Abstract
Mining-related lead (Pb) pollution of the soil poses serious hazards to ecosystems and living organisms, including humans. Improved heavy metal phytoremediation efficacy, achieved by using phytostabilizing plants assisted by plant-growth-promoting (PGP) microorganisms, has been presented as an effective strategy for remediating polluted soils. The objective of this research was to examine the response and potential of the plant-growth-promoting bacterium LMR356, a Rhodococcus qingshengii strain isolated from an abandoned mining soil, under lead stress conditions. Compared to non-contaminated culture media, the presence of lead induced a significant decrease in auxin production (from 21.17 to 2.65 μg mL−1) and phosphate solubilization (from 33.60 to 8.22 mg L−1), whereas other PGP traits increased drastically, such as 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity (from 38.17 to 71.37 nmol mg−1 h−1 α-ketobutyrate), siderophore production (from 69 to 83%), exopolysaccharide production (from 1952.28 to 3637.72 mg mL−1), biofilm formation, and motility. We, therefore, investigated the behavior of Sulla spinosissima L. in the presence or absence of this strain under a variety of experimental conditions. Under hydroponic conditions, Sulla plants showed endurance to varying lead concentrations (500–1000 μM). Inoculation of plants with Rhodococcus qingshengii strain LMR356 enhanced plant tolerance, as demonstrated by the increase in plant biomass (ranging from 14.41 to 79.12%) compared to non-inoculated Pb-stressed and non-stressed control plants. Antioxidant enzyme activities (increasing by −42.71 to 126.8%) and chlorophyll (383.33%) and carotenoid (613.04%) content were also augmented. In addition to its impact on plant lead tolerance, strain LMR356 showed a growth-promoting effect on Sulla plants when cultivated in sterilized non-contaminated sand. Parameters such as plant biomass (16.57%), chlorophyll (24.14%), and carotenoid (30%) contents, as well as ascorbate peroxidase (APX), peroxidase (POD), and catalase (CAT) activities, were all elevated compared to non-inoculated plants. Furthermore, when the same plant species was cultivated in highly polluted soil, inoculation increased plant biomass and improved its physiological properties. These findings demonstrate that LMR356 is a phytobeneficial bacterial strain capable of enhancing Sulla growth under normal conditions and improving its heavy metal tolerance in multi-polluted soils. Thus, it can be considered a promising biofertilizer candidate for growing Sulla spinosissima L. or other selected plants intended for application in restoration and stabilization initiatives aimed at reviving and safeguarding environmentally compromised and polluted soils after mining activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution of soils as a result of anthropogenic activities drastically affects soil functions, microbial activities, biodiversity, and growth, vegetation growth, and biodiversity, in addition to the human food chain (Yaashikaa et al. 2022; Biswal 2022; Ogundola et al. 2022). Lead (Pb) is one of the most toxic non-biodegradable metals; its presence in soil interferes with plants’ functions, including chlorophyll production, cell division, root elongation, seed germination, and plant growth. Exposure of plants to this metal also leads to the production of reactive oxygen species (ROS) and lipid peroxidation, destroying the plant’s cell membrane and organelles. However, the effects depend on Pb levels, exposure time, and plant species (Zulfiqar et al. 2019; Dalyan et al. 2020).
Excessive lead concentration produces many chronic health consequences, damaging cardiovascular, brain, and gastrointestinal systems or even causing the mortality of living species via food chain pollution (Meena et al. 2020; Mousavi et al. 2022; Srivastava et al. 2022; Yang et al. 2022). The bioremediation approach provides effective measures for the treatment of a wide range of pollutants. Among the several known bioremediation processes, phytoremediation, rhizoremediation, and bioremediation by microbes might be effective techniques for reducing soil lead contamination (Saha et al. 2021).
Inherently, contaminated regions harbor microorganisms with a remarkable capacity to endure a wide array of pollutants (Nzila et al. 2016; Oubohssaine et al. 2022a; Chettri et al. 2022; Annamalai et al. 2022; Chatterjee et al. 2022). Specifically, Actinobacteria have been widely identified as potential bioremediation agents among environmental microorganisms. They have been shown to be common in polluted soils and to be capable of metabolizing pollutants to thrive, including pesticides and heavy metals (Mawang et al. 2021; Farda et al. 2022; Behera et al. 2022; Raimondo et al. 2022). In addition to their ability to tolerate heavy metals, they can also contribute to the remediation process through their plant growth-promoting (PGP) properties and heavy metal-induced stress alleviation. The implementation of useful plant growth-promoting rhizobacteria (PGPR) in soil contributes to plant health, development, and nutrition by a variety of processes, including phosphate solubilization, nitrogen fixation, production of phytohormones, ammonia siderophores, and 1-amino-cyclopropane-1-carboxylic acid deaminase (ACCD) (Govindasamy et al. 2022; Oubohssaine et al. 2022a). Furthermore, some Actinobacteria have the ability to chelate excessive Pb and reduce its accessibility by producing active compounds, such as exopolysaccharides (EPS), and forming biofilm under stressful conditions. All of these properties are involved in critical physiological processes that guarantee bacterial survival in the presence of heavy metal contamination (Li et al. 2021a, 2021b; Homero et al. 2021).
One of the most common genera encountered in contaminated soils is Rhodococcus, a promising group of bacteria suitable for bioremediation. Rhodococci are a group of remarkable bacteria known for their extraordinary ability to thrive under mild, moderate, or even severe stresses across diverse environments. They have been discovered in various settings, including those characterized by harsh environmental conditions (Pátek et al. 2021; Oubohssaine et al. 2022a, 2022b). These Gram-positive, aerobic, non-sporulating bacteria possess a high G+C content (Guevara et al. 2019; Donini et al. 2021) and exhibit unique cellular features. Their cell walls contain peptidoglycan, while their cell envelopes consist of arabinogalactan and a lipid bilayer of mycolic acids (Malas 2021). The outer mycomembrane plays a vital role in protecting these cells against various toxic compounds (de Carvalho and da Fonseca 2005; de Carvalho 2012).
Moreover, Rhodococcus strains possess an extensive array of enzymatic activities that prepare them to handle numerous natural and xenobiotic organic compounds, enabling them to degrade substances such as alkanes, cycloalkanes, aromatic compounds, phenols, polycyclic aromatic hydrocarbons, halogenated hydrocarbons, and polychlorinated phenyls (Cappelletti et al. 2019, 2020). For these reasons, Rhodococcus strains are considered one of the best candidates for bioremediation strategies. Furthermore, it can be assumed that the use of tolerant Rhodococcus strains possessing several plant-growth-promoting mechanisms can have a great potential for the enhancement of the efficiency of heavy metal removal from contaminated soils, especially lead, and can serve as an intelligent and eco-friendly way for strategic implementation of phytoremediation interventions.
Here, we set out to examine Sulla spinosissima L.’s ability to phytoremediate heavy metals in association with a selected rhizospheric bacterium, Rhodococcus qingshengii strain LMR356, that was previously isolated from abandoned mining sites situated in Oujda region (Eastern Morocco). This plant species is a perennial native multi-tolerant legume known for its contribution to soil fertility as a result of its symbiotic nitrogen fixation capacity. Furthermore, it exhibits invasive traits as it naturally spreads within the previously mentioned mining sites, attributed to its remarkable ability to withstand and accumulate heavy metals in its roots (Sbabou et al. 2016). Therefore, the current study aimed to (a) examine the plant growth-promoting (PGP) traits of LMR356 in the presence of lead (Pb) pollution, (b) investigate the effects of inoculation with this strain on the growth of Sulla spinosissima L., and (c) reveal its impact on plants growth in a contaminated soil and try to elucidate the mechanistic role of plant growth-promoting rhizobacteria (PGPRs) in enhancing phytoremediation. The final objective is to provide critical knowledge for the prospective scaling-up of this technology to preserve the environment and rehabilitate polluted areas.
Material and methods
Origin of strain LMR356
For this study, we selected a strain isolated from the rhizosphere of Acacia cyanophylla, a legume tree species introduced into an abandoned mining site (Touissit) located in Oujda, Eastern Morocco. Oubohssaine et al. (2022a) previously reported the physico-chemical characteristics of the soil at this site, revealing extensive and varied contamination by several heavy metals, including chromium (Cr), copper (Cu), lead (Pb), zinc (Zn), and arsenic (As).
Molecular identification of strain LMR356
The genomic DNA of the isolate was extracted following the method described by Oubohssaine et al. (2022a, 2022b). Subsequently, the 16S rDNA gene was amplified using the polymerase chain reaction (PCR) technique and specific primers, namely 27f (5-AGAGTTTGATCCTGGCTCAG-3) and FGPS1509 (5-AAGGAGGGGATCCAGCCGCA-3). The PCR products were then sent for nucleotide sequencing at Genoscreen in France. The obtained 16S rDNA sequences were subjected to BLAST analysis in the NCBI database to identify model strains showing high sequence homology. To visualize the evolutionary relationships, a phylogenetic tree was constructed using the MEGA X software and the neighbor-joining method, with bootstrap analysis performed with 1000 repetitions to assess the robustness of the phylogeny.
Behavior of the strain LMR356 faced with lead stress
Pb tolerance of strain LMR356
To assess the strain’s tolerance to lead, we quantified it by measuring the optical density (OD) at 600 nm after 6 days of growth at 28 °C in nutrient broth (NB) medium supplemented with different concentrations of lead (ranging from 0 to 35 mM of Pb (Pb (NO3)2)). The selection of lead concentrations was based on the elevated levels of this metal previously measured at the Touissit site (Oubohssaine et al. 2022a).
Bioaccumulation of Pb
A volume of 100 ml of bacterial cultures, grown in tryptone yeast extract (TY) medium containing 5 g tryptone, 1 g yeast extract, and 0.65 g CaCl2, was prepared in 250 ml flasks. After 48 h of incubation at 37°C, the metal (30 mM of Pb(NO3)2) was introduced into the bacterial cultures, and they were re-incubated under the same conditions. The cell mass was then separated from the medium through centrifugation (8000 rpm for 10 min at 4°C). To distinguish the cells (pellet) from the medium, two specimens of the pellet were washed twice with sterile distilled water (the pellets were re-suspended in sterile distilled water and centrifuged for 6 min at 7000 rpm), while the other two specimens of the pellet were washed twice with 0.25 M of acid ethylenediaminetetraacetic (EDTA) (the pellets were re-suspended in 0.25 M EDTA, left for contact time, and then centrifuged at 7000 rpm for 6 min). After centrifugation, the supernatant represented the membrane compartment (metals extracted from cell membranes by EDTA), while the pellet corresponded to the intracellular compartment.
The pellets of the strain cultivated in the presence of the metal were obtained after centrifugation and dried at 52 °C for 48 h. Subsequently, the dried pellets were subjected to analysis by Inductively coupled plasma - optical emission spectrometry (ICP-OES) after digestion with sulfuric acid (H2SO4), nitric acid (HNO3), and perchloric acid (HClO4) following the method described by El Aafi et al. (2015).
Motility Assay
Following the protocol outlined by Turnbull and Whitchurch (2014), we conducted the bacterial motility assay. The assessment of bacterial motility was performed on a semi-solid tryptone yeast extract (TY) (0.3%) medium under both normal and lead stress conditions (0–30 mM of Pb). The evaluation involved measuring the diameter of the halo formed as an indicator of bacterial motility.
