Abstract
Understanding seasonal variations in water quality is crucial for effective management of freshwater rivers amidst changing environmental conditions. This study employed water quality index (WQI), metal index (MI), and pollution indices (PI) to comprehensively assess water quality and pollution levels in Nyabarongo River of Rwanda. A dynamic driver-pressure-state-impact-response model was used to identify factors influencing quality management. Over 4 years (2018–2021), 69 samples were collected on a monthly basis from each of the six monitoring stations across the Nyabarongo River throughout the four different seasons. Maximum WQI values were observed during dry long (52.90), dry short (21.478), long rain (93.66), and short rain (37.4) seasons, classified according to CCME 2001 guidelines. Ion concentrations exceeded WHO standards, with dominant ions being \({\text{HCO}}_{3}^{-}\) and \({\text{Mg}}^{2+}\). Variations in water quality were influenced by factors such as calcium bicarbonate dominance in dry seasons and sodium sulfate dominance in rainy seasons. Evaporation and precipitation processes primarily influenced ionic composition. Metal indices (MI) exhibited wide ranges: long dry (0.2–433.0), short dry (0.1–174.3), long rain (0.1–223.7), and short rain (0.3–252.5). The hazard index values for Cu2+, Mn4+, Zn2+, and Cr3+ were below 1, ranging from 8.89E − 08 to 7.68E − 07 for adults and 2.30E − 07 to 5.02E − 06 for children through oral ingestion, and from 6.68E − 10 to 5.07E − 07 for adults and 6.61E − 09 to 2.54E − 06 for children through dermal contact. With a total carcinogenic risk of less than 1 for both ingestion and dermal contact, indicating no significant health risks yet send strong signals to Governmental management of the Nyabarongo River. Overall water quality was classified as marginal in long dry, poor in short dry, good in long rain, and poor again in short rain seasons.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ensuring access to clean water is vital for preserving ecosystem health and upholding the functionality of global production systems (Halisçelik and Soytas 2019). Throughout history, rivers have served as foundational elements in the advancement of civilizations, with human settlements heavily reliant on these freshwater reservoirs. However, in the wake of industrialization and intensified agricultural activities, the quality of water in perennial rivers worldwide is witnessing a steady decline (Kumar et al. 2018). This trend is particularly pronounced in Africa, where water pollution has been exacerbated across nearly all river systems since the 1990s, exposing hundreds of millions of people to health risks due to contaminated surface waters (Chen and Shen 2022). Hence, monitoring river water quality serves as an initial stride in formulating strategies for managing freshwater reservoirs to mitigate the risks of waterborne disease.
Monitoring the spatial and temporal dynamics of a set of parameters is a common approach for assessing the surface water quality of a river catchment. Water quality data can include measurements made in the field, water samples collected and analyzed in the laboratory, or information extracted from other data sets or geographic information layers (Basin 2022). This comprehensive approach allows a better understanding of the overall water quality and its impact on ecosystems, human health, and activities. Many countries regularly evaluate water quality in rivers as part of national water monitoring systems (European Environment Agency 2020). These evaluations are conducted through field inventories, which involve the collection and analysis of water samples to assess various parameters that indicate the health and safety of water bodies. For instance, the Environmental Protection Agency (EPA) in the USA conducts the National Water Quality Inventory, which summarizes the findings of both national surveys and site-specific assessments (European Environment Agency 2017). Water quality monitoring efforts by organizations such as the Interstate Commission on the Potomac River Basin (ICPRB) and the US National Park Service (NPS) focus on measuring, analyzing, planning, acting, and educating to ensure the health of rivers and their surrounding ecosystems (European Environment Agency 2020). These efforts include collecting water quality and habitat data, assisting in the establishment of water quality criteria, and developing total maximum daily loads (TMDLs) (Basin 2022).
In many tropical countries, the lack of specific organizations for monitoring water quality in rivers, as well as financial and human resource constraints, can limit the coverage and frequency of field inventories (Kayembe et al. 2018, Camara et al. 2019). The Nyabarongo River, as an essential freshwater source in Rwanda, is facing significant challenges in terms of water quality (Kulimushi et al. 2021). The river is heavily relied upon by the people for various domestic and industrial needs, yet for decades its contamination has been one of Rwanda's most critical water-related issues (Nteziyaremye and Omara 2020, Nteziyaremye et al. 2020). There is an existing paucity of data on the water quality of the Nyabarongo River, making it difficult to determine an appropriate site-specific index for policy and management of the river. Previous studies have shed some light on the ecological status of the river; for example, the bioaccumulation of heavy metals in the West African lungfish (Protopterus annectens) has been examined, and the concentrations were found to be above the threshold limits (Nteziyaremye and Omara 2020, Nteziyaremye et al. 2020). Soils within the Nyabarongo catchment areas were also found to contain high levels of persistent organic pollutants, which can erode into the river during heavy rainfall (Umulisa et al. 2020). To the author’s best knowledge, there is no specific research output quantifying the water quality status of the Nyabarongo River to determine an appropriate site-specific index that could serve as a basis for river policy and management.
Several conceptual frameworks have emerged to examine the impact of human activities on the environment. These include the pressure-state-response (PSR) framework (Li et al. 2021), the driver-pressure-state-impact-response (DPSIR) framework (Qin et al. 2003), the IPAT equation (impact = population × affluence × technology) (Yan Zhou 2016), and the socio-ecological systems framework (Chen et al. 2024). Among these, the DPSIR model stands out for its comprehensive approach to understanding the complex relationship between human actions and the environment, making it particularly valuable for studying seasonal variations in water quality (He et al. 2022). Understanding these seasonal fluctuations in water quality is crucial for assessing their Impacts on human health, aquatic ecosystems, and socioeconomic activities reliant on the river. Applying the DPSIR model allows for the identification of primary factors contributing to water quality decline, evaluating their seasonal impacts, and crafting specific responses like enhancing agricultural methods, implementing watershed management plans, and enacting pollution control measures (Wang et al. 2022b).
