Abstract
Pentachlorophenol (PCP), a persistent organic pollutant, has been banned in many countries, but it is still used in China as a wood preservative, molluscicide, or reagent for fish-pond cleaning, which may pose risks to the ecosystem and humans. However, data on the occurrence of PCP in the environment are scarce in the recent decade. The Yangtze River was regarded as a priority area of PCP pollution according to previous documents. This study aimed to examine the spatial distribution of PCP in the Yangtze River water, the differences in dry and wet seasons, the ecological risk for aquatic organisms, and its removal efficiency in tap water treatment plants. The river water samples (n = 144) were collected from the upper, middle, and lower reaches across ten provinces (or municipalities) in December 2020 and June 2021, respectively. PCP was detected in 88.9% of all the samples, ranging from <MDL to 9.97 ng/L. Spatial distribution differences among the involved provinces were observed, with the highest PCP concentration in the Chongqing section (median: 1.61 ng/L), followed by Hubei (median: 0.23 ng/L), Jiangxi (median: 0.23 ng/L), Shanghai (median: 0.21 ng/L), and other provinces (<0.20 ng/L). The surface water from Qinghai had the lowest concentrations (median: <0.01 ng/L). PCP levels in the Yangtze River water were much lower than those reported in water samples worldwide a decade ago. PCP concentrations in the dry season were higher than in the wet season (p < 0.001). Ecological risk assessment suggested a low risk (RQMax: 0.02) to aquatic organisms posed by PCP in the river. In addition, 100% removal of PCP in tap water treatment plants was observed, while the transformation products of PCP need further studies. The risk of human exposure to PCP through water ingestion only was negligible. However, human exposure risks of PCP in highly contaminated areas still require attention, considering its bioaccumulation and biomagnification in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pentachlorophenol (PCP; 2,3,4,5,6-pentachlorophenol) is a synthetic chemical that has been extensively used as a wood preservative worldwide since its first production in the 1930s (WHO 1987; IARC 2019). Additionally, it could be applied as a herbicide, defoliant, and fungicide in agricultural and domestic fields around the world (FAO 1996). As well, PCP and its sodium salt (Na-PCP) were employed as a molluscicide for controlling schistosomiasis vectors and as a reagent for fish-pond cleaning in aquiculture in China (Hong et al. 2005; Zheng et al. 2012). Hence, PCP has been widely detected in water (Gao et al. 2008), soil (Liu et al. 2006), and air (Zheng et al. 2011, 2012). PCP is classified as a persistent organic pollutant and regulated under Annex A of the Stockholm Convention (Stockholm Convention 2017). It is known for its bioaccumulation in the liver and high toxicity to it (Letcher et al. 2009; USEPA 2010). A large body of literature showed the harmful impact of PCP on the ecosystem, such as honey bees, avian species, and aquatic organisms (WHO 1987; Zha et al. 2006).
PCP was classified as a Group 1 carcinogen, owing to its relationship with non-Hodgkin lymphoma has been well documented (IARC 2019). For general population, exposure to PCP can occur through consumption of contaminated water and food (USEPA 2016a), leading to its occurrence in various human biological samples, such as urine (Honda and Kannan 2018; Schmied-Tobies et al. 2021), hair (Hardy et al. 2021), serum (Carrizo et al. 2008), breast milk, and (maternal and cord blood) plasma (Guvenius et al. 2003). In addition to health risks of occupational PCP exposure, the general population exposed to a relatively high level of PCP were also found to have increased cancer risks (Cheng et al. 2015b; Cui et al. 2017). Moreover, epidemiological evidences suggested that it might increase the risks of gestational diabetes mellitus (Huo et al. 2022), adverse birth outcomes (Guo et al. 2016), and gynecological infertility (Gerhard et al. 1999).
The use of it has been prohibited or restricted in many countries, including China (only permitted for use as a wood preservative) since the 1980s (FAO 1996), resulting in general decline of PCP levels over the past decades worldwide (Zheng et al. 2011). However, with the high prevalence of schistosomiasis around 2000 in traditional epidemic areas of China, PCP was used to eliminate the intermediate host snails (Cui et al. 2017), and therefore relatively higher environmental levels of PCP have been reported in China than in many other countries at that time (Zheng et al. 2012; Cheng et al. 2015a). Currently, the Ministry of Environment Protection of China (MEPC) has included PCP and its salts in the list of priority pollutants (2023 version) (MEPC 2023). The Yangtze River watershed, which holds 40% of the available freshwater resources and serves as source water for over 800 million residents in China (Xing et al. 2020), has been identified as a priority area of PCP pollution previously (Gao et al. 2008). Hence, it is of interest to characterize its contamination level in the Yangtze River again. Additionally, considering the human health risk of PCP via water ingestion, it is essential to know its removal efficiency in drinking water treatment plants, whereas this issue has not been well illustrated in previous studies.
Under this background, the present study was designed to elucidate the spatial distribution of PCP in the Yangtze River water from upstream to downstream and its differences between dry and wet seasons. Then, the potential risk for the aquatic ecosystem in the Yangtze River posed by PCP was assessed. In addition, the fate of PCP during tap water treatment was also characterized to assess human exposure through water ingestion.
Methods
Reagents and chemicals
Standard of PCP and its isotope-labeled standard pentachlorophenol-13C6 (PCP-13C6) were obtained from Toronto Research Chemical Inc. in North York, Ontario, Canada (Table S1). Liquid chromatography-tandem mass spectrometry (LC–MS) grade methanol, water, acetonitrile, and formic acid were purchased from Fisher Chemical, Inc. (Waltham, MA, USA). A Milli-Q IQ7000 (Millipore, Burlington, MA, USA) was used to obtain Milli-Q water.