Exopolysaccharide production and biofilm assay
The strain was initially cultured for 30 h at 28 °C in Man-Rogosa and Sharpe (MRS) broth medium containing sucrose as the carbon source. After centrifugation at 10,000 rpm for 20 min, the supernatant was collected and mixed with three times its volume of cold acetone. The mixture was left overnight at 4 °C for precipitation, resulting in the recovery of the extracellular polysaccharides (EPS). The EPS obtained were then subjected to the phenol sulfuric acid assay to estimate their quantity (DuBois et al. 1956).
To study biofilm formation, pre-sterilized 96-well plates were used following the procedure described by O'Toole (2011) and Sahal et al. (2020). A cell suspension with an optical density of 0.5 at 600 nm was inoculated with 10 μl into each well containing 190 μl of Luria-Bertani (LB) medium, and 200 μl of autoclaved distilled water was added. After 16 h of growth at 37 °C, the cells were immobilized using 99% methanol. Following this, the plates underwent two washes with phosphate buffer saline and were left to air-dry. Subsequently, a 0.2% crystal violet solution was introduced into each well, and after a 5-min incubation, any surplus crystal violet was eliminated through two additional washes, followed by air-drying. To dissolve the crystal violet within the biofilm, 100 μl of 95% ethanol was added to each well, enhancing the reading sensitivity with a spectrophotometer. The growth of the biofilm was monitored by measuring the OD570 using a microplate reader (Rivas et al. 2007; Flores-Treviño et al. 2014; Shukla and Rao 2013; Shukla and Rao 2014).
PGP trait evaluation under normal and lead stress conditions
To assess the production of indole-3-acetic acid (IAA), strain LMR356 was cultured in Yeast Extract Mannitol (YEM) medium supplemented with tryptophan (0.5 mg mL−1), which serves as the precursor of IAA. The quantification of auxin production followed the methods described by Gordon and Weber (1951), Sheng et al. (2008), and Oubohssaine et al. (2022a).
The ability of strain LMR356 to solubilize phosphate was evaluated using a Pikovskaya medium containing 0.5% insoluble phosphate, following the procedures outlined by Pikovskaya (1948) and Oubohssaine et al. (2022a). For the estimation of siderophore production, the Chrome Azurol-S (CAS) analytical method was employed, as described by Modi et al. (1985), Schwyn and Neilands (1987), Manjanatha et al. (1992), and Oubohssaine et al. (2022a).
To measure ACC deaminase activity, the ACC-deaminase activity assay from Penrose and Glick (2003) and Oubohssaine et al. (2022a) was employed. To estimate the ammonium production by strain LMR356, the technique described by Cappuccino and Sherman (1992) was employed. In triplicate, the strain was inoculated into peptone water broth (containing 10 g L−1 Peptone, 5 g L−1 NaCl, and pH 7.0 ± 0.2) and then incubated at 28° ± 2°C with continuous shaking at 200 rpm. Following the incubation period, 1 ml of the cell-free supernatant was combined with 1 ml of Nessler’s reagent.
All the plant growth-promoting (PGP) traits were assessed under both normal and lead stress conditions (30 mM Pb).
Sulla spinosissima experiments
Inoculum and seed preparation
An overnight inoculum of LMR356 was cultured in NB medium, starting with an initial OD600 of 0.05, and incubated at 28 °C with agitation at 180 rpm for 24 h. After incubation, the bacterial culture was harvested by centrifugation at 8000 rpm at 4 °C for 10 min. Afterwards, the supernatant was removed, and the cell pellets were subjected to two washes with sterile distilled water. They were then re-suspended in sterile distilled water to attain an OD600 of 0.1, which corresponds to approximately 108 cells mL−1.
Previously collected seeds of Sulla spinosissima from the Oujda region were manually scarified, then surface sterilized in 70% ethanol for 1 min, followed by five rinses with sterile distilled water. The treated seeds were then germinated on 9% agar plates at 25°C in a dark environment.
Hydroponic experiments
Tolerance of Sulla spinosissima to lead
The primary objective of the initial experiment was to assess the tolerance level of Sulla spinosissima plants to lead (Pb). Uniform and aseptic pre-germinated seeds of Sulla spinosissima were transplanted into vessels filled with nutrient solution following the Hoagland and Arnon (1950) method. The seedlings were allowed to grow under normal conditions for 15 days. Subsequently, the young seedlings were transferred to nutrient solutions containing varying concentrations of Pb(NO3)2 (ranging from 500 to 1000 μM) and were left to grow for 30 days. To ensure controlled growth conditions, the vessels were randomly arranged within a growth chamber set at a temperature of 25/20°C (day/night) with a photoperiod of 16 h of light and 8 h of darkness. To maintain optimal nutrient conditions, the nutrient solutions were renewed every 5 days.
Effect of LMR356 inoculation on Sulla spinosissima
The second experiment aimed to investigate the impact of inoculating the Rhodococcus strain on the growth and development of plants under both normal and Pb-stressed conditions. Three distinct treatments were tested:
-
Sulla plants are grown without Pb and without inoculation (control).
-
Inoculation with Rhodococcus under normal growth conditions at a rate of 108 cells per seedling.
-
Rhodococcus inoculation at the same rate under the previously identified Pb minimal inhibitory concentration for Sulla spinosissima.
All plants were cultivated under the same conditions as described earlier.
Pot experiment
The seedlings were planted in earthen pots of uniform size, filled with sand or soil depending on the specific experiment. The arrangement of the pots followed a completely randomized design (CRD).
Effect of inoculation with LMR356 on Sulla spinosissima growth under normal conditions
To evaluate the influence of the Rhodococcus inoculum on plant growth, the inoculated plants were grown in sand that was autoclaved at 121°C for 1 h. The strain and seeds were prepared following the previously described method.
The plants were cultivated in pots, each containing 100 g of sterilized sand. One plant was placed per pot, and there were 12 repetitions for each treatment. Each plant received an inoculation of 1 mL of the Rhodococcus inoculum at the same rate as before. Additionally, uninoculated control plants were also set up for comparison.
All the pots were placed in a controlled growth chamber, maintaining the same environmental conditions as previously mentioned. Regular watering with a nutritive mineral solution was provided to the plants throughout the 100-day duration of the experiment.
Effect of inoculation with the Rhodococcus strain on Sulla spinosissima growth under lead stressing conditions
In this particular experiment, we utilized the heavily lead-contaminated soil from the Touissit site in the Oujda region, as previously studied by Oubohssaine et al. (2022a). Aseptic pre-germinated seeds were planted in pots filled with this soil, and the experimental conditions were kept consistent with the preceding assay, except for the watering process. In this case, the plants were watered with sterile distilled water instead of the mineral solution. The preparation and application of the bacterial inoculant were carried out in the same manner as in the previous experiment.
Measurements of biomass and chlorophyll/carotenoid content
Following a growth period of 30 days for hydroponic experiments and 100 days for pot assays, the Sulla plants were harvested, and the shoots and roots were carefully separated. In the soil experiment, the roots were meticulously removed from the soil by thorough water washing. Afterward, both the roots and shoots were dried with blotting paper. The length and biomass of the plant shoots and roots were subsequently measured.
A portion of the fresh leaves was utilized to estimate the chlorophyll and carotenoid contents, following the method outlined by Mackinney (1941).
Antioxidant enzyme assays
The measurement of ascorbate peroxidase (APX), catalase (CAT), and peroxidase (POD) activities was conducted using the procedures outlined by Chen and Asada (1989), Nakano and Asada (1981), Aebi (1984), and Chance and Maehly (1955), respectively.
Statistical analysis
The data underwent analysis using the analysis of variance (ANOVA) statistical package within XL STAT for social sciences. Subsequently, multiple treatment levels were compared using the Duncan significant difference test at a significance level of P ≤ 0.05.
Results
Identification of strain LMR356
By comparison of 16S rDNA sequences in BLAST analysis, the strain LMR356 was shown to have 99.7% homology with a Rhodococcus qingshengii strain (GenBank No. NR_115708.1) (Fig. 1). The nucleotide sequence of LMR356 has been registered in the NCBI database with accession number OQ991161.
Rhodococcus strain lead tolerance
As shown in Fig. 2, the growth of strain LMR356 was reduced progressively when lead concentration in the growth medium increased. This strain can tolerate up to 30 mM of Pb, thus the minimal inhibiting concentration (CIM) of strain LMR356 can be established between 30 and 35 mM of Pb.
Bioaccumulation of lead
The primary objective of this study was to assess the bioaccumulation of Pb by the Rhodococcus strain, aiming to characterize the distribution of lead at the subcellular level and evaluate the strain's potential for bioremediation of mining sites. The results obtained showed that the strain accumulated 55 ppm of Pb inside the cell, against 8490 ppm outside of the cell (Table 1).
Motility test
The motility was assessed in the presence of different concentrations of Pb ranging between 0 and 30 mM. Halo measurements indicated that the strains’ motility was significantly increased with the augmentation of the Pb concentration, and the maximum value of 35 mm was measured at 20 mM Pb (Fig. 3).
Exopolysaccharide production and biofilm assay
The phenol-sulphuric acid assay showed that the Rhodococcus strain was able to secrete a high amount of exopolysaccharides under normal conditions (1952.28 mg mL−1). In the presence of 30 mM of lead, EPS production was increased to 3637.72 mg mL−1 (Table 2). The strain was also able to form a biofilm in the presence of Pb that was dependant on the concentration of lead in the medium (10 mM, 20 mM, 25 mM) (Fig. 4).
Plant growth-promoting characteristics
The amounts of IAA produced by the strain LMR356 not exposed and exposed to Pb stress are shown in Table 2. The production of IAA was drastically reduced by the incorporation of lead in the culture medium, 2.65 μg mL−1 of IAA instead of 21.17 μg mL−1. Lead also affected negatively the amount of phosphorus solubilized by the strain grown in PVK liquid medium, 8.22 mg L−1 of P against 33.60 mg L−1 in non-stressed conditions. In contrast to the two previous PGP activities, lead stimulated siderophores production and ACC deaminase activity of the Rhodococcus strain tested (83.7% instead of 69% for siderophores and 71.37 nmol mg−1 h−1 α-ketobutyrate against 38.17 for ACC deaminase). Moreover, ammonium production by the strain was not affected by the presence of lead in the medium. Globally, the results of PGP activity measurements demonstrate that the Rhodococcus LMR356 studied may be a good candidate to be tested with plants growing under normal and Pb-contaminated conditions.
Sulla spinosissima growth under Pb stress in hydroponic conditions
Effect of elevated Pb concentrations on plant growth parameters
During this experiment, Sulla plants were exposed to various concentrations of Pb (500, 750, and 1000 μM) in a hydroponic setup. The results presented in Fig. 5 demonstrate that lead had varying effects on different growth parameters of the plants. The control plants exhibited the highest shoot length, followed by the treatments with 750 μM and 1000 μM of Pb, with shoot lengths of 125 mm plant−1, 80 mm plant−1, and 65 mm plant−1, respectively. However, lead did not have a significant impact on root length, as there were no noticeable differences observed between the control group and both Pb treatments (750 μM and 1000 μM). Interestingly, the plants treated with only 500 μM of Pb displayed the lowest root and shoot lengths, indicating that they were most affected by lead. These results were also reflected in the measurements of shoot and root dry weights.