The main objective of this study was to analyze and depict the fluctuations in water quality attributes across both space and time in the Nyabarongo River situated in Rwanda. Moreover, the study sought to perform a thorough evaluation of water quality utilizing the water quality index (WQI) methodology. Additionally, it endeavored to estimate the potential health hazards stemming from heavy metal contamination in the river. Furthermore, the research aimed to pinpoint and comprehend the primary factors influencing water quality, along with the pressures and impacts on its condition. To accomplish these goals, a DPSIR model was developed to bolster the management system of the river; three indices were utilized, specifically the drinking water quality index (DWQI), metal index (MI), and pollution index (PI) to assess the water quality of the Nyabarongo. Twenty-three water quality parameters were selected, and measurements were conducted monthly at six water quality monitoring stations within the study area. This data collection spanned from January 2018 to December 2021, ensuring a robust dataset for developing a water quality index (WQI) specifically for the Nyabarongo River of Rwanda. The outcomes of this investigation are poised to enhance the understanding of the current water quality status in the Nyabarongo River and facilitate the sustainable management of surface water resources in Rwanda.
Materials and methods
Study area
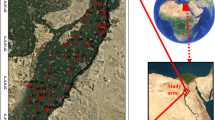
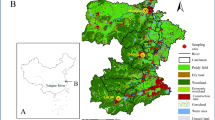
The Nyabarongo River plays a critical role as a primary freshwater source in Rwanda, serving as a crucial dependency for the populace’s various domestic and industrial requirements (Kulimushi et al. 2021). The Nyabarongo River starts from the confluence of the Mbirurume and Mwogo Rivers and ends up at its confluence with the Akanyaru River, emptying into the Akagera River, one of the largest rivers that drain into Lake Victoria (Benjamin 2015). The major tributaries are the Akanyaru River from the South and the Mukungwa River from the north, emerging from the volcanoes. The river catchment area covers 17 districts from all the provinces of the country and the City of Kigali.
Nyabarongo River (Fig. 1) is part of the upper headwaters of the Nile River with an approximate length of 151.5 km and drains a total area of 8478.24 km2 in its catchment zone (Umulisa et al. 2020). The catchment is formed by mountainous terrains with a mean slope of approximately 30% and elevation ranging from 1341 to 4491 m. The geological formation consists of 59% Acrisols, 19.2% Regosols, 9.2% Andolsols, 6.7% Ferralsols, 2.8% Cambisols, 0.8% Histosols, and 0.4% Gleysols (Holzforster and Schmidt 2007). The catchment experiences a tropical climate with two rainfall seasons a year, March to May and September to December. The temperature within the catchment can reach a maximum daily of 25.3 °C and a minimum of 14.6 °C in the western part and 23.6 °C and a minimum daily temperature of 14.0 °C in the southern part (Kwisanga 2017). Precipitation increases with elevation, which varies from about 864 mm in the central plateau across Lake Muhazi and Kigali City to 2258 mm in the mountain ranges over Nyungwe forest and volcano areas (Karamage et al. 2016).
Methodologies
Based on the objectives of this study, a dynamic DPSIR model was utilized to identify factors affecting quality management. Furthermore, the researchers collected water quality data from the period of 2018 to 2021 obtained from six sampling stations to be analyzed. Sixty-nine samples were collected monthly from each station. The stations were chosen strategically in consideration of the pressures on the Nyabarongo.
The sampling regimes and data gathering were concurrent with periodic monitoring activities of the Waste and Sanitation Company (WASAC) of Rwanda to compare results. Additional water quality data for the period of 2018–2020 was provided by WASAC. Water samples were collected throughout the years to coincide with the four major climate seasons of Rwanda in Table 1. Before each sampling event, 25 mL sample bottles were rinsed with dilute ethanol and air-dried overnight at room temperature (± 23 °C). All in situ monitoring instruments were calibrated, and at the site, sample bottles were rinsed with water three times before sampling. The samples were collected to minimize water bubbles collecting in the bottles.
Standard methods prescribed by the American Public Health Association (American Public Health Association 1999) were employed for the analysis of water samples. Water pH and temperature are determined in situ using a Hanna HI9812 combo meter, and in the laboratory, pH of stored water is determined by potentiometry before every analysis. Major cations are analyzed by atomic absorption spectrophotometry (AAS).
-
AAS analytical procedure
-
1.
Sample preparation
Water samples underwent collection following appropriate protocols to prevent contamination. Depending on the targeted cations, some samples were either filtered or subjected to acidification to eliminate interfering substances.
-
2.
Calibration
Calibration standards were established by diluting stock standard solutions of the relevant cations to concentrations spanning the expected range in the samples.
-
3.
Instrument configuration
The AAS instrument underwent calibration using standard solutions specific to each cation. Parameters such as wavelength, lamp current, and slit width were adjusted accordingly.
-
4.
Sample analysis
Samples were introduced into the flame, and the absorbance of each cation’s characteristic wavelength was measured. Utilizing the calibration curve aided in determining the concentration of each cation in the sample.
-
5.
Quality control
Regular checks were conducted on instrument performance using control standards. Additionally, accuracy and precision were verified through replicate analyses and spike tests.
-
Utilized equipment
-
1.
Atomic absorption spectrophotometer
This equipment comprises a light source (hollow cathode lamp or electrodeless discharge lamp), a monochromator, a sample introduction system (flame or graphite furnace), and a detector.
-
2.
Graphite furnace atomizer
Primarily employed for trace analysis due to its heightened sensitivity and reduced interference compared to flame atomization.
-
3.
Sample introduction system
Flame AAS consists of a nebulizer, spray chamber, and burner head. In the case of graphite furnace AAS, it involves a graphite tube and heating system.
-
4.
Calibration standards and reagents
Included stock standard solutions for each targeted cation, dilution solutions, and matrix modifiers for complex samples.
-
Analytical standards
-
1.
Accuracy
Ensured through the analysis of certified reference materials (CRMs) possessing known concentrations of the cations.
-
Precision
Evaluated by conducting replicate analyses of identical samples or by employing control charts.
-
2.
Detection limits
Determined based on signal-to-noise ratios or standard deviation methods for each cation.
-
Interference
Minimized by utilizing suitable matrix modifiers or selecting wavelengths with minimal interference.
-
3.
Quality assurance
Adherence to standardized protocols for quality assurance and quality control (QA/QC) to guarantee result reliability.
Quality assurance and quality control (QA/QC) procedures of analytical and instrument methods were confirmed in the WASAC laboratory which includes 5% repeats, standard calibration curves, and quality control reference standards. Standard curves are generated for every analysis regime. The method detection limits (MDLs) of the reference standard were applied. Table 2 shows general water quality assessment criteria as recommended by the Canadian Council of Ministers Environment (CCME), while Table 4 shows various parameters involved in the WQI assessment.
