Abstract
Polyhydroxybutyrate (PHB) production through CH4 conversion by methanotrophs offers a solution for greenhouse gas emissions and plastic waste concerns. In this study, we aimed to achieve high cell density cultivation of Methylocystis sp. MJC1 for efficient PHB production. Cultivating MJC1 using CH4 and air (3:7, v/v) yielded a final cell density of 52.9 g/L with a 53.7% (28.4 g/L) PHB content after 210 h, showcasing PHB mass production potential. However, long-term cultivation led to a low volumetric productivity of 0.200 g/L/h. To address this, we conducted cultivation at various O2/CH4 ratios using O2 instead of air, which significantly improved the PHB productivity. Under high O2 conditions (O2/CH4 ratio of 1.5), biomass productivity increased 1.51-fold compared to that under low O2 conditions in the same time frame; however, PHB accumulation was delayed. Using an equal ratio of CH4 and O2 induced active cell growth and selective PHB production, achieving the highest PHB productivity (0.365 g/L/h) with a final cell density of 55.9 g/L and PHB content of 61.7% (34.5 g/L) in 162 h. This study highlighted the significance of the O2/CH4 ratio in CH4 conversion and PHB production by M. sp. MJC1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Methane (CH4) is an organic pollutant generated by various human activities, and ranks second only to carbon dioxide (CO2) as one of the most emitted greenhouse gases (Ritchie et al. 2020). In contrast to fully oxidized CO2, CH4, which is the most reduced form of carbon, can be converted into energy and chemical feedstock through appropriate transformation processes (Sobanna et al. 2024; Caballero and Pérez 2013). Hence, there is an urgent need for effective treatments to achieve meaningful greenhouse gas mitigation and explore new carbon resources. However, finding activation pathways for stable C-H bonds in CH4 has proven challenging, aside from gasification or reforming processes that require high temperatures and pressures (Franz et al. 2021). As a result, CH4 has been predominantly employed as a fuel, relying on simple combustion for purposes other than H2 production. An alternative approach is the biological CH4 conversion route, which utilizes methanotrophs (Nguyen et al. 2021). Recent advances in metabolic engineering techniques have enabled the incorporation of foreign biosynthesis pathways into the inherent metabolism of methanotrophs, leading to the production of diverse substances (Nguyen et al. 2020; Gęsicka et al. 2021; Kang et al. 2024).
One of the most actively researched applications of methanotrophs is in the production of biodegradable polymers (Liu et al. 2020). Type II methanotrophs possess a unique polyhydroxybutyrate (PHB) cycle that allows them to accumulate biodegradable polymers under nutrient-limiting conditions without genetic modifications (Karthikeyan et al. 2015; Singh et al. 2022). Moreover, they can produce significant quantities of intracellular products through high cell density cultivation. The use of methanotrophs for CH4 conversion and biodegradable polymer production has already seen some commercialization efforts (Comesaña-Gándara et al. 2022; Koller and Mukherjee 2022).
Despite the advantages of using CH4 as an inexpensive feedstock and its potential application in greenhouse gas conversion, the PHB production process utilizing methanotrophs remains less competitive than conventional PHA production technologies that rely on sugars (Younes et al. 2021; Ahmed et al. 2018). Traditional sugar-based PHA fermentation has a high productivity (> 2 g/L/h) (Kim et al. 1994), with a specific PHA synthesis rate of approximately 0.145 g-PHB/g-cell/h (Du et al. 2001). In contrast, methanotrophs exhibit not only slower intrinsic growth rates compared to established PHA producers, such as Escherichia coli and Cupriavidus necator (Gilman et al. 2015), but also operate at lower growth rates than their inherent growth rates due to gas transfer limitations.
To address these issues, it is of paramount importance to achieve high-cell-density cultures with high PHB content; however, most studies have primarily explored PHB accumulation conditions in small-scale settings, such as flasks or serum bottles (Pieja et al. 2011; Zhang et al. 2017). Notably, the gas composition must be fine-tuned because its impact on both cell growth and PHB accumulation is complex, and the required ratios may vary depending on the stage of cell growth (Rostkowski et al. 2013; Khosravi-Darani et al. 2013; Rodríguez et al 2020). Therefore, there is a pressing need for research at the bioreactor level to allow a comprehensive analysis of the effects of gas substrates while accurately observing gas-transfer phenomena.