Sampling campaign
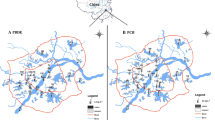
The Yangtze River, the third longest river in the world, is located at a latitude of about 26◦ N to 34◦ N. It is located in the subtropical monsoon climate zone, which is characterized by high temperature and substantial rainfall in summer and reduced rainfall in winter. Hence, the sampling was conducted in December 2020 (dry season) and June 2021 (wet season) to understand the difference between these two seasons. To explore spatial distribution, 72 sampling sites were selected in ten provinces or municipalities (from Qinghai Province to Shanghai municipality) where the mainstream of the Yangtze River flows through (Fig. 1). The sampling sites were selected mainly based on monitoring sites set by the government along the Yangtze River (Table S2). Samples were collected not far from the bank of the Yangtze River. Every composite sample was derived from three samples, which were collected successively at the same sampling site (approximately 1 m below the water surface) and then mixed well. The latitude and longitude of each sampling site are listed in Table S2.
Altogether, 144 surface water samples were collected from these 72 sites (one composite sample from each site, in wet and dry season, respectively). There were 30, 18, and 24 sampling points located in the upstream (from the origin in Qinghai to Yichang, Hubei; Y1–30), midstream (Y31–48), and downstream (from Hukou County, Jiujiang, Jiangxi to the estuary; Y49–72), respectively. Among them, 12 sampling sites (Y5, Y9, Y12, Y15, Y23, Y31, Y35, Y40, Y43, Y57, Y60, and Y70) were located at areas for drinking water sources (labeled in Fig. 1).
To find out the fate of PCP in drinking water treatment, 20 source water and 20 treated water samples (one sample from each water plant in April and August 2021, respectively) were collected from 10 water plants in Wuhan, China. For the source water samples in each month, seven were originated from the Yangtze River's Wuhan section, and three from its largest tributary, the Hanshui River (Fig. S1). Conventional and advanced treatment methods used in the water plants are detailed in Fig. S2.
Before sampling, amber glass bottles (500 mL; pre-cleaned with tap water, methanol, and Milli-Q water) were rinsed with water from the sampling point. Water samples in the water plants were collected after the water flowed for at least three minutes. Then, the water samples were transported to a local laboratory at 4 °C and extracted within 24 h after collection. Milli-Q water was used as blanks.
Pretreatment, instrumental analysis, and quality assurance
Glass fiber filters (GF/F, 0.7 μm, 47 mm diameter; Whatman, Maidstone, UK) were used to remove suspended solids from the Yangtze River water samples and the source water samples from the water plants. After this, 1 ng of the surrogate standard (PCP-13C6, 100 ng/mL) was spiked into each sample. Water samples (500 mL) were loaded onto the Oasis hydrophilic-lipophilic balanced (HLB) solid-phase extraction column (SPE, 500 mg, 6 mL; Waters, Milford, MA, USA), which were pre-treated with 5 mL of acetonitrile and 5 mL of LC-MS grade water. Then, the cartridge was rinsed with 5 mL of LC-MS grade water, and 8 mL of acetonitrile was used to elute PCP. The eluate was collected in a glass tube, dried at 40 °C under a moderate nitrogen stream, and reconstituted with 0.5 mL of 30% acetonitrile in water. The extracts were preserved at –20 °C until analysis. Treated water was extracted directly without filtration or extracted after the free chlorine was quenched by sodium sulfite (Wan et al. 2020).
The extracts were analyzed using an ultra-performance liquid chromatography (ExionLC) coupled with a tandem mass spectrometry system (6500 + , SCIEX, Framingham, MA, USA). An ACQUITY UPLC HSS T3 column (1.8 µm, 2.1 mm × 150 mm, Waters Corporation, Milford, MA, USA) at 40 °C was used for chromatographic separation and 5 μL of the extract was injected. Mobile phase A was 0.5‰ formic acid in water, and phase B was acetonitrile. A gradient elution program (Table S3) was used. The negative multiple reaction monitoring (MRM) parameters are shown in Table S1.
A calibration curve (ranging from 0.01 ng/mL to 10 ng/mL, spiked with 1 ng of surrogate standard for each) was used to quantify PCP. The correlation coefficient (r) was above 0.999. Duplicates (n = 8; variation below 10%), matrix spikes (n = 8; 1 or 10 ng of PCP was spiked), and (procedural and field) blanks (n = 8) were included in every batch of samples (n ≤ 20). Relative recoveries calculated based on matrix-spiked samples ranged from 85.1% to 90.7%. The blank samples contained no detectable PCP. Based on the sample volume and concentration factor, the method detection limit (MDL) for PCP was calculated as 0.01 ng/L at a signal/noise ratio of ≥ 10 (Li et al. 2022).
Ecological risk assessment
The potential ecological risk of PCP detected in the water samples was assessed by the risk quotient (RQ) (Chen et al. 2021). The RQ value was calculated according to the following equation:
where MEC is the measured environmental concentration of PCP in the water samples, and PNEC is the predicted no-effect concentration of PCP. The lowest PNEC value of PCP in freshwater was 0.4 µg/L according to the NORMAN Ecotoxicology Database (NORMAN. 2022). RQ > 1 represents a high risk; 0.1 ≤ RQ ≤ 1 represents a medium risk; RQ < 0.1 represents a low risk (Zhong et al. 2018). General risk (RQMedian) and high risks (RQ95th, RQMax) were calculated with the corresponding concentration of PCP in the water samples.