Effect of lead on Sulla plants grown under hydroponic conditions (growth parameters, total chlorophyll and carotenoids content, and antioxidant enzymes were measured). The growth parameters were determined by calculating the means from 10 replicates. As for the pigment parameters, the results are presented as means ± SE (n = 3). The mean values of antioxidant enzymes were calculated based on four replicates. Subsequently, an ANOVA test was conducted, and the Duncan test revealed significant differences (P < 0.05) among the mean values of different treatments, as denoted by distinct letters
Furthermore, the impact of high Pb concentrations on chlorophyll content was evident, as the production of chlorophyll decreased with increasing Pb concentration. On the other hand, the carotenoid content in the shoots showed an increase with rising Pb concentration, although these differences were not statistically significant.
The measurements of oxidative enzymes in the plants revealed notable variations. Root and leaf APX activities were significantly higher in Sulla plants treated with 1000 μM of Pb, recording 10.58 U mg−1 protein and 2.68 U mg−1 protein, respectively, compared to the control plants with 3 U mg−1 protein and 0.44 U mg−1 protein, respectively. At this Pb concentration, roots’ POD activity was also significantly higher, recording 20.21 U mg−1 protein instead of 3.57 U mg−1 protein in control plants. However, in leaves, the maximum POD activity was recorded in plants treated with 750 μM of Pb, showing 1.52 U mg−1 protein compared to 0.27 U mg−1 protein in control plants. It is noteworthy that all the plants studied did not exhibit any CAT activity at the root level, but in the leaves, higher levels of CAT activity were recorded in plants treated with 750 μM and 1000 μM of lead compared to the control plants (Fig. 5).
Effect of Rhodococcus inoculation on the growth of Sulla spinosissima
In a controlled hydroponic environment, the inoculation of plants with the LMR356 strain exhibited beneficial effects on the plants growing under lead stress, as depicted in Fig. 6. The Pb-stressed inoculated plants demonstrated shoot and root lengths higher than non-inoculated Pb-stressed plants but comparable to the control plants grown under normal conditions. Moreover, the inoculation had a positive influence on the shoot and root dry weights of plants subjected to lead stress, with no significant differences observed between control and inoculated Pb-stressed plants. Additionally, Sulla plants inoculated with strain LMR356 showed partial recovery in chlorophyll and carotenoid content levels compared to plants treated with 1000 μM of Pb, although they performed significantly less efficiently than non-stressed plants.
Effect of Rhodococcus qingshengii LMR356 inoculant on Sulla plants grown under hydroponic conditions in the presence of lead (growth parameters, total chlorophyll and carotenoids content, and antioxidant enzymes, were measured). The growth parameters were determined by calculating the means from 10 replicates. As for the pigment parameters, the results are presented as means ± SE (n = 3). The mean values of antioxidant enzymes were calculated based on four replicates. Subsequently, an ANOVA test was conducted, and the Duncan test revealed significant differences (P < 0.05) among the mean values of different treatments, as denoted by distinct letters
Regarding the enzymatic activities, both root and leaf APX activities were significantly higher in Pb-stressed plants inoculated with strain LMR356, measuring 10.82 U mg−1 protein and 4.96 U mg−1 protein, respectively, compared to non-inoculated non-stressed control plants, which recorded 5 U mg−1 protein and 0.96 U mg−1 protein, respectively. These values were also higher than those of non-inoculated plants treated with 1000 μM of Pb. On the other hand, POD activity was substantially greater in the roots of plants treated with 1000 μM (4.87 U mg−1 protein) compared to both control plants (0.57U mg−1 protein) and those inoculated with LMR356 (2.79 U mg−1 protein). Conversely, in leaves, POD activity was higher in inoculated plants (2.2 U mg−1 protein) than in control plants (0.27 U mg−1 protein). Moreover, CAT activity was not detectable in roots but was measured in leaves of inoculated plants (0.01 U mg−1 protein).
Evaluation of the effect of LMR356 on Sulla plants grown in sand pots
In a controlled environment using sterilized sand, the inoculation with strain LMR356 exhibited a positive influence on Sulla plants. This was evident by the higher shoot dry weights measured (68.07 mg plant−1) compared to the control plants (58.88 mg plant−1). However, it was noted that the root dry weights were lower for the inoculated plants in comparison to the control group. The roots of the latter plants were also longer than inoculated plants, but no significant difference was recorded between the shoot lengths of both types of plants (60 mm plant−1 for inoculated plants versus 54.28 mm plant−1 for the control).
Pigment measurements corresponded to the aboveground biomass, with Sulla-inoculated plants showing higher values of chlorophyll and carotenoids. Additionally, inoculated plants exhibited higher root and leave APX activities (35.19 U mg−1 protein and 15.01 U mg−1 protein, respectively) than control plants (7.82 U mg−1 protein and 12.9 U mg−1 protein, respectively). The same tendency was observed in root and leaf POD activities. As for CAT activities, inoculated plants displayed statistically higher values in leaves than control plants (0.03–0.05 U mg−1 protein). Conversely, the root catalase (CAT) activities in the inoculated plants were found to be statistically indistinguishable from those in the control plants (Fig. 7).
Effect of Rhodococcus qingshengii LMR356 inoculant on Sulla grown in sterilized sand (growth parameters, total chlorophyll and carotenoids content, and antioxidant enzymes were measured). The growth parameters were determined by calculating the means from 14 replicates. As for the pigment parameters, the results are presented as means ± SE (n = 3). The mean values of antioxidant enzymes were calculated based on four replicates. Subsequently, an ANOVA test was conducted, and the Duncan test revealed significant differences (P < 0.05) among the mean values of different treatments, as denoted by distinct letters
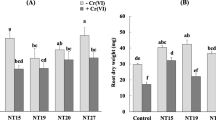
Effect of Rhodococcus inoculation on Sulla spinosissima growth in a metal-contaminated soil
The bacterial isolate LMR356 demonstrated a significant impact on the growth of plants in the highly contaminated soil, as compared to the control non-inoculated plants (Fig. 8). Specifically, Sulla plants inoculated with LMR356 exhibited higher biomass production compared to the control group. This effect was also observed in terms of root biomass, shoot length, and root length. Significant differences were observed in all growth parameters (shoot length, root length, root dry weight) between the control group and the inoculated plants, with a valid significance level of P < 0.05 for all parameters except for shoot dry weight. Moreover, Sulla inoculation also resulted in a significantly higher chlorophyll content (1.49 mg g−1 FW) in comparison with control plants. Conversely, the carotenoid content of inoculated plants was lower than control plants.
Effect of Rhodococcus qingshengii LMR356 inoculant on Sulla plants grown in the heavy metal contaminated soil of Touissit (growth parameters, total chlorophyll and carotenoids content, and antioxidant enzymes were measured). The growth parameters were determined by calculating the means from 14 replicates. As for the pigment parameters, the results are presented as means ± SE (n = 3). The mean values of antioxidant enzymes were calculated based on four replicates. Subsequently, an ANOVA test was conducted, and the Duncan test revealed significant differences (P < 0.05) among the mean values of different treatments, as denoted by distinct letters
Root and leave APX activities were noticeably lower in plants inoculated with LMR356 (47.4 U mg−1 protein and 26.13 U mg−1 protein, respectively) than in control plants (53.8 U mg−1 protein and 30.1 U mg−1 protein, respectively). The same trend was observed for roots and leaves POD activities. However, a different result was recorded for CAT activity, as neither inoculated nor control plants showed any activity in the roots. In contrast, in leaves, the inoculation of plants significantly lowered the CAT activity (0.02 U mg−1 protein) compared with control plants (0.041 U mg−1 protein).
Discussion
Mining sites, whether currently operational or abandoned, exert significant pressure on surrounding ecosystems, leading to pollution and ecological degradation. In Morocco, for instance, approximately 200 abandoned mining sites stand as stark reminders of this environmental challenge. Addressing the contamination of these areas emerges as a paramount concern for nations committed to sustainable development agendas. The remediation of such contaminated sites necessitates robust strategies aimed at mitigating the adverse effects of heavy metal toxicity, thereby safeguarding ecosystems for present and future generations. Among the array of remediation approaches available, biological solutions stand out for their effectiveness and relatively lower environmental risks. Leveraging agro-biotechnological methods, which capitalize on natural processes, offers a promising avenue for the elimination and stabilization of toxic metals in polluted environments. To accomplish phytorestoration of contaminated sites, this bioremediation approach employs either microorganisms or products of their metabolism, or higher plants and their rhizospheric bacteria. With this goal in mind, the present study seeks to explore the capabilities of Pb-tolerant PGPR Rhodococcus qingshengii LMR356 in promoting the growth of Sulla spinosissima and mitigating the effects of Pb-induced stress.
Notably, this strain exhibits multiple characteristics associated with plant growth promotion, enhancing its suitability for assisting in phytoremediation endeavors. Numerous strains within the Rhodococcus genus have been identified as possessing both PGP features and resistance to heavy metals such as lead (Pb). Examples include Rhodococcus erythropolis AV96 (Navazas et al. 2022), Rhodococcus hoagii (MG432495) (Jinal et al. 2019), Rhodococcus sp. NSX2 (Wang et al. 2020), Rhodococcus jostii B12 (Vergani et al. 2019), and Rhodococcus sp. (González Henao and Ghneim-Herrera 2021). These findings underscore the potential of Rhodococcus strains as valuable allies in the quest for sustainable remediation of metal-contaminated sites.
Lead tolerance of Rhodococcus qingshengii LMR356 and possible involved mechanisms
The strain under the current investigation exhibited pronounced resistance to lead at a concentration of 30 mM. However, it was observed that bacterial survival declined progressively under the influence of lead stress, aligning with earlier research findings (Gikas et al. 2009). Lead is recognized for its capacity to instigate structural destabilization of the cytoplasmic membrane, thereby leading to a reduction in the bacterial population. Moreover, the slowing of the onset of growth is a very common aspect of the effects of sub-lethal metal concentrations on bacteria (Aljerf 2018).
According to Li et al. (2021a, 2021b), exopolysaccharide (EPS) production is one of the mechanisms of heavy metal tolerance in bacteria. It prevents heavy metals’ entrance into bacterial cells through the nonspecific binding of heavy metals and EPS. This is the case of strain LMR356 that produced a large amount of EPS and was able to form biofilm at 30 mM of lead. EPS may also decrease metal toxicity in plants regarding the adhesive properties of EPS that assist bacteria in combining with soil particles and heavy metals (Zhang et al. 2006; Gupta and Diwan 2016; Bhagat et al. 2021). Polysaccharides, according to BeMiller (2019), are acidic and have a significant affinity to particular ions. These EPS help to build bacterial aggregates, which improve soil aeration and root development (Bhagat et al. 2021).