In this study, we cultivated Methylocystis sp. MJC1, a type II methanotroph with excellent PHB production capabilities (Choi et al. 2021; Naizabekov et al. 2023), in bioreactors to analyze the effects of gas composition. We initially conducted cultivation using CH4 and air as gaseous substrates, with NH4+ ions as the nitrogen source, to explore the high-cell-density cultivation of M. sp. MJC1 and its associated potential for PHB mass production. Subsequently, we investigated CH4 conversion under various CH4/O2 ratios while excluding atmospheric N2 fixation using pure O2 and compared the fermentation performances, aiming to enhance both cell growth and PHB accumulation by optimizing the CH4 to O2 ratio.
Experimental conditions
Microorganism and seed culture conditions
Methylocystis sp. MJC1 is used for CH4 conversion and PHB production. This strain was stored at -80 ℃ with 20% (v/v) glycerol. Before inoculating the fermenter, two successive seed cultures were performed in nitrate mineral salt (NMS) medium (Jo et al. 2020). The composition of the NMS medium used in the seed culture was: KNO3 2 g/L; MgSO4·7H2O 1 g/L; CaCl2·2H2O 0.2 g/L; Fe-EDTA 0.0038 g/L; Na2MoO4·2H2O 0.0006 g/L; trace element stock 100 μL/L (FeSO4·7H2O 5 g/L; ZnSO4·7H2O 4 g/L; MnCl2·7H2O 0.2 g/L; H3BO3 0.15 g/L; CoCl2·6H2O 0.5 g/L; NiCl2·6H2O 0.1 g/L; EDTA 2.5 g/L); phosphate buffer stock 10 mL/L (KH2PO4 26 g/L; Na2HPO4·7H2O 62 g/L); CuSO4·5H2O 2.5 mg/L; and vitamin stock 1 mL/L (Biotin 20 mg/L; Folic acid 20 mg/L; Thiamine HCl 50 mg/L; Ca pantothenate 50 mg/L; Vitamin B12 1 mg/L; Riboflavin 50 mg/L; and Nicotinamide 50 mg/L). In the first seed culture, a 2 mL glycerol stock from -80 °C was inoculated into a 500 ml baffled flask with a butyl-rubber septum containing 50 mL of NMS medium. Gas with a CH4:air ratio of 3:7 (v/v) was supplied to the flask at 600 mL/min for 4 min and the headspace was refreshed after two days. The first seed culture was conducted at 30 °C and 230 rpm in a shaking incubator (Vision Bionex, Republic of Korea) for three days. It was then transferred to a 500 ml baffled flask with a butyl rubber septum containing 100 mL of NMS, with an inoculum size of 10%. The second seed culture for fermenter inoculation was carried out under the same conditions as the first culture (30 °C, 230 rpm) for three days, except for headspace gas replacement every 24 h.
Bioreactor operation
In this study, the experiments were conducted using four different gas compositions, as outlined in Table 1. All batch cultures were initiated with a 10% (v/v) inoculum at an agitation speed of 400 rpm. If the dissolved oxygen (DO) level decreased below 40%, the agitation speed was incrementally increased by 100 rpm until it reached a maximum of 800 rpm. When the DO level decreased below 10% at the maximum rpm, the specified gas mixture was introduced into the bioreactor according to each condition. For all cultures, a modified ammonium mineral salts medium was used, with the following composition: NH4Cl 0.75 g/L; MgSO4·7H2O 1 g/L; CaCl2·2H2O 0.2 g/L; Fe-EDTA 0.0038 g/L; Na2MoO4·2H2O 0.00118 g/L; trace element stock 1 mL/L (FeSO4·7H2O 22.2 g/L; ZnSO4·7H2O 2.6 g/L; MnCl2·7H2O 1.909 g/L; H3BO3 0.12 g/L; CoCl2·6H2O 0.8 g/L; NiCl2·6H2O 0.024 g/L; EDTA 3.3 g/L); phosphate buffer stock 50 mL/L (KH2PO4 26 g/L; Na2HPO4·7H2O 62 g/L); CuSO4·5H2O 5 mg/L; and vitamin stock 1 mL/L (Biotin 20 mg/L; Folic acid 20 mg/L; Thiamine HCl 50 mg/L; Ca pantothenate 50 mg/L; Vitamin B12 1 mg/L; Riboflavin 50 mg/L; and Nicotinamide 50 mg/L). The temperature was maintained at 30 °C, and the pH was kept within the range of 6.4 – 6.6. Initially, the pH was adjusted using 1N H2SO4 and 1N NaOH. Near an OD600 of 3, the alkali supplied was changed to a 16 wt% ammonium hydroxide (NH4OH) solution for pH control and nitrogen supply.