Statistical analysis
SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) were used for statistical analyses and preparing figures. The 25th, 50th, 75th, and 95th percentiles were utilized to describe the distribution of PCP in the river owing to the skewness of its concentrations (Kolmogorov–Smirnov test). The concentrations below the MDL were set as the MDL divided by √2 for the statistical significance analysis because the detection frequency (DF) of PCP (88.9%) was relatively high (Hornung and Reed 1990). Statistical differences among different subgroups (e.g., watersheds, provinces or municipalities, seasons, and urban–rural regions) were assessed using the linear mixed-effects model (LMM) because of repeated seasonal sampling. LMM was also utilized to examine the association between PCP concentrations and water quality indicators. PCP concentrations were ln-transformed to approximate normal distribution of residuals and homogeneity of variance.
Results and discussion
Occurrence of PCP in the Yangtze River
PCP was detected in 88.9% of the 144 surface water samples from the mainstream of the Yangtze River, and the median concentration was 0.17 ng/L (range: <MDL–9.97 ng/L) (Table 1). Among them, the DF of PCP was 87.5% for the samples corresponding to the drinking water sources (n = 24), with a range of <MDL–3.28 ng/L. Generally, PCP levels of the drinking water sources were similar to that of the other water samples (median: 0.17 ng/L vs. 0.19 ng/L) (Table S4). The maximum concentration (9.97 ng/L) observed in this study was below the Maximum Contaminant Level allowed in drinking water (1 µg/L; while its Maximum Contaminant Level Goal for no known or expected risk to health was zero) recommended by the USEPA (2016b) and well below the provisional guideline value in drinking water (9 µg/L) recommended by the WHO (2003) and China (GB 5749–2022).
The data of this study were compared to the corresponding data for typical surface waters (rivers, lakes, and water supply systems) worldwide, as shown in Table 1. The PCP level in the Yangtze River (mean: 0.81 ng/L) in this study was not much different from those recently reported in other surface waters in China, such as those for the Yinma River, China (mean: 2.27 ng/L) (Zhou et al. 2017), three rivers in Tianjin, China (mean: <3.64 ng/L) (Zhong et al. 2018), and Poyang Lake, China (mean: 0.33 ng/L) (Yang et al. 2019). However, the PCP concentrations in this study (median: 0.17 ng/L) were much lower than the environmental levels in China reported over ten years ago, such as those in the seven major watersheds of China (median: 50 ng/L; sampling year not reported, among which the median value in the Yangtze River was 63 ng/L) reported by Gao et al. (2008), major rivers in ten areas of China (median: 60.7 ng/L, among which the median value in the Yangtze River was 89.7 ng/L) sampled in 2003–2004 reported by Jin et al. (2012). Similarly, PCP concentration in the Dongting Lake in this study was thousands of times lower than those sampled in 1998 (median: 0.73 vs. 3760 ng/L) reported by Zheng et al. (2000). PCP concentrations in source water of three regions (Chizhou, Maanshan, and Tongling) in Anhui, China, were also higher (median: 16.76 ng/L; sampled in 2013–2014) (Cui et al. 2017) than those in this study. This declining trend of the PCP concentrations could be mainly attributed to the restrictions on PCP use in past decades. Currently, there is no public data available on PCP consumption volume. However, it has been reported that PCP has been gradually replaced by other effective snail-killing agents (to kill schistosome hosts) in recent years (Coelho and Caldeira 2016).
The data in other countries (except for Brazil) were mostly reported ten years ago (Table 1) since PCP was banned in many countries (FAO 1996). Therefore, the concentrations in this study (range: <MDL–9.97 ng/L) were much lower than those reported in some of them. After 1996, for example, PCP was reported in the range of 500–17,500 μg/L in the Xochimilco wetland, Mexico City, Mexico (2008–2009), as it was irrigated with semi-treated wastewater (Díaz-Torres et al. 2013; Vazquez-Tapia et al. 2022). PCP was also reported with relatively high concentrations in the Vistula and Pilica river, Poland (sampled in 2008; range: 0.03–640 μg/L) (Michalowicz et al. 2011). Nevertheless, PCP was undetectable in certain surface waters, such as those from Greece (1996–1999; MDL: 0.92 μg/L) (Lekkas et al. 2003) and Brazil (2016–2017; MDL: 2 ng/L) (Maynard et al. 2019), which may be related to their detection limits or prohibition of PCP use. It might be necessary to examine the current residue levels of PCP in surface waters from the abovementioned regions of these countries.
Spatial distribution of PCP in the Yangtze River
Table 2 summarizes descriptive statistics of various subgroups. Water samples from the midstream and downstream had higher DFs of PCP (97.2% and 95.8%, respectively) than those from the upstream (78.3%). The DF of water samples from the urban (city) regions was higher than that of the rural (town or village) regions (94.4% vs. 79.6%). PCP was 100% detected in the water samples from four provinces or municipalities (Chongqing, Hubei, Jiangxi, and Shanghai).
PCP concentration ranges in the upper, middle, and lower reaches of the Yangtze River were <MDL–9.97 ng/L, <MDL–4.33 ng/L, and <MDL–4.02 ng/L, respectively. In the urban and rural regions, PCP concentration ranges were <MDL–9.97 ng/L and <MDL–5.70 ng/L. No statistically spatial differences were observed regarding the watersheds (the upstream, midstream, and downstream) or regions (urban and rural) (Table 2). However, the levels of PCP in the River water varied in different provinces or municipalities (p < 0.0001). Surface waters from the Chongqing section (located in the upper reaches) had the highest level of PCP (median: 1.61 ng/L), followed by Hubei (0.23 ng/L, middle reaches), Jiangxi (0.23 ng/L, middle and lower reaches), Shanghai (0.21 ng/L, lower reaches), and other provinces (less than 0.20 ng/L). No PCP was detected (<MDL) in water samples of Qinghai section.
