Abstract
Objective and methods
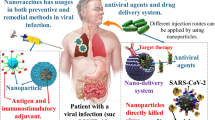
SARS-COV2 (severe acute respiratory syndrome coronavirus 2) has become life-threatening and emerged as a global pandemic in recent years. Although the understanding of coronaviruses has become the focus in almost every research field, efficient early diagnosis and treatment techniques still need improvement. Nanotechnology has presented various solutions to fighting SARS-CoV-2 through infection control measures, detection, therapeutics, and vaccines. More specifically, metallic nanoparticles such as gold, silver, iron, and others made a lot of effort to translate the products for real-time applications to control the pandemic.
Results and conclusion
Owing to their unique characteristics, such as high surface area volume ratio, non-toxic and excellent antiviral properties, metallic nanoparticles are extensively used in developing nanotechnology-based products like disinfection systems, nano-based masks, and other protecting equipment, diagnostic kits, effective therapeutic agents, and vaccines. In this review, we will discuss different metallic nanoparticles, their characteristics, and different approaches of their use to combat SARS-COV2. Thus, this review is highly instructive and helpful in developing strategies based on nanostructures to combat COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
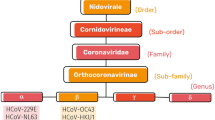
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) threatens global health worldwide. After the World Health Organization reported COVID-19 as a pandemic, the RNA sequence analysis of the infected people and fluid from the bronchoalveolar lavage revealed the presence of an RNA virus [1]. Later after a study of its whole genome sequence, it was reported to belong to the family of SARS (severe acute respiratory syndrome), known as COVID [2]. According to reports, SARS COV is enclosed, known to inhibit-sense RNA with one-strand viruses, and belongs to the group 2b beta coronaviruses. All coronaviruses have a spike (S), membrane (M), envelope (E), viral genome, and nucleocapsid (N) structure. The continuing COVID-19 recurrence has previewed a severe liability to worldwide community health. So far, over sixteen million episodes have been evaluated, and in addition to the humanitarian catastrophe, everywhere in the globe, a financial and social catastrophe has also been brought on by the SARS COV epidemic [3]. An electron micrograph image showed that the viruses contain positive sense viruses made of RNA that range from 60 to 140 nm and have pin-like appendages on their surface that give them the look of crowns (Fig. 1). Four coronaviruses, including HKU1, NL63, 229E, and OC43, have made humans more susceptible [4]. Large droplets released by symptomatic patients' coughing and wheezing are utilized to carry the infection, yet this can also emerge from healthy persons and well before the beginning of symptoms. According to studies, there are not any differences in viral burden between symptomatic and asymptomatic individuals [5]. The reports observed nasal viral loads were greater, while there was no change in viral burden between affected and non-symptomatic people [6]. The virus may survive on structures over days under pleasant environmental circumstances, but standard disinfecting agents, including chlorine bleach, dihydrogen dioxide, etc., could disinfect them [7]. The literature review suggested that SARS-CoV is used to enter the cell through angiotensin-converting enzyme 2 (ACE2) as a receptor, supporting the notion that SARS-CoV originated in bats [8]. Emerging information indicates that SARS-CoV-2 uses the cellular host receptor angiotensin-converting enzyme 2 (ACE2) [9]. The S proteins of the coronavirus fuse to the membrane and bind to host cells via the ACE2 receptor, releasing viral RNA, which is recognized by toll-like receptor TLR 3, TLR 7, TLR 8, and TLR 9 recognition receptors [10]. On the other hand, nanomaterials focus on materials possessing dimensions of less than or equal to one-hundredth of a nanometer [11]. Nanotechnology has played a crucial role in developing the SARS-CoV-2 vaccines [12, 13]. The first vaccines approved by the FDA and EMA were mRNA-based vaccines encoding either the equivalent variation of the S protein (Moderna) or the receptor binding domain of the S protein (Pfizer/BioNTech) [14]. Both Pfizer/BioNTech and Moderna encapsulated mRNAs in lipid nanoparticles for efficient intracellular delivery and to protect them from the extracellular environment. Thus, the use of nanomaterials widely facilitates the fight against COVID-19. In this context, metal and magnetic nanoparticles are among the various nanoparticles used to combat COVID-19. Metallic nanostructures have shown abundant features in the nanotechnology domain, bringing up several new possibilities for research. Metallic nanoparticles are particularly useful for tailoring flexible, functional groups such that they can attach to ligands, antibodies, and medications [4]. Noble metal nanoparticles have a specific position in nanotechnology because of their unique characteristics. The ratio of surface to volume is the most crucial characteristic of nanoparticles since it makes it simple to interact with other particles [15]. So far, several metallic nanoparticles, such as iron oxides, precious metals like gold and silver, nanoshells, and nanocages, have been employed to streamline their application as diagnostic and therapeutic tools [16, 17]. Two distinct approaches are employed for synthesizing metallic nanoparticles: the top–down technique, also known as the dispersion method, and the bottom–up approach, also known as the condensation method [18]. Bottom–up strategies are far more effective and appropriate for producing homogenous nanoparticles that frequently have recognizable dimensions, forms, and organization [19].
