation of acid brown 348 by Fenton-like processes. Asian J Chem 25(4):2246–2250. https://doi.org/10.14233/ajchem.2013.13562
Li R, Wang P, Gu J (2019c) Study on the treatment of Aluminum milling wastewater by electrode-fenton method. IOP Conf Ser Earth Environ Sci 267:042137. https://doi.org/10.1088/1755-1315/267/4/042137
Li Y, Guan Y, Tao C (2011) Influencing factors research of phenol wastewater treatment by cathode electro-fenton method. Appl Inform Commun:726–733. https://doi.org/10.1007/978-3-642-23214-5_92
Li Y, Hsieh W-P, Mahmudov R et al (2013b) Combined ultrasound and Fenton (US-Fenton) process for the treatment of ammunition wastewater. J Hazard Mater 244-245:403–411. https://doi.org/10.1016/j.jhazmat.2012.11.022
Liang J, Komarov S, Hayashi N et al (2007) Improvement in sonochemical degradation of 4-chlorophenol by combined use of Fenton-like reagents. Ultrason Sonochem 14(2):201–207. https://doi.org/10.1016/j.ultsonch.2006.05.002
Lim CH, Ang JJ, Lau S et al (2017) Optimization of hydroxyl radical production using electro-Fenton method for chemical oxygen demand reduction in diluted palm oil mill effluent. Water Environ J 31(4):578–583. https://doi.org/10.1111/wej.12281
Mahmoudi N, Farhadian M, Solaimany Nazar AR et al (2021) Investigation and optimization of the performance of sono-photo-electro-Fenton process for removal of Acid Black 172 and Disperse Blue 56 from polluted water: comparison of the degradation activity with electro-Fenton-based processes. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03296-0
Malakootian M, Moridi A (2017) Efficiency of electro-Fenton process in removing Acid Red 18 dye from aqueous solutions. Process Saf Environ Prot 111:138–147. https://doi.org/10.1016/j.psep.2017.06.008
Mansour D, Fourcade F, Bellakhal N et al (2011) Biodegradability Improvement of Sulfamethazine Solutions by Means of an electro-Fenton Process. Water Air Soil Pollut 223(5):2023–2034. https://doi.org/10.1007/s11270-011-1002-7
Mansour D, Fourcade F, Huguet S et al (2014) Improvement of the activated sludge treatment by its combination with electro Fenton for the mineralization of sulfamethazine. Int Biodeterior Biodegradation 88:29–36. https://doi.org/10.1016/j.ibiod.2013.11.016
Mansour D, Fourcade F, Soutrel I et al (2015) Relevance of a combined process coupling electro-Fenton and biological treatment for the remediation of sulfamethazine solutions -Application to an industrial pharmaceutical effluent. Comptes Rendus Chimie 18(1):39–44. https://doi.org/10.1016/j.crci.2014.05.005
Marselli B, Garcia-Gomez J, Michaud P-A et al (2003) Electrogeneration of Hydroxyl Radicals on Boron-Doped Diamond Electrodes. J Electrochem Soc 150(3):D79. https://doi.org/10.1149/1.1553790
Martínez SS, Bahena CL (2009) Chlorbromuron urea herbicide removal by electro-Fenton reaction in aqueous effluents. Water Res 43(1):33–40. https://doi.org/10.1016/j.watres.2008.09.036
Martínez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ 87(3-4):105–145. https://doi.org/10.1016/j.apcatb.2008.09.017
Martínez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 35(12):1324–1340. https://doi.org/10.1039/b517632h
Moayerikashani M, Soltani SM (2013) Electrocoagulation of a Real Malaysian Leachate Sample Using Al Electrodes to Meet Discharge Standards. Adv Mater Res 1716–1720:622–623. https://doi.org/10.4028/www.scientific.net/amr.622-623.1716
Mohajeri S, Aziz HA, Isa MH et al (2010) Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J Hazard Mater 176(1-3):749–758. https://doi.org/10.1016/j.jhazmat.2009.11.099