Biofilm formation is another characteristic of the studied Rhodococcus strain that is important for lead resistance. In this respect, it was found that higher levels of Pb increased Rhodococcus biofilm development. Studies have reported that bioremediation performed by biofilm cells surpasses that of planktonic cells due to the biofilm cells’ ability to adapt and thrive in stressful conditions, being shielded within the matrix. This phenomenon highlights the multifaceted advantages conferred by biofilm in the context of environmental stressors such as lead contamination (Rodrigues and de Carvalho 2015; Adhami et al. 2017). In addition to EPS and biofilm formation, Pb-tolerant bacterial strains can employ other sophisticated distinct mechanisms to efficiently survive in a Pb-contaminated environment. Several mechanisms have been identified for reducing Pb toxicity, including biosorption, Pb efflux, the formation of metal chelators like metallothionein and siderophores, as well as bioaccumulation (Najm-ul-Seher Ahmad et al. 2021; Pande et al. 2022). The last mechanism was estimated for strain LMR356, and we found that the strain has a high Pb bioaccumulation capacity. The metals associated with the intracellular compartment (cytoplasm) were measured in bacterial pellets (after washing with water) and those associated with the extracellular compartment (membranes and polysaccharides) were determined after washing with EDTA, a chelator that is utilized to capture and remove metallic components adsorbed on the surface of bacterial cells (El Aafi et al. 2015). EDTA washing demonstrated that the overall metal accumulation was consistently higher than the accumulation inside the cell, suggesting that a significant portion of the metal was adsorbed onto the cell surface. This is may be due to the high concentration of polysaccharides produced by the studied strain, where the metals could be trapped and thus adsorbed on these polysaccharides found on the cell surface. El Aafi et al. (2015) reached the same conclusion. These results indicate that the Rhodococcus strain LMR356 is not only capable of tolerating lead but also accumulating high levels of this metal, particularly on the cell surface, which indicates that this strain is suitable for bioremediation in heavy metal-contaminated soils or waters. Alternatively, this type of bacteria can be employed in rhizoremediation by inoculating them to plant roots to increase and improve their tolerance to polluted environments. Moreover, the studied Rhodococcus strain showed a chemotaxis response under Pb stress. Many studies showed similar results such as Rhodococcus sp. BAP-1 (Li et al. 2014), and Rhodococcus ruber and Rhodococcus pyridinivorans (Wang et al. 2018). Aroney et al. (2021) reported that chemotaxis systems, which respond to abiotic stresses, tend to govern bacterial motility, enabling bacteria to migrate toward optimal environments.
Rhodococcus strains not only grew in the presence of lead (Pb) but also in the presence of other heavy metals. In a study conducted by Wevar Oller et al. (2013), Rhodococcus erythropolis AW3 demonstrated growth in high concentrations of arsenite and arsenate. The bacterial strains exhibited resistance of up to 24 mM for arsenite and up to 400 mM for arsenate. In a related study by González Henao and Ghneim-Herrera (2021), a Rhodococcus strain exhibited resistance to high concentrations of arsenite. In another study conducted by our team, Oubohssaine et al. (2022a, 2022b) Rhodococcus strains demonstrated the ability to tolerate high concentrations of lead (Pb), zinc (Zn), and arsenic (As).
Plant growth-promoting traits of Rhodococcus qingshengii LMR356
The strain LMR356 is able to produce significant levels of ammonia, IAA, and siderophores, to solubilize rock phosphate, and synthesize ACC deaminase. Some of these activities were reduced under lead stress, while others were increased and others were not affected.
Phosphorus is an important nutritional source for plants; nevertheless, P deposited in soil is generally inaccessible to plants (He et al. 2021). As a result, isolating bacteria that can use these soil P reserves is of significant interest. Bacteria with P-solubilization ability may solubilize soil inorganic P that plants are unable to utilize, which is an excellent strategy to increase plant P absorption (Ahmad et al. 2022; Adetunji et al. 2022). Strain LMR356 is a phosphate-solubilizing bacteria, but its capacity was reduced under Pb stress. Similar results were reported for a strain of Enterobacter under Cd stress (Li et al. 2022a). The strain LMR356 was also able to produce ammonia even under Pb stress. Rhizobacteria producing ammonia are crucial to plant’s growth and health and play an important role in nutrient cycling (Hayat et al. 2010; Kumar and Verma 2018; Imran et al. 2021).
Among the PGP traits of rhizobacteria, IAA is often researched, especially under stressful conditions. IAA belongs to a significant auxin family and plays a pivotal role in plant root initiation, cell division, and cell enlargement (Borah et al. 2019; Wang et al. 2022a; Wang et al. 2022b; Robas Mora et al. 2022). As a result, PGPR that produces IAA can significantly enhance plant biomass, stimulate rooting and germination, and foster the growth of root hairs and cotyledon cells. The production of IAA is associated with root growth and structural changes in response to stress (Vacheron et al. 2013; Adeleke et al. 2022). In the current research, IAA secretion by strain LMR356 decreased considerably at 30 mM of lead. Li et al. (2022a) elucidated that heightened levels of lead exposure can induce a reduction in the activity of the IAA synthetase system, accompanied by a depletion of the synthetic tryptophan pool. This dual effect may significantly hinder the biosynthesis of indole-3-acetic acid (IAA), a crucial plant growth regulator. This finding underscores the intricate mechanisms through which lead exerts its inhibitory influence on IAA production, shedding light on potential pathways for mitigating its adverse effects on plant development and physiology. Unlike IAA, siderophores production was promoted when Rhodococcus qingshengii LMR356 was exposed to high amounts of Pb. Similarly, Sinha and Mukherjee (2008) discovered that Pseudomonas aeruginosa KUCd1 produced siderophores in response to high Pb exposure.
ACC deaminase activity is one of the PGP attributes that was enhanced strongly in LMR356 exposed to high amounts of lead. This activity is very important in the context of heavy metal stresses. PGPR isolates degrade ACC, the precursor of ethylene, into ammonia and α-ketobutyrate. As a result, the negative effects of ethylene are reduced, and germination improves, resulting in increased plant development under lead circumstances (Glick et al. 2007; Ghosh et al. 2018; Haldar et al. 2022).
Thus, Rhodococcus qingshengii LMR356 shows different PGP characteristics even when exposed to Pb, which provides this bacterium an edge over other competitors in the rhizosphere of plants (Lopez et al. 2022).
Sulla spinosissima behavior under lead stress in hydroponic system
In situ investigations were performed in hydroponic conditions, utilizing the plant species Sulla spinosissima. This species was selected owing to its inherent capacity to thrive naturally in soils heavily contaminated with multiple heavy metals within the Oujda mining region.
We started by looking at how lead concentration affected plant growth, chlorophyll and carotenoid levels, and antioxidant enzyme activities. We found that as the concentration of lead increased, the growth of Sulla plants decreased as compared to control plants and variable responses across the three concentrations tested (500, 750, and 1000 μM) were recorded for the different parameters used.
Excessive concentrations of heavy metals are widely recognized for their detrimental effects on plant biomass, exerting negative influences across various critical stages, including germination, growth, development, and key physiological processes such as photosynthesis (Yaashikaa et al. 2022; Singhal et al. 2022; Podar and Maathuis 2022). Plants’ fast and rapid synthesis of reactive oxygen species (ROS) following Pb exposure is regarded as an early defense response (Shahid et al. 2014; Berni et al. 2019). The development of Sulla plants can be attributed to a complex interplay involving reactive oxygen species (ROS) and biomolecules, coupled with challenges such as inefficient water and nutrient absorption and electrolyte loss due to altered membrane permeability. The diminished chlorophyll content observed in Sulla plants may result from elevated ROS levels or the inactivation of enzymes crucial for chlorophyll synthesis (Batool et al. 2019). The presence of heavy metals is recognized for its impact on diminishing the levels of photosynthetic pigments, predominantly by influencing the integrity of cell walls and thylakoid membranes, and also through alterations in proteins and DNA caused by ROS interference (Ajmal et al. 2022; Noor et al. 2022; Sharma et al. 2022a). This intricate cascade of events underscores the multifaceted impact of heavy metal exposure on plant physiology, emphasizing the importance of understanding these mechanisms for devising effective strategies to mitigate heavy metal-induced stress in plants like Sulla. Carotenoids content increased in response to Pb stress, which can be explained by the presence of nitrogen, as reported by Gurpreet et al. (2012). Whereas the increase in CAT, APX, and POD activities may be related to the activation of plant defense systems that confer resistance to heavy metals stress in plants (Ajmal et al. 2022). These antioxidant enzymes catalyze free radical conversion, and their increased activity leads to plant bioprotection against abiotic stresses. These findings, together with those of Sbabou et al. (2016) and Lamin et al. (2020), indicate that Sulla plants are phytostabilizing plants due to their root structure, which endures at high lead concentrations.
Rhodococcus qingshengii LMR356 effect on Sulla spinosissima growing under Pb stress in hydroponic
The Rhodococcus strain utilized in this study increased the vegetative development of Sulla plants under 1000 μM Pb as compared to uninoculated plants. Many possible traits of the strain can be implicated in such improvement, IAA biosynthesis, inorganic phosphate solubilization, siderophores production, and others. A reduced level of IAA can impact the bioavailability of heavy metals (HMs) in the rhizosphere microenvironment, leading to an increase in HM absorption (Li et al. 2022a, 2022b) and a significant enhancement in root development (Patten and Glick 2002). Additionally, the application of IAA was found to elevate the activity of H+-ATPase in the root plasma membrane of Medicago sativa L., promoting H+ secretion from root tips and resulting in a lowered rhizosphere pH (Wang et al. 2018). Moreover, P-solubilizing bacteria are involved in the production of organic acids, which leads to a decrease in pH, cation chelation, and competition with phosphate for soil adsorption sites (de Freitas Duarte et al. 2022; Bhardwaj et al. 2022). Additionally, the secretion of organic acids by P-solubilizing bacteria was reported to play a role in the dissolution of HMs (Gupta and Diwan 2016). Various organic acids, including ketogluconic acid, gluconic acid, citric acid, oxalic acid, tartaric acid, succinic acid, and others, undergo protonation and conversion into low-molecular-weight organic acids, potentially leading to alterations in soil pH and redox potential, thereby promoting the dissolution of HMs (Li et al. 2022a). Additionally, the secretion of organic acids by siderophore-producing bacteria can modify the bioavailability of heavy metals like Pb in the rhizosphere (Saha et al. 2016; Podar and Maathuis 2022; Pandey et al. 2022; Sharma et al. 2022b). Moreover, this process may facilitate the interaction of siderophores with heavy metal ions, forming metal-siderophore chelates, which enhance heavy metal activity in the plant rhizosphere, consequently increasing their accumulation (Barman and Jha 2021; Mitra et al. 2021; Wang et al. 2022a). Studies by other researchers have demonstrated that bacterial siderophore synthesis can mitigate oxidative stress and ethylene stress, while also providing essential mineral nutrients for plant growth and development (Nazli et al. 2020a; Devi et al. 2022; Koza et al. 2022). Rhodococcus’ mobility also allows bacterial cells to escape a harsh environment, giving them a survival benefit. According to Bashan and Holguin (1994) and Williams (2011), motility is an active process involved in effective colonization. Bacterial motility toward root exudates and the capacity to form EPS and biofilms are essential for rhizosphere and rhizoplane colonization (Lucero et al. 2020).
Effect of LMR356 on Sulla spinosissima growing in sterilized sand
Inoculation of Sulla plants growing in sterilized sand improved plant growth and chlorophyll and carotenoid content. These findings are consistent with the strain’s in vitro PGP features, in particular improved P bioavailability via inorganic phosphate solubilization, siderophores production that improves plant iron uptake, and phytostimulation by auxin production. Plants that were inoculated displayed shoot lengths and dry weights comparable to those of control plants. The slight rise in antioxidant enzyme activities, such as peroxidase (POD), ascorbate peroxidase (APX), and catalase (CAT), observed in certain instances might be attributed to the stimulation of plant defense mechanisms by the inoculated Rhodococcus strain (Oubohssaine et al. 2022b).