Biochemical and physical methodologies have been created for the creation of metallic nanoparticles. Chemical procedures rely on the diminution of metallic ions or the breakdown of starting materials to form atoms, followed by an accumulation of those atoms. Chemically created nanoparticles typically have a restricted size distribution.
The inhibition of metal ions with chemical reductants or the breakdown of metal precursors with additional energy is the mechanism involved in the chemical synthesis of MNPs. Agents stabilizing colloidal dispersion of MNPs, such as sodium dodecyl sulfate (SDS), polyvinyl pyrrolidine (PVP), trisodium citrate, and -cyclodextrin, are crucial for the production of MNPs with a restricted size range [20]. The physical splitting of massive substances creating nanoparticles is a top–down strategy [21]. However, in the bottom–up technique, atoms or molecules are assembled into ordered nanostructures. Self-assembly is a widely used bottom-up technique due to its high efficiency, scalability, and low cost. Researchers have identified a range of technological and physical methods for synthesis, such as chemical reduction [22], microemulsion [23, 24], thermal decomposition [19, 25], sonochemistry [26, 27], the polyol technique [28, 29], and microwave-assisted MNPs, pulsed electrochemical etching [30], lithography [31], sputtering deposition [31], laser ablation [32, 33], vapor deposition [34], and sol–gel [35]. Metallic nanoparticles have shown prominence in imaging technologies due to their improved Rayleigh scattering, surface-enhanced Raman scattering, and strong plasma absorption [36]. Metallic nanoparticles could combat SARS-CoV-2 by blocking the virus's entry into the host cell or blocking viral surface proteins, thereby reducing viral internalization [37, 38] (Figs. 2 and 3). However, some challenges of metallic nanoparticles also exist, such as particle impermanence, where nanomaterials can change because they are located near high-energy local minima and are thermodynamically unstable. Other challenges include quality degradations during storage, corrosion resistance, and maintaining the size and shape of nanostructures. Due to their strong reactivity, nanoparticles also have a significant risk of contamination due to nitrides, oxides, etc. Also, metallic nanoparticles could sometimes be biologically hazardous due to their toxicity and side effects, such as itching and burning [39].
Gold nanoparticles for COVID-19
Biomedical applications have utilized extensive use of metallic nanoparticles. One of the most feasible methods to use nanostructures to detect viruses is localized surface plasmon resonance (LSPR) using metallic nanoparticles, especially gold. The free electrons on the surface of the metallic nanoparticles oscillate at a particular frequency with the oscillation of the electric field of external light, resulting in LSPRs forming near the particle surface [2]. Thus, metallic nanoparticles have broad applications in biomedical areas due to their exciting features, including optical properties, excellent stability, chemical tunability, biomolecule conjugation, and biocompatibility [40]. Owing to their distinctive optical characteristics, gold nanoparticles (AuNPs) have drawn the most interest of all metallic nanoparticles. Much study has been focused on the optical properties of AuNPs in various sizes and shapes [41].
AuNPs are having many valuable properties like their tuneable shape, size, and optoelectronic characters [42] (Fig. 4). Furthermore, they generally have a size-relative peak for assimilation within 500–550 nm [43]. Surface plasmon resonance (SPR) absorption is responsible for a colloidal gold solution's deep, vibrant color and provides AuNPs with distinctive visual properties [44]. The band surface plasmon resonance (SPR) and significant physical properties of AuNPs are influenced by their design, the solvent, ligand, fundamental strength, environmental conditions, and biology [26]. Raman scattering from an individual molecule’s surface was detected using AuNPs [45].
The diagnosis of COVID-19 is a meaningful step to prevent the wide spread of this life-threatening virus, therefore many nanotechnology-based diagnostics ideas emerge to develop the recognition of SARS-COVID-19 [46]. Because of their responsiveness, particularity, and usability in the initial viral genomic detection, the reverse transcription polymerase chain reaction (RT-PCR) assumes the top prime concern among the several SARS-CoV-2 identification techniques currently available [47, 48]. Although PCR is the principal method for diagnosing SARS-CoV-2, several limitations must be considered, such as the time required to prepare the viral RNA, which reduces diagnostic precision and causes false-positive findings. Numerous serological assays, including the chemiluminescence assay, immunofluorescence assay, immunochromatographic test (ICT), and enzyme-linked immunosorbent assay [49], are used to identify SARS-CoV-2 [50, 51] precisely. Emerging evidence suggested using gold nanomaterials in viral detection techniques such as RT-LAMP and ELISA based on reverse transcription loop-mediated isothermal amplification in addition to RT-PCR [52, 53]. The immunochromatographic test (ICT), also known as the lateral flow immunoassay, is another crucial method for the detection of SARS-CoV-2 diagnostic method. This quick, fast, and user-friendly bedside test technique of recognition uses a tiny volume of biological fluids (10–20 L) and does not require a laboratory or qualified professionals.
In addition to the LSPR properties, the colorimetric properties of AuNPs have also been used for responsiveness to the existence/absence of the analyte. In one report, a red color appears due to the interaction of the labeled AuNPs with the analytes [54]. In another report, a bluish color appears due to the change in the wavelength of the light absorbed by the NPs due to the aggregation of the AuNPs [31].