In addition to the standard analytical procedures described, we calculated the charge balance error (CBE) for each water sample to assess the accuracy of our ionic concentration measurements. The CBE ranged from − 4 to + 5%, which is within the ± 5% range considered acceptable for hydro chemical analyses. All statistical tests in this study were performed with a significance level set at 0.05, corresponding to a 95% confidence level.
In this study, we conducted T-tests to analyze the temporal variation in water quality parameters over a 4-year period. The results revealed a T-test value of 0.85, indicating significant differences in water quality among the years assessed.
This suggests notable shifts in water quality over the study period. Such findings underscore the necessity for ongoing monitoring efforts and targeted interventions to mitigate environmental risks and safeguard water resources.
Indexing approach
Different approaches, which were governed by three factors including parameter selection, quality function calculation (sub-index), and accumulation of sub-indices, were applied using arithmetic equations and compared to evaluate the suitability of collected water samples along the Nyabarongo River (Mohseni et al. 2022). The suitability of surface water for drinking and its sensitivity to pollution were investigated using WQI, MI, and PI, which were calculated using physical and chemical properties.
For potable water quality assessment, the findings on 23 physicochemical parameters of collected samples were considered. The physical and chemical parameters were weighted based on their significance to the total quality of water. The WQI reflects the total water quality of water variables based on a combination of water characteristics and their utilization in the ecosystem (Brown et al. 1972) as indicated in Eq. (1);
According to the WHO (WHO 2017), the estimated value of QiWi is influenced by the concentration of each water component (Ci) and their guideline (Si) for drinking water, as indicated in the following equations:
where Wi is the relative weight and wi is the weight unit of each water component. Equation (4) is applied to determine the wi for each component in accordance with the specified criteria for drinking water
K is the constant of proportionality, which can be computed using the following equation:
To calculate the WQI, a weight for each surface water parameter (w) was assigned for pH, turbidity, TDS, EC, K+, Na+, Mg2+, Ca2+, Cl−, SO42−, HCO3−, CO32−, Al3+, Br−, Cr3+, Cu2+, Fe3+, Mn4+ and Zn2+, CN−, F−, NO3−, and PO43− and the quality rating range (Q) and relative weight (Wi) were estimated. The computed values of the standards, unit weights (wi), and relative weights (Wi) for the surface water parameters are shown in Table 3. The derived WQI was compared to the CCME (Saffran et al. 2001)WQI index.
The pollution indices, such as metal index (MI) and pollution index (PI), were calculated for the concentrations of metals including Al3+, Cr3+, Cu2+, Fe3+, Mn4+, Mg2+, K+, and Zn2+ using Eqs. (6) and (7).
Metal index (MI)
According to Eq. (6), the metal index (MI) was used to analyze the probable impact of metals on public health, which helps in swiftly estimating the overall quality of water (Tamasi and Cini 2004).
where Hc is the concentration of each metal in the collected water sample, Hmax is the maximum limit concentration for each metal, and the subscript i is the ith sample (Ojekunle et al. 2016).
Pollution index (PI)
The PI values were used to evaluate the influence of metal pollution on surface water (Caeiro et al. 2005). According to Eq. 7, the PI is categorized into five groups as shown in Table 4, which reflects the distinct pollution influence from each metal on water quality.
where Ci represents the content of each metal in the collected sample and Si represents the standard limit of each metal in potable water (Caeiro et al. 2005, Goher et al. 2019).
Health risk analysis
Assessing the risk of trace elements in water typically considers direct human intake and skin intake. The toxic effects of exposure to pollutants in adults and children were calculated according to the hazard index (HI) and carcinogenic risks (CR) approach offered by the USEPA.
Oral dose (Ming-s):
Dermal dose (Mder-s):
where Cs is the concentration of pollutants in water (mg kg−1), EF is the exposure frequency (days year−1), ED is the exposure duration (years), IngR is the receptor water ingestion rate (mg day−1), SA is the skin surface area available for exposure (cm2), SL is the water-to-skin adherence factor (mg cm−2 event−1), ABS is the dermal dimensionless factor, BW is the time-averaged body weight (kg), and AT is the average time of noncarcinogenic and carcinogenic risks (days). Specific parameters for the estimation of non-carcinogenic risk are provided in Table 5.
For non-carcinogenic risk (NCR), HI refers to all sum of HQ (hazard quotients) in heavy metals. HI > 1 represents the potential adverse effect on human health. For total carcinogenic risk (TCR) and carcinogenic risk (CR), the values TCR and CR < 10−6, 10−6 < TCR and CR < 10−4, and TCR and 10−4 represent no health risk, no significant health risk, and high health risk, respectively (Zhang et al. 2020). The specific toxicity threshold (RfD, mg kg−1 day−1) and slope factors (SF, mg kg−1 day−1) values are presented in Table 6.
Data analysis
To ensure the appropriateness of statistical methods applied in this study, we conducted Shapiro–Wilk tests to assess the normality of the distribution of data collected from various monitoring stations along the Nyabarongo River. These tests confirmed that the data were normally distributed, which justifies our use of parametric statistical methods for further analyses. Specifically, we employed analysis of variance (ANOVA) to analyze the data distribution across different seasons, assessing the variability in water quality indices. Furthermore, Pearson’s correlation analysis Fig. 6 was utilized to examine the relationships between various water quality parameters. This approach allowed us to rigorously interpret the seasonal fluctuations and inter-parameter dependencies in water quality, critical for understanding the impacts and dynamics within the river system.
Multivariate statistical analyses were performed on the physical and chemical data utilizing OriginPro 2021 (OriginLab, Northampton, USA) to generate statistical summaries of the datasets (Awomuti et al. 2023). Piper trilinear diagram (Piper 1944) and Gibbs diagram (Gibbs 1970) were applied to determine surface water types, geochemical processes, geochemical influencing factors, and the categorization of surface water samples and their controlling mechanisms. Multivariate modeling techniques such as principal component analysis (PCA) are commonly utilized for assessing water quality. Therefore, by minimizing the chemical analysis data into common patterns, the correspondence analysis (CA) was used to identify the physicochemical characteristics based on their similarities (Kumar et al. 2020). The PCA was used to study the association between the physicochemical properties that were responsible for changes in water quality by converting the initial variables into a new set that reflected the effect of significant elements on water quality.
