Abstract
Recently, much research has been oriented towards the influence of different food wastes and agricultural by-products on the final larval biomass and chemical composition of the insect species Hermetia illucens L. (Diptera: Stratiomyidae). However, there is a gap in the literature regarding the possible relationship between the feeding substrate of H. illucens larvae and chitin. In this context, in the present study, larvae of H. illucens derived from two populations (i.e., UNIPI and UTH), were reared on different diets composed of fruits, vegetables, and meat. Based on the results, the larval survival was high for all diets tested. Larval growth in terms of weight gain, larval length, and feed conversion ratio (FCR) depended on the composition of each diet. The chitin and chitosan composition of larvae, reared on different substrates, did not reveal significant differences. Given the fact that the feeding substrate represent a significant cost in the industrial production of insects, its correlation with a high value product (i.e. chitosan) is important. On the other hand, as the prepupal stage of H. illucens is currently used as animal feed, the metabolization of chitin by farmed animals when the larvae or prepupae were offered as feed could have adverse effects. Thus, depending on the final product that is to be produced, industries could benefit from the establishment of a suitable diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid growth of the human population has led to an ever-increasing demand for food and energy resources. An allied outcome of this evolution process was the generation of huge amounts of agro-industrial food waste. The high cost associated with the processing methods is leading modern societies to discard industrial and household food wastes in landfills (Kumar Awasthi et al. 2022). Indicatively, worldwide one-third of edible parts of food produced for human consumption is wasted (FAO 2011). The quantities of food waste produced globally are estimated to be 1.3–1.4 billion tons per year, while, until 2025, the projections show that it will reach as high as 2.6 billion tons (Sinha and Tripath 2021).
On the other hand, agro-industrial and domestic food waste could constitute a valuable feeding substrate for insects. Thus, the development of the insect sector in Europe is a promising solution to the transition to a more sustainable food system (EC 2020). The valorization of food wastes has been studied for several insect species, such as the yellow mealworm, Tenebrio molitor (Coleoptera: Tenebrionidae) (Oonincx et al. 2019; Rumbos et al. 2022); the superworm, Zophobas morio (Coleoptera: Tenebrionidae) (Van Broekhoven et al. 2015); the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae) (Gianotten et al. 2020); and the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae) (Bava et al. 2019; Bosch et al. 2019).
It is necessary to point out that, in the EU, the main obstacles in insect-rearing development are at the normative level. In particular, the most relevant EU regulatory restrictions are related to the type of insect-rearing substrate since insects reared for feed fall under the definition of ‘farmed animal’ (Article 3.6 of Regulation EC n. 1069/2009), which has several consequences for the choice of the organic resource which are allowed to be used as feeding substrates. In the EU, for instance, the use of several substrates such as ruminant proteins, catering waste, meat-and-bone meal, and manure are not allowed for any farmed animals (insects comprise) (Regulation (EU) No. 767/2009). Currently, the only authorized rearing substrate for insects, as farmed animals, are the animal feed materials according to the EU catalogue (Regulation EU No. 68/2013), the food produced for human consumption (excluding meat and fish), but no longer considered as such (due to expiry date or manufacturing or packaging defects); ex-food of plant origin (EU No. 68/2013); ex-food of animal origin (non-PAP) (EU No. 142/2011); and other types of organic waste of vegetable nature such as gardening and forest material (EC No. 767/2009, Annex III; EC No. 68/2013 Directive 2008/98/EC) (Bosch et al. 2019).
The insect species H. illucens is native to the Neotropical region (Brammer and von Dohlen 2007). Nowadays, H. illucens has a cosmopolitan distribution and, in some countries, it is utilized to counteract the environmental detriments caused by food waste, by producing organic fertilizers (Sheppard et al. 1994). Thus, much research has been directed to the influence of different food wastes and agricultural by-products on the growth and performance of H. illucens larvae (Nguyen et al. 2013; Cammack and Tomberlin 2017; Salomone et al. 2017; Barragan‐Fonseca et al. 2018; Gold et al. 2020; Kawasaki et al. 2022;). Most of the studies highlighted the variations in final larval biomass and chemical composition. Researchers also correlate differences in larval growth with chitin content in larvae of H. illucens (Diener et al. 2009; Meneguz et al. 2018), which is one of the most abundant polysaccharides in nature that provides insects with the rigid structure of the exoskeleton. Via deacetylation reaction, chitin could be converted to chitosan. Both chitin and chitosan are biocompatible, biodegradable, and non-toxic biopolymers. The importance of the two biopolymers has been brought to light due to their improved functionality and biodegradability compared to synthetic polymers (Rinaudo 2006). Indicatively, chitin and chitosan are utilized in food processing, packaging, cosmetics, textiles, and drug delivery and their production has recently gained considerable economic importance (Park and Kim 2010; Hamed et al. 2016).