Also, AuNPs have now been usually employed for fast and feasible colorimetric diagnostic testing [55]. In one report, AuNPs are linked to the conjugation pad and tagged with an antigen on the surface that selectively attach with SARS-CoV-2 antibodies (containing IgM and IgG) using lateral flow immunoassay. After that, once the serum travels across the lateral chromatographic flow, antibodies bound to the antigen engage against the AuNPs and are finally released into the sampling sheet. Owing to the capillary action, AuNP conjugates pulled across the chromatographic strip, producing two lines: the M line for anti-SARS-CoV-2 IgM antibodies and the G line for anti-COVID-19 IgG antibodies [56]. Li and colleagues have disclosed another approach that used samples of blood from patients suffering from COVID-19 with PCR validation to show that the ICT-based detection technique can simultaneously detect antibodies of both kinds with a sensitivity of 88.7% and a specificity of 90.6 [2]. The lateral flow assays (LFAs) based on AuNPs showed fast diagnosis at the bedside [57]. Correspondingly numerous colorimetric analyses based on AuNPs have been formulated [58]. In another report, a diagnostic kit was designed based on AuNP modified with antisense oligonucleotides to detect the gene of the SARS-Cov-2 N protein [59]. Parmanik et al. showed the synthesis of an anti-spike known to inhibit tethered for rapid identification of a specific COVID-19 viral antigen using a simple colorimetric alteration monitoring in 5 min [60]. Activating antigen-presenting cells and assuring controlled antigen release may be utilized as an adjuvant to boost the efficacy of vaccinations. As a result, AuNPs with these adjuvant capabilities can be an invaluable resource for developing vaccines [61].
Silver nanoparticles for COVID-19
AgNPs are considered the most alluring inorganic compounds owing to their environmental friendliness [62]. Because of their distinctive physicochemical characteristics, AgNPs are used in many fields, including healthcare, nutrition, consumer products, and industrial purposes. They have been used for a variety of applications because of their conductivity, optical signals, electrical power, and thermal and biological characteristics, such as antibacterial agents, commercial and healthcare-related products, consumer goods, medical device coatings, optical sensors, and cosmetics, pharmaceutical, and food industries, as well as in diagnostics, orthopedics, drug delivery, as anticancer agents, and ultimately to improve the tumor-killing effects of anticoagulation [63, 64]. AgNPs, specially designed to target viruses, may quickly bind to the glycoproteins on the surface of viruses. AgNPs can directly target virus cells by attacking the viral genome [65]. AgNPs exhibit the most effective antibacterial activity because of their much greater surface area, which provides improved interaction with microbes. AgNPs exhibit antibacterial activity by penetrating bacteria through adherence to the cell membrane [66]. AgNPs also showed anti-fibrotic activity by abrogating inflammatory cytokines and altering their transcriptional activity [67]. AgNPs have solid antiviral properties by binding to viral genomes, inhibiting their activity and viral and cellular proteins involved in replication, blocking the production of offspring virions, and inhibiting viral replication [36]. AgNPs-based sensors have been reported to be employed for accurate viral infection diagnosis and detection [56]. By offering quick, easy-to-use solutions that do not require specialized tools or experienced personnel, AgNPs can help to lessen the effect and burden of illness [68]. The size and shape of AgNPs are critical factors for their antiviral activity. Studies have demonstrated that surfaces with Dimensions within the range of 10 nm are significantly more sensitive [69]. The reports suggested that AgNPs come in direct contact with the viral genome protein found on the surface membrane of the COVID-19 virus [16, 49]. For use against enveloped and non-enveloped feline coronavirus (FCoV) both with and without a protective envelope, graphene oxide (GO) sheets and AgNPs (GO-AgNPs) were developed in the case of covid as well as the infectious bursal disease virus (IBDV) [70]. The primary transmission mode for coronavirus-2 is respiratory aerosols; different PPEs can effectively reduce this transfer. However, the virus might last longer on the common PPEs. Therefore, PPEs with AgNPs, due to their antiviral activity, could be a possible way to stop exposure and viral dissemination [71]. AgNPs can be utilized as nano-based safety gear, sanitizers that prevent viruses from spreading, or nanovaccines that enhance immunity and antigen carriers, among other things [72]. In another study, a multifunctional electrospun poly (methyl methacrylate) (PMMA) nanofiber platform decorated with hydrothermally synthesized ZnO nanorods and in situ synthesized Ag NPs (PMMA/ZnO–Ag NFs) were developed for coating of antiviral mats [73]. To understand how AgNPs could interact with SARS-CoV2 and how they affect viruses that cause respiratory illnesses, several in vitro and in vivo experiments were performed. Teengam et al. reported a colorimetric assay to detect the complementary DNA of the virus using Ag NPs and a pyrrolidinyl peptide nucleic acid (acpcPNA) probe [46]. AgNPs with negative charge aggregate when incubated with positively charged acpcPNA. Since the DNA analyte is not complimentary, the color of the assay shifts toward red. In contrast, since the DNA forms a double-stranded structure with the probe molecule, no aggregation occurs; therefore, yellow appears.