Results and discussion
DPSIR framework of river water quality and policy analysis
The DPSIR dynamic model was applied to determine factors that could influence the quality management of the Nyabarongo River (Wang et al. 2022a). The model utilizes driving forces (D), which define factors/interests that could lead to deterioration of the water quality status of the river; pressures that come about as a result of realizing the driving forces (P); states describe the spatio-temporal conditions of the river as a result of the exerted pressures (S); impacts (I) are the resulting short-long term effects on rivers species and responses are outcomes which can be formulated into policies to control pollution incidences of the river or reduce the effects (R). For the case of the Nyabarongo River, the DPSIR framework is shown in Fig. 7.
Drivers
Various factors have been identified in the literature as driving forces that lead to pressures on the quality of rivers. In the case of the Nyabarongo River, the main drivers identified during the study period were population growth, climate change, settlement, and urbanization around the river. Although the population of Rwanda is small comparable to other African countries, it was considered a key indicator contributing to the deterioration of the river quality. The population has risen gradually from 12,835,028 (2019) to 13,146,362 (2020), 13,461,888 (2021), and 13,776,698 (2022) (Squire and Nkurunziza 2021). With high population growth comes increased consumption and waste generation with improper disposal systems. The urbanization rate with many people resident in Kigali City (which was the closest city to the study area) is a direct correlation that could exert pressures on the river. As a result of urbanization, sewage and wastewater discharge into the Nyabarongo River can provide excess nitrogen and phosphorus, which lowers the water quality through eutrophication processes affecting aquatic life. The need for constant electricity has also seen the construction of hydroelectric dams along the river system which could potentially impact the river water quality (Kulimushi et al. 2021).
Pressure
Several tributaries connect to the Nyabarongo River implying that soil erosion from the basin of these rivers can impact the river of study increasing its suspended sediment composition (Kulimushi et al. 2021). Soil erosion is also formed due to agriculture resulting from the main driving force of the population growth rate, which exerts an influence on the quality and quantity of the river water (Bires and Raj 2020). Because agriculture is the most important sector of the economy of Rwanda, chemical fertilizers used for this agricultural purpose contain phosphates and nitrates, which are known to be major contributors to water contamination. Eutrophication, which enriches the water surface with nutrients, is aided by nitrogen and phosphorus from the environment. The result of eutrophication is a proliferation of algae blooms (Shiferaw and Abebe 2021). Additionally, mismanagement of municipal solid and liquid waste (from domestic and industrial discharges) can result in disposal into the river which influences its quality over time (Omara et al. 2020).
States
The state of the Nyabarongo River is related to the values of river quality. These changes happen in the physical, chemical, and biological conditions of the river over time. In the context of the DPSIR framework, the changes observed in the water quality relate to the change in the existing natural physical, chemical, and biological properties of clean water as a result of one or more pressures. From the results of this study, there were several indications of the change in the quality of the river over the different climate seasons due to different identified pressures. Pressure derived from the driving forces has left the current condition of the river as having high dissolved solids, and high metal concentrations, resulting in some metals being major influences on the river water quality. High agricultural activities due to the increasing population and settlements around the river catchment will potentially result in increased eutrophication through the accumulation of fertilizers. Dead algal mats decaying in the water can cause terrible tastes and aromas, and their breakdown by bacteria depletes dissolved oxygen in the water, resulting in fish kills. Recent reports have also indicated a high risk in the African Lung Fish which is a dominant species of the river (Nteziyaremye and Omara 2020; Nteziyaremye et al. 2020; Omara et al. 2020).
Impact
The effects and impacts caused by the physical, chemical, and biological conditions of the river are directly correlated to human and ecological health risks. While the river provides enormous social and economic benefits, when contamination and pollution incidences increase, the water becomes hazardous to humans eventually depleting the capital goods it offers such as fish kills. Likewise, increased pollution in the river tends to cause a cost rise in the treatment of river water to meet desirable standards for consumption. The high pollution incidence of the river will impact household food consumption, especially access to fish produce. Additionally, with the agricultural system being largely dependent on the river, there is potential long-term bioaccumulation of heavy metals through food webs which will impact the health status of the population at large (Umulisa et al. 2020).
Response
Several studies have indicated the important roles of stakeholders in providing adequate water services. Meanwhile, many countries are burdened with ensuring this service due to broader drivers and pressures. To resolve this, decision-making on water resource management needs to incorporate diverse knowledge in an accessible and meaningful manner (Kaur et al. 2020). This becomes necessary to support policy and its implementation to control factors that result in water quality degradation. In the case of the Nyabarongo River, the current operations of the WASAC can be expanded to include monitoring other rivers that join to exert similar regulatory controls and management practices to reduce water contamination and pollution (Moges et al. 2017). The government should put in restrictions and establishment of buffer zones around the river to avoid frequent agricultural and irrigation activity setups. New government policies on river water management and enforcement of industrial codes of practices are critical. This is necessary to reduce anthropogenic activities around the river (Malekmohammadi and Jahanishakib 2017). Agricultural conservation and land practices must be controlled through farmer-based education and proper spatial land planning. Regulation of operations within the catchment and watershed of the river system is important to reduce erosion of land-based activities into the river. Lastly, other institutions or ownerships should be included in the management of the river water quality by setting up a watershed management committee with clearly stated responsibilities. This will improve integration between various institutions and specify the owners who are responsible for maintaining the quality of the river.
Physical and chemical properties
The physicochemical characteristics of Nyabarongo River water have been investigated in some parts as helpful criteria for understanding the state of water geochemistry and related regulatory processes that play critical roles in the evolution of water quality (Karamage et al. 2016). Figure 2 and Table 7 show the behavior of water quality data across the seasons and statistical overview of datasets from the investigated surface water respectively. From Fig. 2, much of the dataset across the monitoring season within the years was distributed across the lower and upper percentile ranges presenting a general normal distribution. In the dry long season, the datasets were mostly left skewed except for datasets within June 2019 and August 2021 which were rightly skewed. In the dry short season, the datasets did not display any form of centrality. On average, the datasets from 2018 were left skewed as observed in the previous season, while the distribution was rightly skewed across most of the previous periods. The observations were similar to the long rain and short rain seasons.