Cultivation of MJC1 using CH4 and air
In condition I, utilizing CH4 and air, cultivation was conducted in a 5 L fermentor (BioCNS, Republic of Korea) with a working volume of 3 L. After agitation reached 800 rpm, whenever DO dropped below 10%, a CH4:Air ratio of 3:7 was maintained, increasing aeration by 100 mL/min each time to provide a maximum of 0.5 vvm of gas.
Cultivation of MJC1 using CH4 and O2 with Ar balance
Cultivations under conditions II, III, and IV, using CH4 and pure O2 with Ar balance, was carried out in 4 L fermentors (Injae Bio-Tech, Republic of Korea) with a working volume of 2.5 L. Pure O2 was used instead of air, and CH4 and O2 were supplied at ratios of 1:0.5 (condition II), 1:1.5 (condition III), and 1:1.0 (condition IV). In all conditions, the gas flow rate started at 0.2 vvm, and the agitation was increased to 800 rpm depending on the DO level, with the flow rate reaching a maximum of 0.5 vvm when the DO dropped below 10%.
Analytical methods
Cell mass and PHB contents
Cell mass was determined by measuring the optical density (OD) at 600 nm using a UV–visible spectrophotometer (Biochrom WPA Lightwave 3, Biochrom, UK), and the relationship between OD and dry cell weight was established. The PHB content was measured using the GC-FID method, as previously described (Khang et al. 2021). The composition of the solution used for the PHB analysis is as follows: CH3OH, 970 mL/L; H2SO4, 30 mL/L; and C6H5COOH, 8 g/L. Furthermore, 10 mg of dried cell pellet was suspended in 1 mL of the above solution and 2 mL of chloroform followed by a trans-esterification reaction overnight at 95 °C. After the completion of reaction, 1 mL of distilled water was added to each sample. Phase-separated samples were obtained from the chloroform layer and analyzed for PHB content using a GC-FID (Young In Chromass, Republic of Korea) with a J&W DB-WAX 123–7033 column (Agilent, USA). All analyses were performed in triplicate.
Ammonium ion concentration
The ammonium ion (NH4+) concentration in the culture medium was measured using Horiba LAQUA F-72 (Horiba, Japan) with an ammonia electrode, 5002S-10C (Horiba), and 100 mg/L ammonium ion standard solution (Horiba).
Off-gas analysis
The off-gas composition of the fermenter was analyzed using a gas chromatograph (7890N, Agilent, USA) with Carboxen 1000 column (Sigma-Aldrich, USA) and Agilent J&W 122–5532 column (Agilent, USA). The analysis was conducted using a thermal conductivity detector (TCD) and flame ionization detector (FID). Initially, oven temperature was held at 40 °C for 8.5 min, then increased to 200 °C at 50 °C/min and held at 200 °C for 5.1 mim. The injector and detector were kept at 250 and 200 °C, respectively. Helium was used as a carrier gas at a flow rate of 30 mL/min.
Results and Discussion
Cultivation of M. sp. MJC1 using CH4 and air as gaseous substrates (condition I)
We cultivated M. sp. MJC1 using CH4 and air at a 3:7 (v/v) ratio (O2/CH4 = 0.5), which is commonly used in methanotroph culture (Nguyen et al. 2020) in a 5 L bioreactor. As shown in Fig. 1, after 210 h of cultivation, the final cell density reached 52.9 g/L, and the polyhydroxybutyrate (PHB) content at this point was 53.7% (28.4 g/L), confirming the potential for high-density cultivation and PHB production by M. sp. MJC1. The volumetric productivity of PHB in the production phase was 0.200 g/L/h. We observed that nitrogen supply by adding an NH4OH solution for pH control was effective for cell growth, and as nitrogen became depleted, PHB production ensued.