Impact of inoculation on Sulla spinosissima growth in multi-heavy metal-polluted soil
The Touissit mine soil used in this experiment is heavily contaminated with various heavy metals, particularly As and Pb (Oubohssaine et al. 2022a). Inoculation significantly enhanced shoot and root lengths, as well as the chlorophyll content of the plants growing in this soil. Numerous studies have provided evidence that the inoculation with PGPR strains can elevate the concentration of photosynthetic pigments under conditions of heavy metal stress (Jinal et al. 2019; Zainab et al. 2021; Kaur et al. 2021; Navazas et al. 2022). This effect may be attributed to increased nutrient absorption through phosphate solubilization by the inoculated strain and the release of active substances that play a critical role in the production of photosynthetic pigments (Santoyo et al. 2021; Saeed et al. 2021; Das et al. 2022). Carotenoids, renowned for their ability to scavenge ROS, play a crucial role in shielding the plant’s photosynthetic machinery from photo-oxidative disruptions (Khanna et al. 2019; García-Caparrós et al. 2021; Zandi and Schnug 2022; Kaur and Goyal 2022). Furthermore, characteristics such as phosphate solubilization, siderophore production, IAA (Indole-3-acetic acid) synthesis, EPS (extracellular polymeric substances) production, biofilm development, and nutrient availability contribute to strain’s capacity to support plant growth under heavy metal stress conditions (Khoshru et al. 2020; Nazli et al. 2020b; Syed et al. 2021; Zainab et al. 2021; Singh et al. 2022).
The outstanding performance of LMR356 observed in different plant inoculation experiments (hydroponic or pot) may be attributed to its origin and properties. Being isolated from Touissit’s soil, the strain is well-adapted to polluted environments, tolerating high levels of lead. In the context of this investigation, the level of Pb in soils appears to be a crucial factor. Strain LMR356 produces IAA, ACC deaminase, and EPS, and it has the ability to solubilize phosphate, form biofilm, and exhibit mobility.
When comparing our study with others, conducted under multi-heavy metal-contaminated conditions of Touissit’s soil, the results demonstrate that plants inoculated with a Pb-tolerant PGPR Rhodococcus strain exhibited reduced antioxidant enzyme activities, especially in the aerial parts of the plants. It is well recognized that plants employ a detoxifying antioxidative system consisting of various antioxidant enzymes, such as APX, CAT, and POD, to maintain an optimal level of ROS. The activity of these enzymes can vary depending on factors such as metal concentration, exposure duration, metal ion, and plant species (Sharma et al. 2019; Raza et al. 2021; Hasanuzzaman et al. 2021).
Inoculated plants retained high levels of biomass, chlorophyll, and carotenoid content. Consequently, the decrease in antioxidant enzyme activities may be linked to the action of tolerant PGPR Rhodococcus in the rhizosphere in relation to heavy metals, as documented in a study by Li et al. (2022b). In recent studies, the importance of interactions between plants and tolerant PGPR in heavy metal-contaminated soils has been emphasized, as they can expedite phytoremediation and safeguard plants against the detrimental effects of metals (Deb et al. 2020; Yan et al. 2020; Zainab et al. 2020; Zainab et al. 2021; Oladoye et al. 2022).
In a study by Wang et al. (2022c), it was demonstrated that phytoextraction of cadmium (Cd), zinc (Zn), and lead (Pb) using Vetiveria zizanioides, Brassica juncea, Lolium perenne, Solanum nigrum, and Sedum alfredii was significantly enhanced by inoculation with ABA-catabolizing Rhodococcus qingshengii. This strain notably improved the biomass of the aforementioned hyperaccumulators, thus markedly enhancing the capacity for remediation of Cd, Zn, Pb, and copper (Cu). In another study conducted by Kathi (2022), the phytoextraction of heavy metals and crude oil contaminants from the soil was assessed using the native grass Cynodon dactylon. The results indicated that crude oil contamination caused a reduction in plant biomass across all treatments. The degradation order of metals was found to be Pb, Zn, Cu, and Cd. Higher percentages of degradation of heavy metals and crude oil were observed in Rhodococcus ruber-inoculated treatments compared to other treatments, attributed to the effectiveness of the combination of R. ruber and C. dactylon in soil.
Lu et al. (2020) also confirmed that R. qingshengii improved the phytoextraction efficacy of Cd, Zn, and nickel (Ni). Du et al. (2022) demonstrated that Rhodococcus qingshengii facilitates the phytoextraction of Zn, Cd, Ni, and Pb from soils by Sedum alfredii Hance. Two Rhodococcus erythropolis strains (S4 and S10) isolated from the rhizosphere of Mesembryanthemum crystallinum treated with 10 mM Cd confirmed their positive impact on plant growth and biomass yield, mainly through phosphate and zinc solubilization (Supel et al. 2022). All these studies collectively demonstrate the ability of the genus Rhodococcus to remediate heavy metal-contaminated soils.
Same, our study demonstrates that inoculating with a locally selected Pb-tolerant PGPR Rhodococcus strain promotes the growth of Sulla plants in highly polluted soil, suggesting it as a promising alternative for soil remediation. The inoculation of Sulla plants with this Rhodococcus strain counterbalanced the adverse impacts of lead stress and led to an increase in physiological parameters, including chlorophyll content. These findings align with previous research, which demonstrated that PGPB inoculation promotes enhanced chlorophyll production and accelerates plant growth (Oubohssaine et al. 2022b).
Conclusions
Our study presents compelling evidence of the efficacy of utilizing a multi PGP traits Rhodococcus strain, sourced from a metal-polluted habitat, as a bio-inoculant for enhancing the growth of Sulla spinosissima in heavy metal-contaminated soils. By demonstrating significant improvements in various growth parameters, including biomass production, photosynthetic pigment levels, and antioxidant enzyme activity, our findings underscore the potential of such microbial interventions in facilitating phytoremediation processes. Moreover, our results emphasize the importance of selecting indigenous microbial strains that exhibit resilience to heavy metal stress and possess diverse phytobeneficial properties. This approach not only enhances the efficacy of bio-inoculants but also ensures their compatibility with local environmental conditions, thereby promoting sustainable remediation practices. Furthermore, our study contributes to the growing body of knowledge on the application of plant growth-promoting rhizobacteria (PGPR) in phytoremediation. By highlighting the unique capabilities of PGPR in mitigating heavy metal toxicity and improving plant health, we advocate for the integration of microbial-based strategies into broader remediation initiatives. Importantly, our research underscores the novelty and significance of these findings within the context of environmental science and biotechnology. By elucidating the practical benefits of PGPR inoculation in heavy metal-contaminated soils, we offer valuable insights that can inform future research and guide the development of innovative remediation technologies.
In conclusion, our study not only advances our understanding of microbial-assisted phytoremediation but also underscores the critical role of indigenous microbial communities in sustainable environmental management. By harnessing the potential of microbial bio-inoculants, we can pave the way for more effective and environmentally friendly approaches to soil remediation and ecosystem restoration.
Data availability
Authors confirm that all relevant data are included in the article. Materials are available from the corresponding author upon reasonable request.
References
Adeleke BS, Fadiji AE, Ayilara MS, Igiehon ON, Nwachukwu BC, Babalola OO (2022) Strategies to enhance the use of endophytes as bioinoculants in agriculture. Horticulturae 8:498. https://doi.org/10.3390/horticulturae8060498
Adetunji CO, Anani OA, Thangadurai D, Islam S (2022) Application of phosphate solubilizing microorganisms for effective production of next-generation biofertilizer: a panacea for sustainable organic agriculture, in: organic farming for sustainable development. Apple Academic Press
Adhami E, Aghaei S, Zolfaghari MR (2017) Evaluation of heavy metals resistance in biofilm cells of native Rhodococcus spp. isolated from soil. Arch Hyg Sci 6:235–243. https://doi.org/10.29252/ArchHygSci.6.3.235
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad A, Moin SF, Liaqat I, Saleem S, Muhammad F, Mujahid T, Zafar U (2022) Isolation, solubilization of inorganic phosphate, and production of organic acids by individual and co-inoculated microorganisms. Geomicrobiol J 0:1–11. https://doi.org/10.1080/01490451.2022.2124329
Ajmal AW, Yasmin H, Hassan MN, Khan N, Jan BL, Mumtaz S (2022) Heavy metal–resistant plant growth–promoting Citrobacter werkmanii strain WWN1 and Enterobacter cloacae strain JWM6 enhance wheat (Triticum aestivum L.) growth by modulating physiological attributes and some key Aantioxidants under multi-metal stress. Front Microbiol 13. https://doi.org/10.3389/fmicb.2022.815704 eCollection 2022
Aljerf L (2018) A gateway to metal resistance: bacterial response to heavy metal toxicity in the biological environment. Ann Adv Chem:032–044. https://doi.org/10.29328/journal.aac.1001012
Annamalai J, Ummalyma SB, Pandey A (2022) Chapter 5 - recent trends in the microbial degradation and bioremediation of emerging pollutants in wastewater treatment system. In: Shah MP, Rodriguez-Couto S, Kapoor RT (eds) Development in wastewater treatment research and processes. Elsevier, pp 99–125. https://doi.org/10.1016/B978-0-323-85657-7.00012-2
Aroney STN, Poole PS, Sánchez-Cañizares C (2021) Rhizobial chemotaxis and motility systems at work in the soil. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.725338
Barman D, Jha DK (2021) Metallotolerant microorganisms and microbe-assisted phytoremediation for a sustainable clean environment. In: Singh RP, Manchanda G, Bhattacharjee K, Panosyan H (eds) Microbes in microbial communities: Ecological and applied perspectives. Springer, Singapore, pp 307–336. https://doi.org/10.1007/978-981-16-5617-0_15