The overall behavior and distribution of water quality data across the four seasons. The outcomes indicate the water quality in general is better during the long rainy season possibly due to the high precipitation effect and constant dissolution of water chemical constituents. The river hence becomes self-cleaning to reduce its contamination and pollution levels while the opposite could occur in the dry seasons
Several water quality indicators, including pH, turbidity, and EC, is critical in expressing surface water quality. Therefore, these measures are the primary indicator metrics for assessing surface water quality and its degradation as a result of polluting activities. Water pH has a profound effect on the state such as metal dissolution, hardness, and alkalinity. The survival of most aquatic species is also impacted by pH as their metabolic activities are pH-dependent (Nteziyaremye and Omara 2020, Nteziyaremye et al. 2020). The obtained analytical datasets showed that pH had a mean of 8.7 ± 0.9 which indicated the water samples across the monitoring periods were virtually alkaline. The pH was slightly above WHO’s standard limit of 8.5 for surface water bodies. Anthropogenic, climatic changes, and eutrophication could have significantly affected the water pH. The solubility of chemical constituents in particular metals is predominantly controlled by water pH, water temperature, and river flow. Lowered pH tends to increase competition between metal species and hydrogen ions for biding sites, while a decreased pH could cause the dissolution of metal carbonate complexes hence releasing free metal ions into the river column. (Kopáček et al. 2021).
Turbidity is a measure of the murkiness of water. The mean concentration (5.9 ± 1.6 NTU) was slightly above the WHO’s limit standard of 5 NTU. The high murkiness of the river could be associated with regular erosion comprising silt, clay, organic matter, and plant debris into the river. Typically, transparency increases in correspondence to phases of seasonal succession (Sommer et al. 2012); in this case, high transparency occurs in the long dry and short dry seasons. TDS and EC are directly related in revealing levels of dissolved solids and the corresponding electrical potential of the water. Therefore, high TDS directly infers high EC (Gad et al. 2020). From the datasets obtained across the monitoring seasons, the mean TDS value was (302.4 ± 169.2 mg/L) and EC was (547.9 ± 153.3 µS/cm3). These were below (WHO 2017) standard limits of 500 mg/L and 400 µS/cm3 which reflected a fresh water type. Nutrients were characterized by NO3−, SO42 − , and PO42− with mean concentrations of 12 ± 5.1 mg/L, 11.6 ± 2.5 mg/L, and 7.4 ± 5.2 mg/L. The measured concentrations were below (WHO 2022) standard limits of 50 mg/L, 250 mg/L, and 50 mg/L, respectively. Ionic concentrations on average were above (WHO 2017) limit and increased in order HCO3− > Mg2+ > K+ > Cl− > Ca2+ > Br− > Na+ > Cu2+ > F− > Zn2+ > CN− > Cr3+ > Fe3+ > Mn4+ > CO32−. The results revealed that HCO3− (123.4 ± 13.8) was the dominant anion and Mg2+ (15.3 ± 9.2) was the dominant cation which could influence water controlling processes.
Geochemical facies and controlling mechanisms
To better reveal an understanding of the geochemical mechanism influencing water quality and hydro chemical data of the Nyabarongo River, the water quality datasets were further analyzed by trilinear plots. Piper diagram shows the relative proportions of major ions in the water samples. The colors green, blue, and red typically represent different hydro-chemical facies, (green: calcium bicarbonate type, blue: sodium sulfate type, red: mixed type). Piper diagrams could reveal the prevalent cations and anions in the investigated water samples to define any geochemical facies. From Fig. 3, it is seen that in the dry long and short seasons, the water quality was most formed by calcium bicarbonate composition. In the long rain season, the water quality was formed by sodium sulfate composition, similar to the short rain season. The observations indicated that the geological weathering process could be highly influential in establishing the water chemistry of the Nyabarongo River. This was found in similar studies like that of Regina Irunde et al. (2022).
Piper diagram showing the hydro-chemical facies of water samples from the Nyabarongo River across different seasons (green: calcium bicarbonate type, blue: sodium sulfate type, red: mixed type). The figure portrays that in the long and short dry seasons, the water quality was most formed by calcium bicarbonate composition. In the long rain season, the water quality was formed by sodium sulfate composition, similar to the short rain season. The observations indicated that the geological weathering process could be highly influential in establishing the water chemistry of the Nyabarongo River
To further confirm this, the Gibbs diagram in Fig. 4 was used to understand the relationship between water chemical components relying on the association between TDS, Na+ /(Na+ + Ca2+), and Cl−/(Cl− + HCO3−). The degree and quality of characteristics of the water are identified from three distinct fields, namely, precipitation dominance, evaporation dominance, and rock-water interaction dominance where all ions were expressed in in meq/l in this study. The surface water quality datasets were distributed along the evaporation field which was influential in determining the factors controlling surface water quality. High evaporation rates as controlling mechanisms of the water formation could potentially contribute to high ionic concentrations investigated as similarly reported by (Giri and Singh 2014).
Gibbs plot of water quality formation and lithological characteristics. This was used to understand the relationship between water chemical components relying on the association between TDS, Na+/(Na+ + Ca2+), and Cl−/(Cl− + HCO3−). High evaporation rates as controlling mechanisms of the water formation could potentially contribute to high ionic concentrations
Water quality index
The WQI model was applied to assess surface water quality and measure the potability of the Nyabarongo River for drinking water purposes and values across the monitoring seasons shown in Table 8. The maximum computed WQI from the water quality samples across the monitoring seasons was 52.90 (dry long), 21.478 (dry short), 93.66 (long rain), and 37.4 (short rain). Based on CCME 2001, the water quality across the monitoring seasons of the years could be described as marginal (WQI 45–65) for the dry long seasons, poor (WQI 0–44) for the dry short period, good (WQI: 80–94) for the long rains season, and poor (WQI 0–44) for the short rain season. The outcomes indicate the water quality in general is better during the long rainy season possibly due to the high precipitation effect and constant dissolution of water chemical constituents (Das et al. 2022). The river hence becomes self-cleaning to reduce its contamination and pollution levels while the opposite could occur in the dry seasons.
Pollution and metal indices
Pollution indices (PIs) describe an efficient approach to characterize the quality of drinking water based on specified metal parameters (Kachroud et al. 2019). The metal indices (MI) of the water samples across the seasons varied from 0.2 to 433.0 for dry long, 0.1 to 174.3 for dry short, 0.1 to 223.7 for long rain, and 0.3 to 252.5 for short rain. According to the categorization of PI levels, the PI values indicated that the samples were not affected by K+ and Cr3+, (PI < 1.0) across all seasons but were strongly affected by Cu2+ (PI = 3–5) across all seasons and moderately affected by Al3+ (PI = 2–3), as presented in Table 9. However, the effects of Mn4+, and Zn2+, varied across the seasons, while Fe3+ (PI > 5) severely affected the water quality across the monitoring period as presented in Table 9. The high impact of Fe3+ could be associated with the lithogenic process of bedrock weathering over time (Xiao et al. 2015). Overall, it can be found that PI significantly influences the water quality across different climate seasons.