Based on comparing the behavior of the virus to that of related viral infections in animal model studies, it may be inferred that using AgNPs as a possible treatment for SARS-CoV2 would work well [74]. According to Jeremiah et al. (2020), the activity of SARS-CoV-2 was effectively inhibited by AgNP with a diameter of 10 nm at values between 1 and 10 ppm. In vitro, SARS-CoV-2-exposed cells were used to test the virucidal effectiveness and stability of 10 distinct AgNPs with various surface treatments and particle diameters [75]. The AgNPs could inhibit SARS-CoV-2 activity, with various morphological modifications and particle sizes providing different virucidal effects, with 50 nm BPEI displaying the most potent antiviral effect. They concluded that each AgNP type’s effectiveness and the accompanying potential difference had a positive correlation (r2 = 0.82) and can be used in the medication for COVID-19 [76]. According to a study done by Horacio et al. (2021), AgNPs have been examined in vitro and have been found to have a suppressive impact on infected cells from cultivated SARS-CoV-2. AgNPs can produce free radicals and reactive oxygen species (ROS), which cause cells to die through apoptosis, preventing viral replication [77]. A study suggested that nanobiocide, which consists only of nanostructured materials, provides more potent disinfection than conventional disinfectants for long-term consistency and lesser side effects [19]. To supply preventive impact against SARS-CoV-2, TPNT1, a composite of metal nanoparticles made of AuNP (1 ppm), Ag-NP (5 ppm), ZnO-NP (60 ppm), and ClO2 (42.5 ppm), is a water-based solution, can restrict virus replication [65].
Iron oxide nanoparticles in COVID-19
Iron is one of the most recent transitional metals discovered in the earth's crust, serving as the cornerstone of existing infrastructure [78]. Iron oxides are regularly used and widely distributed for nanoparticle synthesis because they are cost-effective and essential in various biological and environmental processes. Maghemite (Fe2O3) and magnetite (Fe3O4) nanostructures with different sizes and shapes make up iron oxide nanoparticles widely used in magnetic data storage, biosensing, drug delivery, and other fields [15, 79, 80]. These nanoparticles gained much attention in biomedical engineering and the diagnostics industry due to the advantages of non-toxicity and superparamagnetic characteristics, such as basic separation strategies based on surface area and volume ratio.
Chemical and mechanochemical techniques have created iron oxide nanoparticles, including Coprecipitation, hydrothermal, reverse micelle, template-assisted, and sol–gel synthesis [36]. Switching the precursor iron salts and employing the same synthetic techniques make it feasible to synthesize many types of iron oxide, including nanorods, hollow spheres, nanoclusters, and deformed cubes. These innovative methods are simple to use, affordable, and manage shape sustainably [81]. The synthesis process and the ratio of extract to salt determine the ability to produce metal nanoparticles with different shapes and characteristics [4]. Some primary benefits of using iron oxide nanoparticles as nanostructures for biomedical studies include their biocompatibility at medium dosages and their capability to be manufactured in various sizes and forms with the possibility for biofunctionalization [38]. IONPS has shown antiviral activity against dengue, rotavirus, influenzas, HIV, and SARS-COV [38, 39, 82,83,84]. According to a study done by Marte et al., there is a possibility of treating SARS COV-2, Along with iron oxyhydroxide nanoparticles (IOHNPs) coated with sucrose (Venofer) or iron salt (IONPs) coated with biocompatible compounds such as dimercaptosuccinic acid (DMSA), 3-aminopropyl triethoxysilane (APS), or carboxy dextran (FeraSpinTM R) are also available [28]. Photodynamic therapy (PDT) could serve as one of the other treatment approaches to combat SARS-CoV-2. The primary sources of photosensitizer activation are reactive oxygen species (ROS), which can affect the SARS-CoV-19 genome, protein molecules, and viral capsid [28, 85]. IONPs are biodegradable and have received FDA approval to cure anemia [86]. For their antiviral activity. Yasmine et al. conducted computational docking to determine how IONPs (Fe2O3 and Fe3O4) interact with the spike protein of the SARS-CoV-2 receptor-binding domain (S1-RBD) [87], which is crucial for the virus's entrance to target cell receptors [9, 24]. This interconnection of IONPs with viral protein leads to the inactivation of the virus, which could be considered a treatment strategy for SARS-COV-2. IONPs possess significant antiviral activity and thus can be used for coating any surface or equipment to prohibit the viral contagion ventilators, which could prevent the spread of the disease [13]. IONPs possess an anti-inflammatory response against human endothelial and muscle cells and are non-toxic [88]. Using these IONPs to identify and treat COVID-19—while using our current understanding of the SARS-CoV-2 virus's invasion and replication cycle [89]. As per the conclusion made by Rushell et al., IONPs containing nanomedicines can have a significant influence against viruses as they could prevent sensitive (bio)pharmaceuticals from degrading in transit and enable the tailored of living viruses [90]. Due to the paramagnetic properties of IONPs (γ-Fe2O3/Fe2O3/Fe3O4), they can be utilized to prevent the multiplication of the viral genome [42].