As reported by many authors, the H. illucens instars currently used as animal feed are prepupae (Panikkar et al. 2022; Kroeckel et al. 2012). However, the metabolization of chitin by farmed animals when the larvae or prepupae were offered as feed could be problematic (Lee et al. 2022). From an industrial point of view, the extraction of chitin and chitosan from H. illucens prepupae, pupae, and exuviae, is of considerable economic importance. Thus, both sectors could benefit from the establishment of a suitable diet which, as mentioned above, may alternate the growth and chemical composition of different life stages of H. illucens. Therefore, the present study aims to evaluate the growth and performance of H. illucens larvae reared on diets composed of organic waste allowed by EU legislation, compared to a meat-based diet reported in the bibliography as unsuitable and not allowed by EU legislation as well as to investigate the possibility of chitin and chitosan production from prepupae to be influenced by the diet.
Materials and methods
Hermetia illucens rearing
Experiments were performed using two populations of H. illucens, one reared at the Laboratory of Entomology at the Department of Agriculture, Food, and Environment of the University of Pisa (Italy) (UNIPI population) and the other one at the Laboratory of Entomology and Agricultural Zoology of the University of Thessaly (Greece) (UTH population). The two populations were maintained under the same laboratory conditions (28 °C, 60% RH, and photoperiod 16:8 = L:D). The protocol utilized for the rearing of both populations was the same. In detail, the eggs were removed after two days from the oviposition support (a groove of wooden support (20 × 3 × 1 cm)) with a wet brush with a fine tip and moved into plastic boxes (29 × 18 × 9 cm) containing an artificial diet composed of chicken feed and water at a ratio of 40:60, respectively. Each plastic box was covered with gauze to ensure humidity. Once the first pupae appeared, the box was positioned inside a cage (47.5 × 47.5 × 93 cm model BugDorm-4M4590DH) for the emergence of the new adults. The cage was provided with water and sugar ad libitum and a plant branch to increase the surface area available for adults to lean on and perform their “lekking” behaviour for mating.
Diet composition and chemical analyses
Both H. illucens populations (UNIPI and UTH) were fed with five different diets:( a) control diet, based on poultry feed and water at 40:60 w/w; (b) fruit diet, 75% of fruits (apple, orange, and banana at a 1:1:1 ratio w/w) and 25% of control diet; (c) vegetable diet, 75% of vegetables (celery, sweet pepper, and potatoes at a 1:1:1 ratio w/w) and 25% of control diet; (d) meat diet, 75% of poultry meat and 25% of control diet; (e) mixed diet, i.e. control diet, fruit diet, vegetable diet, and meat diet at 1:1:1:1 ratio w/w. Fruits and vegetables were packaging waste from the local supermarkets, washed under tap water for 5 min, and left to air dry before use. Diets were ground by a kitchen grinder, operating at 800 W for 4 min.
The different kinds of diets were chemically analysed following the procedures specified in AOAC (2000) (i.e. crude protein (method 984.13) and ether extract (method 920.39) (Tognocchi et al. 2023)). The contents in moisture, proteins, and lipids are reported in Table 1.
Larval performance
Thirty 6-days-old larvae of H. illucens were weighted as a group and introduced in a polyethylene (PE) cup (5 cm diameter × 8 cm height), filled with 25 g of each diet. Five replicates for each diet were performed for a total of 150 larvae per diet. The cups were maintained under the same laboratory conditions described above (28 °C, 60% RH, and photoperiod 16:8 = L:D). Larval survival, weight, and length were recorded every 3 days until the larvae were 18 days old. Each group of larvae was counted and weighed with an analytical balance and then the individual larval weight was calculated. The length of each one of the larvae was measured with a ruler. The larval growth rate was calculated when the larvae were 15 days old. In fact, after that, the mature larvae stop eating to prepare to moult and, from then on, their weight starts to decrease (Bosch et al. 2019; Miranda et al. 2020). For this reason, the feed conversion ratio (FCR) was calculated 15 days after the start of the bioassay using the following formula:
The FCR indicates the proportion of digested feed assimilated and converted into body mass. The calculation of FCR was performed on as is basis. Low values of FCR indicate a high efficiency of substrate conversion to biomass, while high values of FCR indicate that the feed has been digested, but having a little nutrient value, a large part was excreted (Nyakeri et al. 2017).