Principal component analysis
PCA findings for the physicochemical properties obtained from the surface water samples are shown in Fig. 5. The first principal component (PC1) explained 24.6% of the variation in the datasets, while the second principal component (PC2) explained 23.2% of total variation. Along PC1, Cl−, TDS, SO42−, PO43−−, Mg2+, K+, and Na+ could reveal their concentrations were contributed by similar sources (Samtio et al 2022). Cr3+, Br−, and NO3−, on the other hand, were inversely related which could be contributed by anthropogenic sources. NO3− for instance could be contributed by fertilizer runoff from farming activities along the river and other household depositions of greywater. Metals including Mn4+, Cu2+, CN−, and Fe3+ could explain their sources were possibly generated from the rivers’ baseline conditions (Wang et al. 2017).
Pearson correlation analysis
Pearson correlation analysis was performed to further reveal the relationship between measured physicochemical properties shown in Fig. 6. In the dry long season, significant negative associations were observed between pH and NO3− (r = − 0.97, p = 0.026) as well as between pH and Fe3+ (r = − 0.99, p = 0.0049). Ca2+ and HCO3− also displayed a strong negative correlation (r = − 0.95, p = 0.644) as well between Na+ and HCO3− (r = 0.99, p = 0.00033). The negative correlation between various parameters could originate from different factors including biological processes, such as photosynthesis and microbial activities. Geological factors like calcium carbonate-rich formations and anthropogenic influences such as agricultural runoff further affect the water quality. Additionally weathering processes of rocks and minerals in the river catchment area release ions into the water which makes strong connection with the Rwanda Water Board Annual Report 2021 (Portal 2021). On other hand, Fe3+ correlated positively with NO3− at r = 0.97, p = 0.024, while a similar strong correlation between Cu2+ and PO43− were determined at r = 0.97, p = 0.024. Fe3+, Cu2+, NO3−, and PO43− are typical constituents of fertilizer and pesticides and hence could be a major reason for their association (Stackpoole et al. 2019). Similar strong positive correlations between Br− and CN− with PO42− were at r = 0.99, p = 0.053 and r = 0.98, p = 0.016, respectively. Amongst the cations, Mg2+ displayed strong association with K+ (r = 0.98, p = 0.014). In the dry short season, a similar trend of strong positive and negative associations was established between the various ionic components. The association between EC and TDS was displayed with a strong negative correlation at r = − 0.97, p = 0.023. While HCO3− also correlated with EC at r = 0.95, p = 0.045 and HCO3− with TDS at r = 0.96, p = 0.035. Mg2+ displayed a strong correlation with pH at R2 = 0.95, p = 0.046, while Mn also showed a similarly strong positive correlation with Na+ (r = 0.98, p = 0.010). Zn2+ correlated positively with Al3+ (r = 0.99, p = 0.038) and Cu2+ (r = 0.98, p = 0.041). In the long rainy season, a similar trend of strong positive associations within the cations was determined. Al3+ correlated positively with Ca2+ (r = 0.99, p = 0.00078) and K+ (r = 0.99, p = 0.00064). Similar observations were made with Cu2+ and Ca2+, K+ at r = 0.95, p = 0.04 and r = 0.96, p = 0.034, respectively. Likewise, Fe3+ and Mn4+ correlated strongly with Ca2+ and K+ at r = 0.99), respectively. Strong negative associations were on the other hand established between Cl− and EC (R2 = − 0.97, p = 0.026) and NO3− (r = − 0.99, p = 0.0066). In the short rainy season, there was a very less strong correlation with the different chemical constituents. Similarly, pH positively correlated with Ca2+ (r = 0.95, p = 0.04), while Na+ showed a positive correlation with NO3− (r = 0.99, p = 0.069). CN− and Cr− were strongly correlated negatively with turbidity (r = 0.96, p = 0.039) and (r = 0.98, p = 0.012), respectively. Both Na+ and F− correlated positively with HCO3− at r = 0.99, p = 0.006 and r = 0.98, p = 0.011. The strong high correlation within the chemical constituents could infer they were typically originating from identical sources across the varying seasons. Nonetheless, some more information on the source and pathways of heavy metals could be relevant to provide the correlation between the inter-elements (Zhang et al. 2018).
Non-carcinogenic and carcinogenic human health risk assessment
Non-carcinogenic and carcinogenic risk of heavy metals through oral ingestion (Ming) and dermal contact (Mdermal) to adults and children were estimated and results are presented in Tables 10 and 11, respectively. It is observed that the hazard index (HI) of four heavy metals, Cu, Mn, Zn, and Cr, were below the standards, i.e., HI < 1, and ranged from 8.89E − 08 to 7.68E − 07 for oral ingestion in adults, and 2.30E − 07 to 5.02E − 06 for oral ingestion in children. For dermal contact, the HI in adults ranged from 6.68E − 10 to 5.07E − 07, while in children 6.61E − 09 to 2.54E − 06. Due to the lack of estimated slope factors of other heavy metals, the carcinogenic risk of Cr2+ was estimated. The results indicated a total carcinogenic risk (TCR) of less than 1 for both oral ingestion and dermal contact (TCR < 10−6 and TCR < 10−7) indicating no health risk. Nonetheless, it is important that the concentration of heavy metals be constantly monitored and enhance the water treatment system.