Mollah MYA, Schennach R, Parga JR et al (2001) Electrocoagulation (EC) — science and applications. J Hazard Mater 84(1):29–41. https://doi.org/10.1016/s0304-3894(01)00176-5
Mounia AY, Djilali Z (2012) Electrochemical incineration of refractory organic pollution by electrochemically generated fenton’s reagent. J Chil Chem Soc 57(4):1388–1393. https://doi.org/10.4067/s0717-97072012000400012
Moussavi G, Bagheri A, Khavanin A (2012) The investigation of degradation and mineralization of high concentrations of formaldehyde in an electro-Fenton process combined with the biodegradation. J Hazard Mater 237-238:147–152. https://doi.org/10.1016/j.jhazmat.2012.08.022
Moussavi G, Khosravi R, Farzadkia M (2011) Removal of petroleum hydrocarbons from contaminated groundwater using an electrocoagulation process: batch and continuous experiments. Desalination 278(1-3):288–294. https://doi.org/10.1016/j.desal.2011.05.039
Muruganandham M, Swaminathan M (2004) Decolourisation of Reactive Orange 4 by Fenton and photo-Fenton oxidation technology. Dyes Pigments 63(3):315–321. https://doi.org/10.1016/j.dyepig.2004.03.004
Nazari R, Rajić L, Xue Y et al (2018) Degradation of 4-Chlorophenol in Aqueous Solution by Sono- Electro-Fenton Process. Int J Electrochem Sci:9214–9230. https://doi.org/10.20964/2018.09.46
Nguyen DDD, Phan Quang HH, Nguyen XH et al (2021) The treatment of real dyeing wastewater by the electro-Fenton process using drinking water treatment sludge as a catalyst. RSC Adv 11(44):27443–27452. https://doi.org/10.1039/d1ra04049a
Olvera-Vargas H, Rouch J-C, Coetsier C et al (2018) Dynamic cross-flow electro-Fenton process coupled to anodic oxidation for wastewater treatment: application to the degradation of acetaminophen. Sep Purif Technol 203:143–151. https://doi.org/10.1016/j.seppur.2018.03.063
Oturan MA, Sirés I, Oturan N et al (2008) Sonoelectro-Fenton process: A novel hybrid technique for the destruction of organic pollutants in water. J Electroanal Chem 624(1-2):329–332. https://doi.org/10.1016/j.jelechem.2008.08.005
Oturan N, Hamza M, Ammar S et al (2011) Oxidation/mineralization of 2-Nitrophenol in aqueous medium by electrochemical advanced oxidation processes using Pt/carbon-felt and BDD/carbon-felt cells. J Electroanal Chem 661(1):66–71. https://doi.org/10.1016/j.jelechem.2011.07.017
Ou B, Wang J, Wu Y et al (2019) Reuse of PANI wastewater treated by anodic oxidation/electro-Fenton for the preparation of PANI. Chemosphere 125689. https://doi.org/10.1016/j.chemosphere.2019.125689
Ouarda Y, Tiwari B, Azaïs A et al (2018) Synthetic hospital wastewater treatment by coupling submerged membrane bioreactor and electrochemical advanced oxidation process: kinetic study and toxicity assessment. Chemosphere 193:160–169. https://doi.org/10.1016/j.chemosphere.2017.11.010
Ouarda Y, Trellu C, Lesage G et al (2020) Electro-oxidation of secondary effluents from various wastewater plants for the removal of acetaminophen and dissolved organic matter. Sci Total Environ 140352. https://doi.org/10.1016/j.scitotenv.2020.140352
Özcan A, Şahin Y, Savaş Koparal A et al (2008) Carbon sponge as a new cathode material for the electro-Fenton process: Comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium. J Electroanal Chem 616(1-2):71–78. https://doi.org/10.1016/j.jelechem.2008.01.002
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109(12):6541–6569. https://doi.org/10.1021/cr9001319