Platinum nanoparticles in COVID-19
Platinum nanoparticles (PtNPs) with specific sizes, nanostructures, and morphologies are widely used in several biotechnological and pharmaceutical sectors, such as photothermal treatment, medical implants, medication delivery, and advanced diagnostic with various agents [42, 91,92,93]. They are also utilized in multiple biological sectors, including they are also involved in forming biofuels [94], vitamins, and fats [95]. They are also used in environmentally friendly technologies such as solar energy [96] and wastewater treatment [33, 94].
PtNPs manufacturing for biomedical applications is primarily influenced by their physicochemical characteristics and their dispersion and durability in a biological context since these aspects are crucial in determining the level of their safety, bioavailability, and pharmacokinetics properties [80]. A series of traditional approaches (physical and chemical routes) have been utilized to create PtNPs, including laser blasting [28], ultraviolet illumination [94], ion inseminate [97], microwave processing [98], thermal breakdown [99], chemical redox reaction, etc. [100].
PtNPs have been significantly employed as an antiviral agent and a carrier for the controlled delivery of antiviral drugs [25, 101, 102]. Fortin et al. reported using PtNPs as a molecular marker for detecting SARS-COV2 via RT-PCR in different samples [81]. In another report, it has been suggested that PtNPs can be used in developing vaccine production for SARS-COV2 and biomarkers for disease prevention and curative purposes [88]. Another research group investigated the use of specific antibodies coated with PtNPs with excellent binding and specificity toward p24. It determined the essential bigger nanoparticle size regimes required for effective proliferation and effectiveness in LFIA to diagnose SARS-COV2virus [103].
Discussion
The COVID-19 pandemic has shown several specific problems that nanostructures have been able to address. Nanostructures generally enable effective pathogen-combating agents such as vaccines, therapeutics, sensors, and other protective devices. However, addressing widespread challenges associated with nanomaterials and nanotechnology is equally essential. With this review, we aimed to summarize recent initiatives involving nanostructure applications, specifically metal nanostructures, against this pandemic. For practical applications, we need to focus our research on the biocompatibility of these products. To provide uniform standards for the fabrication of nanomaterials, their activity and toxicity testing must be evaluated in collaboration with regulatory policies. Long-term research on sustainability and its implications on the environment is necessary. Large-scale production capacity is another obstacle that needs to be addressed for the commercialization of therapeutics, sensors, and equipment based on nanotechnology. We are optimistic that these obstacles can be solved and that nanotechnology-based approaches will lead to significant advancements in diagnosing, treating, and preventing harmful pathogens.
Conclusion
The COVID-19 global pandemic gave unprecedented challenges to the entire society and ecosystem. There is a need for research from diverse fields at a common platform to address such complex challenges. So far, nanostructures provide efficient vaccines, therapeutics, diagnostics, and protective equipment to combat such pathogens. However, there are some bottlenecks that we need to solve for the practical applications of nano-based strategies. As for practical and potential applications, research is still needed to assess their biocompatibility, toxicity, safety profiles, and feasibility for bulk production. Moreover, regulatory agencies should also focus on the standard regulations for fabricating nanomaterials for clinical translation. In addition, the long-term effects of nanotechnology-based strategies on environmental sustainability should also be assessed.
References
Ashok G, Paul JJ, Thusnavis MB, Packiavathy SV, Gautam S (2023) Internet of Things (IoT) based automated sanitizer dispenser and COVID-19 statistics reporter in a post-pandemic world. Health Technol 13(2):327–341
Liu W, Liu L, Kou G, Zheng Y, Ding Y et al (2020) Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J Clin Microbiol 6:e00461-e520
Gautam S (2020) The influence of COVID-19 on air quality in India: a boon or inutile. Bull Environ Contam Toxicol 104(6):724–726
Singh A, Gaud B, Jaybhaye S (2020) Optimization of synthesis parameters of silver nanoparticles and its antimicrobial activity. Mater Sci Energy Technol 3:232–236
Jayakumar, J, Ebanesar A, Gautam S (2023) Predictive analysis, diagnosis of COVID-19 through computational screening and validation with spectro photometrical approach. Toxicol Environ Health Sci 1–9.