Determination of chitosan yield
The same described rearing protocol was used for all five different diets to obtain 50 g of prepupae that were frozen, dried at 75 °C until constant weight, grounded and then stored at − 20 °C for chitosan production.
Chitin extraction and chitosan production were performed according to Hahn et al. (2020) with some modifications, as follows. The demineralization process of the dried raw powder was carried out by stirring at room temperature with 0.5 M formic acid (1 g DW:10 mL). After 30 min, the demineralized biomass was filtered using non-woven tissues and washed with distilled water to reach pH neutrality, and finally, it was oven-dried until complete desiccation. These washing and drying steps were repeated also after the deproteinization (2 M sodium hydroxide stirred for 2 h at 80 °C; 1 g DW 10 mL) and deacetylation process (12 M sodium hydroxide stirred for 3 h at 90 °C; 60 g DW 800 mL).
The yield of chitin and chitosan (%) was determined according to the following equation:
Chitosan production was performed in two replicates for each diet and the two populations.
Statistical data analysis
To compare the different growth traits (larval weight and length) and the FCR, data were analysed by ANOVA with a model that considered the fixed effect of the different diets (control, fruit, vegetable, meat and mixed), the different populations (UNIPI and UTH), and their interaction. Statistical differences between groups were assessed using Tukey’s test; “p” values are presented in order to declare the existence or not of statistically significant differences between tested groups. The analyses were conducted with JMP software (SAS Institute, Inc., Cary, NC, USA). The same ANOVA model was used to analyse data relating to chitin and chitosan yield.
The progression of the population survival during the experiments was estimated by Kaplan–Meier survival analyses. Survival differences among treatments were assessed by a log-rank test. The values “χ2” and “p” are represented below and help to determine whether there’s a significant difference in survival rates between different groups or categories in the data. The Kaplan–Meier analyses were performed by the SPSS 22.0 software (IBM SPSS Statistics, Armonk, North Castle, NY, USA).
Results
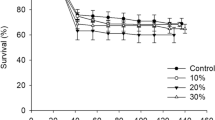
The survival analysis showed no overall differences in the larvae mortality depending on the population (χ2 = 0.043, p = 0.835) with survival rates ranging from 94 to 100% (Fig. 1). As for the diets, the highest survival rates were recorded for the control (100% survival) and the vegetable diets (97.3 and 98.6%, for UNIPI and UTH populations, respectively) with no significant difference between control and vegetable diets (χ2 = 0.043, p = 0.835).
Survival of Hermetia illucens larvae from two different populations (i.e., UNIPI and UTH population) fed on five different diets (i.e., control, fruit, vegetable, meat, and mixed diet) over a time period of 15 days. More specifically, the purple line represents the control diet, the yellow line represents the vegetable diet, the brown line represents the mix diet, the blue line represents the fruit diet, and the green line represents the meat diet
The lowest larval survival (94%) was observed in larvae fed on the meat (UNIPI, UTH) and the mixed diets (UNIPI). In general, significant differences compared to the control diet were recorded for the fruit diet (χ2 = 5.642, p = 0.18), meat diet (χ2 = 12.363, p < 0.001), and mixed diet (χ2 = 6.472, p = 0.011), while vegetable diet showed significant differences only compared to the meat diet (χ2 = 6.748, p = 0.009).