Petrucci E, Da Pozzo A, Di Palma L (2016) On the ability to electrogenerate hydrogen peroxide and to regenerate ferrous ions of three selected carbon-based cathodes for electro-Fenton processes. Chem Eng J 283:750–758. https://doi.org/10.1016/j.cej.2015.08.030
Pokhrel N, Vabbina PK, Pala N (2016) Sonochemistry: Science and Engineering. Ultrason Sonochem 29:104–128. https://doi.org/10.1016/j.ultsonch.2015.07.023
Possetto D, Natera J, Sancho MI et al (2018) Bioallethrin degradation by photo-Fenton process in acetonitrile/water and aqueous β-cyclodextrin solutions. J Photochem Photobiol A Chem 365:103–109. https://doi.org/10.1016/j.jphotochem.2018.07.036
Poza-Nogueiras V, Rosales E, Pazos M et al (2018) Current advances and trends in electro-Fenton process using heterogeneous catalysts – a review. Chemosphere 201:399–416. https://doi.org/10.1016/j.chemosphere.2018.03.002
Primo O, Rivero MJ, Ortiz I (2008) Photo-Fenton process as an efficient alternative to the treatment of landfill leachates. J Hazard Mater 153(1-2):834–842. https://doi.org/10.1016/j.jhazmat.2007.09.053
Rahmani AR, Nematollahi D, Azarian G et al (2015) Activated sludge treatment by electro-Fenton process: parameter optimization and degradation mechanism. Korean J Chem Eng 32(8):1570–1577. https://doi.org/10.1007/s11814-014-0362-2
Ranjit PJD, Palanivelu K, Lee C-S (2008) Degradation of 2,4-dichlorophenol in aqueous solution by sono-Fenton method. Korean J Chem Eng 25(1):112–117. https://doi.org/10.1007/s11814-008-0020-7
Raschitor A, Llanos J, Rodrigo MA et al (2019) Combined electrochemical processes for the efficient degradation of non-polar organochlorine pesticides. J Environ Manag 248:109289. https://doi.org/10.1016/j.jenvman.2019.109289
Rekik R, Hamza M, Jaziri M et al (2017) Electrochemical oxidation of vanillic acid by electro-Fenton process: toward a novel route of protocatechuic acid electrosynthesis. Arab J Chem. https://doi.org/10.1016/j.arabjc.2017.05.001
Ren G, Zhou M, Liu M et al (2016) A novel vertical-flow electro-Fenton reactor for organic wastewater treatment. Chem Eng J 298:55–67. https://doi.org/10.1016/j.cej.2016.04.011
Riesz P, Kondo T (1992) Free radical formation induced by ultrasound and its biological implications. Free Radic Biol Med 13(3):247–270. https://doi.org/10.1016/0891-5849(92)90021-8
Rocha JHB, Solano AMS, Fernandes NS et al (2011) Electrochemical Degradation of Remazol Red BR and Novacron Blue C-D Dyes Using Diamond Electrode. Electrocatalysis 3(1):1–12. https://doi.org/10.1007/s12678-011-0070-1
Rodrigo MA, Cañizares P, Sánchez-Carretero A et al (2010) Use of conductive-diamond electrochemical oxidation for wastewater treatment. Catal Today 151(1-2):173–177. https://doi.org/10.1016/j.cattod.2010.01.058
Şahinkaya S (2013) COD and color removal from synthetic textile wastewater by ultrasound assisted electro-Fenton oxidation process. J Ind Eng Chem 19(2):601–605. https://doi.org/10.1016/j.jiec.2012.09.023
Salgado P, Melin V, Contreras D et al (2013) Fenton Reaction Driven by Iron Ligands. J Chil Chem Soc 58(4):2096–2101. https://doi.org/10.4067/s0717-97072013000400043
Sancheti SV, Gogate PR (2017) A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason Sonochem 36:527–543. https://doi.org/10.1016/j.ultsonch.2016.08.009
Sevimli MF, Deliktaş E, Şahinkaya S et al (2014) A comparative study for treatment of white liquor by different applications of Fenton process. Arab J Chem 7(6):1116–1123. https://doi.org/10.1016/j.arabjc.2012.12.015
Sirés I, Brillas E (2012) Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ Int 40:212–229. https://doi.org/10.1016/j.envint.2011.07.012