Time profiles of (a) cell mass (solid circles), NH4+ concentration (solid triangles), (b) residual cell mass (blank circles), PHB concentration (blank squares), and (c) CH4 (solid line) and O2 (dotted line) consumption rate, CO2 evolution rate (dot-dashed line), the ratio of CH4 consumption to O2 consumption (dashed line) and CO2 evolution to O2 consumption (long dashed line) in the cultivation of Methylocystis sp. MJC1 using CH4 and air
Examining the semilog plot of cell growth in Fig. 5 and Table 1, it is evident that MJC1 cells exhibited exponential growth for up to 54 h, with a specific growth rate of 0.0993 h−1, after which cell growth was significantly retarded. This deceleration was attributed to gas transfer limitations rather than nitrogen depletion because the NH4+ ion concentration was 220 mg/L. PHB accumulation commenced within 72 h, which was similar to the nitrogen depletion point.
According to the analysis of the off-gas from the bioreactor (Fig. 1(c)), a noticeable decrease in CH4 and O2 consumption rates was observed at approximately 56 h, coinciding with the transition to the gas transfer limitation phase. The ratio of the O2 to CH4 consumption rates remained at approximately 1.16 mol/mol until approximately 66 h, after which it decreased to approximately 1.06 mol/mol. The respiratory quotient (RQ), the ratio of CO2 evolution to O2 consumption, slightly increased from 0.38 to 0.44 throughout the cultivation period. The O2:CH4 ratio fed into the reactor was 0.5, which is significantly lower than the consumed O2:CH4 ratio. This disparity suggests that as the cultivation progressed, O2 acted as a limiting substrate. It is necessary to conduct cultivation experiments at various gas ratios to balance CH4 and O2 consumption according to the process demands.
The cell mass was estimated based on the nitrogen balance using the nitrogen content in the microbial cells, the amount of supplied NH4OH solution, and the NH4+ ion concentration in the culture medium (dotted line in Fig. 1(b)). The estimated values closely matched the cell mass for up to 72 h, but a significant discrepancy became evident thereafter. Comparing the estimated values with the residual cell mass (blank circle in Fig. 1(a)), the difference remained after excluding the PHB concentration from the total cell mass. This discrepancy can be attributed to atmospheric N2 fixation. Type II methanotrophic bacteria, including Methylocystis sp., are capable of N2 fixation (Karthikeyan et al. 2015). While fixation has the advantage of not requiring liquid nitrogen supplementation, it demands significant reducing power and energy, potentially lowering the CH4 to biomass yield per unit of CH4 and complicating the precise induction of PHB synthesis. Therefore, we conducted cultivations with pure O2 to eliminate the influence of N2 in M. sp. MJC1 cultivation.
High cell density cultivation of M. sp. MJC1 using CH4 and pure O2
To elucidate the effects of the CH4 to O2 ratio in the feed gas on cell growth and PHB synthesis of the methanotroph through CH4 conversion, we conducted CH4 conversion experiments employing various O2/CH4 ratios using a 4 L bioreactor (2.5 L working volume). Cultivation was performed under conditions of low oxygen (condition II, O2/CH4 = 0.5), high oxygen (condition III, O2/CH4 = 1.5), and equal proportions of CH4 and O2 (condition IV, O2/CH4 = 1.0). Argon, with the same composition as N2 in air, was used to maintain a constant CH4 proportion in the feed gas.
Cultivation under low O2 condition (condition II)
Using the same CH4 to O2 ratio as in the previous experiment (O2/CH4 = 0.5), we cultivated M. sp. MJC1 using CH4 and pure O2 with argon balance. After 95 h of cultivation, the cell density reached 28.8 g/L, with a PHB content of 30.3% (8.73 g/L). PHB production occurred slightly earlier than when air was used. Moreover, the specific growth rates at the exponential growth phase and gas transfer limitation phase were somewhat higher than those with air as the oxygen source, reaching 0.100 h−1 and 0.0224 h−1, respectively (Fig. 2, Table 1). When compared to a cell concentration of 24.2 g/L and PHB content of 17.3% (4.19 g/L) achieved in 102-h cultivation with air-based fermentation, it was evident that the use of pure O2 significantly outperformed, indicating that the reducing power and energy used for N2 fixation were redirected toward PHB synthesis. Consequently, the volumetric productivity of PHB reached 0.273 g/L/h, resulting in a 136.5% improvement in performance.