The larval weight and length on the different diets for both populations were compared (Figs. 2 and 3). For both populations, larvae ceased feeding and stopped gaining weight after 9 days (15-day-old larvae). Concerning the final individual larval weight and length, the two-way ANOVA showed a highly significant interaction between populations and diet treatments (p < 0.001 and p < 0.01, for the final individual larval weight and length, respectively) (Table 2). In particular, concerning the average larval weight for the UNIPI population, larvae reared on the control, and the fruit, diets gave better results and were statistically different compared to the vegetable, meat, and mixed diets. In contrast, for the UTH population, the control, meat, and mixed diets gave better results, statistically different, than the vegetable and fruit diets. However, among the two populations, no statistical differences have been evidenced comparing the weight of the larvae fed on the same kind of diet except the weight of the larvae reared on the meat diet. In particular, for these, the larval weights differed between the two populations, with the UNIPI population larvae weight being statistically lower than the UTH one. As for larval length, a statistical difference was recorded for the UNIPI larvae reared on the meat diet, shorter than the others. It should be noted that the larvae fed on the control diet had the highest weight (p < 0.001, 0.20 ± 0.006 g) and larval length (p < 0.01, 1.76 ± 0.016 g) compared to the other diets for both populations. Moreover, our results indicated that for both populations, the highest FCR was recorded for the meat diet. The two-way ANOVA indicated that the interaction between the populations and the dietary treatments was not significant. Both FCRs of the two populations showed significant differences between the meat diet and the other diets (p < 0.001) (Table 3).
Finally, the final biomass recovery of chitin and chitosan is reported in Table 4. Our results did not reveal the statistically significant influence of the diet factor on the yield of both chitin (p = 0.571) and chitosan (p = 0.713). The population factor also showed no significant influence on the final yield (p = 0.449 and p = 0.642 for chitin and chitosan, respectively). The same occurred for the interaction between the diet and the population factors (p = 0.641 and p = 0.699 for chitin and chitosan, respectively).
Discussion
The present study investigates the survival, growth performance, and food conversion efficiency of larvae as well as the chitin and chitosan composition of prepupae of two populations (UTH and UNIPI) of the insect species H. illucens, reared on different organic waste-based diets. From our results, the high survival rates (> 90%) for all tested diets indicate that H. illucens larvae can thrive in various organic wastes. Similar results also reported by other authors (Nguyen et al. 2013; Cammack and Tomberlin 2017; Salomone et al. 2017; Barragan-Fonseca et al. 2018; Gold et al. 2020; Kawasaki et al. 2022), including kitchen and vegetable wastes (Oonincx et al. 2019; Chia et al. 2020). On the other hand, our data showed that, while larval survival was high for all diets tested (Fig. 1), larval weight gain, larval length, and feed conversion ratio, strongly depended on the composition of each diet (Table 2; Table 3). Moreover, regarding the larval survival, the results of both populations (UNIPI and UTH) reared on a meat-based diet are in agreement with Kawasaki et al. (2022) and Candian et al. (2023), who reported the lowest larval survival when larvae were fed on a meat-based diet (77% and 88% of larval survival respectively). In another study, Zhou et al. (2013) reported differences concerning populations of different geographical origins in terms of waste conversion efficiency and development time. Similarly, for T. molitor, it has also been reported that insect populations from different geographical regions perform differently when feeding on the same diet (Rumbos et al. 2021; Adamaki-Sotiraki et al. 2022). For most of the tested diets, our results did not reveal statistical differences between the two tested populations which were fed on the same diet, except the meat diet. However, each population utilized in this study (UNIPI and UTH populations), performed better in different diets. For instance, besides the control diet, the UNIPI population gave heavier larvae on the fruit diet (0.185 ± 0.014 g), while the heavier larvae for the UTH population were produced on meat and mixed diets (0.177 ± 0.003 g and 0.177 ± 0.009 g, respectively). Based on the above, it is apparent that through a diet with a variety of different nutrient ingredients, larvae may develop faster and gain more weight than via diets with high protein content. It is possible that the results of our study may have influenced from the fact that the diets were not only organic waste, but also contained 25% poultry feed. This approach was followed in order to reduce the moisture content of the diets, as according to Montevecchi et al. (2023) excessive amounts of moisture increase larval survival. In the present study, the highest final individual larval weight was recorded, in descending order, for control, fruit, meat, and mixed diets. Regarding the larval performance obtained with the control diets, the mean individual larval weight (0.187 ± 0.006 g and 0.213 ± 0.006 g, UTH and UNIPI populations respectively) is in agreement with Bava et al. (2019), who reported 0.23 ± 0.2 g/larva. Our data are also in