Sirés I, Brillas E, Oturan MA et al (2014) Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res 21(14):8336–8367. https://doi.org/10.1007/s11356-014-2783-1
Sun M, Zhang G, Liu Y et al (2015) Highly Efficient AuPd/Carbon Nanotube Nanocatalysts for the Electro-Fenton Process. Chem Eur J 21(20):7611–7620. https://doi.org/10.1002/chem.201406676
Teymori M, Khorsandi H, Aghapour AA et al (2019) Electro-Fenton method for the removal of Malachite Green: effect of operational parameters. Appl Water Sci 10(1). https://doi.org/10.1007/s13201-019-1123-5
Thiam A, Zhou M, Brillas E et al (2014) Two-step mineralization of Tartrazine solutions: Study of parameters and by-products during the coupling of electrocoagulation with electrochemical advanced oxidation processes. Appl Catal B Environ 150-151:116–125. https://doi.org/10.1016/j.apcatb.2013.12.011
Ting W-P, Lu M-C, Huang Y-H (2009) Kinetics of 2,6-dimethylaniline degradation by electro-Fenton process. J Hazard Mater 161(2-3):1484–1490. https://doi.org/10.1016/j.jhazmat.2008.04.119
Tiwari BK (2015) Ultrasound: A clean, green extraction technology. TrAC Trends Anal Chem 71:100–109. https://doi.org/10.1016/j.trac.2015.04.013
Trabelsi F, Aït-Lyazidi H, Ratsimba B et al (1996) Oxidation of phenol in wastewater by sonoelectrochemistry. Chem Eng Sci 51(10):1857–1865. https://doi.org/10.1016/0009-2509(96)00043-7
Umar M, Aziz HA, Yusoff MS (2010) Trends in the use of Fenton, electro-Fenton and photo-Fenton for the treatment of landfill leachate. Waste Manag 30(11):2113–2121. https://doi.org/10.1016/j.wasman.2010.07.003
Vasconcelos VM, Ponce-de-León C, Nava JL et al (2016) Electrochemical degradation of RB-5 dye by anodic oxidation, electro-Fenton and by combining anodic oxidation–electro-Fenton in a filter-press flow cell. J Electroanal Chem 765:179–187. https://doi.org/10.1016/j.jelechem.2015.07.040
Wang A, Li Y-Y, Ru J (2010) The mechanism and application of the electro-Fenton process for azo dye Acid Red 14 degradation using an activated carbon fibre felt cathode. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.2450
Wang H, Zhao Z, Zhang X et al (2020) Rapid decomplexation of Ni-EDTA by microwave-assisted Fenton reaction. Chem Eng J 381:122703. https://doi.org/10.1016/j.cej.2019.122703
Wang Q, Liu M, Zhao H et al (2019) Efficiently degradation of perfluorooctanoic acid in synergic electrochemical process combining cathodic electro-Fenton and anodic oxidation. Chem Eng J 122071. https://doi.org/10.1016/j.cej.2019.122071
Wang W, Lu Y, Luo H et al (2017) Effect of an improved gas diffusion cathode on carbamazepine removal using the electro-Fenton process. RSC Adv 7(41):25627–25633. https://doi.org/10.1039/c7ra03793g
Wang Y, Meng G, Shan M et al (2020b) Treatment of high-ammonia-nitrogen landfill leachate nanofiltration concentrate using an Fe-loaded Ni-foam-based electro-Fenton cathode. J Environ Chem Eng 104243. https://doi.org/10.1016/j.jece.2020.104243
Wang Y, Zhou X, Jiang N et al (2020a) Treatment of Biotreated Coking Wastewater by a Heterogeneous Electro-Fenton Process Using a Novel Fe/Activated Carbon/Ni Composite Cathode. Int J Electrochem Sci:4567–4585. https://doi.org/10.20964/2020.05.70
Wood RJ, Lee J, Bussemaker MJ (2017) A parametric review of sonochemistry: control and augmentation of sonochemical activity in aqueous solutions. Ultrason Sonochem 38:351–370. https://doi.org/10.1016/j.ultsonch.2017.03.030