Time profiles of (a) cell mass (solid circles), NH4+ concentration (solid triangles), (b) residual cell mass (blank circles), and PHB concentration (blank squares) in the cultivation of Methylocystis sp. MJC1 using CH4 and O2 with the O2/CH4 ratio of 0.5. The dotted line indicates the residual cell mass estimated by nitrogen balance
Cultivation under high O2 condition (condition III)
We cultivated M. sp. MJC1 cells using a CH4: O2 ratio of 1:1.5 for the same duration as the experiment performed under the low O2 condition. This O2/CH4 ratio of 1.5 corresponds to the stoichiometric ratio in the serine cycle, which is the core metabolic pathway of type II methanotrophs (Karthikeyan et al. 2015). After 95 h of cultivation, the cell concentration reached 43.4 g/L, with a PHB content of 20.3% (8.81 g/L) (Fig. 3). In terms of cell growth, at the low O2 condition, a final cell mass of 28.8 g/L was obtained, while at high O2 condition, 1.51 times more biomass could be produced. This was due to the increase in specific growth rates during the initial exponential growth phase and the gas transfer limitation phase, which were 0.111 h−1 and 0.0286 h−1, respectively, faster than the 0.100 h−1 and 0.0224 h−1 for the low O2 condition (Table 1). Particularly during the gas transfer limitation phase, it was evident that a high specific growth rate of 0.0515 h−1 was maintained for up to 68 h of cultivation, indicating a significantly more efficient substrate supply compared to the low O2 condition. The final PHB content was 20.3%, much lower compared to 30.3% of the low O2 condition, as the accumulation of PHB occurred later because of active cell growth.
Time profiles of (a) cell mass (solid circles), NH4+ concentration (solid triangles), (b) residual cell mass (blank circles), and PHB concentration (blank squares) in the cultivation of Methylocystis sp. MJC1 using CH4 and O2 with the O2/CH4 ratio of 1.5. The dotted line indicates the residual cell mass estimated by nitrogen balance
Furthermore, Fig. 3(b) shows that the estimated values from the nitrogen balance closely reflected the residual cell mass, confirming that the discrepancies between the estimated and actual values in cultivation using air were due to N2 fixation. This suggests that cell mass can be predicted efficiently from simple calculations using the NH4OH feeding amount and the NH4+ ion concentration in the reactor during pH–stat cultivation without N2 fixation.
Cultivation with equal proportion of CH4 and O2 (condition IV)
The initial step in CH4 assimilation by aerobic methanotrophs is methanol production via CH4 oxidation, in which CH4 and O2 react in a 1:1 stoichiometric ratio. Considering this as the rate-limiting step, we conducted M. sp. MJC1 cell cultivation using pure O2 while supplying CH4 and O2 in equal proportions. Figure 4 shows that after 97.5 h of cultivation, the cell concentration reached 34.8 g/L, with a PHB content of 25.3% (8.78 g/L).
Time profiles of (a) cell mass (solid circles), NH4+ concentration (solid triangles), (b) residual cell mass (blank circles), and PHB concentration (blank squares) in the cultivation of Methylocystis sp. MJC1 using CH4 and O2 with the O2/CH4 ratio of 1.0. The dotted line indicates the residual cell mass estimated by nitrogen balance
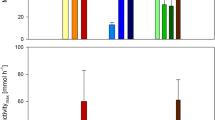
Figure 5 compares the performances of M. sp. MJC1 cultivation under each O2/CH4 condition using pure O2. As shown in Fig. 5, the PHB concentration at the end of 95 h cultivation was comparable for each condition: 8.73 g/L (condition II), 8.79 g/L (condition III), and 8.78 g/L (condition IV). However, in terms of productivity after the onset of PHB production, the condition with equal oxygen and methane ratios demonstrated the highest productivity of 0.288 g/L/h (condition IV), followed by the condition with sufficient oxygen (condition III) of 0.277 g/L/h, and the condition with insufficient oxygen (condition II) of 0.273 g/L/h, respectively (Table 1).