agreement with Jucker et al. (2017), who reported that larvae reared on fruit diets and vegetable diet had lower weights than those reared on mixed (fruit and vegetable) diet. The meat-based diet appears to be unsuitable, as evidenced by the UNIPI results which are in line with those of Gobbi et al. (2013) and Kawasaki et al. (2022) which show the lowest prepupal rate (23%) and adults’ weight (8–9%) if compared with the control diet (32% of prepupal rate and 15–16% of adults’ weight, respectively). Along the same lines, Nguyen et al. (2013) investigated larval development on six different feeding substrates (i.e. fruits, vegetables, liver, manure, fish rendering, and kitchen waste). Their results showed that larvae fed on kitchen waste gained the highest weight. The authors highlight that equilibrium between calories, fat, and protein is important for H. illucens larvae diets. In a few relevant works, Lee et al. (2014) opine that vegetable diets contain fibres, and Barbi et al. (2020) report that fruits are high in carbohydrates. However, neither vegetables nor fruits are high in protein and should be combined with proteinaceous sources to produce fast-growing larvae with high body weight. Yet, exceeding proportions of protein in diets may diminish larval growth (Nguyen et al. 2013; Kawasaki et al. 2022). Interestingly, Barbi et al. (2020) reported that larval growth and development time were optimized by a diet composed of a variety of different fruits, but larvae fed on diets with specific fruits, i.e. melon and exotic fruit, performed better. The authors reported that melon contains a high percentage of fibres, and exotic fruit is rich in polyphenols and carbohydrates. Our data also showed a connection between FCR and diet, which is also supported by the statistical analysis which revealed that FCR was not affected by population but was affected by diet. Indicatively, for larvae fed on meat-based diets, the highest FCR was recorded for both populations, thus resulting in the least suitable diet. The present study confirms previous studies reporting that the composition of the substrate has an important effect on the final growth performance of H. illucens larvae (Nguyen et al. 2013; Jucker et al. 2017; Meneguz et al. 2018; Barbi et al. 2020; Chia et al. 2020; Kawasaki et al. 2022). Interestingly, in contrast to our results, Montevecchi et al. (2023) reported a correlation between larval weight and the protein quantity present in the substrate. The authors utilized a variety of kitchen leftovers. It is evident that, as additional scientific data emerge, it becomes apparent that the growth of H. illucens larvae when fed a mixed diet substrate is influenced not only by protein quantity, but also by protein quality.
Apart from feeding substrates, there is a growing interest of scientific and industrial stakeholders on chitin and chitosan in insects due to its various applications (Mohan et al. 2020; Aranaz et al. 2021). Combining these two subjects, in one of the few available studies, Meneguz et al. (2018) investigated the chitin content of H. illucens prepupae that derived from larvae reared on fruit and vegetable diets, as well as on brewery and winery by-products. The researchers reported that there is a correlation between the chitin content of prepupae and the development time of larvae but not with the substrate. Similar results were reported by Diener et al. (2009), who observed that the larvae that grew slower, and gained less weight had high chitin content. Our results are in line with the above-mentioned studies, as in both populations (UNIPI and UTH), no influence of the 5 different rearing substrates was found on the chitin content extracted from H. illucens prepupae. Given the fact that the feeding substrate represent a significant cost in the industrial production of insects, its correlation with a high value products (i.e. chitosan) should be further investigated. Moreover, potentially, the prospect of biomass increase during H. illucens larvae production will also lead to increased chitin derived from insects. Thus, additional research should be focused on the potential correlation of the feeding substrate and the chitin composition of insects. On the other hand, as the prepupal stage of H. illucens is currently used as animal feed, the metabolization of chitin by farmed animals when the larvae or prepupae were offered as feed could have adverse effects (Lee et al. 2022). Thus, depending on the final product that is to be produced, industries could benefit from the establishment of a suitable diet.
Conclusions
In conclusion, the larvae reared on the fruits, vegetables, and mixed diets showed efficient conversion of the substrate to body weight, as indicated by the low FCR indices, with no statistical difference between the diets or between the two populations. The results thus confirm that larvae reared on vegetable and fruit waste, legally permitted in the EU, are suitable for high-performance mass production of BSF prepupae as animal feed. The present study is among the few highlighting the need to investigate the connection of H. illucens larval growth performance on different organic wastes with the chitin and chitosan content of the prepupae. Although, none of the different substrates affected the chitin and chitosan content in either population, in order for the industries to increase the availability of chitin, the correlation of the diet provided to chitin production should be further investigated.
Data availability
Data will be made available on request.