Zhao Z, Cui H, Song W, Ru X, Zhou W, et al (2020) A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. Mol Biol
Kampf G, Todt D, Pfaender S, Steinmann E (2020) Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 3:246–251
Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX et al (2003) Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science 5643:276–278
Zou L, Ruan F, Huang M, Liang L, Huang H et al (2020) SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 12:1177–1179
Onofrio L, Caraglia M, Facchini G, Margherita V, Placido SD et al (2020) Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA 8:FSO605
Salata O (2004) No title found. J Nanobiotechnol 1:3
Khurana A, Allawadhi P, Khurana I, Allwadhi S, Weiskirchen R et al (2021) Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 38:101142
Nel AE, Miller JF (2021) Nano-enabled COVID-19 vaccines: meeting the challenges of durable antibody plus cellular immunity and immune escape. ACS Nano 4:5793–5818
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S et al (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 5:403–416
Hayashi Y, Matsuzawa M, Yamaguchi J, Yonehara S, Matsumoto Y et al (2006) Large nonlinear effect observed in the enantiomeric excess of proline in solution and that in the solid state. Angew Chem 28:4709–4713
Mody VV, Nounou MI, Bikram M (2009) Novel nanomedicine-based MRI contrast agents for gynecological malignancies. Adv Drug Deliv Rev 10:795–807
Praetorius N, Mandal T (2007) Engineered nanoparticles in cancer therapy. Recent Pat Drug Deliv Formul 1:37–51
Isaacoff BP, Brown KA (2017) Progress in top-down control of bottom-up assembly. Nano Lett 11:6508–6510
Khoshnevisan K, Maleki H, Baharifar H (2021) Nanobiocide based-silver nanomaterials upon coronaviruses: approaches for preventing viral infections. Nanoscale Res Lett 1:100
Laurent S, Forge D, Port M, Roch A, Robic C et al (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 6:2064–2110
Chandrakala V, Aruna V, Angajala G (2022) Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater 6:1593–1615
Khan JA, Kudgus RA, Szabolcs A, Dutta S, Wang E et al (2011) Designing nanoconjugates to effectively target pancreatic cancer cells in vitro and in vivo. PLoS ONE 6:e20347
Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, Ou-Yang Y-S et al (2010) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 4:1115–1122
Yıldırım ÖA, Durucan C (2010) Synthesis of zinc oxide nanoparticles elaborated by microemulsion method. J Alloys Compd 2:944–949
Calderón L, Harris R, Cordoba-Diaz M, Elorza M, Elorza B et al (2013) Nano and microparticulate chitosan-based systems for antiviral topical delivery. Eur J Pharm Sci 1–2:216–222
Yeh Y-C, Creran B, Rotello VM (2012) Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale 6:1871–1880
Li C, Shuford KL, Park Q-H, Cai W, Li Y et al (2007) High-yield synthesis of single-crystalline gold nano-octahedra. Angew Chem Int Ed 18:3264–3268
Darroudi M, Ahmad MB, Zamiri R, Abdullah AH, Ibrahim NA et al (2011) Preparation and characterization of gelatin mediated silver nanoparticles by laser ablation. J Alloys Compd 4:1301–1304
Li M, Cushing SK, Wu N (2015) Plasmon-enhanced optical sensors: a review. Analyst 2:386–406
Tapasztó L, Dobrik G, Lambin P, Biró LP (2008) Tailoring the atomic structure of graphene nanoribbons by scanning tunnelling microscope lithography. Nat Nanotechnol 7:397–401
Nikbakht H, Gill P, Tabarraei A, Niazi A (2014) Nanomolecular detection of human influenza virus type A using reverse transcription loop-mediated isothermal amplification assisted with rod-Shaped gold nanoparticles. RSC Adv 26:13575
Manawi Y, Ihsanullah SA, Al-Ansari T, Atieh M (2018) A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 5:822
Komarneni S, Katsuki H (2002) Nanophase materials by a novel microwave-hydrothermal process. Pure Appl Chem 9:1537–1543
Sreekanth TVM, Nagajyothi PC, Muthuraman P, Enkhtaivan G, Vattikuti SVP et al (2018) Ultra-sonication-assisted silver nanoparticles using panax ginseng root extract and their anti-cancer and antiviral activities. J Photochem Photobiol B 188:6–11
Nissinen T, Ikonen T, Lama M, Riikonen J, Lehto V-P (2016) Improved production efficiency of mesoporous silicon nanoparticles by pulsed electrochemical etching. Powder Technol 288:360–365
Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E et al (2020) The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials 8:1566
Vahedifard F, Chakravarthy K (2021) Nanomedicine for COVID-19: the role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Mater 1:75–99
Al-Hatamleh MAI, Hatmal MM, Alshaer W, Rahman ENSEA, Mohd-Zahid MH et al (2021) COVID-19 infection and nanomedicine applications for development of vaccines and therapeutics: an overview and future perspectives based on polymersomes. Eur J Pharmacol 896:173930
Gutierrez L, Li X, Wang J, Nangmenyi G, Economy J et al (2009) Adsorption of rotavirus and bacteriophage MS2 using glass fiber coated with hematite nanoparticles. Water Res 20:5198–5208
Koohi SR, Derakhshan MA, Faridani F, Muhammad Nejad S, Amanpour S et al (2018) Plasmonic photothermal therapy of colon cancer cells utilising gold nanoshells: an in vitro study. IET Nanobiotechnol 2:196–200
Kubo R (1962) Electronic properties of metallic fine particles. I J Phys Soc Jpn 6:975–986
Zhou J, Kroll AV, Holay M, Fang RH, Zhang L (2020) Biomimetic nanotechnology toward personalized vaccines. Adv Mater 13:1901255
Jain PK, Lee KS, El-Sayed IH, El-Sayed MA (2006) Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J Phys Chem B 14:7238–7248
Link S, El-Sayed MA (1999) Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phys Chem B 40:8410–8426