Comparison of cell growth and PHB contents under various O2/CH4 ratios: (a) semilog plot of the cell mass increase relative to the initial cell mass under condition I (black circles), condition II (white circles), condition III (light gray circles), and condition IV (dark gray circles); and (b) final cell mass (blue bar) and PHB contents (red bar)
The improvement in performance of condition IV compared to the low O2 condition (condition II), can be attributed to two factors. From the perspective of cell growth, the initial specific growth rate was 0.114 h−1, which was faster than the 0.100 h−1 under low O2 condition (Table 1), allowing cells to grow sufficiently. Based on these effects, even with a somewhat lower specific growth rate in the later stages, a higher cell concentration could be obtained despite the shorter cultivation time compared with air-based fermentation.
Additionally, effective nitrogen limitation was introduced during the late stages of cultivation, primarily to promote PHB synthesis over cell growth. The values of the residual cell mass estimated from the nitrogen balance closely followed the actual values and showed no further increase after 72 h (Fig. 4(b)), indicating that the biomass increase in the later stages was primarily due to PHB accumulation.
The reason for the higher PHB productivity compared to the high O2 condition with excellent cell growth at the same time point (95 h) was that the initiation of PHB accumulation was advanced. As is evident from Fig. 3 and 4, at the same CH4 to O2 ratio, the residual cell mass was approximately 25 g/L, whereas it was approximately 36 g/L under high O2 condition. When considering cell growth alone, the high O2 condition appeared to be superior. However, under these conditions, gaseous substrates are predominantly utilized for cell proliferation rather than for PHB accumulation. In contrast, under the equal-ratio condition, selective PHB synthesis was initiated earlier, allowing for higher PHB content and productivity during the same time frame. In high O2 condition, active cell growth led to a more significant NH4+ supply, suggesting that nitrogen limitation was not completely induced. The volume of NH4OH solution supplied was approximately 66 mL in this equal-ratio condition, whereas it was 99 mL in the high O2 condition, indicating additional cell growth.
Considering these points, cultivations were continued until the stationary phase of biomass production to observe the behavior of PHB production under conditions III and IV. Condition III, where sufficient oxygen was supplied, was superior in total biomass as expected (Fig. 6). However, the PHB content was below 45%, resulting in a maximum PHB concentration of only 28.0 g/L. Additionally, in the stationary phase, it was observed that M. sp. MJC1 cells consumed accumulated PHB. In contrast, although condition IV exhibited a slower overall cell growth rate, PHB synthesis continued steadily, resulting in a final PHB content of 61.7% and a PHB concentration of 34.5 g/L, which outperformed the result of condition III. When O2 and CH4 were equally supplied, the volumetric productivity during PHB production reached 0.365 g/L/h, achieving the highest productivity under all conditions.
In conclusion, the CH4 to O2 ratio is crucial for CH4 conversion and PHB production by M. sp. MJC1 considering both biomass production and PHB content. The PHB content plays a vital role not only in determining the amount of PHB produced but also in influencing downstream PHB purification costs (Wang et al. 2014).
Conclusion
In this study, we investigated the effects of CH4 to O2 ratios in a bioreactor for the high cell density culture of methanotrophs and mass production of polyhydroxybutyrate (PHB). Using CH4 and air as carbon sources, M. sp. MJC1 strain achieved a cell concentration of 52.9 g/L and a PHB content of 53.7% (28.4 g/L), demonstrating the simultaneous achievement of high cell density cultivation and elevated PHB content. Nonetheless, long-term cultivation, exceeding 200 h, resulted in a low volumetric productivity of 0.200 g/L/h.
To address this issue, we employed pure O2 instead of air to prevent CH4 spills for atmospheric N2 fixation, and appropriately induced nitrogen-limiting conditions to elevate the PHB content. We also adjusted the CH4 to O2 ratio and found that it significantly influenced both cell growth and PHB content, and thus, overall PHB productivity. When CH4 and O2 were supplied in equal proportions, we achieved a cell concentration of 55.9 g/L, a PHB content of 61.7% (34.5 g/L), and a PHB productivity of 0.365 g/L/h (at PHB production phase) in just 162 h. However, it is essential to consider the cost increases and safety concerns associated with the use of pure O2. Further research on methods, such as economic O2 enriching systems and the deactivation of nitrogenase in N2-containing environments to prevent nitrogen fixation is warranted. Furthermore, selecting an appropriate switching time between the cell growth and PHB production phases and its implementation are expected to enhance productivity.
Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.