References
Adamaki-Sotiraki C, Rumbos C, Athanassiou C (2022) Strain effect on the adult performance of the yellow mealworm, Tenebrio molitor L. J Insects Food Feed: 1–10. https://doi.org/10.3920/JIFF2021.0207
Aranaz I, Alcántara AR, Civera MC, Arias C, Elorza B, Heras Caballero A, Acosta N (2021) Chitosan: an overview of its properties and applications. Polymers 13:3256. https://doi.org/10.3390/polym13193256
AOAC (2000) Official Methods of Analysis, 17th edn. The Association of Official Analytical Chemists, Gaithersburg, MD, USA. Methods 984.13 and 920.39
Barbi S, Macavei LI, Fuso A, Luparelli AV, Caligiani A, Ferrari AM, Maistrello L, Montorsi M (2020) Valorization of seasonal agri-food leftovers through insects. Sci Total Environ 709:136209. https://doi.org/10.1016/j.scitotenv.2019.136209
Barragan-Fonseca KB, Dicke M, van Loon JJ (2018) Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol Exp Appl 166:761–770. https://doi.org/10.1111/eea.12716
Bava L, Jucker C, Gislon G, Lupi D, Savoldelli S, Zucali M, Colombini S (2019) Rearing of Hermetia illucens on different organic by-products: influence on growth, waste reduction, and environmental impact. Animals 9:289. https://doi.org/10.3390/ani9060289
Bosch G, Van Zanten H, Zamprogna A, Veenenbos M, Meijer N, Van der Fels-Klerx H, Van Loon J (2019) Conversion of organic resources by black soldier fly larvae: legislation, efficiency and environmental impact. J Clean Prod 222:355–363. https://doi.org/10.1016/j.jclepro.2019.02.270
Brammer CA, von Dohlen CD (2007) Evolutionary history of Stratiomyidae (Insecta: Diptera): the molecular phylogeny of a diverse family of flies. Mol Phylogenetics Evol 43:660–673. https://doi.org/10.1016/j.ympev.2006.09.006
Cammack JA, Tomberlin JK (2017) The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 8:56. https://doi.org/10.3390/insects8020056
Candian V, Meneguz M, Tedeschi R (2023) Immune responses of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae) reared on catering waste. Life 13:213. https://doi.org/10.3390/life13010213
Chia SY, Tanga CM, Osuga IM, Cheseto X, Ekesi S, Dicke M, van Loon JJ (2020) Nutritional composition of black soldier fly larvae feeding on agro-industrial by-products. Entomol Exp Appl 168:472–481. https://doi.org/10.1111/eea.12940
Diener S, Zurbrügg C, Tockner K (2009) Conversion of organic material by black soldier fly larvae: establishing optimal feeding rates. Waste Manag Res 27:603–610. https://doi.org/10.1177/0734242X09103838
EC (2020) European Commission (EC) (2020). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: a farm to fork strategy for a fair, healthy and environmentally-friendly food system COM/2020/381 final. Available at: https://ec.europa.eu/food/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf
FAO (2011) Global food losses and food waste, causes and prevention. Rome: Food and Agriculture Organization of the United Nations. https://www.fao.org/3/i2697e/i2697e.pdf
Gianotten N, Soetemans L, Bastiaens L (2020) Agri-food side-stream inclusions in the diet of Alphitobius diaperinus part 1: impact on larvae growth performance parameters. Insects 11:79. https://doi.org/10.3390/insects11020079
Gobbi P, Martinez-Sanchez A, Rojo S (2013) The effects of larval diet on adult life-history traits of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Eur J Entomol 110: 461. http://www.eje.cz/pdfs/110/3/461ISSN1210-5759
Gold M, Cassar CM, Zurbrügg C, Kreuzer M, Boulos S, Diener S, Mathys A (2020) Biowaste treatment with black soldier fly larvae: increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag 102:319–329. https://doi.org/10.1016/j.wasman.2019.10.036
Hahn T, Roth A, Ji R, Schmitt E, Zibek S (2020) Chitosan production with larval exoskeletons derived from the insect protein production. J Biotech 310:62–67. https://doi.org/10.1016/j.jbiotec.2019.12.015
Hamed I, Özogul F, Regenstein JM (2016) Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci Technol 48:40–50. https://doi.org/10.1016/j.tifs.2015.11.007