Kneipp K, Wang Y, Kneipp H, Perelman LT, Itzkan I et al (1997) Single molecule detection using surface-enhanced raman scattering (SERS). Phys Rev Lett 9:1667–1670
Talebian S, Wallace GG, Schroeder A, Stellacci F, Conde J (2020) Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat Nanotechnol 8:618–621
Clem AL, Sims J, Telang S, Eaton JW, Chesney J (2007) Virus detection and identification using random multiplex (RT)-PCR with 3’-locked random primers. Virol J 1:65
Jung JY, Yoon HK, An S, Lee JW, Ahn E-R et al (2018) Rapid oral bacteria detection based on real-time PCR for the forensic identification of saliva. Sci Rep 1:10852
Xiang J, Yan M, Li H, Liu T, Lin C, et al (2020) Evaluation of enzyme-linked immunoassay and colloidal gold-immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19). Epidemiology
Santiago I (2020) Trends and innovations in biosensors for COVID-19 mass testing. ChemBioChem 20:2880–2889
Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q et al (2020) Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci 5:591–605
Huang X, Zhao Z, Fan J, Tan Y, Zheng N (2011) Amine-assisted synthesis of concave polyhedral platinum nanocrystals having 411 high-index facets. J Am Chem Soc 13:4718–4721
Gao Y, Yan L, Huang Y, Liu F, Zhao Y et al (2020) Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 6492:779–782
Glynou K, Ioannou PC, Christopoulos TK, Syriopoulou V (2003) Oligonucleotide-functionalized gold nanoparticles as probes in a dry-reagent strip biosensor for DNA analysis by hybridization. Anal Chem 16:4155–4160
Ventura BD, Cennamo M, Minopoli A, Campanile R, Censi SB et al (2020) Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sens 10:3043–3048
Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M et al (2020) Diagnosing COVID-19: the disease and tools for detection. ACS Nano 4:3822–3835
Loynachan CN, Thomas MR, Gray ER, Richards DA, Kim J et al (2018) Platinum nanocatalyst amplification: redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 1:279–288
Khan J, Rasmi Y, Kırboğa KK, Ali A, Rudrapal M et al (2022) Development of gold nanoparticle-based biosensors for COVID-19 diagnosis. Beni-Suef Univ J Basic Appl Sci 1:111
Moitra P, Alafeef M, Dighe K, Frieman MB, Pan D (2020) Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 6:7617–7627
Pramanik A, Gao Y, Patibandla S, Mitra D, McCandless MG et al (2021) The rapid diagnosis and effective inhibition of coronavirus using spike antibody attached gold nanoparticles. Nanoscale Adv 6:1588–1596
Dykman LA, Staroverov SA, Fomin AS, Gabalov KP (2021) The potential of gold nanoparticles for coronavirus diagnosis and prophylaxis. In: Tuchin VV, Genina EA, eds. Saratov fall meeting 2020: optical and nanotechnologies for biology and medicine. SPIE, Saratov, Russian Federation, p 40
Barcikowski S, Devesa F, Moldenhauer K (2009) Impact and structure of literature on nanoparticle generation by laser ablation in liquids. J Nanoparticle Res 8:1883–1893
Gurunathan S, Park JH, Han JW, Kim J-H (2015) Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: targeting p53 for anticancer therapy. Int J Nanomed 10:4203
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR et al (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett 10:515–519
Chernousova S, Epple M (2013) Silver as Antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed 6:1636–1653
Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V et al (2011) Silver nanoparticles as potential antiviral agents. Molecules 10:8894–8918
Wong KKY, Cheung SOF, Huang L, Niu J, Tao C et al (2009) Further evidence of the anti-inflammatory effects of silver nanoparticles. ChemMedChem 7:1129–1135
Cohen J (2020) Lab’s scramble to spot hidden coronavirus infections. Science
Marimuthu S, Antonisamy AJ, Malayandi S, Rajendran K, Tsai P-C et al (2020) Silver nanoparticles in dye effluent treatment: a review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J Photochem Photobiol B 205:111823
Chen Y-N, Hsueh Y-H, Hsieh C-T, Tzou D-Y, Chang P-L (2016) Antiviral activity of graphene-silver nanocomposites against non-enveloped and enveloped viruses. Int J Environ Res Public Health 4:430
AbdEllah NH, Gad SF, Muhammad K, E Batiha G, Hetta HF (2020) Nanomedicine as a promising approach for diagnosis, treatment, and prophylaxis against COVID-19. Nanomedicine 21:2085–2102
Eustis S, El-Sayed MA (2006) Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev 3:209–217
Karagoz S, Kiremitler NB, Sarp G, Pekdemir S, Salem S et al (2021) Antibacterial, antiviral, and self-cleaning mats with sensing capabilities based on electrospun nanofibers decorated with ZnO nanorods and Ag nanoparticles for protective clothing applications. ACS Appl Mater Interfaces 4:5678–5690
Pilaquinga F, Morey J, Torres M, Seqqat R, Piña MDLN (2021) Silver nanoparticles as a potential treatment against SARS-COV-2: a review. WIREs Nanomed Nanobiotechnol 13(5):e1707
Jeremiah SS, Miyakawa K, Morita T, Yamaoka Y, Ryo A (2020) Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem Biophys Res Commun 1:195–200
He Q, Lu J, Liu N, Lu W, Li Y et al (2022) Antiviral properties of silver nanoparticles against SARS-CoV-2: effects of surface coating and particle size. Nanomaterials 6:990
Allawadhi P, Singh V, Khurana A, Khurana I, Allwadhi S et al (2021) Silver nanoparticle based multifunctional approach for combating COVID-19. Sens Int 2:100101
Huber D (2005) Synthesis, properties, and applications of iron nanoparticles. Small 5:482–501
Fernández-Remolar DC (2015) Iron oxides, hydroxides and oxy-hydroxides. In: Gargaud M, Irvine WM, Amils R, Cleaves HJ, Pinti DL, et al., (eds.) Encyclopedia of astrobiology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 1268–1270.