The maximum concentration (9.97 ng/L) was found in the water of the Fengshou Dam, Chongqing, in December. In addition, the Poyang Lake and Dongting Lake (two of the largest lakes in China), once the critical schistosome epidemic areas, had been reported to be heavily contaminated by PCP (Zheng et al. 2000). In this survey, PCP had mean concentrations of 2.02 ng/L and 0.73 ng/L, respectively, in water samples from the outlets of the Poyang Lake (Jiangxi Province) and Dongting Lake (Hunan Province).
The spatial differences may be related to different purposes of using PCP. Eliminating snails in the traditional schistosomiasis epidemic areas (Hubei, Jiangxi, Poyang Lake, Dongting Lake) may explain why these locations had relatively higher concentrations than other sites (Zheng et al. 2000, 2012; Zhang et al. 2021). However, Chongqing is not an endemic area for schistosomiasis, and the high PCP concentrations here may be related to the following reasons. On one hand, besides wood preservatives, there are several other industries where PCP may be used in Chongqing, such as tanneries, shipbuilding factories, and paper manufacturing industries (Gao et al. 2008; Wang and Sun 2009). On the other hand, the continued use of PCP in aquaculture may also be a contributing factor. Several articles reported that PCP and Na-PCP had been abused as a reagent for fish-pond cleaning (to kill mussels and shellfish) in China, considering their low cost and high efficiency (Hong et al. 2005; Ge et al. 2007; Cheng et al. 2015a), even though such application of PCP had been banned for many years (FAO 1996). Many types of aquatic products were detected with PCP in Chongqing (AMR 2022a, b), implicating that PCP may still be contaminating small ponds and some aquaculture facilities. Aquaculture spreads throughout every district of Chongqing, and the largest aquaculture base in southwest China (Changshou Lake) is located in Chongqing (Chen et al. 2023). Therefore, possible abuse of PCP in aquaculture throughout China merits further attention.
Differences of PCP in dry and wet seasons
During December 2020 (dry season) and June 2021 (wet season), the PCP levels in the Yangtze River water were significantly different (Fig. 2); higher concentrations were observed in December (range: <MDL–9.97; median: 0.33 ng/L) than those in June (range: <MDL–1.86; median: 0.14 ng/L) (p < 0.001) (Table S5). For different watersheds, seasonal differences were found in the upstream (December vs. June: 1.30 vs. 0.11; p < 0.01) and midstream (0.93 vs. 0.13; p < 0.001) of the Yangtze River (Table S5; Fig. 2A). As shown in Fig. 2B, seasonal differences were only found in Chongqing and Hubei sections of the Yangtze River. Such differences were supposed to be mainly related to the seasonality of actual PCP application; as a wood preservative and reagent for fish-pond cleaning, PCP was used more frequently in the fall and winter. Winter has historically been the prime season for logging, and PCP is used to preserve them longer (FAO 1996; Dahlgren et al. 2007). Also, aquatic farmers usually clean fish ponds with PCP during winter (Tang 2008). On the other hand, the relatively low flow rate of the Yangtze River water in December (Table S6) may cause poorer transportation and dilution of PCP. Similarly, in Ge et al. (2007)'s report, average PCP concentration in shrimp (from Huiming Fish Market, Jiangsu, China) was also found to be higher in December 2003 [0.30 μg/kg wet weight (ww)] compared to that in June 2003 (0.25 μg/kg ww). It implied that such differences between dry and wet seasons could be associated with continuous/stable release of PCP from the sediment but less precipitation in dry season.
The concentrations of PCP in the upper, middle, and lower reaches of the Yangtze River, China, in the wet (June) and dry (December) seasons (A); median concentrations of PCP in river sections of ten provinces in two seasons (B). Statistical differences among different subgroups were assessed using the linear mixed-effects model. The band inside the box of (A) shows the median (50th percentile); the bottom and top of the box are the first (25th) and the third (75th) quartiles; the whiskers represent the minimum and the maximum values. ** represents p < 0.001; * represents p < 0.01
Moreover, the association between PCP concentrations and certain water quality indicators (e.g., pH, conductivity, transparency, dissolved oxygen, total nitrogen, ammonia nitrogen, total phosphorus, permanganate index) were explored. The available water quality indicators at the monitoring sites along the Yangtze River are presented in Table S7. The results of the LMM analyses suggested significant associations of water temperature (negative), dissolved oxygen (positive), conductivity (negative), and total phosphorus (positive) with PCP contamination (p < 0.05) (Table S8). The strongest association was observed between total phosphorus and PCP concentrations, which implied that (agricultural, municipal, and industrial) wastewater discharge may most heavily impact the level of PCP in the river (Conley et al. 2009).
Risk assessment for aquatic ecosystem
As shown in Table S9, the highest risks in both wet and dry seasons were found in the Chongqing section of the river (RQMax in the dry season: 0.02, in Fengshou Dam; RQMax in the wet season: 0.005, in Zhutuo Town, Chongqing). In the entire Yangtze River mainstream, the RQs of <0.1 indicated that PCP was unlikely to pose severe hazards to aquatic organisms, which was consistent with the conclusions of Jin et al. (2012) and Xing et al. (2012). Nevertheless, the hazards of PCP should not be ignored as this assessment method is only appropriate for conservative screening-level and preliminary risk assessment (Jin et al. 2012). Plants can bioconcentrate PCP from the environment and pass it to animals and human beings through the food chain (Wang and Sun 2009). Letcher et al. (2009) reported biomagnification of PCP. Thus, PCP may be a potential stress factor to the health of animals, which deserves further studies. Besides, there are a large number of contaminants in the river water (Li et al. 2022; Shi et al. 2023). Co-occurrence of other compounds such as chlorophenol congeners in the river might influence organisms jointly (Zhong et al. 2010); thus, the present assessment might lead to underestimation of the potential risk.