Bashan Y, Holguin G (1994) Root-to-root travel of the beneficial bacterium Azospirillum brasilense. Appl Environ Microbiol 60:2120–2131. https://doi.org/10.1128/aem.60.6.2120-2131.1994
Batool A, Akram NA, Cheng ZG, Lv GC, Ashraf M, Afzal M, Xiong JL, Wang JY, Xiong YC (2019) Physiological and biochemical responses of two spring wheat genotypes to non-hydraulic root-to-shoot signalling of partial and full root-zone drought stress. Plant Physiol Biochem 139:11–20. https://doi.org/10.1016/j.plaphy.2019.03.001
Behera HT, Mojumdar A, Ray L (2022) Chapter 9 - Biology, genetic aspects and oxidative stress response of actinobacteria and strategies for bioremediation of toxic metals. In: Das S, Dash HR (eds) Microbial biodegradation and bioremediation (Second Edition). Elsevier, pp 181–192. https://doi.org/10.1016/B978-0-323-85455-9.00004-7
BeMiller JN (2019) Chapter 4 - Polysaccharides: occurrence, structures, and chemistry. In: JN BM (ed) Carbohydrate Chemistry for Food Scientists (Third Edition). AACC International Press, pp 75–101. https://doi.org/10.1016/B978-0-12-812069-9.00004-2
Berni R, Luyckx M, Xu X, Legay S, Sergeant K, Hausman JF, Lutts S, Cai G, Guerriero G (2019) Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ Exp Bot 161:98–106. https://doi.org/10.1016/j.envexpbot.2018.10.017
Bhagat N, Raghav M, Dubey S, Bedi N (2021) Bacterial exopolysaccharides: insight into their role in plant abiotic stress tolerance 31: 1045–1059. https://doi.org/10.4014/jmb.2105.05009
Bhardwaj S, Kaushal R, Jhilta P, Rana A, Dipta B (2022) Phosphate solubilizing microorganisms: potential bioinoculants for sustainable agriculture. In: Prasad R, Zhang SH (eds) Beneficial Microorganisms in Agriculture, Environmental and Microbial Biotechnology. Springer Nature, Singapore, pp 131–159. https://doi.org/10.1007/978-981-19-0733-3_5
Biswal T (2022) Phytoremediation of soils contaminated with heavy metals: techniques and strategies. In: Malik JA (ed) Advances in bioremediation and phytoremediation for sustainable soil management: principles, monitoring and remediation. Springer International Publishing, Cham, pp 31–55. https://doi.org/10.1007/978-3-030-89984-4_3
Borah A, Das R, Mazumdar R, Thakur D (2019) Culturable endophytic bacteria of Camellia species endowed with plant growth promoting characteristics. J Appl Microbiol 127:825–844. https://doi.org/10.1111/jam.14356
Cappelletti M, Zampolli J, Di Gennaro P, Zannoni D (2019) Genomics of Rhodococcus. In: Alvarez HM (ed) Biology of Rhodococcus. Springer International Publishing, Cham, pp 23–60. https://doi.org/10.1007/978-3-030-11461-9_2
Cappelletti M, Presentato A, Piacenza E, Firrincieli A, Turner RJ, Zannoni D (2020) Biotechnology of Rhodococcus for the production of valuable compounds. Appl Microbiol Biotechnol 104:8567–8594. https://doi.org/10.1007/s00253-020-10861-z
Cappuccino JC, Sherman N (1992) Microbiology: a laboratory manual, 3rd edn. Benjamin/Cummings Pub. Co., New York, pp 125–179
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chatterjee S, Kumari S, Rath S, Das S (2022) Chapter 1 - Prospects and scope of microbial bioremediation for the restoration of the contaminated sites. In: Das S, Dash HR (eds) Microbial Biodegradation and Bioremediation (Second Edition). Elsevier, pp 3–31. https://doi.org/10.1016/B978-0-323-85455-9.00011-4
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:897–998
Chettri D, Sharma B, Verma AK (2022) Chapter 12 - Advancement in microbial bioremediation. In: Rodriguez-Couto S, Shah MP (eds) Development in Wastewater Treatment Research and Processes. Elsevier, pp 243–262. https://doi.org/10.1016/B978-0-323-85839-7.00001-3
Dalyan E, Yüzbaşıoğlu E, Akpınar I (2020) Physiological and biochemical changes in plant growth and different plant enzymes in response to lead stress. In: Gupta DK, Chatterjee S, Walther C (eds) Lead in plants and the environment, radionuclides and heavy metals in the environment. Springer International Publishing, Cham, pp 129–147. https://doi.org/10.1007/978-3-030-21638-2_8
Das PP, Singh KR, Nagpure G, Mansoori A, Singh RP, Ghazi IA, Kumar A, Singh J (2022) Plant-soil-microbes:a tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ Res 214:113821. https://doi.org/10.1016/j.envres.2022.113821
De Carvalho CCCR (2012) Adaptation of Rhodococcus erythropolis cells for growth and bioremediation under extreme conditions. Res Microbiol 163:125–136. https://doi.org/10.1016/j.resmic.2011.11.003
De Carvalho CCCR, da Fonseca MMR (2005) The remarkable Rhodococcus erythropolis. Appl Microbiol Biotechnol 67:715–726. https://doi.org/10.1007/s00253-005-1932-3
de Freitas Duarte N, Oliveira Paiva CA, Pagano MC, Correa EJA (2022) Chapter 15 - phosphate solubilization by microorganisms. In: Singh H, Vaishnav A (eds) New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier, pp 257–282. https://doi.org/10.1016/B978-0-323-85163-3.00019-3
Deb VK, Rabbani A, Upadhyay S, Bharti P, Sharma H, Rawat DS, Saxena G (2020) Microbe-assisted phytoremediation in reinstating heavy metal-contaminated sites: concepts, mechanisms, challenges, and future perspectives. In: Arora PK (ed) Microbial technology for health and environment, microorganisms for sustainability. Springer, Singapore, pp 161–189. https://doi.org/10.1007/978-981-15-2679-4_6
Devi R, Kaur T, Kour D, Hricovec M, Mohan R, Yadav N, Rai PK, Rai AK, Yadav A, Kumar M, Yadav AN (2022) Microbes-mediated alleviation of heavy metal stress in crops: current research and future challenges. J App Biol Biotech:25–37. https://doi.org/10.7324/JABB.2022.10s203
Donini E, Firrincieli A, Cappelletti M (2021) Systems biology and metabolic engineering of Rhodococcus for bioconversion and biosynthesis processes. Folia Microbiol 66:701–713. https://doi.org/10.1007/s12223-021-00892-y
Du S, Lu Q, Liu L, Wang Y, Li J (2022) Rhodococcus qingshengii facilitates the phytoextraction of Zn, Cd, Ni, and Pb from soils by Sedum alfredii Hance. J Hazard Mater 424:127638. https://doi.org/10.1016/j.jhazmat.2021.127638
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. ACS Publications. https://doi.org/10.1021/ac60111a017
El Aafi N, Saidi N, Maltouf AF, Perez-Palacios P, Dary M, Brhada F, Pajuelo E (2015) Prospecting metal-tolerant rhizobia for phytoremediation of mining soils from Morocco using Anthyllis vulneraria L. Environ Sci Pollut Res Int 22:4500–4512. https://doi.org/10.1007/s11356-014-3596-y
Farda B, Djebaili R, Vaccarelli I, Del Gallo M, Pellegrini M (2022) Actinomycetes from caves: an overview of their diversity, biotechnological properties, and insights for their use in soil environments. Microorganisms 10:453. https://doi.org/10.3390/microorganisms10020453
Flores-Treviño S, Gutiérrez-Ferman JL, Morfín-Otero R, Rodríguez-Noriega E, Estrada-Rivadeneyra D, Rivas-Morales C, Llaca-Díaz JM, Camacho-Ortíz A, Mendoza-Olazarán S, Garza-González E (2014) Stenotrophomonas maltophilia in Mexico: antimicrobial resistance, biofilm formation and clonal diversity. J Med Microbiol 63:1524–1530. https://doi.org/10.1099/jmm.0.074385-0
García-Caparrós P, De Filippis L, Gul A, Hasanuzzaman M, Ozturk M, Altay V, Lao MT (2021) Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot Rev 87:421–466. https://doi.org/10.1007/s12229-020-09231-1
Ghosh PK, De TK, Maiti TK (2018) Role of ACC deaminase as a stress ameliorating enzyme of plant growth-promoting rhizobacteria useful in stress agriculture: a review. In: Meena VS (ed) Role of rhizospheric microbes in soil, Stress management and agricultural sustainability, vol 1. Springer, Singapore, pp 57–106. https://doi.org/10.1007/978-981-10-8402-7_3
Gikas P, Sengor SS, Ginn T, Moberly J, Peyton B (2009) The effects of heavy metals and temperature on microbial growth and lag. Glob NEST J 11(3):325–332
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339
González Henao S, Ghneim-Herrera T (2021) Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Front Environ Sci:9. https://doi.org/10.3389/fenvs.2021.604216
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195
Govindasamy V, George P, Ramesh SV, Sureshkumar P, Rane J, Minhas PS (2022) Characterization of root-endophytic actinobacteria from cactus (Opuntia ficus-indica) for plant growth promoting traits. Arch Microbiol 204:150. https://doi.org/10.1007/s00203-021-02671-2
Guevara G, Castillo Lopez M, Alonso S, Perera J, Navarro-Llorens JM (2019) New insights into the genome of Rhodococcus ruber strain Chol-4. BMC Genomics 20:332. https://doi.org/10.1186/s12864-019-5677-2
Gupta P, Diwan B (2016) Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep (Amst) 13:58–71. https://doi.org/10.1016/j.btre.2016.12.006
Gurpreet S, Rajneesh KA, Rajendra SR, Mushtaq A (2012) Effect of lead and nickel toxicity on chlorophyll and proline content of Urd (Vigna mungo L.) seedlings. Int J Plant Physiol Biochem 4:136–141. https://doi.org/10.5897/IJPPB12.005
Haldar S, Mondal S, Kumari A, Ghosh A, Chattopadhyay D, Ghosh A (2022) Chapter 16 - Rhizosphere microbiome engineering. In: Joshi S, Pandey A, Sirohi R, Park SH (eds) Current developments in biotechnology and bioengineering. Elsevier, pp 377–396. https://doi.org/10.1016/B978-0-323-88504-1.00014-5
Hasanuzzaman M, Parvin K, Bardhan K, Nahar K, Anee TI, Masud AAC, Fotopoulos V (2021) Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 10:2537. https://doi.org/10.3390/cells10102537
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion : a review. Ann Microbiol 60:579–598. https://doi.org/10.1007/s13213-010-0117-1
He H, Wu M, Su R, Zhang Z, Chang C, Peng Q, Dong Z, Pang J, Lambers H (2021) Strong phosphorus (P)-zinc (Zn) interactions in a calcareous soil-alfalfa system suggest that rational P fertilization should be considered for Zn biofortification on Zn-deficient soils and phytoremediation of Zn-contaminated soils. Plant Soil 461:119–134. https://doi.org/10.1007/s11104-020-04793-w
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil; Circular 347. California Agricultural Experiment Station, Berkeley, CA, USA
Homero U, Tortella G, Sandoval E, Cuozzo SA (2021) Extracellular polymeric substances (EPS) produced by Streptomyces sp. biofilms: chemical composition and anticancer properties. Microbiol Res 253:126877. https://doi.org/10.1016/j.micres.2021.126877
Imran A, Hakim S, Tariq M, Nawaz MS, Laraib I, Gulzar U, Hanif MK, Siddique MJ, Hayat M, Fraz A, Ahmad M (2021) Diazotrophs for lowering nitrogen pollution crises: looking deep into the roots. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.637815