Conclusion
In this study, a DPSIR model was developed to identify primary factors contributing to water quality decline, evaluate their seasonal impacts, and craft specific responses (Fig. 7). The results from this study applied statistical approaches to evaluate the water quality of the Nyabarongo River and established a drinking water quality index. The acceptability of the water quality differed across the four main climatic seasons existing in Rwanda, ranging from poor to good, and marginal according to the Canadian Council of Ministers of Environment (Saffran et al. 2001). The water chemistry was found to mainly be formed out of evaporation of lithological constituents. Multivariate analysis could reveal that the water quality is influenced by both anthropogenic (agricultural, industrial discharges, household discharges, poor sanitation) and possible long-term natural causes (lithological weathering of the river bedrock). Ion concentrations surpassed the guidelines set by the World Health Organization (WHO), with prevalent ions identified as bicarbonate (HCO3−) and magnesium (Mg2+). The fluctuations in water quality across seasons were driven by factors such as increased prevalence of calcium bicarbonate during dry periods and elevated presence of sodium sulfate during rainy periods. Pollution indices were associated with Fe3+ and Mg2+ concentrations. Health risk assessment for both adults and children revealed that heavy metals in rivers may not pose significant threats, yet send strong signals to governmental management since the Nyabarongo River is a major source of drinking water in the country. The results obtained are significant inputs towards developing drinking water quality guidelines in Rwanda and contributing towards meeting SDG 6.
Data availability
Not applicable.
References
American Public Health Association A (1999) Standard method for examination of water and wastewater. American public health association, american water works association, water environment federation. https://dl.icdst.org/pdfs/files/9b8d1f12b54f3154c8c648e0ec810185.pdf
Awomuti A et al (2023) Towards adequate policy enhancement: an AI-driven decision tree model for efficient recognition and classification of EPA status via multi-emission parameters. City and Environment Interactions 20:100127
Basin (2022) Drinking water and water resources. Interstate commission on the potomac river basin. https://www.potomacriver.org/about-icprb/
Benjamin G (2015) Assessment of micro to small hydropower potential sites of Nyabarongo river basin of Rwanda. Ethiopia, Arba Minch University, MSc
Bires Z, Raj S, (2020) Tourism as a pathway to livelihood diversification: evidence from biosphere reserves, Ethiopia. Tour Manag 81:104159. https://doi.org/10.1016/j.tourman.2020.104159
Brown RM et al (1972) A water quality index — crashing the psychological barrier. Indicators of Environmental Quality, Boston, MA, Springer, US
Caeiro S et al (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Ind 5(2):151–169
Camara J, Abdullah AFB et al (2019) Spatiotemporal assessment of water quality monitoring network in a tropical river. Environ Monit Assess 191:729
Chen K, Gao Shen (2022) Assessment of urban river water pollution with urbanization in East Africa. Environ Sci Pollut R 29:40812–40825
Chen Y, Yu P, Wang L, Chen Y, Chan EHW (2024). The impact of rice-crayfish field on socio-ecological system in traditional farming areas: implications for sustainable agricultural landscape transformation. J Clean Prod 434:139625. https://doi.org/10.1016/j.jclepro.2023.139625
Das M, Semy K, Kuotsu K (2022) Seasonal monitoring of algal diversity and spatiotemporal variation in water properties of Simsang river at South Garo Hills, Meghalaya, India. Sustain Water Resour Manag 8:16. https://doi.org/10.1007/s40899-022-00611-6
European Environment Agency (2017) Surface water quality monitoring - summary. The UK and internationalmonitoring programmes. European Environment Agency. https://www.eea.europa.eu/publications/92-9167-001-4/page008.html
European Environment Agency (2020) Surface water quality monitoring summary—France. from https://www.eea.europa.eu/publications/92-9167-001-4/page008.html. Accessed Dec 2023
Gad M et al (2020) Combining water quality indices and multivariate modeling to assess surface water quality in the Northern Nile Delta. Egypt. Water 12(8):2142
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Giri S, Singh AK (2014) Assessment of surface water quality using heavy metal pollution index in Subarnarekha River. India Water Qual Expo Health 5(4):173–182
Goher ME et al (2019) Water quality status and pollution indices of Wadi El-Rayan lakes, El-Fayoum. Egypt Sustain Water Resour Manag 5(2):387–400
Halisçelik E, Soytas MA (2019) Sustainable development, vol 27, no. 4. Wiley Online Library. https://doi.org/10.1002/sd.1869
Holzforster F, Schmidt U (2007) Anatomy of a river drainage reversal in the Neogene Kivu-Nile Rift. Quat Sci Rev 26(13–14):1771–1789
Irunde R, Ijumulana J, Ligate F, Maity JP, Ahmad A, Mtamba JOD, Mtalo F, Bhattacharya P (2022) Arsenic in Africa: potential sources, spatial variability, and the state of the art for arsenic removal using locally available materials. Groundwater Sustain Dev 18. https://api.semanticscholar.org/CorpusID:247486565
Kachroud M, Trolard F, Kefi M, Jebari S, Bourri G (2019) Water quality indices: challenges and application limits in the literature. Water 11(2). https://api.semanticscholar.org/CorpusID:133798554
Karamage F et al (2016) USLE-based assessment of soil erosion by water in the Nyabarongo river catchment. Rwanda Int J Environ Health Res 13(8):835
Kaur M et al (2020) Investigating the impacts of urban densification on buried water infrastructure through DPSIR framework. J Clean Prod 259:120897
Kayembe JM, Sivalingam P, Salgado CD, Maliani J, Ngelinkoto P, Otamonga J-P, Mulaji CK, Mubedi JI, Poté J (2018) Assessment of water quality and time accumulation of heavy metals in the sediments of tropical urban rivers: case of Bumbu River and Kokolo Canal, Kinshasa City, Democratic Republic of the Congo. J Afr Earth Sci 147:536–543. https://doi.org/10.1016/j.jafrearsci.2018.07.016
Kopáček J et al (2021) Biogeochemical causes of sixty-year trends and seasonal variations of river water properties in a large European basin. Biogeochemistry 154(1):81–98
Kulimushi L et al (2021) Erosion risk assessment through prioritization of sub-watersheds in Nyabarongo river catchment. Rwanda Environ Challenges 5:100260
Kumar V et al (2020) Assessment of heavy-metal pollution in three different Indian water bodies by combination of multivariate analysis and water pollution indices. Um Ecol Risk Assess 26(1):1–16