The fate of PCP in water plants and the human exposure risk
In the samples of source water taken from the water plants in Wuhan, the DF of PCP was 85.0%, with a range of <MDL–8.10 ng/L (Table S10). The spiked surrogate standard PCP-13C6 would disappear if the free chlorine was not quenched and PCP was not detected in any treated water samples, implying that it might have been transformed in the water treatment processes of the water plants. Similarly, Stackelberg et al. (2004) reported that PCP was detected in 33% of sampled stream and raw water supplying a U.S. water treatment facility prior to treatment (range: <0.1–0.3 μg/L), and it was not detected in finished water. No data on PCP transformation during chlorination were available yet. In Anotai et al. (2007)'s report, the primary intermediates were found to be tetrachlorophenols and phenol during treatment of PCP-contaminated wastewater by ozonation. Whereas, Hong and Zeng (2002) found that ozonation of PCP could produce intermediates such as tetrachloro-p-benzoquinone (TCBQ; with the lowest PNEC of 190 ng/L) and tetrachloro-p-hydroquinone (TCHQ; with the lowest PNEC of 48 ng/L) (NORMAN. 2022). An animal experiment revealed that TCBQ and TCHQ were able to generate oxidative DNA damage and might contribute to the carcinogenicity of PCP (Dahlhaus et al. 1996). Thus, what transformation products of PCP can be found in drinking water and surface water should be confirmed in future studies and their concentrations need to be measured.
Contamination of PCP in sediment [because it is a weakly polar chemical with a high logKow value of 5.12 (Table S1), which means it tends to be absorbed by the sediment] of the Yangtze River is of concern in future studies. The finding of 100% removal of PCP in the water plants suggested that the risk caused by PCP itself via water consumption is negligible for humans. Whether there exist any hazards posed by the transformation products of PCP should be considered in future studies, which is one of the limitations of this paper. Second, PCP exposure levels in real life should not be neglected because of its bioaccumulation and the fact that the general population may ingest PCP through other pathways, such as ingestion of foodstuffs (Coad and Newhook 1992). Other limitations of this study included that seasonal variations were not well illustrated since only two months were included for analysis, and the sampling size for every section of the river may not be sufficient, which needs to be increased in future studies. Future studies need to care about adverse health effects related to both PCP and its transformation products, and establish an integrated ecological risk assessment model. Also, PCP levels in other environmental media and its exposure levels in people from contaminated areas like Chongqing Municipality and Hubei Province deserve more attention.
Conclusions
In this study, the distribution characteristics of PCP in the Yangtze River water, from upstream to downstream, were described. Spatial variations among various provinces along the river were observed for the first time, with the highest PCP concentrations in Chongqing section, followed by that in Hubei, Jiangxi, and Shanghai sections. The dry season witnessed higher concentrations and detection frequencies of PCP. PCP in the Yangtze River water posed a low threat (RQMax: 0.02) to aquatic organisms. The exposure risk of PCP through tap water ingestion only was negligible for humans. However, ecological risks and human exposure levels in highly contaminated areas may still require attention. In addition, the occurrence of PCP transformation products in tap water and the related health risk needed to be investigated in future studies.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
AMR (2022a) Chongqing Dadukou District Administration for Market Regulation on 55 batches of food safety sampling notice (No. 7 of 2022). Administration for Market Regulation of Dadukou District. Accessed at April 16, 2023. http://scjgj.cq.gov.cn/zz/ddkq/zfxxgkml/spypjglyjczwgkzl/jdjc/jcjg_215168/202206/t20220615_10816523.html
AMR (2022b) Chongqing Liangping District Market Supervision Administration on 68 batches of food safety sampling notice (No. 3 of 2022). Administration for Market Regulation of Liangping District. Accessed at April 16, 2023. http://www.cqlp.gov.cn/scjgj/zwgk_29120/jczwgk/jdjc/jcjg/202204/t20220414_10618221.html
Anotai J, Wuttipong R, Visvanathan C (2007) Oxidation and detoxification of pentachlorophenol in aqueous phase by ozonation. J Environ Manage 85:345–349. https://doi.org/10.1016/j.jenvman.2006.10.001
Bradley PM, Journey CA, Romanok KM, Barber LB, Buxton HT, Foreman WT, Furlong ET, Glassmeyer ST, Hladik ML, Iwanowicz LR, Jones DK, Kolpin DW, Kuivila KM, Loftin KA, Mills MA, Meyer MT, Orlando JL, Reilly TJ, Smalling KL, Villeneuve DL (2017) Expanded Target-Chemical Analysis Reveals Extensive Mixed-Organic-Contaminant Exposure in U.S. Streams. Environ Sci Technol 51:4792–4802. https://doi.org/10.1021/acs.est.7b00012
Carrizo D, Grimalt JO, Ribas-Fito N, Torrent M, Sunyer J (2008) Pentachlorobenzene, hexachlorobenzene, and pentachlorophenol in children’s serum from industrial and rural populations after restricted use. Ecotoxicol Environ Saf 71:260–266. https://doi.org/10.1016/j.ecoenv.2007.08.021