Wu T Y, Guo N, Teh C Y, et al. Advances in Ultrasound Technology for Environmental Remediation. SpringerBriefs in Molecular Science, 2013. https://doi.org/10.1007/978-94-007-5533-8
Xia G, Lu Y, Xu H (2015) Electrogeneration of hydrogen peroxide for electro-Fenton via oxygen reduction using polyacrylonitrile-based carbon fiber brush cathode. Electrochim Acta 158:390–396. https://doi.org/10.1016/j.electacta.2015.01.102
Xu A, Han W, Li J et al (2016) Electrogeneration of hydrogen peroxide using Ti/IrO2 –Ta2O5 anode in dual tubular membranes Electro-Fenton reactor for the degradation of tricyclazole without aeration. Chem Eng J 295:152–159. https://doi.org/10.1016/j.cej.2016.03.001
Yan L, Wang Y, Li J et al (2016) Reduction of Chemical Oxygen Demand from Refinery Wastewater by Three-Dimensional Electrode-Electro-Fenton Process. Bull Chem Soc Jpn 89(1):50–57. https://doi.org/10.1246/bcsj.20150250
Yasman Y, Bulatov V, Gridin VV et al (2004) A new sono-electrochemical method for enhanced detoxification of hydrophilic chloroorganic pollutants in water. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2003.10.004
Yuan S, Lu X (2005) Comparison treatment of various chlorophenols by electro-Fenton method: relationship between chlorine content and degradation. J Hazard Mater 118(1-3):85–92. https://doi.org/10.1016/j.jhazmat.2004.08.025
Zazou H, Afanga H, Akhouairi S et al (2019) Treatment of textile industry wastewater by electrocoagulation coupled with electrochemical advanced oxidation process. J Water ProcEng 28:214–221. https://doi.org/10.1016/j.jwpe.2019.02.006
Zhang H, Choi HJ, Huang C-P (2005) Optimization of Fenton process for the treatment of landfill leachate. J Hazard Mater 125(1-3):166–174. https://doi.org/10.1016/j.jhazmat.2005.05.025
Zhang H, Fei C, Zhang D et al (2007) Degradation of 4-nitrophenol in aqueous medium by electro-Fenton method. J Hazard Mater 145(1-2):227–232. https://doi.org/10.1016/j.jhazmat.2006.11.016
Zhang H, Zhang D, Zhou J (2006) Removal of COD from landfill leachate by electro-Fenton method. J Hazard Mater 135(1-3):106–111. https://doi.org/10.1016/j.jhazmat.2005.11.025
Zhang W, Chen J, Wang J et al (2021) Impact of Active Chlorines and •OH Radicals on Degradation of Quinoline Using the Bipolar Electro-Fenton Process. Water. https://doi.org/10.3390/w13020128
Zhao X, Zhang B, Liu H et al (2012) Transformation characteristics of refractory pollutants in plugboard wastewater by an optimal electrocoagulation and electro-Fenton process. Chemosphere 87(6):631–636. https://doi.org/10.1016/j.chemosphere.2012.01.054
Zheng M, Yang Y, Qiao S et al (2021) A porous carbon-based electro-Fenton hollow fiber membrane with good antifouling property for microalgae harvesting. J Membr Sci 626:119189. https://doi.org/10.1016/j.memsci.2021.119189
Funding
Funding information is not applicable.
Author information
Authors and Affiliations
Contributions
Chongjie Xia: Investigation, Writing - Original Draft, Writing – Editing. Xinjun Shen*(Corresponding Author): Writing - Review & Editing
Corresponding author
Ethics declarations
Ethical approval
Not application.
Consent for publication
All authors have agreed to participate and consent to the publication of this work.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Weiming Zhang
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, C., Shen, X. Analysis of factors influencing on Electro-Fenton and research on combination technology (II): a review. Environ Sci Pollut Res 31, 46910–46948 (2024). https://doi.org/10.1007/s11356-024-34159-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34159-z