Jucker C, Erba D, Leonardi MG, Lupi D, Savoldelli S (2017) Assessment of vegetable and fruit substrates as potential rearing media for Hermetia illucens (Diptera: Stratiomyidae) larvae. Environ Entomol 46:1415–1423. https://doi.org/10.1093/ee/nvx154
Kawasaki K, Ohkawa M, Zhao J, Yano K (2022) Effect of dietary meat content on weight gain, mortality, and pre-pupal rate in black soldier fly (Hermetia illucens) larvae. Insects 13:229. https://doi.org/10.3390/insects13030229
Kroeckel S, Harjes AG, Roth I, Katz H, Wuertz S, Susenbeth A, Schulz C (2012) When a turbot catches a fly: evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute - growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 364:345–352. https://doi.org/10.1016/j.aquaculture.2012.08.041
Kumar Awasthi M, Paul A, Kumar V, Sar T, Kumar D, Sarsaiya S, Liu H, Zhang Z, Binod P, Sindhu R, Kumar V, Taherzadeh MJ (2022) Recent trends and developments on integrated biochemical conversion process for valorization of dairy waste to value added bioproducts: a review. Bioresour Technol 344:126193. https://doi.org/10.1016/j.biortech.2021.126193
Lee CM, Lee YS, Seo SH, Yoon SH, Kim SJ, Hahn BS, Sim JS, Koo BS (2014) Screening and characterization of a novel cellulase gene from the gut microflora of Hermetia illucens using the metagenomic library. J Microbiol Biotechnol 24:1196–1206. https://doi.org/10.4014/jmb.1405.05001
Lee JH, Kim TK, Cha JY, Jang HW, Yong HI, Choi YS (2022) How to develop strategies to use insects as animal feed: digestibility, functionality, safety, and regulation. J Anim Sci Technol 64:409. https://doi.org/10.5187/jast.2022.e27
Meneguz M, Schiavone A, Gai F, Dama A, Lussiana C, Renna M, Gasco L (2018) Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J Sci Food Agric 98:5776–5784. https://doi.org/10.1002/jsfa.9127
Miranda CD, Cammack JA, Tomberlin JK (2020) Mass production of the black soldier fly, Hermetia illucens (L.), (Diptera: Stratiomyidae) reared on three manure types. Animals 10:1243. https://doi.org/10.3390/ani10071243
Mohan K, Ganesan AR, Muralisankar T, Jayakumar R, Sathishkumar P, Uthayakumar V, ... Revathi N (2020) Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci Technol 105:17–42. https://doi.org/10.1016/j.tifs.2020.08.016
Montevecchi G, Macavei LI, Zanelli E, Benassi G, Pinotti G, D’Arco S, ... & Antonelli A (2023) Seasonal variability of the HO.RE.CA. food leftovers employed as a feeding substrate for black soldier fly (Hermetia illucens L.) larvae and effects on the rearing performance. Sustain Chem Pharm 33:101061. https://doi.org/10.1016/j.scp.2023.101061
Nguyen TT, Tomberlin JK, Vanlaerhoven S (2013) Influence of resources on Hermetia illucens (Diptera: Stratiomyidae) larval development. J Med Entomol 50:898–906. https://doi.org/10.1603/me12260
Nyakeri EM, Ogola HJO, Ayieko MA, Amimo FA (2017) Valorisation of organic waste material: growth performance of wild black soldier fly larvae (Hermetia illucens) reared on different organic wastes. J Insects Food Feed 3:193–202. https://doi.org/10.3920/JIFF2017.0004
Oonincx DG, van Broekhoven S, van Huis A, van Loon JJ (2019) Correction: Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS One 14:e0222043. https://doi.org/10.1371/journal.pone.0144601
Panikkar P, Parakkandi J, Khan F, Das BK, Udayakumar A, Eregowda VM, Yandigeri M (2022) Use of black soldier fly (Hermetia illucens) prepupae reared on organic waste as feed or as an ingredient in a pellet-feed formulation for Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res 29:72968–72978. https://doi.org/10.1007/s11356-022-20926-3
Park BK, Kim MM (2010) Applications of chitin and its derivatives in biological medicine. Int J Mol Sci 11:5152–5164. https://doi.org/10.3390/ijms11125152
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
Rumbos C, Adamaki-Sotiraki C, Gourgouta M, Karapanagiotidis I, Asimaki A, Mente E, Athanassiou C (2021) Strain matters: strain effect on the larval growth and performance of the yellow mealworm, Tenebrio molitor L. J Insects Food Feed 7:1195–1205. https://doi.org/10.3920/JIFF2021.0035