Kumar B, Singh UK, Ojha SN (2018) Evaluation of geochemical data of Yamuna River using WQI and multivariate statistical analyses: a case study. Int J River Basin Manag 17(2):143–155. https://doi.org/10.1080/15715124.2018.1437743
Kwisanga JMP (2017) Assessing flood risk and developing a framework for a mitigation strategy under current and future climate scenarios in Nyabarongo Upper Catchment, Rwanda. https://repository.pauwes-cop.net
Li W, Qi J, Huang S, Fu W, Zhong L, He B-J (2021) A pressure-state-response framework for the sustainability analysis of water national parks in China. Ecol Indic 131:108127. https://doi.org/10.1016/j.ecolind.2021.108127
Malekmohammadi B, Jahanishakib F (2017) Vulnerability assessment of wetland landscape ecosystem services using driver-pressure-state-impact-response (DPSIR) model. Ecol Ind 82:293–303
Moges M et al (2017) Water quality assessment by measuring and using Landsat 7 ETM+ images for the current and previous trend perspective: Lake Tana Ethiopia. J Water Resource Prot 9:1564–1585
Mohseni U et al (2022) An innovative approach for groundwater quality assessment with the integration of various water quality indexes with GIS and multivariate statistical analysis—a case of Ujjain City. Waer. Cons. Sci & Eng, India
He N, Zhou Y, Li W, Li Q, Zuo Q, Liu J, Li M (2022) Spatiotemporal evaluation and analysis of cultivated land ecological security based on the DPSIR model in Enshi autonomous prefecture, China, vol. 145. Elsevier, United Kingdom. https://www.sciencedirect.com/journal/ecologica
Nteziyaremye P, Omara T (2020) Bioaccumulation of priority trace metals in edible muscles of West African lungfish (Protopterus annectens Owen, 1839) from Nyabarongo River. Rwanda Cogent Environ Sci 6(1):1779557
Nteziyaremye P, Omara T, Li F (2020) Bioaccumulation of priority trace metals in edible muscles of West African lungfish (Protopterus annectens Owen, 1839) from Nyabarongo River, Rwanda. Cogent Environ Sci 6(1). https://doi.org/10.1080/23311843.2020.1779557
Ojekunle O et al (2016) Evaluation of surface water quality indices and ecological risk assessment for heavy metals in scrap yard neighbourhood. Springerplus 5:560
Omara T, Nteziyaremye P, Akaganyira S et al (2020) Physicochemical quality of water and health risks associated with consumption of African lung fish (Protopterus annectens) from Nyabarongo and Nyabugogo rivers, Rwanda. BMC Res Notes 13:66. https://doi.org/10.1186/s13104-020-4939-z
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS-Trans Am Geophys Union 25(6):914–928
Portal RW (2021) Surface water monitoring report. from https://waterportal.rwb.rw. Accessed Feb 2024
Qin X, Hu X, Xia W (2003) Investigating the dynamic decoupling relationship between regional social economy and lake water environment: the application of DPSIR-Extended Tapio decoupling model. J Environ Manag 345:118926. https://doi.org/10.1016/j.jenvman.2023.118926. Accessed 1 Nov 2023
Saffran KS, Cash KJ, Hallard KA, Wright R (2001) Canadian water quality guidelines for the protection of aquatic life CCME water quality index 1.0 User's Manual. https://api.semanticscholar.org/CorpusID:2789561
Samtio R, Rajper KH, Hakro AAaD et al (2022) Impact of water–sediment interaction on hydrogeochemical signature of dug well aquifer by using geospatial and multivariate statistical techniques of Islamkot sub-district, Tharparkar district, Sindh, Pakistan. Arab J Geosci 15:167. https://doi.org/10.1007/s12517-022-09436-1
Shiferaw M, Abebe R (2021) A spatial analysis and modeling study of sedimentation impacts on dams found in south Gondar zone Ethiopia. Modeling Earth Syst Environ 7(4):2225–2230
Sommer U et al (2012) Beyond the plankton ecology group (PEG) model: mechanisms driving plankton succession. Annu Rev Ecol Evol Syst 43(1):429–448
Squire JNT, Nkurunziza J (2021) Urban waste management in post-genocide Rwanda: an empirical survey of the City of Kigali. J Asian Afr Stud 57(4):760–772
Stackpoole SM et al (2019) Variable impacts of contemporary versus legacy agricultural phosphorus on US river water quality. Proc Natl Acad Sci 116(41):20562–20567
Tamasi G, Cini R (2004) Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci Total Environ 327(1):41–51
Umulisa V et al (2020) First evaluation of DDT (dichlorodiphenyltrichloroethane) residues and other Persistence Organic Pollutants in soils of Rwanda: Nyabarongo urban versus rural wetlands. Ecotoxicol Environ Saf 197:110574
Wang J et al (2017) Multivariate statistical evaluation of dissolved trace elements and a water quality assessment in the middle reaches of Huaihe River, Anhui, China. Sci Total Environ 583:421–431
Wang B et al (2022a) A SEEC model based on the DPSIR framework approach for watershed ecological security risk assessment: a case study in Northwest China. Water 12:1534
Wang B, Yu F, Teng Y, Cao G, Zhao D, Zhao M (2022b) A SEEC model based on the DPSIR framework approach for watershed ecological security risk assessment: a case study in Northwest China. Water 14:106. https://doi.org/10.3390/w14010106
WHO (2017) Guidelines for drinking-water quality: 4th edition, incorporating the 1st addendum. World Health Organization. https://www.who.int/publications/i/item/9789241549950. Accessed 24 Apr 2017
Xiao Y-H et al (2015) Iron as a source of color in river waters. Sci Total Environ 536:914–923
Yan Zhou LZ (2016) Impact analysis of the implementation of cleaner production for achieving the low-carbon transition for SMEs in the Inner Mongolian coal industry. Cleaner Production 127:418–424
Zhang Z et al (2018) Assessment of heavy metal contamination, distribution and source identification in the sediments from the Zijiang River, China. Sci Total Environ 645:235–243
Zhang R et al (2020) Health risk assessment of heavy metals in agricultural soils and identification of main influencing factors in a typical industrial park in northwest China. Chemosphere 252:126591
Funding
Financial support for this study was provided by Prof. Fengting Li and the National Key Technologies R&D Program of China (grant no. 2019YFC1904002).
Author information
Authors and Affiliations
Contributions
Mycline Umuhoza: conceptualization, investigation, data curation and analysis, writing—original draft preparation, review and editing; Dongjie Niu: review and editing; Prof. Fengting Li: funding acquisition and supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Consent to participate
All authors agreed to participate in this research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Umuhoza, M., Niu, D. & Li, F. Exploring seasonal variability in water quality of Nyabarongo River in Rwanda via water quality indices and DPSIR modelling. Environ Sci Pollut Res 31, 44329–44347 (2024). https://doi.org/10.1007/s11356-024-34015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-34015-0