Jinal HN, Gopi K, Prittesh P, Kartik VP, Amaresan N (2019) Phytoextraction of iron from contaminated soils by inoculation of iron-tolerant plant growth-promoting bacteria in Brassica juncea L. Czern. Environ Sci Pollut Res 26:32815–32823. https://doi.org/10.1007/s11356-019-06394-2
Kathi S (2022) Phytoremediation of heavy metals and petroleum hydrocarbons using Cynodon dactylon (L.) Pers. In: Kathi S, Devipriya S, Thamaraiselvi K (eds) Cost effective technologies for solid waste and wastewater treatment, Advances in environmental pollution research. Elsevier, pp 135–145. https://doi.org/10.1016/B978-0-12-822933-0.00005-X
Kaur H, Goyal N (2022) Chapter3 - Biochemical adaptations in plants under heavy metal stress: a revisit to antioxidant defense network. In: Aftab T, Hakeem K (eds) Metals metalloids soil plant water systems. Academic Press, pp 51–90. https://doi.org/10.1016/B978-0-323-91675-2.00001-9
Kaur T, Devi R, Kour D, Yadav A, Yadav AN, Dikilitas M, Abdel-Azeem AM, Ahluwalia AS, Saxena AK (2021) Plant growth promoting soil microbiomes and their potential implications for agricultural and environmental sustainability. Biologia 76:2687–2709. https://doi.org/10.1007/s11756-021-00806-w
Khanna K, Jamwal VL, Gandhi SG, Ohri P, Bhardwaj R (2019) Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci Rep 9:5855. https://doi.org/10.1038/s41598-019-41899-3
Khoshru B, Mitra D, Khoshmanzar E, Myo EM, Uniyal N, Mahakur B, Mohapatra PKD, Panneerselvam P, Boutaj H, Alizadeh M, Cely MVT, Senapati A, Rani A (2020) Current scenario and future prospects of plant growth-promoting rhizobacteria: an economic valuable resource for the agriculture revival under stressful conditions. J Plant Nutr 43:3062–3092. https://doi.org/10.1080/01904167.2020.1799004
Koza NA, Adedayo AA, Babalola OO, Kappo AP (2022) Microorganisms in plant growth and development: roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms 10:1528. https://doi.org/10.3390/microorganisms10081528
Kumar A, Verma JP (2018) Does plant—microbe interaction confer stress tolerance in plants: a review. Microbiol Res 207:41–52. https://doi.org/10.1016/j.micres.2017.11.004
Lamin H, Alami S, Bouhnik O, Bennis M, Benkritly S, Abdelmoumen H, Bedmar EJ, Idrissi MME (2020) Identification of the endosymbionts from Sulla spinosissima growing in a lead mine tailing in Eastern Morocco as Mesorhizobium camelthorni sv. aridi. J Appl Microbiol 130:948–959
Li Y, Wang H, Hua F, Su M, Zhao Y (2014) Trans-membrane transport of fluoranthene by Rhodococcus sp. BAP-1 and optimization of uptake process. Bioresour Technol 155:213–219. https://doi.org/10.1016/j.biortech.2013.12.117
Li Y, Xin M, Xie D, Fan S, Ma J, Liu K, Yu F (2021a) Variation in extracellular polymeric substances from Enterobacter sp. and their Pb2+ adsorption behaviors. ACS Omega 6:9617–9628. https://doi.org/10.1021/acsomega.1c00185
Li W, Liu M, Siddique MS, Graham N, Yu W (2021b) Contribution of bacterial extracellular polymeric substances (EPS) in surface water purification. Environ Pollut 280:116998. https://doi.org/10.1016/j.envpol.2021.116998
Li Y, Mo L, Zhou X, Yao Y, Ma J, Liu K, Yu F (2022a) Characterization of plant growth-promoting traits of Enterobacter sp. and its ability to promote cadmium/lead accumulation in Centella asiatica L. Environ Sci Pollut Res 29:4101–4115. https://doi.org/10.1007/s11356-021-15948-2
Li Q, Xing Y, Huang B, Chen X, Ji L, Fu X, Li T, Wang J, Chen G, Zhang Q (2022b) Rhizospheric mechanisms of Bacillus subtilis bioaugmentation-assisted phytostabilization of cadmium-contaminated soil. Sci Total Environ 825:154136. https://doi.org/10.1016/j.scitotenv.2022.154136
Lopez AMQ, Silva ALDS, Maranhão FCDA, Ferreira LFR (2022) Plant growth promoting bacteria: aspects in metal bioremediation and phytopathogen Management. In: Kumar A (ed) Microbial Biocontrol: Sustainable Agriculture and management: Volume 1. Springer International Publishing, Cham, pp 51–78. https://doi.org/10.1007/978-3-030-87512-1_3
Lu Q, Weng Y, You Y, Xu Q, Li H, Li Y, Liu H, Du S (2020) Inoculation with abscisic acid (ABA)-catabolizing bacteria can improve phytoextraction of heavy metal in contaminated soil. Environ Pollut 257:113497. https://doi.org/10.1016/j.envpol.2019.113497
Lucero CT, Lorda GS, Ludueña LM, Anzuay MS, Taurian T (2020) Motility and biofilm production involved in the interaction of phosphate solubilizing endophytic strains with peanut, maize and soybean plants. Rhizosphere 15:100228. https://doi.org/10.1016/j.rhisph.2020.100228
Mackinney Q (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Malas AA (2021) Encapsulation of bacteriophages to improve effective delivery to the intracellular bacterial pathogens (thesis). University of Leicester. https://doi.org/10.25392/leicester.data.17105639.v1
Manjanatha MG, Loynachan TE, Atherly AG (1992) Tn5 mutagenesis of Chinese Rhizobium fredii for siderophore overproduction. Soil Biol Biochem 24:151–155
Mawang CI, Azman AS, Fuad ASM, Ahamad M (2021) Actinobacteria: an eco-friendly and promising technology for the bioaugmentation of contaminants. Biotechnol Rep 32:e00679. https://doi.org/10.1016/j.btre.2021.e00679
Meena V, Dotaniya ML, Saha JK, Das H, Patra AK (2020) Impact of lead contamination on agroecosystem and human health. In: Gupta DK, Chatterjee S, Walther C (eds) Lead in Plants and the Environment. Springer International Publishing, Cham, pp 67–82. https://doi.org/10.1007/978-3-030-21638-2_4
Mitra A, Chatterjee S, Kataki S, Rastogi RP, Gupta DK (2021) Bacterial tolerance strategies against lead toxicity and their relevance in bioremediation application. Environ Sci Pollut Res 28:14271–14284. https://doi.org/10.1007/s11356-021-12583-9
Modi M, Shah KS, Modi VV (1985) Isolation and characterization of catechol-like siderophore from cowpea Rhizobium RA-1. Arch Microbiol 141:156–158
Mousavi SM, Brodie G, Payghamzadeh K, Raiesi T, Kumar A (2022) Lead (Pb) bioavailability in the environment, its exposure and effect. JAEHR:10. https://doi.org/10.32598/JAEHR.10.1.1256
Najm-ul-Seher Ahmad M, Ahmad I, Nazli F, Mumtaz MZ, Latif M, Al-Mosallam MS, Alotaibi FS, Dewidar AZ, Mattar MA, El-Shafei AA (2021) Lead-tolerant Bacillus strains promote growth and antioxidant activities of spinach (Spinacia oleracea) treated with sewage water. Agronomy 11:2482. https://doi.org/10.3390/agronomy11122482
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Navazas A, Mesa V, Thijs S, Fuente-Maqueda F, Vangronsveld J, Peláez AI, Cuypers A, González A (2022) Bacterial inoculant-assisted phytoremediation affects trace element uptake and metabolite content in Salix atrocinerea. Sci Total Environ 820:153088. https://doi.org/10.1016/j.scitotenv.2022.153088
Nazli F, Mustafa A, Ahmad M, Hussain A, Jamil M, Wang X, Shakeel Q, Imtiaz M, El-Esawi MA (2020a) A review on practical application and potentials of phytohormone-producing plant growth-promoting rhizobacteria for inducing heavy metal tolerance in crops. Sustainability 12:9056. https://doi.org/10.3390/su12219056
Nazli F, Jamil M, Hussain A, Hussain T (2020b) Exopolysaccharides and indole-3-acetic acid producing Bacillus safensis strain FN13 potential candidate for phytostabilization of heavy metals. Environ Monit Assess 192:1–16. https://doi.org/10.1007/s10661-020-08715-2
Noor I, Sohail H, Sun J, Nawaz MA, Li G, Hasanuzzaman M, Liu J (2022) Heavy metal and metalloid toxicity in horticultural plants: tolerance mechanism and remediation strategies. Chemosphere 303:135196. https://doi.org/10.1016/j.chemosphere.2022.135196
Nzila A, Razzak SA, Zhu J (2016) Bioaugmentation: an emerging strategy of industrial wastewater treatment for reuse and discharge. IJERPH 13:846. https://doi.org/10.3390/ijerph13090846
Ogundola AF, Adebayo EA, Ajao SO (2022) 2 - Phytoremediation: the ultimate technique for reinstating soil contaminated with heavy metals and other pollutants. In: Kumar V, Shah MP, Shahi SK (eds) Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water. Elsevier, pp 19–49. https://doi.org/10.1016/B978-0-323-85763-5.00012-X
Oladoye PO, Olowe OM, Asemoloye MD (2022) Phytoremediation technology and food security impacts of heavy metal contaminated soils: a review of literature. Chemosphere 288:132555. https://doi.org/10.1016/j.chemosphere.2021.132555
O'Toole GA (2011) Microtiter dish biofilm formation assay. J Vis Exp 47:2437
Oubohssaine M, Dahmani I, Sbabou L, Bruneel O, Aurag J (2022a) The rhizosphere of Sulla spinosissima growing in abandoned mining soils is a reservoir of heavy metals tolerant plant growth-promoting rhizobacteria. ISBAB 39:102236. https://doi.org/10.1016/j.bcab.2021.102236
Oubohssaine M, Sbabou L, Aurag J (2022b) Native heavy metal-tolerant plant growth promoting rhizobacteria improves Sulla spinosissima (L.) growth in post-mining contaminated soils. Microorganisms 10:838. https://doi.org/10.3390/microorganisms10050838
Pande V, Pandey SC, Sati D, Bhatt P, Samant M (2022) Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front Microbiol 13:824084. https://doi.org/10.3389/fmicb.2022.824084
Pandey AK, Zorić L, Sun T, Karanović D, Fang P, Borišev M, Wu X, Luković J, Xu P (2022) The anatomical basis of heavy metal responses in legumes and their impact on plant–rhizosphere interactions. Plants 11:2554. https://doi.org/10.3390/plants11192554
Pátek M, Grulich M, Nešvera J (2021) Stress response in Rhodococcus strains. Biotechnol Adv 53:107698. https://doi.org/10.1016/j.biotechadv.2021.107698
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801. https://doi.org/10.1128/AEM.68.8.3795-3801.2002
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya 17:362–370
Podar D, Maathuis FJM (2022) The role of roots and rhizosphere in providing tolerance to toxic metals and metalloids. Plant Cell Environ 45:719–736. https://doi.org/10.1111/pce.14188
Raimondo EE, Bigliardo AL, González SK, Saez JM, Polti MA, Benimeli CS (2022) Bioaugmentation of biomixtures with consortia of actinobacteria and fungi for improving pesticides removal. In: Udayanga D, Bhatt P, Manamgoda D, Maria SJ (eds) Mycoremediation protocols, Springer Protocols Handbooks. Springer US, New York, NY, pp 221–235. https://doi.org/10.1007/978-1-0716-2006-9_19
Raza A, Hussain S, Javed R, Hafeez MB, Hasanuzzaman M (2021) Antioxidant defense systems and remediation of metal toxicity in plants. In: Hasanuzzaman M (ed) Approaches to the Remediation of Inorganic Pollutants. Springer, Singapore, pp 91–124. https://doi.org/10.1007/978-981-15-6221-1_6
Rivas L, Dykes GA, Fegan N (2007) A comparative study of biofilm formation by Shiga toxigenic Escherichia coli using epifluorescence microscopy on stainless steel and a microtitre plate method. J Microbiol Methods 69:44–51. https://doi.org/10.1016/j.mimet.2006.11.014