Rumbos C, Oonincx D, Karapanagiotidis I, Vrontaki M, Gourgouta M, Asimaki A, Mente E, Athanassiou C (2022) Agricultural by-products from Greece as feed for yellow mealworm larvae: circular economy at a local level. J Insects Food Feed 8:9–22. https://doi.org/10.3920/JIFF2021.0044
Salomone R, Saija G, Mondello G, Giannetto A, Fasulo S, Savastano D (2017) Environmental impact of food waste bioconversion by insects: application of life cycle assessment to process using Hermetia illucens. J Clean Prod 140:890–905. https://doi.org/10.1016/j.jclepro.2016.06.154
Sheppard DC, Newton GL, Thompson SA, Savage S (1994) A value added manure management system using the black soldier fly. Bioresour Technol 50:275–279. https://doi.org/10.1016/0960-8524(94)90102-3
Sinha S, Tripath P (2021) Trends and challenges in valorisation of food waste in developing economies: a case study of India. Case Stud Chem Environ Eng 4:100162. https://doi.org/10.1016/j.cscee.2021.100162
Tognocchi M, Conte G, Rossi E, Perioli R, Mantino A, Serra A, Mele M (2023) Characterization of polar and non-polar lipids of Hermetia illucens and Tenebrio molitor meals as animal feed ingredients. Anim Feed Sci Technol 295:115524. https://doi.org/10.1016/j.anifeedsci.2022.115524
Van Broekhoven S, Oonincx DG, Van Huis A, Van Loon JJ (2015) Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J Insect Physiol 73:1–10. https://doi.org/10.1016/j.jinsphys.2014.12.005
Zhou F, Tomberlin JK, Zheng L, Yu Z, Zhang J (2013) Developmental and waste reduction plasticity of three black soldier fly populations (Diptera: Stratiomyidae) raised on different livestock manures. J Med Entomol 50:1224–1230. https://doi.org/10.1603/ME13021
Acknowledgements
Sincere thanks for their patience go to the staff of the Carrefour Express supermarket where the meat and vegetable scraps used during the experiment were purchased.
Funding
This research was granted by the PRIMA program, project FEDKITO. The PRIMA program is supported by the European Union and by the Italian Ministero dell’Università e della Ricerca.
Author information
Authors and Affiliations
Contributions
Adamaki-Sotiraki C., Abenaim L, Mannucci A., Rumbos I.C., Bedini S., Castagna A., Conte G., Tognocchi M., Dolianitis V., Athanassiou G.C., and Conti B. revised the content and all agreed to submit the manuscript. Prior to submission, Adamaki-Sotiraki C., Abenaim L, Mannucci A., Rumbos I.C., Bedini S., Castagna A., Conte G., Tognocchi M., Dolianitis V., Athanassiou G.C., and Conti B. contributed to the conceptualization of the idea as well as to the design of the work and analysis of data. Moreover, Adamaki-Sotiraki C., Abenaim L, Mannucci A., Rumbos I.C., Bedini S., Castagna A., Conte G., Tognocchi M., Dolianitis V., Athanassiou G.C., and Conti B. approved the publication of this version and declared to be accountable for all aspects of the work in ensuring that inquiries regarding the accuracy or integrity of all parts of this work are investigated and resolved appropriately.
Corresponding author
Ethics declarations
Ethical approval
Research activities in the present study involved invertebrate animals, in particular insects. Insect mass rearing was performed taking into consideration insect welfare, treating insects as sentient beings and ensuring insect well-being. Particularly, trials were conducted with the following insect species: Hermetia illucens (Diptera: Stratiomyidae). No ethical restrictions are applied for experimental and practical tests having as subject the above cited species. All the trials that involved insects were conducted by UNIPI and UTH.
Consent to participate
No human subjects were involved; thus, this part is not applicable.
Consent for publication
No human subjects were involved; thus, this part is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adamaki-Sotiraki, C., Abenaim, L., Mannucci, A. et al. Performance of Hermetia illucens (Diptera: Stratiomyidae) larvae reared on organic waste diets and pupal chitin and chitosan yield. Environ Sci Pollut Res 31, 37366–37375 (2024). https://doi.org/10.1007/s11356-024-33545-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-33545-x