Crist RM, Grossman JH, Patri AK, Stern ST, Dobrovolskaia MA et al (2013) Common pitfalls in nanotechnology: lessons learned from NCI’s nanotechnology characterization laboratory. Integr Biol 1:66–73
Fortin J-P, Wilhelm C, Servais J, Ménager C, Bacri J-C et al (2007) Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J Am Chem Soc 9:2628–2635
Murugan K, Wei J, Alsalhi MS, Nicoletti M, Paulpandi M et al (2017) Magnetic nanoparticles are highly toxic to chloroquine-resistant Plasmodium falciparum, dengue virus (DEN-2), and their mosquito vectors. Parasitol Res 2:495–502
Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD (2019) Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv 5:2673–2702
Kumar R, Nayak M, Sahoo GC, Pandey K, Sarkar MC et al (2019) Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J Infect Chemother 5:325–329
Wiehe A, O’Brien JM, Senge MO (2019) Trends and targets in antiviral phototherapy. Photochem Photobiol Sci 11:2565–2612
Maisch T (2007) Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci 2:83–91
Coyne DW (2009) Ferumoxytol for treatment of iron deficiency anemia in patients with chronic kidney disease. Expert Opin Pharmacother 15:2563–2568
Weiss C, Carriere M, Fusco L, Capua I, Regla-Nava JA et al (2020) Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 6:6383–6406
Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A et al (2020) The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 78:185–193
Russell P, Esser L, Hagemeyer CE, Voelcker NH (2023) The potential impact of nanomedicine on COVID-19-induced thrombosis. Nat Nanotechnol 1:11–22
Johnstone TC, Suntharalingam K, Lippard SJ (2016) The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev 5:3436–3486
Wang Z, Chen L, Huang C, Huang Y, Jia N (2017) Albumin-mediated platinum nanocrystals for in vivo enhanced computed tomography imaging. J Mater Chem B 19:3498–3510
Doherty RE, Sazanovich IV, McKenzie LK, Stasheuski AS, Coyle R et al (2016) Photodynamic killing of cancer cells by a Platinum(II) complex with cyclometallating ligand. Sci Rep 1:22668
Liu Y, Chen S, Zhong L, Wu G (2009) Preparation of high-stable silver nanoparticle dispersion by using sodium alginate as a stabilizer under gamma radiation. Radiat Phys Chem 4:251–255
Madsen AT, Ahmed EH, Christensen CH, Fehrmann R, Riisager A (2011) Hydrodeoxygenation of waste fat for diesel production: study on model feed with Pt/alumina catalyst. Fuel 11:3433–3438
Kasem KK (2012) Role of platinum in photoelectrochemical studies related to solar energy harvesting. Platin Met Rev 4:221–228
Popok VN, Stepanov AL, Odzhaev VB (2005) Synthesis of silver nanoparticles by the ion implantation method and investigation of their optical properties. J Appl Spectrosc 2:229–234
Wani IA, Ganguly A, Ahmed J, Ahmad T (2011) Silver nanoparticles: ultrasonic wave assisted synthesis, optical characterization and surface area studies. Mater Lett 3:520–522
Shim I-K, Lee YI, Lee KJ, Joung J (2008) An organometallic route to highly monodispersed silver nanoparticles and their application to ink-jet printing. Mater Chem Phys 2–3:316–321
Dong C, Zhang X, Cai H (2014) Green synthesis of monodisperse silver nanoparticles using hydroxy propyl methyl cellulose. J Alloys Compd 583:267–271
Poon W-L, Alenius H, Ndika J, Fortino V, Kolhinen V et al (2017) Nano-sized zinc oxide and silver, but not titanium dioxide, induce innate and adaptive immunity and antiviral response in differentiated THP-1 cells. Nanotoxicology 7:936–951
Miyako E, Nagata H, Hirano K, Sakamoto K, Makita Y et al (2008) Photoinduced antiviral carbon nanohorns. Nanotechnology 7:075106
Cuevas-Ferrando E, Randazzo W, Pérez-Cataluña A, Falcó I, Navarro D et al (2021) Platinum chloride-based viability RT-qPCR for SARS-CoV-2 detection in complex samples. Sci Rep 1:18120
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Istuti Saraswat, Sarmistha Saha, Anuja Mishra declare that we have no conflict of interest.
Ethical approval
This article does not contain any studies with human subjects or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saraswat, I., Saha, S. & Mishra, A. A review of metallic nanostructures against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Toxicol. Environ. Health Sci. 15, 315–324 (2023). https://doi.org/10.1007/s13530-023-00182-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-023-00182-9