Chen Y, Zhang J, Dong Y, Duan T, Zhou Y, Li W (2021) Phenolic compounds in water, suspended particulate matter and sediment from Weihe River in Northwest China. Water Sci Technol 83:2012–2024. https://doi.org/10.2166/wst.2021.119
Chen AL, Xu FQ, Su X, Zhang FP, Tian WC, Chen SJ, Gou F, Xing ZL, Xiang JX, Li J, Zhao TT (2023) Water microecology is affected by seasons but not sediments: A spatiotemporal dynamics survey of bacterial community composition in Lake Changshou-The largest artificial lake in southwest China. Mar Pollut Bull 186:114459. https://doi.org/10.1016/j.marpolbul.2022.114459
Cheng P, Zhang Q, Shan X, Shen D, Wang B, Tang Z, Jin Y, Zhang C, Huang F (2015b) Cancer risks and long-term community-level exposure to pentachlorophenol in contaminated areas, China. Environ Sci Pollut Res Int 22:1309–1317. https://doi.org/10.1007/s11356-014-3469-4
Cheng C, Xiang H, Dai X, Liu C, Zeng Y, Gong X (2015a) Occurrence, Management, and Source Attribution of Pentachlorophenol Water Environment of China [in Chinese]. Asian J Androl 10, 71–79. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=STDL201506009&DbName=CJFQ201502015
Coad S, Newhook RC (1992) PCP exposure for the Canadian general population: a multimedia analysis. J Expo Anal Environ Epidemiol 2:391–413. https://pubmed.ncbi.nlm.nih.gov/1483028/. Accessed 28 June 2023
Coelho P, Caldeira RL (2016) Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect Dis Poverty 5:57. https://doi.org/10.1186/s40249-016-0153-6
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Ecology Controlling Eutrophication: Nitrogen and Phosphorus. Science 323:1014–1015. https://doi.org/10.1126/science.1167755
Cui Y, Liang L, Zhong Q, He Q, Shan X, Chen K, Huang F (2017) The association of cancer risks with pentachlorophenol exposure: Focusing on community population in the areas along certain section of Yangtze River in China. Environ Pollut 224:729–738. https://doi.org/10.1016/j.envpol.2016.12.011
Dahlgren J, Takhar H, Schecter A, Schmidt R, Horsak R, Paepke O, Warshaw R, Lee A, Anderson-Mahoney P (2007) Residential and biological exposure assessment of chemicals from a wood treatment plant. Chemosphere 67:S279-285. https://doi.org/10.1016/j.chemosphere.2006.05.109
Dahlhaus M, Almstadt E, Henschke P, Lüttgert S, Appel KE (1996) Oxidative DNA lesions in V79 cells mediated by pentachlorophenol metabolites. Arch Toxicol 70:457–460. https://doi.org/10.1007/s002040050299
Díaz-Torres E, Gibson R, González-Farías F, Zarco-Arista AE, Mazari-Hiriart M (2013) Endocrine Disruptors in the Xochimilco Wetland, Mexico City. Water Air Soil Pollut 224:1586. https://doi.org/10.1007/s11270-013-1586-1
FAO (1996) Decision guidance documents: Pentachlorophenol and its salts and esters. Food and Agriculture Organization of the United Nations. Accessed at June 27, 2022. http://www.pic.int/Portals/5/DGDs/DGD_Pentachlorophenol_EN.pdf
Gao J, Liu L, Liu X, Zhou H, Huang S, Wang Z (2008) Levels and spatial distribution of chlorophenols - 2,4-dichlorophenol, 2,4,6-trichlorophenol, and pentachlorophenol in surface water of China. Chemosphere 71:1181–1187. https://doi.org/10.1016/j.chemosphere.2007.10.018
GB 5749–2022. China's national sanitary standard for drinking water (GB 5749–2022). People's Republic of China. Accessed at January 12, 2024. https://dghb.dg.gov.cn/attachment/0/168/168129/3983117.pdf
Ge J, Pan J, Fei Z, Wu G, Giesy JP (2007) Concentrations of pentachlorophenol (PCP) in fish and shrimp in Jiangsu Province, China. Chemosphere 69:164–169. https://doi.org/10.1016/j.chemosphere.2007.04.025
Gerhard I, Frick A, Monga B, Runnebaum B (1999) Pentachlorophenol exposure in women with gynecological and endocrine dysfunction. Environ Res 80:383–388. https://doi.org/10.1006/enrs.1998.3934
Guo J, Wu C, Lv S, Lu D, Feng C, Qi X, Liang W, Chang X, Xu H, Wang G, Zhou Z (2016) Associations of prenatal exposure to five chlorophenols with adverse birth outcomes. Environ Pollut 214:478–484. https://doi.org/10.1016/j.envpol.2016.04.074
Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K (2003) Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect 111:1235–1241. https://doi.org/10.1289/ehp.5946
Hardy EM, Dereumeaux C, Guldner L, Briand O, Vandentorren S, Oleko A, Zaros C, Appenzeller BMR (2021) Hair versus urine for the biomonitoring of pesticide exposure: Results from a pilot cohort study on pregnant women. Environ Int 152:106481. https://doi.org/10.1016/j.envint.2021.106481
Honda M, Kannan K (2018) Biomonitoring of chlorophenols in human urine from several Asian countries, Greece and the United States. Environ Pollut 232:487–493. https://doi.org/10.1016/j.envpol.2017.09.073
Hong PK, Zeng Y (2002) Degradation of pentachlorophenol by ozonation and biodegradability of intermediates. Water Res 36:4243–4254. https://doi.org/10.1016/s0043-1354(02)00144-6
Hong HC, Zhou HY, Luan TG, Lan CY (2005) Residue of pentachlorophenol in freshwater sediments and human breast milk collected from the Pearl River Delta, China. Environ Int 31:643–649. https://doi.org/10.1016/j.envint.2004.11.002
Hornung R, Reed L (1990) Estimation of Average Concentration in the Presence of Nondetectable Values. Appl Occup Environ Hyg 5:46–51. https://doi.org/10.1080/1047322X.1990.10389587