Robas Mora M, Jiménez Gómez PA, González Reguero D, Probanza Lobo A (2022) Effect of plant growth-promoting bacteria on biometrical parameters and antioxidant enzymatic activities of Lupinus albus var. Orden Dorado under mercury stress. Front Microbiol 13. https://doi.org/10.3389/fmicb.2022.891882
Rodrigues CJC, de Carvalho CCCR (2015) Rhodococcus erythropolis cells adapt their fatty acid composition during biofilm formation on metallic and non-metallic surfaces. FEMS Microbiol Ecol 91:135. https://doi.org/10.1093/femsec/fiv135
Saeed Q, Xiukang W, Haider FU, Kučerik J, Mumtaz MZ, Holatko J, Naseem M, Kintl A, Ejaz M, Naveed M, Brtnicky M, Mustafa A (2021) Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: a comprehensive review of effects and mechanisms. Int J Mol Sci 22:10529. https://doi.org/10.3390/ijms221910529
Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P (2016) Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res 23:3984–3999. https://doi.org/10.1007/s11356-015-4294-0
Saha L, Tiwari J, Bauddh K, Ma Y (2021) Recent developments in microbe–plant-based bioremediation for tackling heavy metal-polluted soils. Front Microbiol 12:731723. https://doi.org/10.3389/fmicb.2021.731723
Sahal G, Woerdenbag HJ, Hinrichs WLJ, Visser A, Tepper PG, Quax WJ, van der Mei HC, Bilkay IS (2020) Antifungal and biofilm inhibitory effect of Cymbopogon citratus (lemongrass) essential oil on biofilm forming by Candida tropicalis isolates; an in vitro study. J Ethnopharmacol 246:112188. https://doi.org/10.1016/j.jep.2019.112188
Santoyo G, Urtis-Flores CA, Loeza-Lara PD, Orozco-Mosqueda MD, Glick BR (2021) Rhizosphere colonization determinants by plant growth-promoting Rhizobacteria (PGPR). Biology 10:475. https://doi.org/10.3390/biology10060475
Sbabou L, Idir Y, Bruneel O, Quere AL, Aurag J, Bena G, Filali-Maltouf A (2016) Characterization of root-nodule bacteria isolated from Hedysarum spinosissimum L, growing in mining sites of Northeastern region of Morocco. SOJ Microbiol Infect Dis 4:1–8
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E (2014) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. In: Whitacre DM (ed) Reviews of environmental contamination and toxicology, Reviews of environmental contamination and toxicology, vol 232. Springer International Publishing, Cham, pp 1–44. https://doi.org/10.1007/978-3-319-06746-9_1
Sharma P, Jha AB, Dubey RS (2019) Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In: Handbook of Plant and Crop Stress, Fourth edn. CRC Press
Sharma A, Kapoor D, Gautam S, Landi M, Kandhol N, Araniti F, Ramakrishnan M, Satish L, Singh VP, Sharma P, Bhardwaj R, Tripathi DK, Zheng B (2022a) Heavy metal induced regulation of plant biology: recent insights. Physiol Plant 174:e13688. https://doi.org/10.1111/ppl.13688
Sharma P, Chaturvedi P, Chandra R, Kumar S (2022b) Identification of heavy metals tolerant Brevundimonas sp. from rhizospheric zone of Saccharum munja L. and their efficacy in in-situ phytoremediation. Chemosphere 295:133823. https://doi.org/10.1016/j.chemosphere.2022.133823
Sheng X, He L, Wang Q, Ye H, Jiang C (2008) Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil. J Hazard Mater 155:17–22
Shukla SK, Rao TS (2013) Effect of calcium on Staphylococcus aureus biofilm architecture: a confocal laser scanning microscopic study. Colloids Surf B 103:448
Shukla SK, Rao TS (2014) Calcium-mediated modulation of Staphylococcal bacterial biofilms. Indian J Geomarine Sci 43:2107
Singh P, Singh RK, Zhou Y, Wang J, Jiang Y, Shen N, Wang Y, Yang L, Jiang M (2022) Unlocking the strength of plant growth promoting Pseudomonas in improving crop productivity in normal and challenging environments: a review. J Plant Interact 17:220–238. https://doi.org/10.1080/17429145.2022.2029963
Singhal RK, Kumar M, Bose B, Mondal S, Srivastava S, Dhankher OP, Tripathi RD (2022) Heavy metal (loid)s phytotoxicity in crops and its mitigation through seed priming technology. Int J Phytoremediation 0:1–20. https://doi.org/10.1080/15226514.2022.2068502
Sinha S, Mukherjee SK (2008) Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56:55–60. https://doi.org/10.1007/s00284-007-9038-z
Srivastava N, Chattopadhyay J, Yashi A, Rathore T (2022) Heavy metals removal techniques from industrial wastewater. In: Roy S, Garg A, Garg S, Tran TA (eds) Advanced industrial wastewater treatment and reclamation of water: comparative study of water pollution during pre-industrial, Industrial period and prospect of wastewater treatment for water resource conservation. Springer International Publishing, Cham, pp 87–101. https://doi.org/10.1007/978-3-030-83811-9_5
Supel P, Śliwa-Cebula M, Miszalski Z, Kaszycki P (2022) Cadmium-tolerant rhizospheric bacteria of the C3/CAM intermediate semi-halophytic common ice plant (Mesembryanthemum crystallinum L.) grown in contaminated soils. Front Plant Sci:13. https://doi.org/10.3389/fpls.2022.820097
Syed A, Zeyad MT, Shahid M, Elgorban AM, Alkhulaifi MM, Ansari IA (2021) Heavy metals induced modulations in growth, physiology, cellular viability, and biofilm formation of an identified bacterial isolate. ACS Omega 6:25076–25088. https://doi.org/10.1021/acsomega.1c04396
Turnbull L, Whitchurch CB (2014) Motility assay: twitching motility. In: Filloux A, Ramos JL (eds) Pseudomonas methods and protocols, methods in molecular biology. Springer, New York, NY, pp 73–86. https://doi.org/10.1007/978-1-4939-0473-0_9
Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C (2013) Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 4:356. https://doi.org/10.3389/fpls.2013.00356
Vergani L, Mapelli F, Suman J, Cajthaml T, Uhlik O, Borin S (2019) Novel PCB-degrading Rhodococcus strains able to promote plant growth for assisted rhizoremediation of historically polluted soils. PLoS One 14:e0221253. https://doi.org/10.1371/journal.pone.0221253
Wang H, Hu J, Xu K, Tang X, Xu X, Shen C (2018) Biodegradation and chemotaxis of polychlorinated biphenyls, biphenyls, and their metabolites by Rhodococcus spp. Biodegradation 29:1–10. https://doi.org/10.1007/s10532-017-9809-6
Wang L, Lin H, Dong Y, Li B, He Y (2020) Effects of endophytes inoculation on rhizosphere and endosphere microecology of Indian mustard (Brassica juncea) grown in vanadium-contaminated soil and its enhancement on phytoremediation. Chemosphere 240:124891. https://doi.org/10.1016/j.chemosphere.2019.124891
Wang Y, Huang W, Ali SW, Li Y, Yu F, Deng H (2022a) Isolation, identification, and characterization of an efficient siderophore producing bacterium from heavy metal contaminated soil. Curr Microbiol 79:227. https://doi.org/10.1007/s00284-022-02922-5
Wang Y, Narayanan M, Shi X, Chen X, Li Z, Natarajan D, Ma Y (2022b) Plant growth-promoting bacteria in metal-contaminated soil: current perspectives on remediation mechanisms. Front Microbiol:13. https://doi.org/10.3389/fmicb.2022.966226
Wang Y, Li Z, Wu J, Liu H, Sun X, Liu L, Du S (2022c) Abscisic acid-catabolizing bacteria: a useful tool for enhancing phytoremediation. Sci Total Environ 812:151474. https://doi.org/10.1016/j.scitotenv.2021.151474
Wevar Oller AL, Talano MA, Agostini E (2013) Screening of plant growth-promoting traits in arsenic-resistant bacteria isolated from the rhizosphere of soybean plants from Argentinean agricultural soil. Plant Soil 369:93–102. https://doi.org/10.1007/s11104-012-1543-6
Williams TC (2011) Chemotaxis: types, clinical significance, and mathematical models, cell biology research progress. Nova Science Publishers, New York
Yaashikaa PR, Kumar PS, Jeevanantham S, Saravanan R (2022) A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ Pollut 301:119035. https://doi.org/10.1016/j.envpol.2022.119035
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci:11. https://doi.org/10.3389/fpls.2020.00359
Yang Y, Huang B, Xu J, Li Z, Tang Z, Wu X (2022) Heavy metal domestication enhances beneficial effects of arbuscular mycorrhizal fungi on lead (Pb) phytoremediation efficiency of Bidens parviflora through improving plant growth and root Pb accumulation. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-18588-2
Zainab N, Amna Din BU, Javed MT, Afridi MS, Mukhtar T, Kamran MA, Khan AA, Ali J, Jatoi WN, Munis MF, Chaudhary HJ (2020) Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol Biochem 152:90–99. https://doi.org/10.1016/j.plaphy.2020.04.039
Zainab N, Amna Khan AA, Azeem MA, Ali B, Wang T, Shi F, Alghanem SM, Hussain Munis MF, Hashem M, Alamri S, Abdel Latef AAH, Ali OM, Soliman MH, Chaudhary HJ (2021) PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. in industrially contaminated soils. Agronomy 11:1820. https://doi.org/10.3390/agronomy11091820
Zandi P, Schnug E (2022) Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology 11:155. https://doi.org/10.3390/biology11020155
Zhang D, Wang J, Pan X (2006) Cadmium sorption by EPSs produced by anaerobic sludge under sulfate-reducing conditions. J Hazard Mater 138:589–593. https://doi.org/10.1016/j.jhazmat.2006.05.092
Zulfiqar U, Farooq M, Hussain S, Maqsood M, Hussain M, Ishfaq M, Ahmad M, Anjum MZ (2019) Lead toxicity in plants: impacts and remediation. J Environ Manag 250:109557. https://doi.org/10.1016/j.jenvman.2019.109557
Acknowledgments
The authors express their gratitude to Mohammed V University in Rabat and IRD-France for their support within the framework of the LMI project (Laboratoire Mixte International) “Biotechnologie Microbienne et Végétale.”
Funding
Partial financial support for this research was provided by Mohammed V University in Rabat and IRD-France through the LMI project (Laboratoire Mixte International) “Biotechnologie Microbienne et Végétale.”
Author information
Authors and Affiliations
Contributions
Investigation, methodology, data curation, writing—original draft, and formal analysis, Malika Oubohssaine; conceptualization, review and editing, and funding acquisition, Laila Sbabou; conceptualization, writing—review and editing, project administration, and funding acquisition, Jamal Aurag. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Manyun Zhang
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oubohssaine, M., Sbabou, L. & Aurag, J. Potential of the plant growth-promoting rhizobacterium Rhodococcus qingshengii LMR356 in mitigating lead stress impact on Sulla spinosissima L.. Environ Sci Pollut Res 31, 46002–46022 (2024). https://doi.org/10.1007/s11356-024-34150-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34150-8