References
Ahmed T, Shahid M, Azeem F et al (2018) Biodegradation of plastics: current scenario and future prospects for environmental safety. Environ Sci Pollut Res 25:7287–7298. https://doi.org/10.1007/s11356-018-1234-9
Caballero A, Pérez PJ (2013) Methane as raw material in synthetic chemistry: the final frontier. Chem Soc Rev 42:8809–8820. https://doi.org/10.1039/C3CS60120J
Choi M, Yun T, Song MJ, Kim J, Lee BH, Löffler FE, Yoon S (2021) Cometabolic vinyl chloride degradation at acidic pH catalyzed by acidophilic methanotrophs isolated from alpine peat bogs. Environl Sci Technol 55:5959–5969. https://doi.org/10.1021/acs.est.0c08766
Comesaña-Gándara B, García-Depraect O, Santos-Beneit F et al (2022) Recent trends and advances in biogas upgrading and methanotrophs-based valorization. Chem Eng J Adv 11:100325. https://doi.org/10.1016/j.ceja.2022.100325
Du G, Chen J, Yu J, Lun S (2001) Continuous production of poly-3-hydroxybutyrate by Ralstonia eutropha in a two-stage culture system. J Biotechnol 88:59–65. https://doi.org/10.1016/S0168-1656(01)00266-8
Franz R, Uslamin EA, Pidko EA (2021) Challenges for the utilization of methane as a chemical feedstock. Mendeleev Commun 31:584–592. https://doi.org/10.1016/j.mencom.2021.09.002
Gęsicka A, Oleskowicz-Popiel P, Łężyk M (2021) Recent trends in methane to bioproduct conversion by methanotrophs. Biotechnol Adv 53:107861. https://doi.org/10.1016/j.biotechadv.2021.107861
Gilman A, Laurens LM, Puri AW et al (2015) Bioreactor performance parameters for an industrially-promising methanotroph Methylomicrobium buryatense 5GB1. Microb Cell Fact 14:182. https://doi.org/10.1186/s12934-015-0372-8
Jo SY, Rhie MN, Jung SM, Sohn YJ, Yeon YJ, Kim MS, Park C, Lee J, Park SJ, Na JG (2020) Hydrogen production from methane by Methylomonas sp. DH-1 under micro-aerobic conditions. Biotechnol Bioproc Eng 25:71–77. https://doi.org/10.1007/s12257-019-0256-6
Kang NK, Chau THT, Lee EY (2024) Engineered methane biocatalysis: strategies to assimilate methane for chemical production. Curr Opin Biotechnol 85:103031. https://doi.org/10.1016/j.copbio.2023.103031
Karthikeyan OP, Chidambarampadmavathy K, Cirés S, Heimann K (2015) Review of sustainable methane mitigation and biopolymer production. Crit Rev Environ Sci Technol 45:1579–1610. https://doi.org/10.1080/10643389.2014.966422
Khang TU, Kim MJ, Yoo JI, Sohn YJ, Jeon SG, Park SJ, Na JG (2021) Rapid analysis of polyhydroxyalkanoate contents and its monomer compositions by pyrolysis-gas chromatography combined with mass spectrometry (Py-GC/MS). Int J Biol Macromol 174:449–456. https://doi.org/10.1016/j.ijbiomac.2021.01.108
Khosravi-Darani K, Mokhtari ZB, Amai T, Tanaka K (2013) Microbial production of poly (hydroxybutyrate) from C1 carbon sources. Appl Microbiol Biotechnol 97:1407–1424. https://doi.org/10.1007/s00253-012-4649-0
Kim BS, Lee SC, Lee SY, Chang HN, Chang YK, Woo SI (1994) Production of poly (3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol Bioeng 43:892–898. https://doi.org/10.1002/bit.260430908
Koller M, Mukherjee A (2022) A new wave of industrialization of PHA biopolyesters. Bioeng 9:74. https://doi.org/10.3390/bioengineering9020074
Liu LY, Xie GJ, Xing DF, Liu BF, Ding J, Ren NQ (2020) Biological conversion of methane to polyhydroxyalkanoates: current advances, challenges, and perspectives. Environ Sci Ecotechnol 2:100029. https://doi.org/10.1016/j.ese.2020.100029
Naizabekov S, Hyun SW, Na JG, Yoon S, Lee OK, Lee EY (2023) Comparative genomic analysis of Methylocystis sp. MJC1 as a platform strain for polyhydroxybutyrate biosynthesis. Plos One 18:e0284846. https://doi.org/10.1371/journal.pone.0284846