Huo Y, Wan Y, Huang Q, Wang A, Mahai G, He Z, Xu S, Xia W (2022) Pentachlorophenol exposure in early pregnancy and gestational diabetes mellitus: A nested case-control study. Sci Total Environ 831:154889. https://doi.org/10.1016/j.scitotenv.2022.154889
IARC (2019) Pentachlorophenol and some related compounds. International Agency for Research on Cancer. Accessed at June 27, 2022. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Pentachlorophenol-And-Some-Related-Compounds-2019
Jin X, Gao J, Zha J, Xu Y, Wang Z, Giesy JP, Richardson KL (2012) A tiered ecological risk assessment of three chlorophenols in Chinese surface waters. Environ Sci Pollut Res Int 19:1544–1554. https://doi.org/10.1007/s11356-011-0660-8
Lekkas T, Kostopoulou M, Petsas A, Vagi M, Golfinopoulos S, Stasinakis A, Thomaidis N, Pavlogeorgatos G, Kotrikla A, Gatidou G, Xylourgidis N, Kolokythas G, Makri C, Babos D, Lekkas DF, Nikolaou A (2003) Monitoring priority substances of directives 76/464/EEC and 2000/60/EC in Greek water bodies. J Environ Monit 5:593–597. https://doi.org/10.1039/b304809h
Letcher RJ, Gebbink WA, Sonne C, Born EW, McKinney MA, Dietz R (2009) Bioaccumulation and biotransformation of brominated and chlorinated contaminants and their metabolites in ringed seals (Pusa hispida) and polar bears (Ursus maritimus) from East Greenland. Environ Int 35:1118–1124. https://doi.org/10.1016/j.envint.2009.07.006
Li S, Wan Y, Wang Y, He Z, Xu S, Xia W (2022) Occurrence, spatial variation, seasonal difference, and ecological risk assessment of organophosphate esters in the Yangtze River, China: From the upper to lower reaches. Sci Total Environ 851:158021. https://doi.org/10.1016/j.scitotenv.2022.158021
Liu Y, Wen B, Shan XQ (2006) Determination of pentachlorophenol in wastewater irrigated soils and incubated earthworms. Talanta 69:1254–1259. https://doi.org/10.1016/j.talanta.2005.12.051
Maynard IFN, Cavalcanti EB, da Silva LL, Martins EAJ, Pires MAF, de Barros ML, Cardoso E, Marques MN (2019) Assessing the presence of endocrine disruptors and markers of anthropogenic activity in a water supply system in northeastern Brazil. J Environ Sci Health A Tox Hazard Subst Environ Eng 54:891–898. https://doi.org/10.1080/10934529.2019.1606574
MEPC (2023) List of key control new pollutants (2023 version). Ministry of Environment Protection of the People’s Republic of China. Accessed at Jan. 15, 2023. http://www.gov.cn/zhengce/2022-12/30/content_5734728.htm
Michalowicz J, Stufka-Olczyk J, Milczarek A, Michniewicz M (2011) Analysis of annual fluctuations in the content of phenol, chlorophenols and their derivatives in chlorinated drinking waters. Environ Sci Pollut Res Int 18:1174–1183. https://doi.org/10.1007/s11356-011-0469-5
Muir J, Eduljee G (1999) PCP in the freshwater and marine environment of the European Union. Sci Total Environ 236:41–56. https://doi.org/10.1016/s0048-9697(99)00281-8
NORMAN (2022) NORMAN Ecotoxicology Database — Lowest PNECs. Accessed at April 12, 2023. https://www.norman-network.com/nds/ecotox/lowestPnecsIndex.php
Schmied-Tobies MIH, Murawski A, Schmidt L, Rucic E, Schwedler G, Apel P, Goen T, Kolossa-Gehring M (2021) Pentachlorophenol and nine other chlorophenols in urine of children and adolescents in Germany - Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Environ Res 196:110958. https://doi.org/10.1016/j.envres.2021.110958
Shi Y, Wan Y, Wang Y, Li Y, Xu S, Xia W (2023) Fipronil and its transformation products in the Yangtze River: Assessment for ecological risk and human exposure. Chemosphere 320:138092. https://doi.org/10.1016/j.chemosphere.2023.138092
Stackelberg PE, Furlong ET, Meyer MT, Zaugg SD, Henderson AK, Reissman DB (2004) Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Sci Total Environ 329:99–113. https://doi.org/10.1016/j.scitotenv.2004.03.015
Stockholm Convention, D. (2017) The Listing of POPs in the Stockholm Convention. Accessed at June 27, 2022. http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx
Tang F (2008) Fish pond cleaning techniques in winter [in Chinese]. Mod Agric Sci Techno, 291. https://kns.cnki.net/kcms2/article/abstract?v=Sea5Lprb1OpaGlXQKVyDXUrx5X0gcfy9LuTtVKhDiPsges4Y6nGsCvzL4cHfnDSkgYxiEojZocBBh3U8wLJ9qvcbfVkTKwpF09hMGE9ASyQo2iEcH4HswCjS1hRk4gm&uniplatform=NZKPT&language=CHS. Accessed 15 April 2023
USEPA (2010) Toxicological Review of Pentochlorophenal (CASRN 87–86–5). U.S Environmental Protection Agency. Accessed at April 16, 2023. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100SLDU.txt
USEPA (2016a) Pentachlorophenol. U.S Environmental Protection Agency. Accessed at June 27, 2022. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100ZBEU.txt
USEPA (2016b) Table of regulated drinking water contaminants. National primary drinking water regulations. Ground water and drinking water. Washington (DC), USA: United States Environmental Protection Agency. Accessed at January 12, 2024. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations
Vazquez-Tapia I, Salazar-Martinez T, Acosta-Castro M, Melendez-Castolo KA, Mahlknecht J, Cervantes-Aviles P, Capparelli MV, Mora A (2022) Occurrence of emerging organic contaminants and endocrine disruptors in different water compartments in Mexico - A review. Chemosphere 308:136285. https://doi.org/10.1016/j.chemosphere.2022.136285