Nguyen DTN, Lee OK, Lim C, Lee J, Na JG, Lee EY (2020) Metabolic engineering of type II methanotroph, Methylosinus trichosporium OB3b, for production of 3-hydroxypropionic acid from methane via a malonyl-CoA reductase-dependent pathway. Metab Eng 59:142–150. https://doi.org/10.1016/j.ymben.2020.02.002
Nguyen DTN, Lee OK, Nguyen TT, Lee EY (2021) Type II methanotrophs: a promising microbial cell-factory platform for bioconversion of methane to chemicals. Biotechnol Adv 47:107700. https://doi.org/10.1016/j.biotechadv.2021.107700
Pieja AJ, Rostkowski KH, Criddle CS (2011) Distribution and selection of poly-3-hydroxybutyrate production capacity in methanotrophic proteobacteria. Microb Ecol 62:564–573. https://doi.org/10.1007/s00248-011-9873-0
Ritchie H, Rosado P, Rose M (2020) Greenhouse gas emissions. Our World in Data. https://ourworldindata.org/greenhouse-gas-emissions. Accessed 26 October 2023
Rodríguez Y, Firmino PIM, Pérez V, Lebrero R, Muñoz R (2020) Biogas valorization via continuous polyhydroxybutyrate production by Methylocystis hirsuta in a bubble column bioreactor. Waste Manag 113:395–403. https://doi.org/10.1016/j.wasman.2020.06.009
Rostkowski KH, Pfluger AR, Criddle CS (2013) Stoichiometry and kinetics of the PHB-producing Type II methanotrophs Methylosinus trichosporium OB3b and Methylocystis parvus OBBP. Bioresour Technol 132:71–77. https://doi.org/10.1016/j.biortech.2012.12.129
Singh R, Ryu J, Kim SW (2022) An overview on methanotrophs and the role of Methylosinus trichosporium OB3b for biotechnological applications. Biotechnol Bioprocess Eng 27:468–481. https://doi.org/10.1007/s12257-022-0046-4
Sobanaa M, Prathiviraj R, Selvin J, Prathaban M (2024) A comprehensive review on methane’s dual role: effects in climate change and potential as a carbon–neutral energy source. Environ Sci Pollut Res 31:10379–10394. https://doi.org/10.1007/s11356-023-30601-w
Wang Y, Yin J, Chen GQ (2014) Polyhydroxyalkanoates, challenges and opportunities. Curr Opin Biotechnol 30:59–65. https://doi.org/10.1016/j.copbio.2014.06.001
Younes S, Awad D, Kassab E, Haack M, Schuler C, Mehlmer N, Brueck T (2021) Systems biology engineering of the pantothenate pathway to enhance 3HB productivity in Escherichia coli. Biotechnol Bioprocess Eng 26:621–629. https://doi.org/10.1007/s12257-021-0033-1
Zhang T, Zhou J, Wang X, Zhang Y (2017) Coupled effects of methane monooxygenase and nitrogen source on growth and poly-β-hydroxybutyrate (PHB) production of Methylosinus trichosporium OB3b. J Environ Sci 52:49–57. https://doi.org/10.1016/j.jes.2016.03.001
Funding
This work was supported by the C1 Gas Refinery Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Science and ICT (MSIT) (NRF-2015M3D3A1A01064926) and SMILE ERC at UNIST through NRF funded by the MSIT (NRF-2020R1A5A1019631).
Author information
Authors and Affiliations
Contributions
Conceptualization: Jeong-Geol Na; Methodology: Hyo Jin Hong, Ji Sung Hyung; Formal analysis and investigation: Hyo Jin Hong, Ji Sung Hyung, Jinwon Lee, Jeong-Geol Na; Writing – original draft preparation: Hyo Jin Hong, Jeong-Geol Na; Writing – review and editing: Jinwon Lee, Jeong-Geol Na; Funding acquisition: Jeong-Geol Na; Resources: Jinwon Lee; Supervision: Jeong-Geol Na.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
All authors have studied the manuscript thoroughly and consented to the publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hong, H.J., Hyung, J.S., Lee, J. et al. Effects of methane to oxygen ratio on cell growth and polyhydroxybutyrate synthesis in high cell density cultivation of Methylocystis sp. MJC1. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33524-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-33524-2