Wan Y, Han Q, Wang Y, He Z (2020) Five degradates of imidacloprid in source water, treated water, and tap water in Wuhan, central China. Sci Total Environ 741:140227. https://doi.org/10.1016/j.scitotenv.2020.140227
Wang X, Sun L (2009) Pentachlorophenol Pollution: Status Quo and Studies on Its Degradation and Fate [in Chinese]. Environ Sci Technol 32:8. https://kns.cnki.net/kcms2/article/abstract?v=T-ziT3f7Rg-Hk7QVgHCNhONwq1qI-Y692V0Ymu2DOgNkyBETYhLZlWtQlCv2NJW6BMjZk46mdZk31GOD0iRJtiAsXxOR-VGHeCzXujIV47J2vjWoh11LvFRUK0d3xo03&uniplatform=NZKPT&language=CHS. Accessed 28 June 2023
WHO (1987) Pentachlorophenol. World Health Organization. Accessed at June 26, 2022. https://apps.who.int/iris/handle/10665/38414
WHO (2003) Pentachlorophenol in drinking-water: background document for development of WHO Guidelines for Drinking-water Quality. World Health Organization. Accessed at January 12, 2024. https://www.who.int/docs/default-source/wash-documents/wash-chemicals/pentachlorophenol-background-document.pdf?sfvrsn=3c173c85_4
WQP (2021) Pentachlorophenol. Water quality portal. Water Quality Data. United States Geological Survey (USGS). https://www.waterqualitydata.us/portal/. Accessed 4 Feb 2024
Xing L, Liu H, Giesy JP, Zhang X, Yu H (2012) Probabilistic ecological risk assessment for three chlorophenols in surface waters of China. J Environ Sci (china) 24:329–334. https://doi.org/10.1016/s1001-0742(11)60779-1
Xing L, Tao M, Zhang Q, Kong M, Sun J, Jia S, Liu CH (2020) Occurrence, spatial distribution and risk assessment of organophosphate esters in surface water from the lower Yangtze River Basin. Sci Total Environ 734:139380. https://doi.org/10.1016/j.scitotenv.2020.139380
Yang P, Chen X, Zhang X, Huang L, Zeng X (2019) Pollution characteristics of pentachlorophenol in multi-environment medium of Poyang Lake wetland during low water periods [in Chinese]. Environ Pollut Control 41:261–265. https://doi.org/10.15985/j.cnki.1001-3865.2019.03.002
Zha J, Wang Z, Schlenk D (2006) Effects of pentachlorophenol on the reproduction of Japanese medaka (Oryzias latipes). Chem Biol Interact 161:26–36. https://doi.org/10.1016/j.cbi.2006.02.010
Zhang L, Xu Z, Yang F, Dang H, Li Y, Lu S, Cao C, Xu J, Li S, Zhou X (2021) Endemic status of schistosomiasis in People’s Republic of China in 2020 [in Chinese]. Chin J Schisto Contral 33:225–233. https://doi.org/10.16250/j.32.1374.2021109
Zheng MH, Zhang B, Bao ZC, Yang H, Xu XB (2000) Analysis of pentachlorophenol from water, sediments, and fish bile of Dongting lake in China. Bull Environ Contam Toxicol 64:16–19. https://doi.org/10.1007/s001289910003
Zheng W, Wang X, Yu H, Tao X, Zhou Y, Qu W (2011) Global trends and diversity in pentachlorophenol levels in the environment and in humans: a meta-analysis. Environ Sci Technol 45:4668–4675. https://doi.org/10.1021/es1043563
Zheng W, Yu H, Wang X, Qu W (2012) Systematic review of pentachlorophenol occurrence in the environment and in humans in China: not a negligible health risk due to the re-emergence of schistosomiasis. Environ Int 42:105–116. https://doi.org/10.1016/j.envint.2011.04.014
Zhong W, Wang D, Xu X, Luo Q, Wang B, Shan X, Wang Z (2010) Screening level ecological risk assessment for phenols in surface water of the Taihu Lake. Chemosphere 80:998–1005. https://doi.org/10.1016/j.chemosphere.2010.05.036
Zhong W, Wang D, Wang Z (2018) Distribution and potential ecological risk of 50 phenolic compounds in three rivers in Tianjin, China. Environ Pollut 235:121–128. https://doi.org/10.1016/j.envpol.2017.12.037
Zhou M, Zhang J, Sun C (2017) Occurrence, Ecological and Human Health Risks, and Seasonal Variations of Phenolic Compounds in Surface Water and Sediment of a Potential Polluted River Basin in China. Int J Environ Res Public Health 14. https://doi.org/10.3390/ijerph14101140
Acknowledgements
We thank all the participants and co-workers for their assistance in sampling. Yanjian Wan is grateful to Dr. Kurunthachalam Kannan for mentoring. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the department.
Funding
This study was funded by the Natural Science Foundation of Hubei, China (grant number 2022CFB485), the Health Commission of Hubei Province Scientific Research Project (grant numbers WJ2019H307 and WJ2019H303), and the National Natural Science Foundation of China (grant numbers 42277428 and 21407117).
Author information
Authors and Affiliations
Contributions
Fengting Yang (Conceptualization, Investigation, Writing – Original Draft)
Yanjian Wan (Conceptualization, Investigation, Writing – Review & Editing)
Yan Wang (Conceptualization, Investigation)
Shulan Li (Conceptualization, Investigation)
Shunqing Xu (Resources, Writing – Review & Editing)
Wei Xia (Conceptualization, Investigation, Writing – Review & Editing)
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent to publish
All authors have read and approved this manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Hongwen Sun
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fengting Yang and Yanjian Wan are co-first author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, F., Wan, Y., Wang, Y. et al. Occurrence of pentachlorophenol in surface water from the upper to lower reaches of the Yangtze River and treated water in Wuhan, China. Environ Sci Pollut Res 31, 25589–25599 (2024). https://doi.org/10.1007/s11356-024-32821-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32821-0